Abstract
Object
Mild traumatic brain injury (TBI) has been proposed as a risk factor for development of Alzheimer’s disease, Parkinson’s disease, depression, and other illnesses. This study’s objective was to determine the association of prior mild TBI with subsequent diagnosis (i.e., at least one year post-injury) of neurologic or psychiatric disease.
Methods
All studies from 1995–2012 reporting TBI as a risk factor for diagnoses of interest were identified by searching PubMed, study references, and review articles. Reviewers abstracted the data and assessed study design and characteristics.
Results
57 studies met inclusion criteria. A random effects meta-analysis revealed a significant association of prior TBI with subsequent neurologic and psychiatric diagnosis. The pooled odds ratio (OR) for TBI on development of any illness was 1.67 (95% CI 1.44–1.93, p<.001). Prior TBI was independently associated with both neurologic [OR 1.55 (95% CI 1.31–1.83, p<.001)] and psychiatric [OR 2.00 (95% CI 1.50–2.66, p<.001)] outcomes. Analyses of individual diagnoses found higher odds of Alzheimer’s disease, Parkinson’s disease, mild cognitive impairment, depression, mixed affective disorders, and bipolar disorder in individuals with previous TBI compared to those without TBI. This association was present when examining only studies of mild TBI and when considering the influence of study design and characteristics. Analysis of a subset of studies found no evidence that multiple TBIs were associated with higher odds of disease than a single TBI.
Conclusions
History of TBI, including mild TBI, is associated with the development of neurologic and psychiatric illness. This indicates that either TBI is a risk factor for heterogeneous pathologic processes or that TBI may contribute to a common pathologic mechanism.
Keywords: dementia, psychiatry, head injury, meta-analysis
INTRODUCTION
Since the 1928 description of the “punch drunk” condition,48 there has been speculation about a connection between traumatic brain injury (TBI) and late-life neurologic or psychiatric illness. Though this syndrome was later referred to as “dementia pugilistica” because it was thought to uniquely affect boxers,14 an accumulation of cases in recent years have suggested that repeated brain injury in other sports such as football, soccer, and wrestling might also predispose to neurodegenerative disease52 and that non-sports-related TBI, such as that sustained on the battlefield, can lead to this same illness.30 It has recently been proposed that a history of even minor brain injury may predispose certain individuals to develop this pathologic process, now referred to as “chronic traumatic encephalopathy” or CTE52. The presentation of CTE is variable and may include neurologic and/or psychiatric manifestations. The current CTE literature suggests two common syndromes: a behavior and mood predominant illness, frequently accompanied by paranoia, which would be diagnosed as psychiatric illness and a predominantly cognitive disorder that is frequently diagnosed as Alzheimer’s disease 82. A third syndrome, which was emphasized by the prior literature on boxers includes motor dysfunction with parkinsonism14. Some CTE cases have also been described with motor neuron disease53, 54. Epidemiological study of CTE has been significantly limited since it is a pathological, rather than clinical diagnosis, and its presence can only be definitively confirmed after death. There is accumulating evidence, however, that CTE may be a pathologic process that unites seemingly disparate clinical syndromes and reflects a shared vulnerability to cognitive-behavioral-motor dysfunction. Recent studies have found support for a relationship between TBI and risk for later development of these individual neurologic and psychiatric syndromes. Since James Parkinson himself theorized a causative link to trauma in 1817, there has been continuing debate regarding the relationship between TBI and Parkinson’s disease,19 with many17, 29, 87 but not all3, 43, 49 studies finding a positive association. Epidemiological studies investigating the risk of Alzheimer’s disease after TBI have also shown mixed results. Meta-analyses of these studies in 199158 and in 200324 have shown an elevated risk. Prior TBI has also been associated with a significantly elevated risk of frontotemporal dementia70 and although a prior meta-analysis of the risk of TBI on development of amyotrophic lateral sclerosis (ALS) showed a mildly elevated risk,11 others have disputed the connection.93 Although psychiatric symptoms (e.g., depression and anxiety) are common acutely after TBI,6, 35, 40 whether there are protracted psychiatric sequelae from earlier-life TBI remains poorly understood.96
Our aim was to determine the association of mild TBI with the later development of those neurologic and psychiatric illnesses that have previously been linked to TBI and are potential manifestations of CTE. To investigate the wide range of disorders associated with prior TBI, we reviewed the literature examining TBI and subsequent neurologic or psychiatric diagnoses and performed a meta-analysis according to current guidelines56, 84. Consistent with the notion that mild TBI may make certain individuals vulnerable to a number of neurologic and psychiatric conditions, we hypothesized that there would be an association between all diagnoses and a history of TBI, including mild TBI.
METHODS
Identification of the studies
Searches were conducted in Medline (1995 to February 2012) using a comprehensive search strategy. We used two components in each search: component A identified papers with keywords “craniocerebral trauma,” “head injury,” “brain injury,” or “concussion.” This was combined with component B or component C. Component B identified papers pertaining to the neurologic disorders of interest (i.e., “neurodegenerative diseases," "mild cognitive impairment," "Alzheimer," "Parkinson," "frontotemporal dementia," "amyotrophic lateral sclerosis," "vascular dementia," or "dementia"), and component C identified papers pertaining to the psychiatric illnesses of interest (i.e., "anxiety disorders," "mood disorders," or "schizophrenia and disorders with psychotic features"). We limited our search to papers in English and humans.
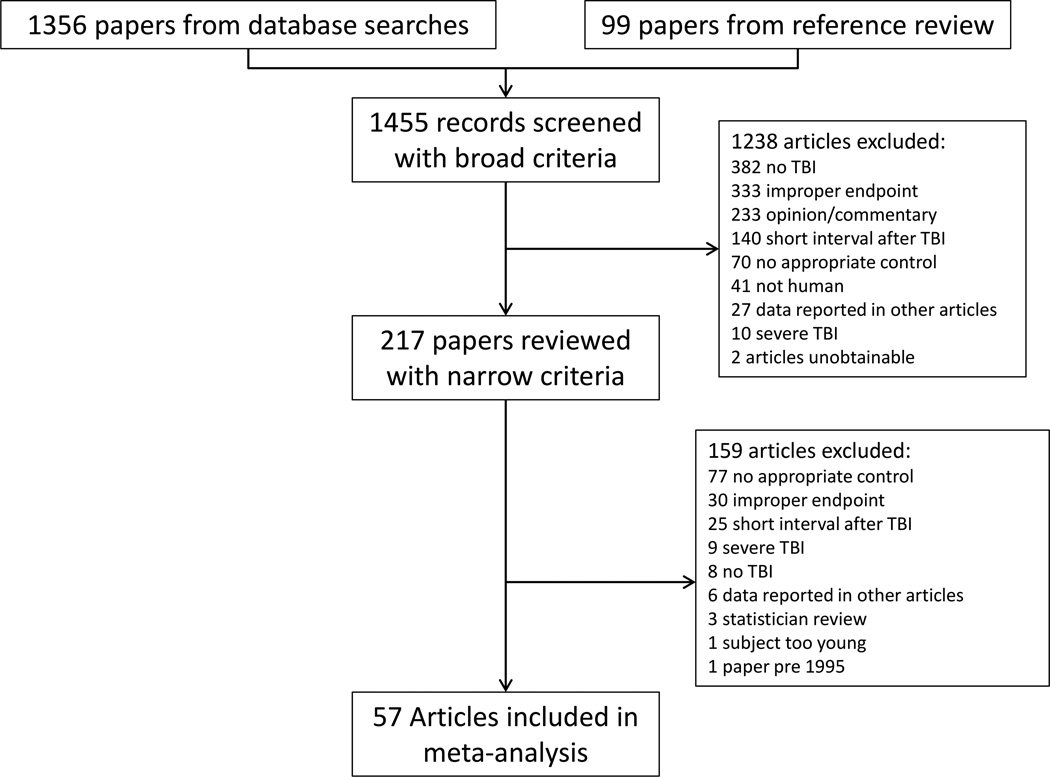
Three additional steps were taken to ensure search comprehensiveness: (1) references from included papers were reviewed, (2) to avoid any bias toward positive results inherent in the search strategy an additional search for “risk factors” for each diagnosis was performed to capture studies with weak or null findings that did not include our search terms in their title, abstract or keywords, (3) the citation lists in review papers were examined. For papers in which the required metrics were not easily identified the authors were contacted. A pair of reviewers (a neurologist and a neuropsychologist) discussed all papers at each stage of the process (Figure 1). Concordance between the reviewers for determining study inclusion was high; in cases of disagreement, studies were discussed until a consensus decision was reached. Ethics committee approval was not needed for this study as it included only analysis of previously published data.
Figure 1.
Flow chart depicting study identification and screening
Broad inclusion criteria
We first applied broad inclusion criteria (developed by a team of expert neurologists, neurosurgeons, and neuropsychologists) to select papers for further review.
Original, peer-reviewed research articles (no case reports)
Adult subjects over 18 years of age at the time of evaluation (not TBI)
Presence of TBI without accompanying structural lesion (e.g., subdural hematoma or penetrating brain injury). Though our goal was to specifically examine the effect of mild TBI, in order to capture all pertinent studies, at this search stage we broadly included studies employing the various definitions and labels that are used to refer to minor head trauma (e.g., concussion).
Presence of neurologic or psychiatric diagnosis
TBI occurred before the diagnosis of the neurologic or psychiatric disorder (with at least 12 months between the TBI and outcome diagnosis, if specified)
Narrow inclusion criteria
Papers that met broad inclusion criteria were next reviewed in detail. In addition to ensuring adherence to broad criteria, we also confirmed that they met narrow inclusion criteria. If some subjects in a study were reported to have structural lesions, but they could be separated from those without lesions, we only included subjects with mild TBI.
Presence of neurologic or psychiatric disorder. For neurologic disorders, studies must have utilized consensus diagnostic criteria or clinical evaluation. For psychiatric disorders, diagnoses were based on either criteria (e.g., DSM-IV) or scores from standardized measures (e.g., Beck Depression Inventory).
Inclusion of a control group. Included studies were cross-sectional, cohort, or case-control studies in which all subjects underwent identical assessment and diagnostic procedures.
