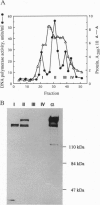
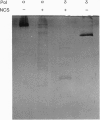
Abstract
We have purified a DNA polymerase activity from 0- to 2-hr embryos of Drosophila melanogaster to near homogeneity. The purified enzyme consists of a single 120-kDa polypeptide, which contains polymerase and 3'-->5' exonuclease activities. Exonuclease activity is inhibited by deoxynucleoside triphosphates, suggesting that the polymerase and exonuclease activities are coupled. The polymerase is more active with poly(dA-dT) than with activated DNA or poly(dA)/oligo(dT) as template. It shows a low degree of processivity with poly(dA)/oligo(dT). The polymerase is sensitive to aphidicolin and carbonyldiphosphonate but resistant to N2-[p-(n-butyl)phenyl]-2-deoxyguanosine triphosphate, 2-[p-(n-butyl)anilino]-2-deoxyadenosine triphosphate, and dideoxythymidine triphosphate. The 120-kDa polypeptide can be distinguished from the large subunit of Drosophila DNA polymerase alpha on the basis of the peptides generated by partial cleavage with N-chlorosuccinimide and by its failure to react with a monoclonal antibody directed against the large subunit of DNA polymerase alpha. The DNA polymerase is inhibited by 200 mM NaCl and is unable to use poly(rA)/oligo(dT) as a template, thus differentiating it from DNA polymerase gamma. On the basis of these properties, we propose that the DNA polymerase that we have purified from 0- to 2-hr Drosophila melanogaster embryos is DNA polymerase delta.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bambara R. A., Jessee C. B. Properties of DNA polymerases delta and epsilon, and their roles in eukaryotic DNA replication. Biochim Biophys Acta. 1991 Jan 17;1088(1):11–24. doi: 10.1016/0167-4781(91)90147-e. [DOI] [PubMed] [Google Scholar]
- Bauer G. A., Burgers P. M. The yeast analog of mammalian cyclin/proliferating-cell nuclear antigen interacts with mammalian DNA polymerase delta. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7506–7510. doi: 10.1073/pnas.85.20.7506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bialek G., Nasheuer H. P., Goetz H., Grosse F. Exonucleolytic proofreading increases the accuracy of DNA synthesis by human lymphocyte DNA polymerase alpha-DNA primase. EMBO J. 1989 Jun;8(6):1833–1839. doi: 10.1002/j.1460-2075.1989.tb03578.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenthal A. B., Kriegstein H. J., Hogness D. S. The units of DNA replication in Drosophila melanogaster chromosomes. Cold Spring Harb Symp Quant Biol. 1974;38:205–223. doi: 10.1101/sqb.1974.038.01.024. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bravo R., Frank R., Blundell P. A., Macdonald-Bravo H. Cyclin/PCNA is the auxiliary protein of DNA polymerase-delta. Nature. 1987 Apr 2;326(6112):515–517. doi: 10.1038/326515a0. [DOI] [PubMed] [Google Scholar]
- Brooke R. G., Singhal R., Hinkle D. C., Dumas L. B. Purification and characterization of the 180- and 86-kilodalton subunits of the Saccharomyces cerevisiae DNA primase-DNA polymerase protein complex. The 180-kilodalton subunit has both DNA polymerase and 3'----5'-exonuclease activities. J Biol Chem. 1991 Feb 15;266(5):3005–3015. [PubMed] [Google Scholar]
- Burgers P. M. Eukaryotic DNA polymerases alpha and delta: conserved properties and interactions, from yeast to mammalian cells. Prog Nucleic Acid Res Mol Biol. 1989;37:235–280. doi: 10.1016/s0079-6603(08)60700-x. [DOI] [PubMed] [Google Scholar]
- Burgers P. M. Saccharomyces cerevisiae replication factor C. II. Formation and activity of complexes with the proliferating cell nuclear antigen and with DNA polymerases delta and epsilon. J Biol Chem. 1991 Nov 25;266(33):22698–22706. [PubMed] [Google Scholar]
- Cotterill S. M., Reyland M. E., Loeb L. A., Lehman I. R. A cryptic proofreading 3'----5' exonuclease associated with the polymerase subunit of the DNA polymerase-primase from Drosophila melanogaster. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5635–5639. doi: 10.1073/pnas.84.16.5635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fansler B. S., Loeb L. A. Sea urchin nuclear DNA polymerase. Methods Enzymol. 1974;29:53–70. doi: 10.1016/0076-6879(74)29009-8. [DOI] [PubMed] [Google Scholar]
- Goulian M., Herrmann S. M., Sackett J. W., Grimm S. L. Two forms of DNA polymerase delta from mouse cells. Purification and properties. J Biol Chem. 1990 Sep 25;265(27):16402–16411. [PubMed] [Google Scholar]
- Kaguni L. S., Lehman I. R. Eukaryotic DNA polymerase-primase: structure, mechanism and function. Biochim Biophys Acta. 1988 Jul 13;950(2):87–101. doi: 10.1016/0167-4781(88)90001-2. [DOI] [PubMed] [Google Scholar]
