Abstract
Anxiety disorders are characterized by enhanced reactivity to threat, and event-related potentials (ERPs) are useful neural measures of the dynamics of threat processing. In particular, the late positive potential (LPP) is an ERP component that reflects sustained attention towards motivationally salient information. Previous studies in adults suggest that the LPP is enhanced to threatening stimuli in anxiety but blunted in depression; however, very little work has evaluated the LPP to threat in anxious youth. We measured the LPP during an emotional face-matching task in youth (age 7–19) with current anxiety disorders (n = 53) and healthy controls with no history of psychopathology (n = 37). We evaluated group differences, as well as the effect of depressive symptoms on the LPP. Youth with anxiety disorders exhibited enhanced LPPs to angry and fearful faces 1000–2000 ms after stimulus onset. Higher depressive symptoms were associated with reduced LPPs to angry faces across both groups. Enhanced LPPs to threatening faces were most apparent for social anxiety disorder, as opposed to generalized anxiety or separation anxiety disorder. Results suggest the LPP may be a useful neural measure of threat reactivity in youth with anxiety disorders and highlight the importance of accounting for symptoms of both depression and anxiety when examining emotional processing.
Keywords: anxiety, youth, event-related potentials, late positive potential, emotional faces, depression
Anxiety disorders are one of the most common classes of psychological disorders in children and adolescents, and predict increased rates of both anxiety and depressive disorders in adulthood (Weems & Silverman, 2013). Identifying core emotional processes associated with psychopathology may aid in the identification of etiological mechanisms and treatment targets (Kring, 2010; Rottenberg & Johnson, 2007). Emotional processes may be particularly relevant for understanding psychological disorders from a developmental perspective and lead to more effective early interventions for child and adolescent psychopathology (Suveg, Southam-Gerow, Goodman, & Kendall, 2007).
Anxiety disorders have been characterized by heightened attention towards and reactivity to threat. For example, both youth and adults with anxiety exhibit behavioral patterns of attentional biases towards threatening stimuli, such as angry or fearful faces (e.g., Bar-Haim, Lamy, Pergamin, Bakermans-Kranenburg, & van Ijzendoorn, 2007; Mogg, Garner, & Bradley, 2007; Roy et al., 2008). In addition, pathophysiological models of anxiety implicate neural regions known for their role in processing threat. For example, functional magnetic resonance imaging (fMRI) work has found heightened amygdala activation and abnormalities in amygdala-prefrontal cortex connectivity in anxious children, adolescents, and adults (Birbaumer et al., 1998; Blackford & Pine, 2012; Monk et al., 2008; Phan, Fitzgerald, Nathan, & Tancer, 2006; Prater, Hosanagar, Klumpp, Angstadt, & Phan, 2013; Shin et al., 2005; Strawn et al., 2014; Thomas et al., 2001). In addition to fMRI, event-related potential (ERP) measures of threat reactivity could represent a complementary probe of neural physiology.
ERP methods are economical, easily assessed in youth (Banaschewski & Brandeis, 2007), and may be particularly useful as neural measures of attention towards and reactivity to emotional information. In particular, the late positive potential (LPP) is a slow wave ERP component appearing as early as 200–300 ms after stimulus onset as a positivity for emotional compared to neutral stimuli (Cuthbert, Schupp, Bradley, Birbaumer, & Lang, 2000). The LPP appears to reflect sustained attention towards emotional stimuli and activation of motivational systems in response to salient information (Cuthbert et al., 2000; Hajcak, Weinberg, MacNamara, & Foti, 2011), and has been linked to activation in the visual cortex, as well as subcortical structures including the amygdala, ventral striatum/nucleus accumbens, anterior cingulate, and anterior insula (Liu, Huang, McGinnis-Deweese, Keil, & Ding, 2012; Sabatinelli, Keil, Frank, & Lang, 2013; Sabatinelli, Lang, Keil, & Bradley, 2007). The LPP has demonstrated good two-year stability across development, and may be a more reliable measure of emotional reactivity in children than reaction time (RT) or accuracy measures (Kujawa, Klein, & Proudfit, 2013).
In adult anxiety, the LPP appears to track heightened reactivity towards threat, particularly stimuli that are relevant to subject-specific fears. For example, enhanced LPPs to spider images have been observed in adults with spider phobias (Leutgeb, Schäfer, & Schienle, 2009; Michalowski et al., 2009). In addition, social anxiety has been associated with increased LPPs to threatening faces and to faces overall, regardless of valence (Moser, Huppert, Duval, & Simons, 2008; Mühlberger et al., 2009), and generalized anxiety disorder (GAD) has been characterized by enhanced LPPs to aversive pictures (MacNamara & Hajcak, 2010).
Given the early onset of anxiety disorders (Kessler et al, 2005), characterizing neural markers of emotional reactivity from early in development may aid in understanding etiology and developmental course. Children with spider phobias have been found to exhibit heightened LPPs to spider stimuli (Leutgeb, Schäfer, Köchel, Scharmüller, & Schienle, 2010), but it remains unclear whether other types of anxiety in children and adolescents are characterized by an enhanced LPP to threat. As with specific phobia, social anxiety disorder, generalized anxiety disorder (GAD), and separation anxiety disorder have an earlier age of onset than other internalizing disorders, are among the most common forms of psychopathology in children and adolescents, and often continue into adulthood (Mohatt, Bennett, & Walkup, 2014). The LPP could be a valid neural index of threat processing in youth with social anxiety disorder, GAD, and/or separation anxiety disorder, but most of the existing literature on the LPP and anxiety has focused on adults and often only a single disorder.
Though comorbidity of anxiety and depressive disorders is common and both classes of disorder share a number of etiological influences (Goldberg, Krueger, Andrews, & Hobbs, 2009), evidence from a variety of laboratory measures suggests that depression may be characterized by decreased reactivity to both negative and positive information (Bylsma, Morris, & Rottenberg, 2008; Rottenberg, Gross, & Gotlib, 2005). That is, while anxiety may be marked by heightened attention towards and defensive responding in the context of threat, depression may be better characterized by withdrawal and disengagement from emotional changes in the environment. Consistent with this, there is evidence that depression in adults and risk for depression in children is associated with reduced LPPs to emotional stimuli (Foti, Olvet, Klein, & Hajcak, 2010; Kayser, Bruder, Tenke, Stewart, & Quitkin, 2000; Kujawa, Hajcak, Torpey, Kim, & Klein, 2012; Proudfit, Bress, Foti, Kujawa & Klein, in press), suggesting that depression and anxiety may have distinct effects on threat processing. Previous work has yet to delineate the effects of symptoms of both anxiety and depression on the LPP.