TBI preceded neurologic or psychiatric diagnosis. We excluded studies that reported that the diagnosis of the neurologic or psychiatric disorder had been made less than 12 months post-TBI. For studies in which the date of the TBI was not reported, we included studies of subjects with neurologic or psychiatric illness who were asked about TBI earlier in life.
No redundant subjects across studies. In cases where multiple papers used the same study cohort we included the most recent papers to capture the largest sample size. If multiple outcome diagnoses were reported in one paper, we included each odds ratio (OR) if the diagnoses were mutually exclusive. If the diagnoses were not mutually exclusive, in the analyses that examined the association of TBI with any neurologic or psychiatric outcome, we chose the broader diagnosis (e.g., dementia was preferred over Alzheimer’s disease) or, if that distinction was not possible, we chose the diagnosis with the larger number of subjects.
Assessment of study characteristics
We recorded additional data regarding factors that could influence the relationship between TBI and outcome diagnoses. These included (1) the rigor with which each study employed a 12-month TBI-outcome diagnosis interval, (2) the TBI characteristics required in each study (e.g., whether subjects met accepted criteria for mild TBI or had any individual symptoms such as loss of consciousness), (3) whether the TBI diagnosis was based on patient or informant self-report as opposed to being made by a clinician, derived from medical records, or based on diagnostic criteria, (4) the study design (cohort, case-control, or cross-sectional), and (5) whether information was provided regarding the number of TBIs sustained by each subject. These data were used in subgroup analyses geared towards assessing whether study characteristics influenced the meta-analysis results.
Statistical Analysis
Primary analyses
The effect of interest for this meta-analysis was the pooled OR. For the majority of the studies (51/57), unadjusted ORs were directly calculated from data extraction. Standard errors were calculated from the logarithm of the OR to allow for symmetry of the estimate on both sides of unity.23 Where sample sizes were not available the published unadjusted ORs were used. We then applied standard meta-analytic techniques,34 including weighted estimates of the pooled OR with a 95% confidence interval (CI). For those studies where the raw cell frequencies did not exist and only the standard error of the OR was available, to provide appropriate weighting of the study in the meta-analysis, the standard error of the OR was transformed to the standard error of the logarithm of the OR by linear interpolation. To determine whether there was significant variation among studies, tests of heterogeneity were performed.34 All analyses were conducted using SAS v9.3 (Cary, NC).
Subgroup analyses
Since the overall analysis was inclusive of various TBI definitions and study characteristics, we next conducted seven additional subgroup analyses to examine whether our results differed when pooling studies with more uniformity of TBI assessment, TBI diagnostic criteria, and study design. When possible, we selected out only those subjects from the total study that met criteria for each subgroup analysis. The result is that for some studies a different number of subjects was included in the overall analysis compared to each subgroup analysis.
Subgroup 1: Effect of time interval between TBI and diagnosis
-
1.
Clearest interval - To ensure that studies with less stringent guidelines about timing of TBI were not significantly impacting our results, we excluded studies with the possibility that some subjects had a less than 12 month interval between TBI and diagnosis.
Subgroups 2–4: Effect of TBI features and severity
-
2.
Brief loss of consciousness – This subgroup included only studies that required that loss of consciousness not exceed 30 minutes. This is the maximum duration established in the mild TBI criteria of the American Congress of Rehabilitation Medicine, Centers for Disease Control, and World Health Organization10, 42, 59.
-
3.
Required loss of consciousness – We included only studies that required brain injury with loss of consciousness. This subgroup considered the effect of TBI with a uniform minimum severity.
-
4.
Any mild TBI feature – In order to exclude extremely mild or asymptomatic brain injury we performed an analysis including only those studies that required the brain injury be accompanied by any one (or more than one) common feature of mild TBI, including loss of consciousness, post-traumatic amnesia, Glasgow Coma Scale (GCS) score ≥13, focal neurological deficit, altered mental status, brain injury requiring medical care, or symptoms of the postconcussive syndrome (e.g., headache, dizziness, nausea, photo- or phonophobia, fatigue, sleep difficulty, blurred vision).
Subgroups 5–6: Effect of study design
-
5.
Excluding self-report - In order to assess the impact of recall bias we conducted an additional analysis excluding studies with self-reported TBI.
-
6.
Cohort studies - To eliminate recall bias we also performed an analysis including only cohort studies (rather than cross-sectional or case-control).
Subgroup 7: Effect of number of TBI
-
7.
Repeated injury - Because we were also interested in whether there is a dose effect of TBI on development of later illness, we conducted an additional analysis in which we calculated the odds of neurologic or psychiatric diagnosis in subjects with more than one TBI compared to a single TBI using a subset of studies that provided this information.
Publication bias analysis
To assess for the effect of publication bias on our results we used the Egger method18 to examine whether the logarithm of the included ORs are predicted by the standard error, which reflects the sample size. We visually examined funnel plots of OR against sample size and the logarithm of the OR against the standard error of the logarithm of the OR and quantified the degree of bias by multiple regression. Using standard error rather than sample size in funnel plots may provide a more accurate visual depiction of whether bias is present83.
RESULTS
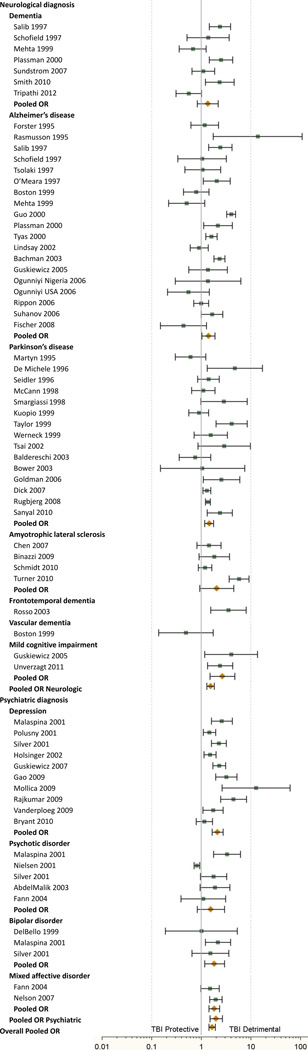
57 papers met narrow inclusion criteria and were used in the meta-analyses.3, 11, 17, 29, 43, 49, 70, 87 1, 2, 5, 7, 8, 9, 15, 16, 20, 22, 25, 27, 31, 32, 33, 37, 45, 47, 51, 55, 57, 60, 61, 62, 63, 64, 65, 67, 68, 69, 71, 72, 73, 74, 75, 76, 79, 80, 81, 85, 86, 90, 91, 92, 93, 94, 95, 97, 98 Among the included papers, a sufficient number were found to apply meta-analytic methods for the diagnoses of dementia, Alzheimer’s disease, Parkinson’s disease, ALS, mild cognitive impairment, depression, psychotic disorders, bipolar disorder, and mixed affective disorder (a combined group of depression and anxiety). Insufficient numbers of studies were found to calculate a pooled OR for frontotemporal dementia, vascular dementia, or anxiety disorders. There was significant heterogeneity among studies (Q = 381.99, df = 58, p<.001), justifying the use of the random effects meta-analysis.
Prior TBI was associated with development of any of the neurologic and psychiatric illnesses of interest [OR 1.67 (95% CI 1.44–1.93), p<.0001]. This association was found for both neurologic [OR 1.55 (95% CI 1.31–1.83, p<.0001)] and psychiatric [OR 2.00 (95% CI 1.50–2.66, p<.0001)] disease in individuals with TBI, and was also found in the following diagnoses: Alzheimer’s disease [OR 1.40 (95% CI 1.02–1.90, p<.05)], Parkinson’s disease [OR 1.45 (95% CI 1.18–1.78, p<.001)], mild cognitive impairment [OR 2.69 (95% CI 1.51–4.77, p<.001)], depression [OR 2.14 (95% CI 1.65–2.77, p<.0001)], bipolar disorder [OR 1.85 (95% CI 1.17–2.94, p<.01)], and mixed affective disorder [OR 1.84 (95% CI 1.50–2.66, p<.0001)]. See Table 1 and Figure 2.
Table 1.