- Kaguni L. S., Rossignol J. M., Conaway R. C., Lehman I. R. Isolation of an intact DNA polymerase-primase from embryos of Drosophila melanogaster. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2221–2225. doi: 10.1073/pnas.80.8.2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee S. H., Pan Z. Q., Kwong A. D., Burgers P. M., Hurwitz J. Synthesis of DNA by DNA polymerase epsilon in vitro. J Biol Chem. 1991 Nov 25;266(33):22707–22717. [PubMed] [Google Scholar]
- Lischwe M. A., Ochs D. A new method for partial peptide mapping using N-chlorosuccinimide/urea and peptide silver staining in sodium dodecyl sulfate-polyacrylamide gels. Anal Biochem. 1982 Dec;127(2):453–457. doi: 10.1016/0003-2697(82)90203-2. [DOI] [PubMed] [Google Scholar]
- Mitsis P. G., Kowalczykowski S. C., Lehman I. R. A single-stranded DNA binding protein from Drosophila melanogaster: characterization of the heterotrimeric protein and its interaction with single-stranded DNA. Biochemistry. 1993 May 18;32(19):5257–5266. doi: 10.1021/bi00070a038. [DOI] [PubMed] [Google Scholar]
- Morrison A., Araki H., Clark A. B., Hamatake R. K., Sugino A. A third essential DNA polymerase in S. cerevisiae. Cell. 1990 Sep 21;62(6):1143–1151. doi: 10.1016/0092-8674(90)90391-q. [DOI] [PubMed] [Google Scholar]
- Ng L., Prelich G., Anderson C. W., Stillman B., Fisher P. A. Drosophila proliferating cell nuclear antigen. Structural and functional homology with its mammalian counterpart. J Biol Chem. 1990 Jul 15;265(20):11948–11954. [PubMed] [Google Scholar]
- O'Donnell M. E., Elias P., Lehman I. R. Processive replication of single-stranded DNA templates by the herpes simplex virus-induced DNA polymerase. J Biol Chem. 1987 Mar 25;262(9):4252–4259. [PubMed] [Google Scholar]
- Olson M. W., Kaguni L. S. 3'-->5' exonuclease in Drosophila mitochondrial DNA polymerase. Substrate specificity and functional coordination of nucleotide polymerization and mispair hydrolysis. J Biol Chem. 1992 Nov 15;267(32):23136–23142. [PubMed] [Google Scholar]
- Peck V. M., Gerner E. W., Cress A. E. Delta-type DNA polymerase characterized from Drosophila melanogaster embryos. Nucleic Acids Res. 1992 Nov 11;20(21):5779–5784. doi: 10.1093/nar/20.21.5779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prelich G., Tan C. K., Kostura M., Mathews M. B., So A. G., Downey K. M., Stillman B. Functional identity of proliferating cell nuclear antigen and a DNA polymerase-delta auxiliary protein. Nature. 1987 Apr 2;326(6112):517–520. doi: 10.1038/326517a0. [DOI] [PubMed] [Google Scholar]
- Read S. M., Northcote D. H. Minimization of variation in the response to different proteins of the Coomassie blue G dye-binding assay for protein. Anal Biochem. 1981 Sep 1;116(1):53–64. doi: 10.1016/0003-2697(81)90321-3. [DOI] [PubMed] [Google Scholar]
- Shivji K. K., Kenny M. K., Wood R. D. Proliferating cell nuclear antigen is required for DNA excision repair. Cell. 1992 Apr 17;69(2):367–374. doi: 10.1016/0092-8674(92)90416-a. [DOI] [PubMed] [Google Scholar]
- So A. G., Downey K. M. Eukaryotic DNA replication. Crit Rev Biochem Mol Biol. 1992;27(1-2):129–155. doi: 10.3109/10409239209082561. [DOI] [PubMed] [Google Scholar]
- Syväoja J., Suomensaari S., Nishida C., Goldsmith J. S., Chui G. S., Jain S., Linn S. DNA polymerases alpha, delta, and epsilon: three distinct enzymes from HeLa cells. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6664–6668. doi: 10.1073/pnas.87.17.6664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan C. K., Castillo C., So A. G., Downey K. M. An auxiliary protein for DNA polymerase-delta from fetal calf thymus. J Biol Chem. 1986 Sep 15;261(26):12310–12316. [PubMed] [Google Scholar]
- Wang T. S. Eukaryotic DNA polymerases. Annu Rev Biochem. 1991;60:513–552. doi: 10.1146/annurev.bi.60.070191.002501. [DOI] [PubMed] [Google Scholar]
- Wang Z., Wu X., Friedberg E. C. DNA repair synthesis during base excision repair in vitro is catalyzed by DNA polymerase epsilon and is influenced by DNA polymerases alpha and delta in Saccharomyces cerevisiae. Mol Cell Biol. 1993 Feb;13(2):1051–1058. doi: 10.1128/mcb.13.2.1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiser T., Gassmann M., Thömmes P., Ferrari E., Hafkemeyer P., Hübscher U. Biochemical and functional comparison of DNA polymerases alpha, delta, and epsilon from calf thymus. J Biol Chem. 1991 Jun 5;266(16):10420–10428. [PubMed] [Google Scholar]
- Wilson S., Abbotts J., Widen S. Progress toward molecular biology of DNA polymerase beta. Biochim Biophys Acta. 1988 Feb 28;949(2):149–157. doi: 10.1016/0167-4781(88)90078-4. [DOI] [PubMed] [Google Scholar]