The current study is part of a treatment study modeled after the design of the Child/Adolescent Anxiety Multimodal Study to evaluate three of the most common anxiety disorders in children and adolescents (social anxiety disorder, separation anxiety disorder, and GAD) across a broad age range (Walkup et al., 2008). The primary goal of the current study was to evaluate the LPP to emotional faces in youth with anxiety disorders. To this end, we measured the LPP during an emotional face-matching task in healthy controls and anxious youth. Facial expressions are commonly used as stimuli in fMRI and behavioral studies of youth anxiety disorders (Bar-Haim et al., 2007; Monk, 2008), as they are particularly relevant for adaptive social behavior and developmentally appropriate emotional stimuli. We hypothesized that youth with anxiety disorders would exhibit enhanced LPPs to threatening emotional faces (i.e., angry or fearful faces) but not to pleasant (i.e., happy) faces. Given evidence of distinct effects of depression on the LPP (Foti et al., 2010; Kayser et al., 2000; Kujawa, Hajcak et al., 2012), we also evaluated effects of depressive symptoms, hypothesizing that depressive symptoms would be associated with blunted LPPs to emotional faces. Due to normative developmental changes in the LPP (Hajcak & Dennis, 2009; Kujawa, Klein, & Hajcak, 2012; Kujawa, Klein et al., 2013), we controlled for age in all analyses. Lastly, given limited previous work evaluating the LPP in youth anxiety disorders, additional exploratory analyses examined whether enhanced LPPs to threat were specific to certain anxiety disorders or to clinical anxiety more generally and/or whether enhanced LPPs were more evident in more severe anxiety.
Methods
Participants
Participants were recruited through outpatient clinics, flyers and postings in communities in Ann Arbor, MI (University of Michigan; UM) and Chicago, IL (University of Illinois at Chicago; UIC). Youth between the ages of 7 and 19 with current anxiety disorders or no history of psychopathology were eligible to participate. To be eligible for inclusion, participants were required to be free of developmental or intellectual disabilities, lifetime schizophrenia, lifetime bipolar disorder and severe current depression or suicidal ideation. At the time of the electroencephalographic (EEG) assessment, participants were not taking any psychotropic medications or undergoing psychotherapy. Inclusion criteria for the anxiety disorders (AD) group required a current diagnosis of social anxiety disorder, separation anxiety disorder, and/or GAD. Participants in the healthy control (HC) group had no current or lifetime history of axis I disorders.
EEG data were available for a total of 104 youth. Participants with excessively noisy EEG data (n = 11) or less than 70% accuracy on the task overall (n = 2) were excluded from analyses. In addition, one participant was missing data on depressive symptoms and excluded. Thus, the final sample consisted of 90 participants (53 AD, 37 HC). The final sample was 54.4% female and had a mean age of 13.93 (SD = 3.86; range: 7–19 years). With regard to race, the sample was 58.4% Caucasian, 15.7% African American, 15.7% Latino, and 10.1% Asian or Pacific Islander.
This study was approved by the UM and UIC Institutional Review Boards, and informed consent was obtained from all participants, as well as parents of youth under age 18.
Measures
Diagnostic Interview
Participants were interviewed using the Kiddie Schedule of Affective Disorders and Schizophrenia (K-SADS; Kaufman et al., 1997) to assess current and lifetime diagnoses of Axis I disorders. For younger children, both the parent and participants were interviewed simultaneously, and for older adolescents, only the participant was interviewed. Typically, youth under the age of 14 were interviewed with parents, while youth age 14 and older were interviewed alone; however, the decision of whether to include a parent was made for individual participants depending on developmental level, insight into symptoms, and participant’s comfort with the parent participating in the interview. All interviews were administered by Masters-level or Doctorate-level clinicians. Participants in the AD group with comorbid disorders were included if the clinician determined the anxiety disorder to be the primary diagnosis (see Participant Characteristics).
Anxiety Severity
To assess severity of anxiety symptoms, participants were administered the Pediatric Anxiety Rating Scale (PARS; Research Units on Pediatric Psychopharmacology Anxiety Study Group, 2002), a clinician-rated measure of the severity of anxiety symptoms that has demonstrated adequate reliability and validity. Masters-level clinicians rated overall severity on a 6-point scale across seven dimensions (number of symptoms, frequency, overall severity, physical symptom severity, avoidance, interference at home, and interference outside of the home), with these dimensions then combined to form a total PARS score. PARS data were missing for three participants. Internal consistency for PARS total scores was excellent (Cronbach’s α = .95).
Depressive Symptoms
Participants completed the Children’s Depression Inventory (CDI; Kovacs, 1992) to assess for current depressive symptoms. The CDI has demonstrated acceptable reliability and validity for youth between the ages of 7 and 17 (Kovacs, 1992). The majority of the current sample (74.4%) was 17 years old or younger, and in order to avoid confounding age effects with a different measure, we administered the CDI to all participants. Internal consistency for the CDI was excellent (Cronbach’s α = .90).
Emotional Face-Matching Task
Participants completed an emotional face-matching task (Hariri, Tessitore, Mattay, Fera, & Weinberger, 2002), which has been used to assess ERPs to threat in adult anxiety (Labuschagne et al., 2010; MacNamara, Post, Kennedy, Rabinak, & Phan, 2013). The task involved the presentation of three images in a triangular arrangement for 3000 ms, and participants were instructed to select one of the two images at the bottom of the screen that matched the image centered at the top of the screen. The task included both face matching and shape matching trials. On face matching trials, one emotional face was presented at the top of the screen (fearful, happy, or angry face), and the face of a different actor with a similar expression and a face with a neutral expression were presented at the bottom of the screen. Participants selected which face at the bottom of the screen matched the expression of the face at the top. Shape matching trials were included to ERPs in a non-emotional condition, and required participants to match geometric shapes. The task was divided into two blocks with each block consisting of 12 trials for each condition: shapes, angry, fearful and happy, presented in random order. The intertrial interval varied between 1000 and 3000 ms during which time a white fixation cross was presented on a black background. Participants performed six practice trials prior to beginning the experiment.