Individual and pooled odds ratios for all included studies
| Study | Cases (# with TBI/ # without TBI) |
Controls (# with TBI/ # without TBI) |
OR | 95% CI |
|---|---|---|---|---|
| Neurological diagnosis | ||||
| Dementia | ||||
| Salib 1997b,c,d,e | 96/266 | 23/153 | 2.40 | 1.46–3.95 |
| Schofield 1997b,e,g | 6/41 | 21/198 | 1.38 | 0.52–3.61 |
| Mehta 1999d,e,g | 11/118 | 788/5728 | 0.68 | 0.36–1.26 |
| Plassman 2000b,e,f,g | 28/26 | 520/1202 | 2.49 | 1.45–4.29 |
| Sundstrom 2007b,e,h | 25/156 | 46/316 | 1.10 | 0.65–1.86 |
| Smith 2010e | 31/14 | 154/164 | 2.36 | 1.21–4.60 |
| Tripathi 2012e | 22/128 | 35/115 | 0.56 | 0.31–1.02 |
| Pooled OR | 1.36 | 0.84–2.19 | ||
| Alzheimer’s disease | ||||
| Forster 1995b | 25/84 | 22/87 | 1.18 | 0.62–2.25 |
| Rasmusson 1995b,d,e | 20/48 | 1/33 | 13.75 | 1.76–107.53 |
| Salib 1997a | 53/145 | 23/153 | 2.43 | 1.42–4.17 |
| Schofield 1997a | 4/34 | 23/205 | 1.05 | 0.34–3.22 |
| Tsolaki 1997 | 14/47 | 15/54 | 1.07 | 0.47–2.45 |
| O’Meara 1997b,e | 32/317 | 16/326 | 2.06 | 1.11–3.82 |
| Boston 1999 | 30/192 | 23/117 | 0.79 | 0.44–1.43 |
| Mehta 1999a | 6/85 | 788/5728 | 0.51 | 0.22–1.18 |
| Guo 2000e | 394/1782 | 127/2313 | 4.03 | 3.27–4.96 |
| Plassman 2000a | 17/18 | 520/1202 | 2.18 | 1.12–4.27 |
| Tyas 2000 | 203/821 | 93/605 | 1.61 | 1.23–2.10 |
| Lindsay 2002g | 28/151 | 603/2963 | 0.91 | 0.60–1.38 |
| Bachman 2003e | 397/1538 | 84/760 | 2.34 | 1.82–3.00 |
| Guskiewicz 2005e | 15/7 | 1148/732 | 1.37 | 0.56–3.37 |
| Ogunniyi Nigeria 2006g | 2/60 | 11/450 | 1.36 | 0.30–6.30 |
| Ogunniyi USA 2006g | 5/84 | 37/344 | 0.55 | 0.21–1.45 |
| Rippon 2006e | 72/78 | 648/700 | 1.00 | 0.71–1.40 |
| Suhanov 2006d,e | 46/214 | 30/230 | 1.65 | 1.00–2.71 |
| Fischer 2008g | 4/86 | 37/352 | 0.44 | 0.15–1.27 |
| Pooled OR | 1.40 | 1.02–1.90 | ||
| Parkinson’s disease | ||||
| Martyn 1995e | 11/156 | 35/301 | 0.61 | 0.30–1.23 |
| De Michele 1996d,e | 13/103 | 3/113 | 4.75 | 1.32–17.16 |
| Seidler 1996e | . | . | 1.40 | 0.85–2.30 |
| McCann 1998d,e | . | . | 1.10 | 0.64–1.90 |
| Smargiassi 1998d,e | 13/73 | 5/81 | 2.88 | 0.98–8.49 |
| Kuopio 1999d,e,h | 39/84 | 84/162 | 0.90 | 0.56–1.42 |
| Taylor 1999b,e | 35/105 | 11/136 | 4.12 | 2.00–8.50 |
| Werneck 1999 | 17/75 | 14/96 | 1.55 | 0.72–3.35 |
| Tsai 2002b,e | 11/19 | 5/25 | 2.89 | 0.86–9.75 |
| Baldereschi 2003d,e,g | 8/105 | 403/3980 | 0.75 | 0.36–1.56 |
| Bower 2003b,c,e,f | 2/183 | 2/193 | 1.05 | 0.15–7.57 |
| Goldman 2006b,c,e,h | 20/73 | 9/84 | 2.56 | 1.10–5.96 |
| Dick 2007d,e | . | . | 1.30 | 1.09–1.55 |
| Rugbjerg 2008b,e,f | 409/13194 | 1513/66792 | 1.37 | 1.22–1.53 |
| Sanyal 2010 | 27/148 | 25/325 | 2.37 | 1.33–4.23 |
| Pooled OR | 1.45 | 1.18–1.78 | ||
| Amyotrophic lateral sclerosis | ||||
| Chen 2007b,e,h | 24/85 | 42/213 | 1.43 | 0.82–2.51 |
| Binazzi 2009b | 16/61 | 23/162 | 1.85 | 0.91–3.73 |
| Schmidt 2010b,e,h | 84/157 | 185/412 | 1.19 | 0.87–1.64 |
| Turner 2010b,e,f,g | 41/34 | 106552/511831 | 5.79 | 3.68–9.13 |
| Pooled OR | 2.07 | 0.94–4.56 | ||
| Frontotemporal dementia | ||||
| Rosso 2003b,e | 19/61 | 10/114 | 3.55 | 1.55–8.11 |
| Vascular dementia | ||||
| Boston 1999 | 3/31 | 23/117 | 0.49 | 0.14–1.75 |
| Mild cognitive impairment | ||||
| Guskiewicz 2005e | 19/3 | 450/286 | 4.03 | 1.18–13.73 |
| Unverzagt 2011g | . | . | 2.40 | 1.34–4.30 |
| Pooled OR | 2.69 | 1.51–4.77 | ||
| Pooled OR Neurologic | 1.55 | 1.31–1.83 | ||
| Psychiatric diagnosis | ||||
| Depression | ||||
| Malaspina 2001 | 107/661 | 22/355 | 2.61 | 1.62–4.21 |
| Polusny 2001b,c,e,g | . | . | 1.47 | 1.10–1.97 |
| Silver 2001e | 40/243 | 321/4430 | 2.27 | 1.60–3.23 |
| Holsinger 2002b,c,e,f,g | 96/160 | 387/974 | 1.51 | 1.14–2.00 |
| Guskiewicz 2007e | 206/63 | 1272/893 | 2.30 | 1.71–3.08 |
| Gao 2009 | 38/497 | 28/1174 | 3.21 | 1.95–5.28 |
| Mollica 2009d,e | 10/3 | 6/23 | 12.78 | 2.65–61.56 |
| Rajkumar 2009d,e | 19/108 | 33/840 | 4.48 | 2.46–8.15 |
| Vanderploeg 2009b,e,g | 36/43 | 242/505 | 1.75 | 1.09–2.79 |
| Bryant 2010b,c,e,f,g | 56/265 | 77/419 | 1.15 | 0.79–1.68 |
| Pooled OR | 2.14 | 1.65–2.77 | ||
| Psychotic disorder | ||||
| Malaspina 2001 | 22/107 | 22/355 | 3.32 | 1.77–6.23 |
| Nielsen 2001b,e,f | 278/7854 | 3394/78710 | 0.82 | 0.72–0.93 |
| Silver 2001a | 12/89 | 349/4584 | 1.77 | 0.96–3.27 |
| AbdelMalik 2003b | 23/44 | 22/80 | 1.90 | 0.95–3.79 |
| Fann 2004a | . | . | 1.10 | 0.39–3.10 |
| Pooled OR | 1.57 | 0.83–2.97 | ||
| Bipolar disorder | ||||
| DelBello 1999b,c,d,e,g | 4/17 | 3/13 | 1.02 | 0.19–5.37 |
| Malaspina 2001 | 28/207 | 22/355 | 2.18 | 1.22–3.91 |
| Silver 2001a | 6/51 | 355/4622 | 1.53 | 0.65–3.59 |
| Pooled OR | 1.85 | 1.17–2.94 | ||
| Mixed affective disorder | ||||
| Fann 2004b,f,g | . | . | 1.50 | 0.98–2.30 |
| Nelson 2007b | 76/248 | 318/2045 | 1.97 | 1.49–2.62 |
| Pooled OR | 1.84 | 1.44–2.36 | ||
| Pooled OR Psychiatric | 2.00 | 1.50–2.66 | ||
| Overall Pooled OR | 1.67 | 1.44–1.93 | ||
Studies that were not included in the overall analysis or pooled neurologic/psychiatric analyses because diagnostic groups within the study were not mutually exclusive. Studies that were included in subgroup analyses:
clearest interval between TBI and symptom onset,
meeting mild TBI criteria for loss of consciousness,
requiring loss of consciousness,
requiring at least one mild TBI feature,
TBI diagnoses not based on self-report,
cohort studies, and
analysis of risk of repeated TBI
Figure 2.
Individual and pooled odds ratios for all included studies (to be aligned with Table 1).
Analyses of subgroups revealed a robust relationship between TBI and remote neurologic and psychiatric outcomes. The studies included in each subgroup analysis are specified in Table 1. Table 2 includes the features reported in each study regarding the time interval between TBI and diagnosis, the TBI features and severity, and the study design. Results of the subgroup analyses are reported in Table 3. Overall odds and the independent OR for neurologic, but not psychiatric disease remained significant when only including studies with the clearest greater than 12-month interval between TBI and diagnosis (Subgroup 1). The overall OR was significant among those studies that adhered to mild TBI criteria limiting duration of loss of consciousness to less than 30 minutes (Subgroup 2). The overall OR and OR for any of the studied neurologic and psychiatric diagnoses were also significant when only including studies that required loss of consciousness (Subgroup 3). When including studies that required the presence of at least one mild TBI symptom (Subgroup 4), the overall OR and OR for any of the neurologic and all psychiatric diagnoses of interest remained significant. After eliminating the studies with TBI diagnoses based on self-report (Subgroup 5), the overall OR and OR for neurologic disorders remained significant, though the OR for psychiatric outcomes no longer reached significance. When only cohort studies were included (Subgroup 6), the OR for neurologic outcomes was not significant though the overall OR and OR for psychiatric illness remained significant. The odds were not higher among those that reported more than one TBI compared to those with a single injury (Subgroup 7).
Table 2.