EEG Data Acquisition and Processing
Continuous EEG was recorded using a 34-channel cap (32 channel setup based on 10/20 system with the addition of FCz and Iz) and the BioSemi system (BioSemi, Amsterdam, Netherlands). Electrodes were placed on each of the left and right mastoids, and the electrooculogram (EOG) was recorded from four facial electrodes places approximately 1 cm above and below the right eye and beyond the outer edge of each eye. The data were digitized at 24-bit resolution with a Least Significant Bit (LSB) value of 31.25 nV and a sampling rate of 1024 Hz, using a low-pass fifth order sinc filter with −3dB cutoff point at 208 Hz. As per BioSemi’s design, the voltage from each active electrode was referenced online with respect to a common mode sense active electrode producing a monopolar (non-differential) channel.
Data were processed offline using Brain Vision Analyzer software (Brain Products, Gilching, Germany). Data were converted to a linked mastoid reference, filtered with high-pass and low-pass filters of .01 and 30 Hz, respectively. Data for correct trials only were segmented beginning 200 ms before stimulus onset and continuing for the 3000 ms stimulus duration. Eyeblinks were corrected using the method by Gratton, Coles, and Donchin (1983), and semi-automated artifact rejection procedures removed artifacts with the following criteria: voltage step of more than 50 µV between sample points, a voltage difference of 300 µV within a trial, and a maximum voltage difference of less than .5 µV within 100 ms intervals. Additional artifacts were removed using visual inspection. Participants were required to have a minimum of 12 artifact-free trials in each condition to be included in analyses (Moran, Jendrusina, & Moser, 2013); however, the mean number of artifact-free trials in each condition was 21.75 (SD = 1.70). Trials were baseline corrected using the 200 ms prior to stimulus onset and averaged across each condition (shapes, angry, fearful, happy).
The LPP was maximal across parietal and occipital sites, consistent with previous LPP research with youth (e.g., Kujawa, Weinberg, Hajcak, & Klein, 2013), and scored at a pooling of the following electrodes: O1, O2, Oz, PO3, PO4, P3, P4, and Pz (Figure 1). Similar to a previous study measuring ERPs in the same task (MacNamara et al., 2013), sustained positivities for emotional faces compared to shapes were observable beginning around 500 ms. There is some evidence that rather than a single component, the LPP may be better characterized by a series of overlapping components that peak at earlier and later times across stimulus presentation (Foti, Hajcak, & Dien, 2009; Hajcak et al., 2011). Given the relatively long stimulus duration (i.e., 3000 ms) in the current task, we evaluated the temporal dynamics of emotional face processing in anxiety by dividing the LPP into three windows (early: 500–1000 ms, middle: 1000–2000 ms, late: 2000–3000 ms).
Figure 1.
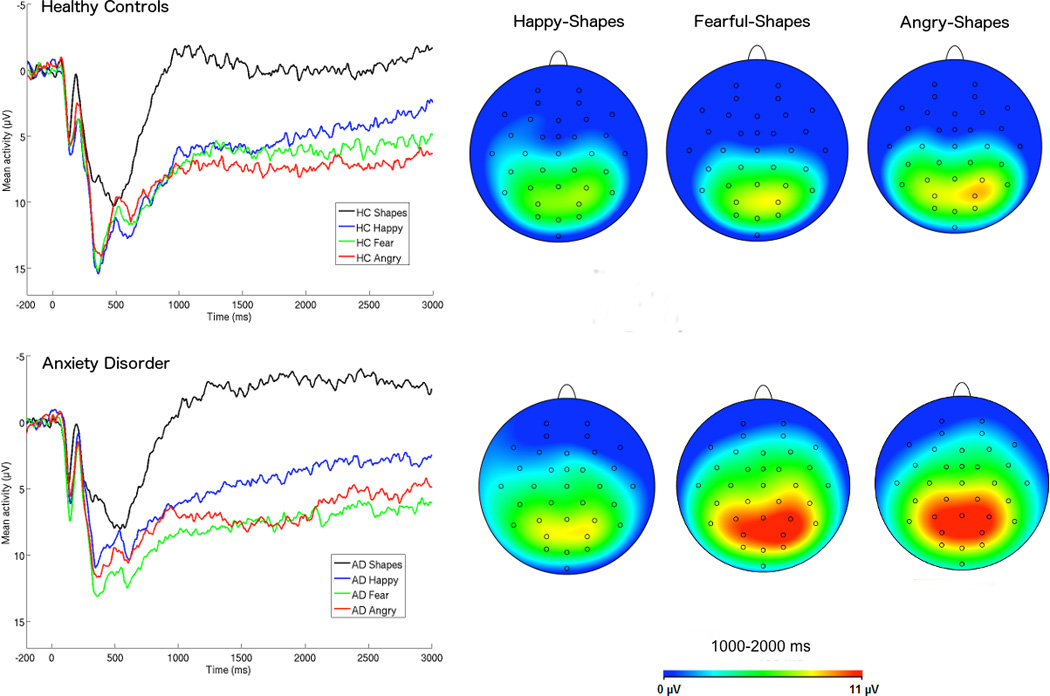
ERPs (negative up) across occipital and parietal electrode sites for healthy control (HC) youth (top) and youth with anxiety disorders (AD; bottom), and scalp distributions depicting the emotional face minus shape difference. AD youth exhibited more positive LPPs to angry and fearful faces, particularly in the middle time window (1000–2000 ms).
Data Analyses
Mixed-design analysis of covariance (ANCOVA) was computed to evaluate the effects of each stimulus type (i.e., angry faces, fearful faces, happy faces, shapes) on reaction time (RT), accuracy, and the LPP. In each analysis, anxiety disorder group (i.e., AD or HC) served as the between–subjects factor. Effects of CDI scores were also examined, and given the broad age range of the sample, age was included as a covariate in all analyses. The three time windows of the LPP were first evaluated using a 4 (stimulus: angry, fearful, happy, shapes) X 3 (time window: early, middle, late) ANCOVA with follow-up tests of significant interactions to examine individual time windows and specific emotional faces controlling for shapes LPP. Greenhouse-Geisser corrections were applied to p values associated with multiple-df repeated-measures comparisons with violations in the assumption of sphericity.