Design and TBI features reported for each included study
| Study | Study design | Age at head injury, mean |
Interval between injury and diagnosis, mean |
Required TBI characteristics |
Additional TBI information |
|---|---|---|---|---|---|
| Neurological diagnosis | |||||
| Dementia | |||||
| Salib (1997) | Case-control | 7.3 years | None given | Grouped by with or without LOC |
|
| Schofield (1997) | Cohort | LOC or PTA | |||
| Mehta (1999) | Cohort | Grouped | LOC | Grouped by LOC< or > 15 minutes |
|
| Plassman (2000) | Cohort | MC and LOC or PTA or nondisplaced skull fracture |
Excluded if penetrated dura or resulted in significant sequelae 3 months after TBI, severity ranked with mild group having LOC or PTA<30 minutes and no skull fracture |
||
| Sundstrom (2007) | Case-control | ≥5 years | MC | ||
| Smith (2010) | Cross-sectional | None given | |||
| Tripathi (2012) | Case-control | LOC or PTA or a symptom of PCS |
|||
| Alzheimer’s disease | |||||
| Forster (1995) | Case-control | Grouped (in adulthood or childhood) |
None given | ||
| Rasmusson (1995) | Case-control | 27.2 in sporadic Alzheimer’s group, 45.2 in familial Alzheimer’s group |
>5 years (mean 33.4 years in sporadic Alzheimer’s group, 18.67 in familial Alzheimer’s group) |
None given | Excluded if head injury resulted in “immediate, permanent cognitive or functional impairment,” head injury with LOC reported separately. Distinction made between mild and severe but not defined. |
| Salib (1997) | Case-control | 7.9 years | None given | Grouped by with or without LOC |
|
| Schofield (1997) | Cohort | 14.5 years | LOC or PTA | ||
| Tsolaki (1997) | Case-control | None given | |||
| O’Meara (1997) | Case-control | 46 (range 10–85) | 34 years (range 1- 72) |
MC or LOC | |
| Boston (1999) | Case-control | None given | |||
| Mehta (1999) | Cohort | Grouped | LOC | Grouped by LOC< or > 15 minutes |
|
| Guo (2000) | Case-control | MC or LOC | |||
| Plassman (2000) | Cohort | MC and LOC or PTA or nondisplaced skull fracture |
Excluded if penetrated dura or resulted in significant sequelae 3 months after TBI, severity ranked with mild group having LOC or PTA<30 minutes and no skull fracture |
||
| Tyas (2000) | Cross-sectional | None given | |||
| Lindsay (2002) | Cohort | None given | Both with and without LOC | ||
| Bachman (2003) | Case-control | MC | |||
| Guskiewicz (2005) | Cross-sectional | AMS and one symptom of PCS |
|||
| Ogunniyi Nigeria (2006) | Cohort | None given | |||
| Ogunniyi USA (2006) | Cohort | None given | |||
| Rippon (2006) | Cross-sectional | LOC or PTA | |||
| Suhanov (2006) | Case-control | LOC | |||
| Fischer (2008) | Cohort | None given | |||
| Parkinson’s disease | |||||
| Martyn (1995) | Case-control | LOC or MC | |||
| De Michele (1996) | Case-control | LOC | |||
| Seidler (1996) | Case-control | PTA or PCS | |||
| McCann (1998) | Case-control | LOC | |||
| Smargiassi (1998) | Case-control | LOC | |||
| Kuopio (1999) | Case-control | None given | Records number with and without LOC and duration of LOC < or > 5 minutes |
||
| Taylor (1999) | Case-control | 16.3 | 36.5 years | LOC or AMS or ND or PCS |
|
| Werneck (1999) | Case-control | None given | |||
| Tsai (2002) | Case-control | 18.5 | 17.2 years | LOC or PTA or PCS or ND |
|
| Baldereschi (2003) | Cohort | LOC | |||
| Bower (2003) | Case-control | >3 years (range 3– 55, median 29 years for TBI of all severities in study) |
PTA | Excluded from this group if LOC>1 minute, PTA>30 minutes, or imaging abnormal. Mild TBI with LOC, moderate, and severe TBI analyzed separately |
|
| Goldman (2006) | Case-control | 25.7 | 36.9 years (range 2–70), separate analysis reported of only those >10 years |
LOC or PTA | |
| Dick (2007) | Case-control | LOC | |||
| Rugbjerg (2008) | Case-control | Grouped, >1 year data used |
MC | Excluded if imaging abnormal | |
| Sanyal (2010) | Case-control | None given | |||
| Amyotrophic lateral sclerosis |
|||||
| Chen (2007) | Case-control | Grouped | Grouped | MC | |
| Binazzi (2009) | Case-control | Grouped | Grouped | None given | |
| Schmidt (2010) | Case-control | Grouped | Grouped (2–80+ years) |
LOC or MC | |
| Turner (2010) | Cohort | MC | |||
| Frontotemporal dementia | |||||
| Rosso (2003) | Case-control | PCS or LOC or PTA | |||
| Vascular dementia | |||||
| Boston (1999) | Case-control | None given | |||
| Mild cognitive impairment |
|||||
| Guskiewicz (2005) | Cross-sectional | AMS and one symptom of PCS |
|||
| Unverzagt (2011) | Cohort | None given | |||
| Psychiatric diagnosis | |||||
| Depression | |||||
| Malaspina (2001) | Case-control | None given | Severity grouped by LOC duration with “severe” TBI having LOC >15 minutes |
||
| Polusny (2001) | Cohort | >1 year (1–2.33 years) |
AMS or LOC | LOC >20 minutes excluded | |
| Silver (2001) | Cross-sectional | LOC or AMS | |||
| Holsinger (2002) | Cohort | 20.9 (includes some not in analysis) |
MC + LOC or PTA or nondisplaced skull fracture |
Excluded if penetrated dura or resulted in significant sequelae 3 months after TBI |
|
| Guskiewicz (2007) | Cross-sectional | AMS and one symptom of PCS |
|||
| Gao (2009) | Cross-sectional | None given | |||
| Mollica (2009) | Cross-sectional | LOC, PTA, and ND | |||
| Rajkumar (2009) | Cross-sectional | LOC | |||
| Vanderploeg (2009) | Cohort | 16 years | LOC or PTA or AMS |
Excluded if admitted to hospital |
|
| Bryant (2010) | Cohort | 37.8 | 1 year | GCS≥13 | Excluded if focal deficit, imaging abnormal, or LOC>30 minutes |
| Psychotic disorder | |||||
| Malaspina (2001) | Case-control | None given | Severity grouped by duration LOC with “severe” TBI having LOC >15 minutes |
||
| Nielson (2001) | Case-control | Grouped (>1 year) | MC | ICD9 code for concussion included, excluded if skull fracture or intracranial hemorrhage |
|
| Silver (2001) | Cross-sectional | LOC or AMS | |||
| AbdelMalik (2003) | Case-control | <17 | Median 12 years | Closed head injuries without intracranial hemorrhage or other immediate sequelae |
|
| Fann (2004) | Cohort | 3 years | By ICD9 codes - Excluded if imaging abnormal or LOC>1 hour |
||
| Bipolar disorder | |||||
| DelBello (1999) | Cross-sectional | 10.7 | 6.3 years | LOC | |
| Malaspina (2001) | Case-control | None given | Severity grouped by duration LOC with “severe” TBI having LOC >15 minutes |
||
| Silver (2001) | Cross-sectional | LOC or AMS | |||
| Mixed affective disorder |
|||||
| Fann (2004) | Cohort | 3 years | By ICD9 codes - Excluded if imaging abnormal or LOC>1 hour |
||
| Nelson (2007) | Cross-sectional | >1 year | None given |
“Grouped” refers to data presented in the paper by stratification or division of subjects into groups without an available mean.
AMS – alteration in mental status, GCS – Glasgow Coma Scale, LOC – loss of consciousness, MC – injury for which medical care was received, ND – Neurological deficit, PCS – post-concussion syndrome (e.g., headache, dizziness, nausea, photo- or phonophobia, fatigue, sleep difficulty, blurred vision), PTA – post-traumatic amnesia, TBI –traumatic brain injury
Table 3.
Results of subgroup analyses
| Analysis | Odds ratio | 95 % confidence interval |
Statistical significance |
|---|---|---|---|
|
Risk of TBI when including only studies with clearest interval on: |
|||
| All neurologic and psychiatric outcomes |
1.75 | 1.43–2.14 | p<.001 |
| All neurologic outcomes | 2.05 | 1.55–2.71 | p<.001 |
| All psychiatric outcomes | 1.38 | 0.95–2.00 | p=.09 |
|
Risk of TBI when including only studies meeting mild TBI requirements for maximum duration of loss of consciousness |
1.54 | 1.18–2.01 | p=.001 |
|
Risk of TBI when including only studies requiring associated loss of consciousness on: |
|||
| All neurologic and psychiatric outcomes |
1.69 | 1.18–2.44 | p<.01 |
| All neurologic outcomes | 1.33 | 1.00–1.75 | p<.05 |
| All psychiatric outcomes | 4.09 | 1.36–12.32 | p=.01 |
|
Risk of TBI when including only studies requiring a mild TBI feature on: |
|||
| All neurololgic and psychiatric outcomes |
1.70 | 1.42–2.05 | p<.0001 |
| All neurologic outcomes | 1.67 | 1.36–2.07 | p<.0001 |
| All psychiatric outcomes | 1.81 | 1.23–2.66 | p<.01 |
|
Risk of TBI when eliminating studies with TBI diagnosis based on self-report on: |
|||
| All neurologic and psychiatric outcomes |
1.62 | 1.14–2.31 | p<.01 |
| All neurologic outcomes | 2.38 | 1.01–5.62 | p<.05 |
| All psychiatric outcomes | 1.18 | 0.81–1.71 | p=.39 |
|
Risk of TBI when including only cohort studies on: |
|||
| All neurologic and psychiatric outcomes |
1.38 | 1.02–-1.87 | p<.05 |
| All neurologic outcomes | 1.27 | 0.72–2.25 | p=.41 |
| All psychiatric outcomes | 1.45 | 1.23–1.71 | p<.0001 |
|
Risk of multiple TBIs vs one TBI on any outcome diagnosis |
1.10 | 0.72–1.70 | p=.65 |
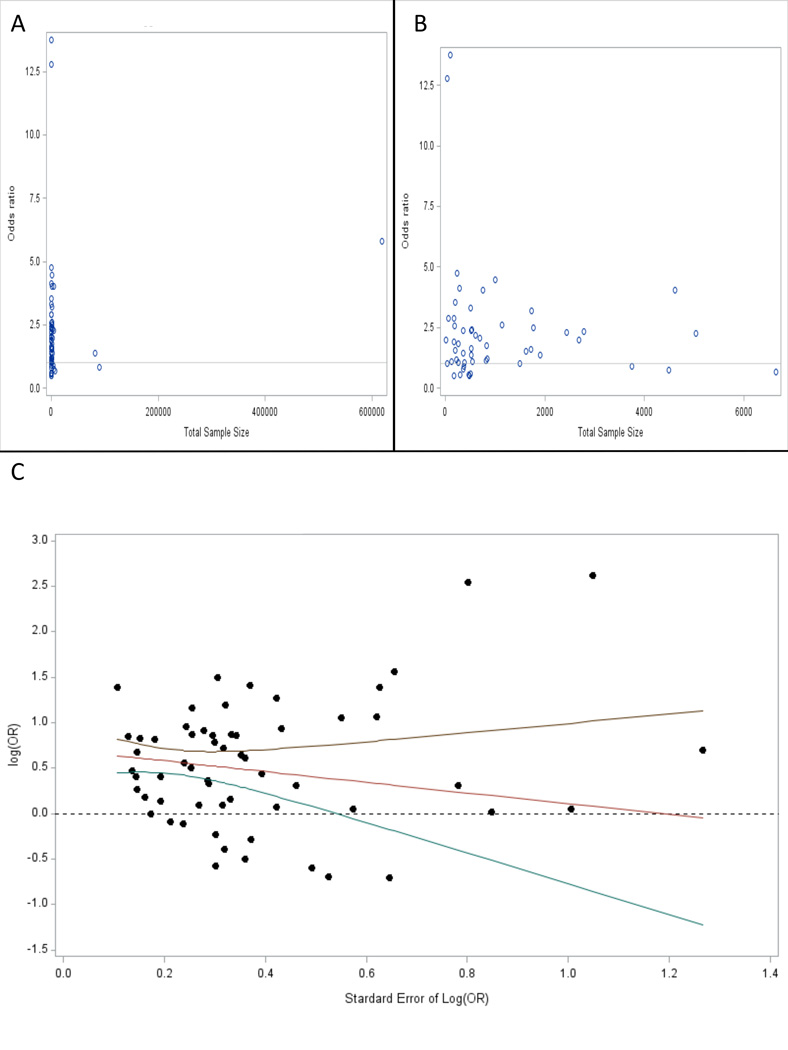
Publication bias analyses did not show evidence of bias in the included studies. Visual inspection of a funnel plot based on sample size showed that three studies with large samples strongly influenced the appearance (Figure 3A). When these studies are removed a more expected funnel shape is appreciated (Figure 3B). Regression indicates that the effects size (the logarithm of the OR) is not significantly predicted by the standard error when all studies are included (F(1,60)=3.08, p=.08) or when the three large sample studies are excluded (F(1,57)=1.11, p=.30, Figure 3C).