Results
Participant Characteristics
In terms of site of data collection, 60% of the sample was recruited at UIC. The sample recruited at UIC included a larger proportion of minority participants, while the UM sample was primarily Caucasian, χ2(3) = 20.78, p < .001. In addition, a greater proportion of youth with anxiety disorders compared to healthy controls were recruited from UM compared to UIC, χ2(1) = 4.41, p = .04. Sites did not differ with regard to distribution of sex or primary diagnoses (ps > .01). T-tests indicated that youth recruited at each site did not differ with regard to any LPP amplitudes, age, CDI, or PARS (ps > .01).
With regard to AD diagnoses, 54.7% had current social anxiety disorder (58.5% lifetime), 9.4% current separation anxiety disorder (17.0% lifetime), and 67.9% current GAD (67.9% lifetime). With regard to comorbid anxiety disorders, 9.4% also had current panic disorder (9.4% lifetime), 5.7% obsessive-compulsive disorder (OCD; 7.5% lifetime), 17.0% specific phobia (20.8% lifetime), and 1.9% current post-traumatic stress disorder (PTSD; 1.9% lifetime). With regard to number of anxiety disorders, 50.9% had one, 32.1% had two, and 17.0% had three current anxiety disorders. In addition, 9.4% of the AD group had current depressive disorders (13.2% lifetime), including major depressive disorder (MDD), dysthymia, and depression not otherwise specified (NOS), and 11.3% had attention deficit hyperactivity disorder (ADHD)1.
As expected, the AD group (M = 22.91, SD = 4.65) had higher PARS total scores than the HC group (M = 1.64, SD = 2.78), t(85) = 24.51, p < .001. In addition, the AD group (M = 13.28, SD = 7.88) had higher CDI scores than the HC group (M = 2.46, SD = 2.93), t(88) = 7.97, p < .001. The groups did not differ with regard to age, t(88) = 0.47, p = .64, distribution of sex, χ2(1) = .00, p = .95, or distribution of race, χ2(3) = 2.63, p = .45.
Behavioral Results
First, a mixed-design ANCOVA was computed for accuracy. The main effect of stimulus was significant, F(3, 258) = 36.75, p < .001, ηp2 = .30. All paired samples t-tests between conditions were significant (p < .05), with accuracy for matching shapes (M = 98.8%, SD = 2.6) > happy faces (M = 96.8%, SD = 6.1) > fearful faces (M = 95.5%, SD = 6.6) > angry faces (M = 86.2%, SD = 8.3). The main effects of group and CDI on accuracy, as well as the interactions with stimulus type were not significant (ps > .46).
RT data were unavailable for 2 participants. Similar to accuracy results, the main effect of stimulus was significant, F(3, 252) = 94.48, p < .001, ηp2 = .53. All paired samples t-tests between conditions were significant (p < .001), with RT for matching angry faces (M = 1429.80, SD = 303.95) > fearful faces (M = 1346.45, SD = 318.20) > happy faces (M = 1268.37, SD = 286.65) > shapes (M = 803.75, SD = 188.60). The main effects of group and CDI, as well as the stimulus X group and stimulus X CDI interactions were not significant (ps > .30).
LPP Results
In the overall ANCOVA, the stimulus X time X group interaction was significant, F(3.50, 516) = 2.47, p = .02, ηp2 = .03, Greenhouse-Geisser corrected p = .05, and follow up analyses evaluated the model in each of the three LPP time windows2.
Early Window
The effect of stimulus was significant in the early time window, F(3, 258) = 4.99, p < .01, ηp2 = .06. The main effects of group and CDI, as well as interactions with stimulus did not reach significance (ps > .17).
Middle Window
The stimulus X anxiety group interaction was significant, F(2.70, 258) = 3.20, Greenhouse-Geisser corrected p = .03, ηp2 = .04. The stimulus X CDI interaction did not reach significance, F(2.70, 258) = 1.90, Greenhouse-Geisser corrected p = .14, ηp2 = .02.
Effects of anxiety group on each emotion type were then evaluated, controlling for shapes LPP. For angry faces, anxiety was associated with an enhanced LPP, F(1, 85) = 6.29, p = .01, η2p = .07 (Figure 1), and depressive symptoms were negatively associated with the angry LPP, F(1, 85) = 6.52, p = .01, η2p = .07, such that greater depressive symptoms predicted a reduced LPP to angry faces (Figure 2). Similar associations were observed for fearful faces, with anxiety associated with an enhanced LPP, F(1, 85) = 4.97, p = .03, η2p = .063, and the negative effect of CDI on the LPP to fearful faces approaching significance (p = .10). The effects of anxiety and CDI were not significant for happy faces (ps > .45).
Figure 2.