Figure 3.
Publication bias analysis. (A) Funnel plot of OR versus total sample size. (B) Funnel plot of OR versus total sample size after excluding the three studies with largest sample sizes (Rugbjerg 2008, Nielson 2001, and Turner 2010). (C) Plot of the logarithm of the OR after excluding the three largest sample size studies compared to the standard error of the logarithm of the OR showing a regression line and 95% confidence interval with slope that is not statistically significantly different from 0.
DISCUSSION
This meta-analysis supports an association between prior TBI and later diagnosis of the relevant neurologic or psychiatric diseases. This association was found independently for both neurologic and psychiatric outcomes. Alzheimer’s disease, Parkinson’s disease, mild cognitive impairment, depression, mixed affective disorders, and bipolar disorder showed a statistically significant association with prior TBI. The magnitude of effect is comparable across diagnoses, with mild cognitive impairment, depression, and bipolar disorder having the highest OR among those results that reached significance. The OR of Alzheimer’s disease in this analysis is comparable to the findings of prior meta-analyses.24, 58 The OR of ALS was among the highest in the study, and there was some evidence of an association of TBI with dementia and psychotic disorders, but these did not reach statistical significance.
The overall combined OR for the selected neurologic and psychiatric illnesses and for neurologic illness independently in individuals with TBI remained significant when including only articles that explicitly specified a minimum 12-month interval between TBI and outcome diagnosis. The magnitude of association with psychiatric illness, however, did not remain significant. These results suggest that there may be a different time course in which psychiatric and neurologic symptoms manifest after TBI. While psychiatric symptoms are common in the acute phase after mild TBI6, 21, 35, 40 and some of these may be short-lived manifestations of the injury, others may reflect a more sustained susceptibility to mental illness. The results of this study suggest that TBI is a risk factor for both remote psychiatric and neurologic disease and are consistent with the possibility that both types of illness arise secondary to a common shared pathologic mechanism.
We conducted additional subgroup analyses to determine whether TBI characteristics or methodological factors would influence our findings. The overall OR of TBI remained significant when including only studies that required adherence to typical loss of consciousness criteria for mild TBI, the presence of any specific mild TBI symptom, or loss of consciousness. Though TBI definitions varied widely among studies, these additional analyses support an association of mild TBI with the studied neurologic and psychiatric outcomes. A significant OR for combined neurologic and psychiatric outcomes was also found when eliminating studies that used self-reported diagnosis of TBI and when including only cohort studies. Though statistical significance was lost when assessing the association with psychiatric outcomes when eliminating self-report and the odds of neurologic outcomes among cohort studies, the magnitudes of the ORs were largely consistent with the main analysis, and the change in significance is likely due to the small number of articles in these analyses and resulting loss of power rather than reflecting a weaker association due to recall bias, though this cannot be excluded. Our analyses also suggest that the finding of an association with TBI is unlikely to be due to publication bias, though low power may affect the publication bias test.
The results of this meta-analysis support an association of illness with a single TBI. A relevant associated question is whether this effect is compounded by multiple TBIs. In our analysis of multiple head traumas, the results do not show strong evidence for elevated odds of illness associated with having more than one head trauma compared to a single TBI. Given that only six studies were included in this analysis, lower power may have influenced these results. More research on the relationship between TBI exposure and diagnostic outcomes is needed.
The magnitude of the OR of TBI in this meta-analysis is relatively modest, but comparable to other strongly implicated risk factors. For example, for Alzheimer’s disease, the Apolipoprotein E e4 allele is associated with an OR of 1.80–9.05,41 and obesity with an OR of 1.80.4 The OR for pesticide exposure and Parkinson’s disease is 1.94.66 Therefore, the presence of a risk factor in an individual does not indicate an inevitable development of disease. The ORs found in this study suggest that others factors modify an individual’s susceptibility to develop a neuropsychiatric disorder after TBI. These factors are largely unknown and worthy of further investigation.
The fact that multiple neurodegenerative and psychiatric diagnoses are associated with the same exposure raises questions about possible mechanisms of shared vulnerability. Trauma could predispose the brain to different types of neurodegeneration through common mechanisms such as oxidative stress and microglial activation77, 99 or induction of plasma proteins associated with degeneration such as MCP-1.36 Trauma might also activate molecular pathways leading to specific degenerative diseases, such as the finding that Alzheimer’s disease-associated proteins including beta amyloid, beta secretase, presenilin-1, and caspase-3 accumulate in axons of brain injured animals.12 Cleaved forms of the tau protein, which is associated with Alzheimer’s disease and frontotemporal lobar degeneration, accumulate after trauma26 and tau abnormalities after trauma have been found to be independent of beta amyloid effects.89 The nature of the TBI could also influence the clinical presentation in an individual. For example, boxers may suffer from more torsional injury that could injury brainstem structures such as the substantia nigra, leading to parkinsonism82. Genetic variation could also help to explain the susceptibility of individuals to late-life effects of TBI. For example, apolipoprotein E, which is associated with risk of Alzheimer’s disease, has shown a variable interaction with mild TBI.50, 63, 88
An alternate explanation for the association across diagnostic groups is that the various clinical presentations could be different expressions of a common pathology28, 78. Although CTE has been described as a distinct pathological process, the clinical characterization is not clearly established, and case reports suggest cognitive, motor, and psychiatric presentations. This phenotypic variability could lead to a diagnosis of dementia, Parkinson’s disease, motor neuron disease, or primary psychiatric illness in different individuals. A study of causes of death among retired National Football League players found a three-fold higher rate of dying from neurodegenerative disease compared to the typical population frequency, with Alzheimer’s disease and ALS being the most overrepresented44, which would be consistent either a shared vulnerability hypothesis across neurodegenerative diseases or a common pathology. This meta-analysis examined clinical, not pathological, studies. Thus, it is unknown whether any of the subjects would have shown characteristic CTE pathology rather than (or in addition to) the more typical neuropathological features associated with their syndromes.
Among the articles that were reviewed, several addressed the association between TBI and clinical outcomes among athletes. These articles assessed the risk of Parkinson’s disease among retired Thai traditional boxers,46 depression and dementia among retired football players,32, 33 ALS or chronic encephalopathy among soccer players,13, 39 and chronic TBI in boxers.38 Only two of the articles32, 33 that directly evaluated TBI in sports met strict inclusion criteria for this study. The ability of this meta-analysis to inform the questions surrounding the long-term consequences of sports-related mild TBI is therefore limited by this lack in the existing literature. Further longitudinal studies among athletes with appropriate control groups, characterization of head injuries (including severity, number, and exposure to repetitive subconcussive trauma), and assessment of late-life neurologic and psychiatric outcomes will be needed to address this question.
Several limitations of this meta-analysis warrant consideration. One is the possible bias of the included studies. We took several steps to mitigate this possibility. Our search strategy included a variety of epidemiological studies that focused on many possible risk factors, not just TBI, thereby capturing negative studies that might otherwise have not been published. Our formal analyses also did not support publication bias. Although the strict inclusion criteria should reduce this possibility, variation in the studies themselves (e.g., different criteria for diagnosis of illness, or co-morbid environmental and genetic factors of the study population) limits the generalizability of the results. Variable study quality could also have resulted in heterogeneity, and it is possible that the presence of other confounding factors could have led to the observed association between TBI and later clinical outcomes. For example, patients who sustain a TBI as a result of a fall or motor vehicle accident may have other medical comorbidities (e.g., vascular disease or substance abuse) or differences in socioeconomic status that could predispose to neurologic or psychiatric illness. Another possibility is that the TBI itself could lead to injury of change in lifestyle that could modify risk of a mood disorder. Finally, ill patients who fall and suffer TBI may also undergo more medical testing and therefore be more likely to receive one of these neurologic or psychiatric diagnoses. Only English language studies were reviewed, which could have led to exclusion of some relevant studies. In spite of our criteria regarding an interval between TBI and illness onset, an alternative explanation for the observed association is that some head injuries may have been early manifestations of neurologic or psychiatric disease rather than an independent predisposing factor for illness. The authors of one of the studies concluded this reverse causality was responsible for their findings. They stratified the interval between TBI and diagnosis and found the association between TBI and Parkinson’s was no longer present when only looking at TBI that occurred greater than 10 years prior to diagnosis71.
A major strength of this meta-analysis was the inclusion of a variety of different neurologic and psychiatric outcomes rather than a single diagnosis. By focusing on diagnoses rather than self-reported symptoms or performance on cognitive tests, this study assessed outcomes of sufficient magnitude to affect quality of life. The included studies also come from countries around the world, allowing for more generalizable results. The literature search was comprehensive, making this a rigorous examination of the topic.
CONCLUSIONS
This study supports an association of TBI, including mild TBI, on subsequent development of neurologic and psychiatric illness, including Alzheimer’s disease, Parkinson’s disease, mild cognitive impairment, depression, mixed affective disorders, and bipolar disorder. Due to limitations and heterogeneity in the existing studies, prospective studies with uniform assessment will be needed to confirm this result and determine the risk conferred by the number and severity of TBI in different settings, such as sports or the military.
Acknowledgments
Dr. Sturm is supported by National Institute on Aging 1K23AG040127. Dr. Peterson is supported by National Cancer Institute Award KM1CA156687. Dr. Boeve receives research support from the National Institute on Aging (P50 AG016574, U01 AG006786, R01 AG032306, and R01 AG041797) and the Mangurian Foundation. Dr. Miller is funded by NIH grants P50AG023501, P01AG019724, P50 AG1657303, and the state of California. Dr. Welsh-Bohmer received funding from the National Institute of Aging (P30 AG28377), from private donors to the Joseph & Kathleen Bryan Alzheimer’s Disease Center at Duke University, and from Takeda and Zinfandel Pharmaceutical companies.
Footnotes
DISCLOSURES
Dr. Perry reports no competing interests. Dr. Pieper reports no competing interests. Mr. Bullock reports no competing interests. Dr. Guskiewicz reports no competing interests. Dr. Berger reports no competing interests. Dr. Kramer reports no competing interests.
REFERENCES
- 1.AbdelMalik P, Husted J, Chow EW, Bassett AS. Childhood head injury and expression of schizophrenia in multiply affected families. Arch Gen Psychiatry. 2003;60:231–236. doi: 10.1001/archpsyc.60.3.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bachman DL, Green RC, Benke KS, Cupples LA, Farrer LA MIRAGE Study Group. Comparison of alzheimer's disease risk factors in white and african american families. Neurology. 2003;60:1372–1374. doi: 10.1212/01.wnl.0000058751.43033.4d. [DOI] [PubMed] [Google Scholar]
- 3.Baldereschi M, Di Carlo A, Vanni P, Ghetti A, Carbonin P, Amaducci L, et al. Lifestyle-related risk factors for parkinson’s disease: A population-based study. Acta Neurol Scand. 2003;108:239–244. doi: 10.1034/j.1600-0404.2003.00128.x. [DOI] [PubMed] [Google Scholar]
- 4.Beydoun MA, Beydoun HA, Wang Y. Obesity and central obesity as risk factors for incident dementia and its subtypes: A systematic review and meta-analysis. Obes Rev. 2008;9:204–218. doi: 10.1111/j.1467-789X.2008.00473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Binazzi A, Belli S, Uccelli R, Desiato MT, Talamanca IF, Antonini G, et al. An exploratory case-control study on spinal and bulbar forms of amyotrophic lateral sclerosis in the province of rome. Amyotroph Lateral Scler. 2009;10:361–369. doi: 10.3109/17482960802382313. [DOI] [PubMed] [Google Scholar]
- 6.Bombardier CH, Fann JR, Temkin NR, Esselman PC, Barber J, Dikmen SS. Rates of major depressive disorder and clinical outcomes following traumatic brain injury. JAMA. 2010;303:1938–1945. doi: 10.1001/jama.2010.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boston PF, Dennis MS, Jagger C. Factors associated with vascular dementia in an elderly community population. Int J Geriatr Psychiatry. 1999;14:761–766. doi: 10.1002/(sici)1099-1166(199909)14:9<761::aid-gps14>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 8.Bower JH, Maraganore DM, Peterson BJ, McDonnell SK, Ahlskog JE, Rocca WA. Head trauma preceding PD: A case-control study. Neurology. 2003;60:1610–1615. doi: 10.1212/01.wnl.0000068008.78394.2c. [DOI] [PubMed] [Google Scholar]
- 9.Bryant RA, O'Donnell ML, Creamer M, McFarlane AC, Clark CR, Silove D. The psychiatric sequelae of traumatic injury. Am J Psychiatry. 2010;167:312–320. doi: 10.1176/appi.ajp.2009.09050617. [DOI] [PubMed] [Google Scholar]
- 10.Carroll L, Cassidy J, Holm L, Kraus J, Coronado V. Methodological issues and research recommendations for mild traumatic brain injury: The WHO collaborating centre task force on mild traumatic brain injury. J Rehabil Med. 2004;43:113–125. doi: 10.1080/16501960410023877. [DOI] [PubMed] [Google Scholar]
- 11.Chen H, Richard M, Sandler DP, Umbach DM, Kamel F. Head injury and amyotrophic lateral sclerosis. Am J Epidemiol. 2007;166:810–816. doi: 10.1093/aje/kwm153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen XH, Siman R, Iwata A, Meaney DF, Trojanowski JQ, Smith DH. Long-term accumulation of amyloid-beta, beta-secretase, presenilin-1, and caspase-3 in damaged axons following brain trauma. Am J Pathol. 2004;165:357–371. doi: 10.1016/s0002-9440(10)63303-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chio A, Benzi G, Dossena M, Mutani R, Mora G. Severely increased risk of amyotrophic lateral sclerosis among italian professional football players. Brain. 2005;128:472–476. doi: 10.1093/brain/awh373. [DOI] [PubMed] [Google Scholar]
- 14.Corsellis JA, Bruton CJ, Freeman-Browne D. The aftermath of boxing. Psychol Med. 1973;3:270–303. doi: 10.1017/s0033291700049588. [DOI] [PubMed] [Google Scholar]
- 15.De Michele G, Filla A, Volpe G, De Marco V, Gogliettino A, Ambrosio G, et al. Environmental and genetic risk factors in parkinson's disease: A case-control study in southern italy. Mov Disord. 1996;11:17–23. doi: 10.1002/mds.870110105. [DOI] [PubMed] [Google Scholar]
- 16.DelBello MP, Soutullo CA, Zimmerman ME, Sax KW, Williams JR, McElroy SL, et al. Traumatic brain injury in individuals convicted of sexual offenses with and without bipolar disorder. Psychiatry Res. 1999;89:281–286. doi: 10.1016/s0165-1781(99)00112-2. [DOI] [PubMed] [Google Scholar]
- 17.Dick FD, De Palma G, Ahmadi A, Scott NW, Prescott GJ, Bennett J, et al. Environmental risk factors for parkinson's disease and parkinsonism: The geoparkinson study. Occup Environ Med. 2007;64:666–672. doi: 10.1136/oem.2006.027003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Factor SA, Sanchez-Ramos J, Weiner WJ. Trauma as an etiology of parkinsonism: A historical review of the concept. Mov Disord. 1988;3:30–36. doi: 10.1002/mds.870030105. [DOI] [PubMed] [Google Scholar]
- 20.Fann JR, Burington B, Leonetti A, Jaffe K, Katon WJ, Thompson RS. Psychiatric illness following traumatic brain injury in an adult health maintenance organization population. Arch Gen Psychiatry. 2004;61:53–61. doi: 10.1001/archpsyc.61.1.53. [DOI] [PubMed] [Google Scholar]
- 21.Fedoroff JP, Starkstein SE, Forrester AW, Geisler FH, Jorge RE, Arndt SV, et al. Depression in patients with acute traumatic brain injury. Am J Psychiatry. 1992;149:918–923. doi: 10.1176/ajp.149.7.918. [DOI] [PubMed] [Google Scholar]
- 22.Fischer P, Zehetmayer S, Jungwirth S, Weissgram S, Krampla W, Hinterberger M, et al. Risk factors for alzheimer dementia in a community-based birth cohort at the age of 75 years. Dement Geriatr Cogn Disord. 2008;25:501–507. doi: 10.1159/000128577. [DOI] [PubMed] [Google Scholar]
- 23.Fleiss JL, Levin BA, Paik MC. Statistical Methods for Rates and Proportions. New York City: Wiley; 2003. [Google Scholar]
- 24.Fleminger S, Oliver DL, Lovestone S, Rabe-Hesketh S, Giora A. Head injury as a risk factor for Alzheimer’s disease: The evidence 10 years on; a partial replication. Journal of Neurology, Neurosurgery & Psychiatry. 2003;74:857–862. doi: 10.1136/jnnp.74.7.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Forster DP, Newens AJ, Kay DW, Edwardson JA. Risk factors in clinically diagnosed presenile dementia of the alzheimer type: A case-control study in northern england. J Epidemiol Community Health. 1995;49:253–258. doi: 10.1136/jech.49.3.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gabbita SP, Scheff SW, Menard RM, Roberts K, Fugaccia I, Zemlan FP. Cleaved-tau: A biomarker of neuronal damage after traumatic brain injury. J Neurotrauma. 2005;22:83–94. doi: 10.1089/neu.2005.22.83. [DOI] [PubMed] [Google Scholar]
- 27.Gao S, Jin Y, Unverzagt FW, Liang C, Hall KS, Ma F, et al. Correlates of depressive symptoms in rural elderly chinese. Int J Geriatr Psychiatry. 2009;24:1358–1366. doi: 10.1002/gps.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gavett BE, Stern RA, Cantu RC, Nowinski CJ, McKee AC. Mild traumatic brain injury: A risk factor for neurodegeneration. Alzheimers Res Ther. 2010;2:18. doi: 10.1186/alzrt42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goldman SM, Tanner CM, Oakes D, Bhudhikanok GS, Gupta A, Langston JW. Head injury and parkinson's disease risk in twins. Ann Neurol. 2006;60:65–72. doi: 10.1002/ana.20882. [DOI] [PubMed] [Google Scholar]