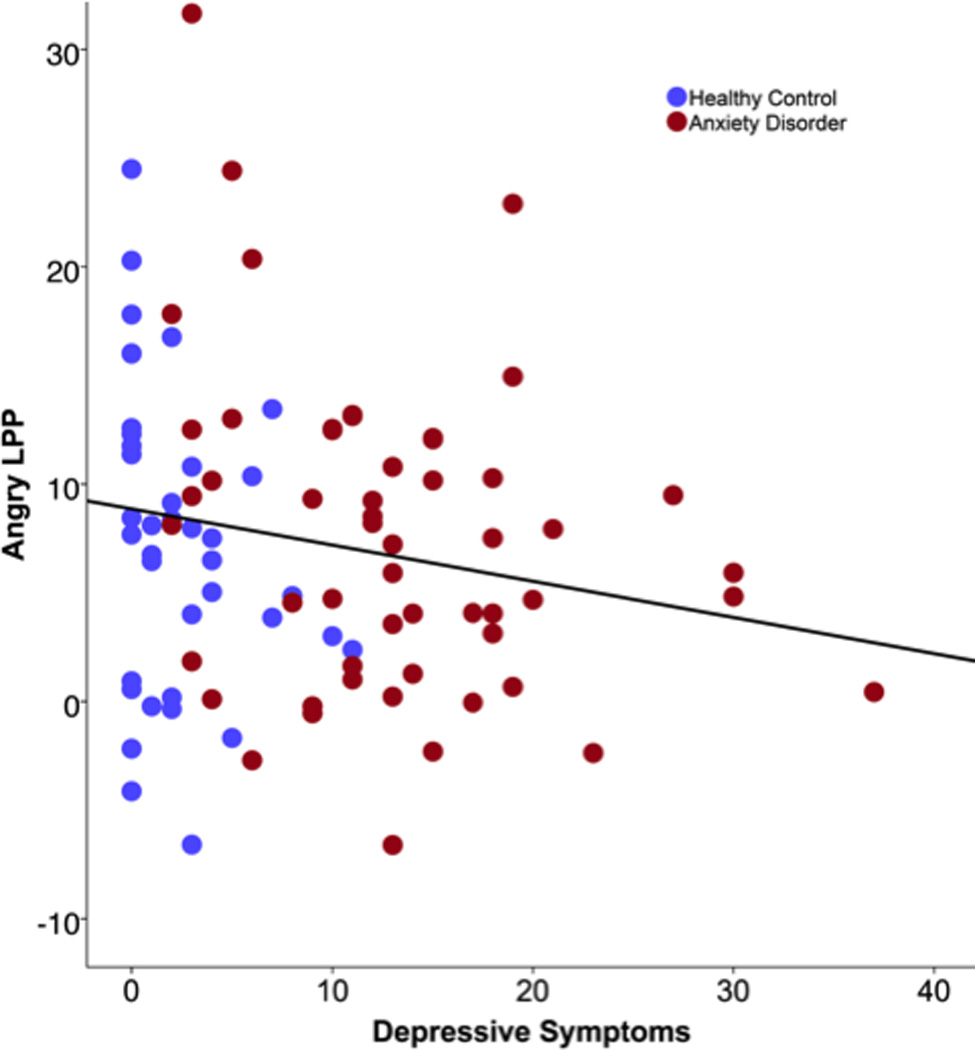
Scatter plot depicting the association between depressive symptoms and the LPP to angry faces 1000–2000 ms after onset. Greater depressive symptoms were associated with a less positive (i.e., blunted) LPP to angry faces when controlling for shapes LPP.
Late Window
The stimulus X anxiety group, F(3, 258) = 2.29, p = .08, η2p = .03, and stimulus X CDI, F(3, 258) = 2.21, p = .09, η2p = .03, interactions did not reach significance in the late LPP window, though the interactions approached significance with comparable patterns of results as observed in the previous time window.
Specific Anxiety Disorders
In order to evaluate whether the anxiety effects were driven by a specific anxiety diagnosis, we computed two additional ANCOVAs examining effects of presence or absence of lifetime social anxiety, separation anxiety disorder, and GAD on the LPP to angry and fearful faces in the middle window where the overall effect of anxiety was most apparent (controlling for CDI, age, and shapes LPP). Interactions between anxiety disorders were not included in the model due to low n in some cells. Social anxiety disorder was associated with an enhanced LPP to angry, F(1, 83) = 10.94, p = .001, η2p = .12, and fearful faces, F(1, 83) = 8.32, p = .01, η2p = .09, but the effects of separation anxiety disorder and GAD did not reach significance (ps > .18).
Anxiety Severity
Lastly, we computed two ANCOVAs to evaluate the effect of PARS total score within the AD group (controlling for CDI, age, and shapes LPP) on the LPP to angry and fearful faces in the middle window. The effect of PARS did not reach significance (ps > .17).
Discussion
We evaluated electrocortical (i.e., LPP) and behavioral measures of processing of emotional faces in youth with current anxiety disorders and healthy controls. Though youth with anxiety disorders did not differ from healthy controls with regard to accuracy and response speed, anxiety disorders were associated with heightened electrocortical activation to threatening (i.e., fearful and angry) faces. Interestingly, effects of anxiety on the LPP were most apparent in relatively late stages of processing (1000–2000 ms after stimulus onset, with a trend for effects in the 2000–3000 ms window). There is evidence that the LPP may reflect a series of overlapping components that peak earlier and later across presentation time (Hajcak et al., 2011; Foti et al., 2009), and the current findings suggest that anxiety disorders in youth are characterized by sustained processing of threatening faces as measured by relatively later portions of the LPP. On the other hand, the extent of depressive symptoms was associated with a blunted LPP to angry faces.
The LPP is thought to reflect elaborative processing of and sustained attention towards motivationally salient information (Hajcak et al., 2011); thus, the current findings indicate that youth with anxiety exhibit enhanced reactivity and sustained attention to threat compared to healthy controls. In addition, these results suggest that enhanced neural reactivity to threatening faces, as measured by the LPP, may be a biomarker for anxiety in youth that is relatively specific to anxiety, rather than depression. Anxious children and adolescents have previously been shown to exhibit attentional biases towards signals of threat in behavioral tasks (Bar-Haim et al., 2007; Roy et al., 2008), as well as heightened neural (e.g., amygdala) reactivity to threat in fMRI studies (Monk, 2008; Strawn et al., 2014); however, to our knowledge, the current study is the first to examine the LPP as a measure of threat reactivity in GAD, social anxiety disorder, and separation anxiety disorder in youth.
The current findings may have important implications for understanding the development of anxiety. That is, there is some evidence that abnormalities in the LPP may be observable among children at high risk for psychopathology prior to the onset of symptoms. For example, young children who exhibit behaviors consistent with fearful temperament styles have been shown to exhibit enhanced LPPs to unpleasant stimuli (Solomon, DeCicco, & Dennis, 2012) and blunted LPPs to emotional faces have been observed in children at high risk for depression (Kujawa, Hajcak et al., 2012). Thus, the LPP may provide a neural measure of patterns of emotional reactivity that contribute to the development of internalizing disorders. Importantly, extent of LPP magnitude to threatening faces was not significantly correlated with anxiety symptom severity in the current study, suggesting that it may reflect relatively trait-like patterns of emotional reactivity rather than a symptom-dependent measure. Consistent with this, there is also some evidence linking enhanced LPPs to threat to greater trait anxiety in adults (Holmes, Nielsen, Tipper, & Green, 2009; Li, Zinbarg, & Paller, 2007). Future research is needed to examine the relationship between an increased LPP to social threat and the development of anxiety, in terms of whether this is a state marker of the disorder or a vulnerability that may play a role in etiology.
While overall effects of anxiety on the LPP to angry and fearful faces were observed, follow up analyses indicated that the effects were primarily driven by social anxiety disorder, rather than GAD or separation anxiety disorder, and were not related to overall anxiety severity. This finding is surprising in that behavioral and fMRI measures of enhanced reactivity towards threat have been observed in a range of anxiety disorders (Bar-Haim et al., 2007; Strawn et al., 2014). At the same time, however, the LPP may be uniquely enhanced for stimuli relevant to the clinical fear. For example, individuals with spider phobias exhibit increased LPPs to spider images but not to more general negative or fearful images (Leutgeb et al., 2009; Michalowski et al., 2009). Thus, it is possible that the unique effects of social anxiety in the current study are due to the use of emotional faces, which serve as potent non-verbal signals of social feedback and thus may be more relevant and salient to fear of social evaluation, and that increased LPPs may be observed in youth with GAD and/or separation anxiety disorder with stimuli that are more relevant to patients’ specific fears. Additional work is needed to evaluate this possibility.
Lastly, the current results indicate that symptoms of depression in both healthy controls and anxious youth may be characterized by reduced engagement with at least some types of emotional stimuli. While this finding is in line with previous findings of blunted LPPs in adult depression and children at high risk for depression (Foti et al., 2010; Kayser et al., 2000; Kujawa, Hajcak et al., 2012; Proudfit et al., in press), it must be interpreted cautiously as the interactions between condition and depressive symptoms did not reach significance in the overall model. Nonetheless, it is important to note that youth with severe depression were excluded from the study; thus, the range of depressive symptoms in the study was somewhat limited, yet associations between depressive symptoms and a blunted LPP to angry faces were still apparent. It is surprising that depressive symptoms did not appear to be associated with reduced LPPs to happy faces; however, it has previously been suggested that happy faces elicit a relatively weak LPP response that limits the ability to detect depression effects (Foti et al., 2010).
Limitations
A few limitations of the current study should be noted. First, though the overall sample size was large enough to detect effects of anxiety disorders on the LPP, the power to detect effects of specific anxiety diagnoses may be somewhat limited and we cannot rule out the possibility that this contributed to the lack of significant effects for GAD or separation anxiety disorder. This may be particularly relevant for separation anxiety disorder, which was less common in the current sample than social anxiety disorder or GAD. Future studies are needed to delineate diagnostic specificity of LPP hyper-reactivity to threatening emotional faces. We also included a broad range of ages, spanning developmental stages from middle childhood to late adolescence/young adulthood. Though we controlled for age in our analyses to account for developmental changes and did not find evidence that age moderated the effects of anxiety on the LPP to angry or fearful faces, longitudinal studies and research designs evaluating samples of children in similar age ranges are needed to clarify the developmental course of these effects. Relatedly, the CDI has not been validated for use with young adults, which may have limited our assessment of depressive symptoms.
Conclusion
The current study is among the first to identify electrocortical measures of increased reactivity to threatening faces in anxiety disorders in youth. Results indicate that the LPP may be a useful measure of threat reactivity associated with anxiety (especially social anxiety) across development and highlight the importance of accounting for comorbid depressive symptoms in examining psychophysiological measures of emotional processing in anxiety.
Acknowledgments
This work was supported by National Institute of Mental Health Grant R01-MH086517 to Christopher S. Monk and K. Luan Phan.
Footnotes
Models were also evaluated excluding youth with depressive disorders and ADHD, and no substantive changes in results were observed.
Because participants were recruited across two sites, we also evaluated the main model controlling for site. Effects of site on the LPP were not significant (ps > .18) and controlling for sites did not substantively change the results. In addition, the model was tested including sex as a between-subjects factor. Effects of sex on the LPP were not significant (ps > .29) and including sex did not change the overall findings.
We also evaluated whether age moderated the effect of anxiety of the LPP to angry and fearful faces in the middle window. The age X anxiety group interactions were not significant (ps > .52).
References
- Banaschewski T, Brandeis D. Annotation: What electrical brain activity tells us about brain function that other techniques cannot tell us – a child psychiatric perspective. Journal of Child Psychology and Psychiatry. 2007;48:415–435. doi: 10.1111/j.1469-7610.2006.01681.x. [DOI] [PubMed] [Google Scholar]
- Bar-Haim Y, Lamy D, Pergamin L, Bakermans-Kranenburg MJ, van Ijzendoorn MH. Threat-related attentional bias in anxious and nonanxious individuals: A meta-analytic study. Psychological Bulletin. 2007;133:1–24. doi: 10.1037/0033-2909.133.1.1. [DOI] [PubMed] [Google Scholar]
- Birbaumer N, Grodd W, Diedrich O, Klose U, Erb M, Lotze M, Flor H. fMRI reveals amygdala activation to human faces in social phobics. Neuroreport. 1998;9:1223–1226. doi: 10.1097/00001756-199804200-00048. [DOI] [PubMed] [Google Scholar]