- 30.Goldstein LE, Fisher AM, Tagge CA, Zhang XL, Velisek L, Sullivan JA, et al. Chronic traumatic encephalopathy in blast-exposed military veterans and a blast neurotrauma mouse model. Sci Transl Med. 2012;4:134ra60. doi: 10.1126/scitranslmed.3003716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guo Z, Cupples LA, Kurz A, Auerbach SH, Volicer L, Chui H, et al. Head injury and the risk of AD in the MIRAGE study. Neurology. 2000;54:1316–1323. doi: 10.1212/wnl.54.6.1316. [DOI] [PubMed] [Google Scholar]
- 32.Guskiewicz KM, Marshall SW, Bailes J, McCrea M, Cantu RC, Randolph C, et al. Association between recurrent concussion and late-life cognitive impairment in retired professional football players. Neurosurgery. 2005;57:719–726. doi: 10.1093/neurosurgery/57.4.719. discussion 719–26. [DOI] [PubMed] [Google Scholar]
- 33.Guskiewicz KM, Marshall SW, Bailes J, McCrea M, Harding HP, Jr, Matthews A, et al. Recurrent concussion and risk of depression in retired professional football players. Med Sci Sports Exerc. 2007;39:903–909. doi: 10.1249/mss.0b013e3180383da5. [DOI] [PubMed] [Google Scholar]
- 34.Hedges LV, Olkin I. Statistical Methods for Meta-Analysis. Orlando, FL: Academic Press; 1985. [Google Scholar]
- 35.Hibbard MR, Uysal S, Kepler K, Bogdany J, Silver J. Axis I psychopathology in individuals with traumatic brain injury. J Head Trauma Rehabil. 1998;13:24–39. doi: 10.1097/00001199-199808000-00003. [DOI] [PubMed] [Google Scholar]
- 36.Ho L, Zhao W, Dams-O'Connor K, Tang CY, Gordon W, Peskind ER, et al. Elevated plasma MCP-1 concentration following traumatic brain injury as a potential "predisposition" factor associated with an increased risk for subsequent development of alzheimer's disease. J Alzheimers Dis. 2012;31:301–313. doi: 10.3233/JAD-2012-120598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Holsinger T, Steffens DC, Phillips C, Helms MJ, Havlik RJ, Breitner JC, et al. Head injury in early adulthood and the lifetime risk of depression. Arch Gen Psychiatry. 2002;59:17–22. doi: 10.1001/archpsyc.59.1.17. [DOI] [PubMed] [Google Scholar]
- 38.Jordan BD, Relkin NR, Ravdin LD, Jacobs AR, Bennett A, Gandy S. Apolipoprotein E epsilon4 associated with chronic traumatic brain injury in boxing. JAMA. 1997;278:136–140. [PubMed] [Google Scholar]
- 39.Jordan SE, Green GA, Galanty HL, Mandelbaum BR, Jabour BA. Acute and chronic brain injury in united states national team soccer players. Am J Sports Med. 1996;24:205–210. doi: 10.1177/036354659602400216. [DOI] [PubMed] [Google Scholar]
- 40.Jorge RE, Robinson RG, Starkstein SE, Arndt SV. Depression and anxiety following traumatic brain injury. J Neuropsychiatry Clin Neurosci. 1993;5:369–374. doi: 10.1176/jnp.5.4.369. [DOI] [PubMed] [Google Scholar]
- 41.Jun G, Naj AC, Beecham GW, Wang LS, Buros J, Gallins PJ, et al. Meta-analysis confirms CR1, CLU, and PICALM as alzheimer disease risk loci and reveals interactions with APOE genotypes. Arch Neurol. 2010;67:1473–1484. doi: 10.1001/archneurol.2010.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kay T, Harrington DE, Adams R, Anderson T, Berrol S, Cicerone K, et al. Definition of mild traumatic brain injury. J Head Trauma Rehabil. 1993;8:86–87. [Google Scholar]
- 43.Kuopio AM, Marttila RJ, Helenius H, Rinne UK. Environmental risk factors in parkinson's disease. Mov Disord. 1999;14:928–939. doi: 10.1002/1531-8257(199911)14:6<928::aid-mds1004>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 44.Lehman EJ, Hein MJ, Baron SL, Gersic CM. Neurodegenerative causes of death among retired national football league players. Neurology. 2012;79:1970–1974. doi: 10.1212/WNL.0b013e31826daf50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lindsay J, Laurin D, Verreault R, Hébert R, Helliwell B, Hill GB, et al. Risk factors for Alzheimer’s disease: A prospective analysis from the canadian study of health and aging. American Journal of Epidemiology. 2002;156:445–453. doi: 10.1093/aje/kwf074. [DOI] [PubMed] [Google Scholar]
- 46.Lolekha P, Phanthumchinda K, Bhidayasiri R. Prevalence and risk factors of parkinson's disease in retired thai traditional boxers. Mov Disord. 2010;25:1895–1901. doi: 10.1002/mds.23210. [DOI] [PubMed] [Google Scholar]
- 47.Malaspina D, Goetz RR, Friedman JH, Kaufmann CA, Faraone SV, Tsuang M, et al. Traumatic brain injury and schizophrenia in members of schizophrenia and bipolar disorder pedigrees. Am J Psychiatry. 2001;158:440–446. doi: 10.1176/appi.ajp.158.3.440. [DOI] [PubMed] [Google Scholar]
- 48.Martland HS. Punch drunk. Journal of the American Medical Association. 1928;91:1103–1107. [Google Scholar]
- 49.Martyn CN, Osmond C. Parkinson's disease and the environment in early life. J Neurol Sci. 1995;132:201–206. doi: 10.1016/0022-510x(95)00148-u. [DOI] [PubMed] [Google Scholar]
- 50.Mayeux R, Ottman R, Maestre G, Ngai C, Tang MX, Ginsberg H, et al. Synergistic effects of traumatic head injury and apolipoprotein-epsilon 4 in patients with alzheimer's disease. Neurology. 1995;45:555–557. doi: 10.1212/wnl.45.3.555. [DOI] [PubMed] [Google Scholar]
- 51.McCann SJ, LeCouteur DG, Green AC, Brayne C, Johnson AG, Chan D, et al. The epidemiology of parkinson's disease in an australian population. Neuroepidemiology. 1998;17:310–317. doi: 10.1159/000026185. [DOI] [PubMed] [Google Scholar]
- 52.McKee AC, Cantu RC, Nowinski CJ, Hedley-Whyte ET, Gavett BE, Budson AE, et al. Chronic traumatic encephalopathy in athletes: Progressive tauopathy after repetitive head injury. J Neuropathol Exp Neurol. 2009;68:709–735. doi: 10.1097/NEN.0b013e3181a9d503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McKee AC, Gavett BE, Stern RA, Nowinski CJ, Cantu RC, Kowall NW, et al. TDP-43 proteinopathy and motor neuron disease in chronic traumatic encephalopathy. J Neuropathol Exp Neurol. 2010;69:918–929. doi: 10.1097/NEN.0b013e3181ee7d85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McKee AC, Stern RA, Nowinski CJ, Stein TD, Alvarez VE, Daneshvar DH, et al. The spectrum of disease in chronic traumatic encephalopathy. Brain. 2013;136:43–64. doi: 10.1093/brain/aws307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mehta KM, Ott A, Kalmijn S, Slooter AJ, van Duijn CM, Hofman A, et al. Head trauma and risk of dementia and alzheimer's disease: The rotterdam study. Neurology. 1999;53:1959–1962. doi: 10.1212/wnl.53.9.1959. [DOI] [PubMed] [Google Scholar]
- 56.Moher D, Liberati A, Tetzlaff J, Altman DG PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mollica RF, Lyoo IK, Chernoff MC, Bui HX, Lavelle J, Yoon SJ, et al. Brain structural abnormalities and mental health sequelae in south vietnamese ex-political detainees who survived traumatic head injury and torture. Arch Gen Psychiatry. 2009;66:1221–1232. doi: 10.1001/archgenpsychiatry.2009.127. [DOI] [PubMed] [Google Scholar]
- 58.Mortimer JA, van Duijn CM, Chandra V, Fratiglioni L, Graves AB, Heyman A, et al. Head trauma as a risk factor for alzheimer's disease: A collaborative re-analysis of case-control studies EURODEM risk factors research group. Int J Epidemiol. 1991;20(Suppl 2):S28–S35. doi: 10.1093/ije/20.supplement_2.s28. [DOI] [PubMed] [Google Scholar]
- 59.National Center for Injury Prevention and Control (US) Report to Congress on Mild Traumatic Brain Injury in the United States: Steps to Prevent a Serious Public Health Problem. Centers for Disease Control and Prevention; 2003. [Google Scholar]
- 60.Nelson LA, Rhoades DA, Noonan C, Manson SM AI-SUPERPFP Team. Traumatic brain injury and mental health among two american indian populations. J Head Trauma Rehabil. 2007;22:105–112. doi: 10.1097/01.HTR.0000265098.52306.a9. [DOI] [PubMed] [Google Scholar]
- 61.Nielsen AS, Mortensen PB, O'Callaghan E, Mors O, Ewald H. Is head injury a risk factor for schizophrenia? Schizophr Res. 2002;55:93–98. doi: 10.1016/s0920-9964(01)00205-5. [DOI] [PubMed] [Google Scholar]
- 62.Ogunniyi A, Hall KS, Gureje O, Baiyewu O, Gao S, Unverzagt FW, et al. Risk factors for incident alzheimer's disease in african americans and yoruba. Metab Brain Dis. 2006;21:235–240. doi: 10.1007/s11011-006-9017-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.O'Meara ES, Kukull WA, Sheppard L, Bowen JD, McCormick WC, Teri L, et al. Head injury and risk of alzheimer's disease by apolipoprotein E genotype. Am J Epidemiol. 1997;146:373–384. doi: 10.1093/oxfordjournals.aje.a009290. [DOI] [PubMed] [Google Scholar]
- 64.Plassman BL, Havlik RJ, Steffens DC, Helms MJ, Newman TN, Drosdick D, et al. Documented head injury in early adulthood and risk of alzheimer's disease and other dementias. Neurology. 2000;55:1158–1166. doi: 10.1212/wnl.55.8.1158. [DOI] [PubMed] [Google Scholar]
- 65.Polusny MA, Kehle SM, Nelson NW, Erbes CR, Arbisi PA, Thuras P. Longitudinal effects of mild traumatic brain injury and posttraumatic stress disorder comorbidity on postdeployment outcomes in national guard soldiers deployed to iraq. Arch Gen Psychiatry. 2011;68:79–89. doi: 10.1001/archgenpsychiatry.2010.172. [DOI] [PubMed] [Google Scholar]