- Blackford JU, Pine DS. Neural substrates of childhood anxiety disorders: A review of neuroimaging findings. Child and Adolescent Psychiatric Clinics of North America. 2012;21:501–525. doi: 10.1016/j.chc.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bylsma LM, Morris BH, Rottenberg J. A meta-analysis of emotional reactivity in major depressive disorder. Clinical Psychology Review. 2008;28:676–691. doi: 10.1016/j.cpr.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Cuthbert BN, Schupp HT, Bradley MM, Birbaumer N, Lang PJ. Brain potentials in affective picture processing: Covariation with autonomic arousal and affective report. Biological Psychology. 2000;52:95–111. doi: 10.1016/s0301-0511(99)00044-7. [DOI] [PubMed] [Google Scholar]
- Foti D, Hajcak G, Dien J. Differentiating neural responses to emotional pictures: evidence from temporal-spatial PCA. Psychophysiology. 2009;46:521–530. doi: 10.1111/j.1469-8986.2009.00796.x. [DOI] [PubMed] [Google Scholar]
- Foti D, Olvet DM, Klein DN, Hajcak G. Reduced electrocortical response to threatening faces in major depressive disorder. Depression and Anxiety. 2010;27:813–820. doi: 10.1002/da.20712. [DOI] [PubMed] [Google Scholar]
- Goldberg DP, Krueger RF, Andrews G, Hobbs MJ. Emotional disorders: Cluster 4 of the proposed meta-structure for DSM-V and ICD-11. Psychological Medicine. 2009;39:2043–2059. doi: 10.1017/S0033291709990298. [DOI] [PubMed] [Google Scholar]
- Gratton G, Coles MG, Donchin E. A new method for off-line removal of ocular artifact. Electroencephalography & Clinical Neurophysiology. 1983;55:468–484. doi: 10.1016/0013-4694(83)90135-9. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Dennis TA. Brain potentials during affective picture processing in children. Biological Psychology. 2009;80:333–338. doi: 10.1016/j.biopsycho.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajcak G, Weinberg A, MacNamara A, Foti D. ERPs and the study of emotion. In: Luck SJ, Kappenman E, editors. Handbook of event-related potential components. New York: Oxford University Press; 2011. [Google Scholar]
- Hariri AR, Tessitore A, Mattay VS, Fera F, Weinberger DR. The amygdala response to emotional stimuli: a comparison of faces and scenes. Neuroimage. 2002;17:317–323. doi: 10.1006/nimg.2002.1179. [DOI] [PubMed] [Google Scholar]
- Holmes A, Nielsen MK, Tipper S, Green S. An electrophysiological investigation into the automaticity of emotional face processing in high versus low trait anxious individuals. Cognitive, Affective, & Behavioral Neuroscience. 2009;9:323–334. doi: 10.3758/CABN.9.3.323. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Kaufman Birmaher B, Brent D, Rao U, Flynn C, Moreci PJ, et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime version (K-SADS-PL): Initial reliability and validity data. Journal of the American Academy of Child & Adolescent Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Kayser J, Bruder GE, Tenke CE, Stewart JW, Quitkin FM. Event-related potentials (ERPs) to hemifield presentations of emotional stimuli: Differences between depressed patients and healthy adults in P3 amplitude and asymmetry. International Journal of Psychophysiology. 2000;36:211–236. doi: 10.1016/s0167-8760(00)00078-7. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Kovacs M. Children's Depression Inventory. Toronto, ON: Multi-Health Systems, Inc.; 1992. [Google Scholar]
- Kring AM. The future of emotion research in the study of psychopathology. Emotion Review. 2010;2:225–228. [Google Scholar]
- Kujawa A, Hajcak G, Torpey D, Kim J, Klein DN. Electrocortical reactivity to emotional faces in young children and associations with maternal and paternal depression. Journal of Child Psychology and Psychiatry. 2012;53:207–215. doi: 10.1111/j.1469-7610.2011.02461.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa A, Klein DN, Hajcak G. Electrocortical reactivity to emotional images and faces in middle childhood to early adolescence. Developmental Cognitive Neuroscience. 2012;2:458–467. doi: 10.1016/j.dcn.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa A, Klein DN, Proudfit GH. Two-year stability of the late positive potential across middle childhood and adolescence. Biological Psychology. 2013;94:290–296. doi: 10.1016/j.biopsycho.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa A, Weinberg A, Hajcak G, Klein DN. Differentiating event-related potential components sensitive to emotion in middle childhood: Evidence from temporal–spatial PCA. Developmental Psychobiology. 2013;55:539–550. doi: 10.1002/dev.21058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labuschagne I, Phan KL, Wood A, Angstadt M, Chua P, Heinrichs M, Nathan PJ. Oxytocin attenuates amygdala reactivity to fear in generalized social anxiety disorder. Neuropsychopharmacology. 2010;35:2403–2413. doi: 10.1038/npp.2010.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leutgeb V, Schäfer A, Köchel A, Scharmüller W, Schienle A. Psychophysiology of spider phobia in 8- to 12-year-old girls. Biological Psychology. 2010;85:424–431. doi: 10.1016/j.biopsycho.2010.09.004. [DOI] [PubMed] [Google Scholar]
- Leutgeb V, Schäfer A, Schienle A. An event-related potential study on exposure therapy for patients suffering from spider phobia. Biological Psychology. 2009;82:293–300. doi: 10.1016/j.biopsycho.2009.09.003. [DOI] [PubMed] [Google Scholar]
- Li W, Zinbarg RE, Paller KA. Trait anxiety modulates supraliminal and subliminal threat: brain potential evidence for early and late processing influences. Cognitive, Affective, & Behavioral Neuroscience. 2007;7:25–36. doi: 10.3758/cabn.7.1.25. [DOI] [PubMed] [Google Scholar]
- Liu Y, Huang H, McGinnis-Deweese M, Keil A, Ding M. Neural substrate of the late positive potential in emotional processing. The Journal of Neuroscience. 2012;32:14563–14572. doi: 10.1523/JNEUROSCI.3109-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacNamara A, Hajcak G. Distinct electrocortical and behavioral evidence for increased attention to threat in generalized anxiety disorder. Depression and anxiety. 2010;27:234–243. doi: 10.1002/da.20679. [DOI] [PubMed] [Google Scholar]
- MacNamara A, Post D, Kennedy AE, Rabinak CA, Phan KL. Electrocortical processing of social signals of threat in combat-related post-traumatic stress disorder. Biological Psychology. 2013;94:441–449. doi: 10.1016/j.biopsycho.2013.08.009. [DOI] [PubMed] [Google Scholar]