- 66.Priyadarshi A, Khuder SA, Schaub EA, Shrivastava S. A meta-analysis of parkinson's disease and exposure to pesticides. Neurotoxicology. 2000;21:435–440. [PubMed] [Google Scholar]
- 67.Rajkumar AP, Thangadurai P, Senthilkumar P, Gayathri K, Prince M, Jacob KS. Nature, prevalence and factors associated with depression among the elderly in a rural south indian community. Int Psychogeriatr. 2009;21:372–378. doi: 10.1017/S1041610209008527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rasmusson DX, Brandt J, Martin DB, Folstein MF. Head injury as a risk factor in alzheimer's disease. Brain Inj. 1995;9:213–219. doi: 10.3109/02699059509008194. [DOI] [PubMed] [Google Scholar]
- 69.Rippon GA, Tang MX, Lee JH, Lantigua R, Medrano M, Mayeux R. Familial alzheimer disease in latinos: Interaction between APOE, stroke, and estrogen replacement. Neurology. 2006;66:35–40. doi: 10.1212/01.wnl.0000191300.38571.3e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rosso SM, Landweer EJ, Houterman M, Donker Kaat L, van Duijn CM, van Swieten JC. Medical and environmental risk factors for sporadic frontotemporal dementia: A retrospective case-control study. J Neurol Neurosurg Psychiatry. 2003;74:1574–1576. doi: 10.1136/jnnp.74.11.1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rugbjerg K, Ritz B, Korbo L, Martinussen N, Olsen JH. Risk of parkinson's disease after hospital contact for head injury: Population based case-control study. BMJ. 2008;337:a2494. doi: 10.1136/bmj.a2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Salib E, Hillier V. Head injury and the risk of alzheimer's disease: A case control study. Int J Geriatr Psychiatry. 1997;12:363–368. doi: 10.1002/(sici)1099-1166(199703)12:3<363::aid-gps515>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 73.Sanyal J, Chakraborty DP, Sarkar B, Banerjee TK, Mukherjee SC, Ray BC, et al. Environmental and familial risk factors of parkinsons disease: Case-control study. Can J Neurol Sci. 2010;37:637–642. doi: 10.1017/s0317167100010829. [DOI] [PubMed] [Google Scholar]
- 74.Schmidt S, Kwee LC, Allen KD, Oddone EZ. Association of ALS with head injury, cigarette smoking and APOE genotypes. J Neurol Sci. 2010;291:22–29. doi: 10.1016/j.jns.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schofield PW, Tang M, Marder K, Bell K, Dooneief G, Chun M, et al. Alzheimer's disease after remote head injury: An incidence study. J Neurol Neurosurg Psychiatry. 1997;62:119–124. doi: 10.1136/jnnp.62.2.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Seidler A, Hellenbrand W, Robra BP, Vieregge P, Nischan P, Joerg J, et al. Possible environmental, occupational, and other etiologic factors for parkinson's disease: A case-control study in germany. Neurology. 1996;46:1275–1284. doi: 10.1212/wnl.46.5.1275. [DOI] [PubMed] [Google Scholar]
- 77.Shitaka Y, Tran HT, Bennett RE, Sanchez L, Levy MA, Dikranian K, et al. Repetitive closed-skull traumatic brain injury in mice causes persistent multifocal axonal injury and microglial reactivity. J Neuropathol Exp Neurol. 2011;70:551–567. doi: 10.1097/NEN.0b013e31821f891f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shively S, Scher AI, Perl DP, Diaz-Arrastia R. Dementia resulting from traumatic brain injury: What is the pathology? Arch Neurol. 2012;69:1245–1251. doi: 10.1001/archneurol.2011.3747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Silver JM, Kramer R, Greenwald S, Weissman M. The association between head injuries and psychiatric disorders: Findings from the new haven NIMH epidemiologic catchment area study. Brain Inj. 2001;15:935–945. doi: 10.1080/02699050110065295. [DOI] [PubMed] [Google Scholar]
- 80.Smargiassi A, Mutti A, De Rosa A, De Palma G, Negrotti A, Calzetti S. A case-control study of occupational and environmental risk factors for parkinson's disease in the emilia-romagna region of italy. Neurotoxicology. 1998;19:709–712. [PubMed] [Google Scholar]
- 81.Smith K, Flicker L, Dwyer A, Atkinson D, Almeida OP, Lautenschlager NT, et al. Factors associated with dementia in aboriginal australians. Aust N Z J Psychiatry. 2010;44:888–893. doi: 10.3109/00048674.2010.491816. [DOI] [PubMed] [Google Scholar]
- 82.Stern RA, Daneshvar DH, Baugh CM, Seichepine DR, Montenigro PH, Riley DO, et al. Clinical presentation of chronic traumatic encephalopathy. Neurology. 2013;81:1122–1129. doi: 10.1212/WNL.0b013e3182a55f7f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sterne JA, Egger M. Funnel plots for detecting bias in meta-analysis: Guidelines on choice of axis. J Clin Epidemiol. 2001;54:1046–1055. doi: 10.1016/s0895-4356(01)00377-8. [DOI] [PubMed] [Google Scholar]
- 84.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: A proposal for reporting meta-analysis of observational studies in epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 85.Suhanov AV, Pilipenko PI, Korczyn AD, Hofman A, Voevoda MI, Shishkin SV, et al. Risk factors for alzheimer's disease in russia: A case-control study. Eur J Neurol. 2006;13:990–995. doi: 10.1111/j.1468-1331.2006.01391.x. [DOI] [PubMed] [Google Scholar]
- 86.Sundstrom A, Nilsson LG, Cruts M, Adolfsson R, Van Broeckhoven C, Nyberg L. Increased risk of dementia following mild head injury for carriers but not for non-carriers of the APOE epsilon4 allele. Int Psychogeriatr. 2007;19:159–165. doi: 10.1017/S1041610206003498. [DOI] [PubMed] [Google Scholar]
- 87.Taylor CA, Saint-Hilaire MH, Cupples LA, Thomas CA, Burchard AE, Feldman RG, et al. Environmental, medical, and family history risk factors for parkinson's disease: A new england-based case control study. Am J Med Genet. 1999;88:742–749. [PubMed] [Google Scholar]
- 88.Terrell TR, Bostick RM, Abramson R, Xie D, Barfield W, Cantu R, et al. APOE, APOE promoter, and tau genotypes and risk for concussion in college athletes. Clin J Sport Med. 2008;18:10–17. doi: 10.1097/JSM.0b013e31815c1d4c. [DOI] [PubMed] [Google Scholar]
- 89.Tran HT, LaFerla FM, Holtzman DM, Brody DL. Controlled cortical impact traumatic brain injury in 3xTg-AD mice causes acute intra-axonal amyloid-beta accumulation and independently accelerates the development of tau abnormalities. J Neurosci. 2011;31:9513–9525. doi: 10.1523/JNEUROSCI.0858-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tripathi M, Vibha D, Gupta P, Bhatia R, Srivastava MV, Vivekanandhan S, et al. Risk factors of dementia in north india: A case-control study. Aging Ment Health. 2012;16:228–235. doi: 10.1080/13607863.2011.583632. [DOI] [PubMed] [Google Scholar]
- 91.Tsai CH, Lo SK, See LC, Chen HZ, Chen RS, Weng YH, et al. Environmental risk factors of young onset parkinson's disease: A case-control study. Clin Neurol Neurosurg. 2002;104:328–333. doi: 10.1016/s0303-8467(02)00027-6. [DOI] [PubMed] [Google Scholar]
- 92.Tsolaki M, Fountoulakis K, Chantzi E, Kazis A. Risk factors for clinically diagnosed alzheimer's disease: A case-control study of a greek population. Int Psychogeriatr. 1997;9:327–341. doi: 10.1017/s104161029700447x. [DOI] [PubMed] [Google Scholar]
- 93.Turner MR, Abisgold J, Yeates DG, Talbot K, Goldacre MJ. Head and other physical trauma requiring hospitalisation is not a significant risk factor in the development of ALS. J Neurol Sci. 2010;288:45–48. doi: 10.1016/j.jns.2009.10.010. [DOI] [PubMed] [Google Scholar]
- 94.Tyas SL, Pederson LL, Koval JJ. Is smoking associated with the risk of developing alzheimer's disease? results from three canadian data sets. Ann Epidemiol. 2000;10:409–416. doi: 10.1016/s1047-2797(00)00061-2. [DOI] [PubMed] [Google Scholar]
- 95.Unverzagt FW, Ogunniyi A, Taler V, Gao S, Lane KA, Baiyewu O, et al. Incidence and risk factors for cognitive impairment no dementia and mild cognitive impairment in african americans. Alzheimer Dis Assoc Disord. 2011;25:4–10. doi: 10.1097/WAD.0b013e3181f1c8b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.van Reekum R, Cohen T, Wong J. Can traumatic brain injury cause psychiatric disorders? J Neuropsychiatry Clin Neurosci. 2000;12:316–327. doi: 10.1176/jnp.12.3.316. [DOI] [PubMed] [Google Scholar]
- 97.Vanderploeg RD, Belanger HG, Curtiss G. Mild traumatic brain injury and posttraumatic stress disorder and their associations with health symptoms. Arch Phys Med Rehabil. 2009;90:1084–1093. doi: 10.1016/j.apmr.2009.01.023. [DOI] [PubMed] [Google Scholar]
- 98.Werneck AL, Alvarenga H. Genetics, drugs and environmental factors in parkinson's diseaseA case-control study. Arq Neuropsiquiatr. 1999;57:347–355. doi: 10.1590/s0004-282x1999000300001. [DOI] [PubMed] [Google Scholar]
- 99.Zhang QG, Laird MD, Han D, Nguyen K, Scott E, Dong Y, et al. Critical role of NADPH oxidase in neuronal oxidative damage and microglia activation following traumatic brain injury. PLoS One. 2012;7:e34504. doi: 10.1371/journal.pone.0034504. [DOI] [PMC free article] [PubMed] [Google Scholar]