- Michalowski JM, Melzig CA, Weike AI, Stockburger J, Schupp HT, Hamm AO. Brain dynamics in spider-phobic individuals exposed to phobia-relevant and other emotional stimuli. Emotion. 2009;9:306–315. doi: 10.1037/a0015550. [DOI] [PubMed] [Google Scholar]
- Mogg K, Garner M, Bradley BP. Anxiety and orienting of gaze to angry and fearful faces. Biological Psychology. 2007;76:163–169. doi: 10.1016/j.biopsycho.2007.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohatt J, Bennett SM, Walkup JT. Treatment of separation, generalized, and social anxiety disorders in youths. American Journal of Psychiatry. 2014;171:741–748. doi: 10.1176/appi.ajp.2014.13101337. [DOI] [PubMed] [Google Scholar]
- Monk CS. The development of emotion-related neural circuitry in health and psychopathology. Development and Psychopathology. 2008;20:1231–1250. doi: 10.1017/S095457940800059X. [DOI] [PubMed] [Google Scholar]
- Monk CS, Telzer EH, Mogg K, Bradley BP, Mai X, Louro HM, Pine DS. Amygdala and ventrolateral prefrontal cortex activation to masked angry faces in children and adolescents with generalized anxiety disorder. Archives of General Psychiatry. 2008;65:568–576. doi: 10.1001/archpsyc.65.5.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran TP, Jendrusina AA, Moser JS. The psychometric properties of the late positive potential during emotion processing and regulation. Brain Research. 2013;1516:66–75. doi: 10.1016/j.brainres.2013.04.018. [DOI] [PubMed] [Google Scholar]
- Moser JS, Huppert JD, Duval E, Simons RF. Face processing biases in social anxiety: An electrophysiological study. Biological Psychology. 2008;78:93–103. doi: 10.1016/j.biopsycho.2008.01.005. [DOI] [PubMed] [Google Scholar]
- Mühlberger A, Wieser MJ, Herrmann MJ, Weyers P, Tröger C, Pauli P. Early cortical processing of natural and artificial emotional faces differs between lower and higher socially anxious persons. Journal of Neural Transmission. 2009;116:735–746. doi: 10.1007/s00702-008-0108-6. [DOI] [PubMed] [Google Scholar]
- Phan KL, Fitzgerald DA, Nathan PJ, Tancer ME. Association between amygdala hyperactivity to harsh faces and severity of social anxiety in generalized social phobia. Biological Psychiatry. 2006;59:424–429. doi: 10.1016/j.biopsych.2005.08.012. [DOI] [PubMed] [Google Scholar]
- Prater KE, Hosanagar A, Klumpp H, Angstadt M, Phan KL. Aberrant amygdala–frontal cortex connectivity during perception of fearful faces and at rest in generalized social anxiety disorder. Depression and Anxiety. 2013;30:234–241. doi: 10.1002/da.22014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proudfit GH, Bress JN, Foti D, Kujawa A, Klein DN. Depression and event-related potentials: Emotional disengagement and reward insensitivity. Current Opinion in Psychology. doi: 10.1016/j.copsyc.2014.12.018. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resarch Units of Pediatric Psychopharmacology Anxiety Study Group. The pediatric anxiety rating scale (PARS): Development and psychometric properties. Journal of the American Academy of Child & Adolescent Psychiatry. 2002;41:1061–1069. doi: 10.1097/00004583-200209000-00006. [DOI] [PubMed] [Google Scholar]
- Rottenberg J, Gross JJ, Gotlib IH. Emotion context insensitivity in major depressive disorder. Journal of Abnormal Psychology. 2005;114:627–639. doi: 10.1037/0021-843X.114.4.627. [DOI] [PubMed] [Google Scholar]
- Rottenberg J, Johnson SL. Emotion and psychopathology: Bridging affective and clinical science. Washington, DC US: American Psychological Association; 2007. [Google Scholar]
- Roy AK, Vasa RA, Bruck M, Mogg K, Bradley BP, Sweeney M, Pine DS. Attention bias toward threat in pediatric anxiety disorders. Journal of the American Academy of Child & Adolescent Psychiatry. 2008;47:1189–1196. doi: 10.1097/CHI.0b013e3181825ace. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatinelli D, Keil A, Frank DW, Lang PJ. Emotional perception: Correspondence of early and late event-related potentials with cortical and subcortical functional MRI. Biological Psychology. 2013;92:513–519. doi: 10.1016/j.biopsycho.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatinelli D, Lang PJ, Keil A, Bradley MM. Emotional perception: Correlation of functional MRI and event-related potentials. Cerebral Cortex. 2007;17:1085–1091. doi: 10.1093/cercor/bhl017. [DOI] [PubMed] [Google Scholar]
- Shin LM, Wright CI, Cannistraro PA, Wedig MM, McMullin K, Martis B, Krangel TS. A functional magnetic resonance imaging study of amygdala and medial prefrontal cortex responses to overtly presented fearful faces in posttraumatic stress disorder. Archives of General Psychiatry. 2005;62:273–281. doi: 10.1001/archpsyc.62.3.273. [DOI] [PubMed] [Google Scholar]
- Solomon B, DeCicco JM, Dennis TA. Emotional picture processing in children: an ERP study. Developmental Cognitive Neuroscience. 2012;2:110–119. doi: 10.1016/j.dcn.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strawn JR, Dominick KC, Patino LR, Doyle CD, Picard LS, Phan KL. Neurobiology of pediatric anxiety disorders. Current Behavioral Neuroscience Reports. 2014;1:154–160. doi: 10.1007/s40473-014-0014-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suveg C, Southam-Gerow MA, Goodman KL, Kendall PC. The role of emotion theory and research in child therapy development. Clinical Psychology: Science & Practice. 2007;14:358–371. [Google Scholar]
- Thomas KM, Drevets WC, Dahl RE, Ryan ND, Birmaher B, Eccard CH, Casey B. Amygdala response to fearful faces in anxious and depressed children. Archives of General Psychiatry. 2001;58:1057–1063. doi: 10.1001/archpsyc.58.11.1057. [DOI] [PubMed] [Google Scholar]
- Walkup JT, Albano AM, Piacentini J, Birmaher B, Compton SN, Sherrill JT, Kendall PC. Cognitive behavioral therapy, sertraline, or a combination in childhood anxiety. The New England Journal of Medicine. 2008;359:2753–2766. doi: 10.1056/NEJMoa0804633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weems CF, Silverman WK. Anxiety disorders. In: Beauchaine TP, Hinshaw SP, editors. Child and adolescent psychopathology. Hoboken, NJ: John Wiley; 2013. pp. 513–541. [Google Scholar]


