Abstract
This research describes the design, deployment, performance, and acceptability of a novel outdoor active air sampler to provide simultaneous measurements of multiple contaminants at timed intervals for the Aggravating Factors of Asthma in Rural Environment (AFARE) study—a longitudinal cohort of 50 children in Yakima Valley, Washington. The sampler was constructed of multiple sampling media connected to individual critical orifices and a rotary vane vacuum pump. It was connected to a timed control valve system to collect 24 hours samples every six days over 18 months. We describe a spatially representative approach with both quantitative and qualitative location criteria to deploy a network of 14 devices at participant residences in a rural region (20 × 60 km). Overall the sampler performed well, as the concurrent mean sample flow rates were within or above the ranges of recommended sampling rates for each exposure metric of interest. Acceptability was high among the study population of Hispanic farmworker participant households. The sampler design may prove useful for future urban and rural community-based studies with aims at collecting multiple contaminant data during specific time periods.
1 Introduction
Communities living in rural agricultural settings may be exposed to an array of biological (e.g. organic dusts from animal and crop products, bacteria, fungi, endotoxins) and non-biological particles and gases (e.g. pesticides, hydrogen sulfide and ammonia).1 In addition, diesel particulate may also be exhausted from farming equipment and nearby road ways where shipping trucks are common.2–4 Paved and unpaved rural roadways are a significant source of fugitive dust in these areas due to re-suspension of particulate matter and wind erosion.5 Most of this dust generation from roads can be defined by road surface (paved, dirt or gravel) and traffic. Children with asthma may be particularly vulnerable to health compromise associated with these potential triggers.
Although many previous studies have examined ambient air triggers of childhood asthma in urban environments using residentially-based sampling campaigns or existing regulatory monitoring systems, there are few data on contaminants in rural and agricultural residential settings. Due to low population densities in these areas, community air monitoring stations are sparsely located and less amenable to application to population based studies. Longitudinal residential based assessments that require repeat visits to capture multiple contaminants and temporal variability are less feasible in rural settings often remotely located from academic centers where investigators are based. We sought to develop a practical method of assessing multiple exposures for a longitudinal cohort study of children with asthma in Yakima Valley, Washington (AFARE – Aggravating Factors of Asthma in a Rural Environment).
The first aim was to design a sampler that optimized the number of simultaneous air measurements of common asthmatic triggers in rural environments without substantial increases in cost or loss of temporal resolution. These measurements will provide the opportunity to evaluate associations with multiple time-resolved estimates of disease status in the AFARE study. The second aim was to use GIS tools to deploy the samplers in a manner that was geographically representative of the AFARE cohort. The third aim was to examine participant acceptability of the outdoor sampling device in the AFARE cohort which is represented largely by a Hispanic farmworker population with relatively high mobility and few homeowners.
2 Sampling methods
2.1 Design
To construct the outdoor sampler, we created a protected sampling chamber in which five active air sampling devices could operate side-by-side. The unit was stored and locked in a waterproof 24 gallon container (Rubbermaid® Actionpacker™) which enhanced portability. It was lined with polyurethane foam to reduce noise. Inside, the chamber was a polytetrafluoroethylene (PFTE) container (33 × 25 × 25 cm) connected to an inlet for outdoor air that was protected from obstruction with wire mesh fitting (see Fig. 1). The air is circulated and flows through an outlet connected to an oil-less rotary vane vacuum pump (GAST Model 0523) after being pulled through five active air sampling (AAS) tubes. Although the pump relies on internal intake and filtration, additional steps were taken to lessen exhaust through additional side vents and a localized exhaust fan.6
Fig. 1.
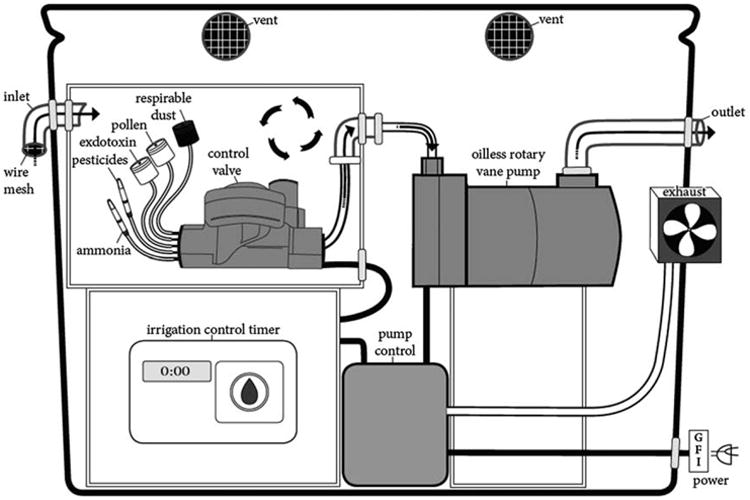
AFARE household monitor.
After literature review of contaminants of concern unique to Washington State agriculture (see Table 3 for references); we decided the sampler needed to be designed to perform at the required flow rates consistent for measurements of total dust (e.g. fugitive dust), respirable dust (≤4 um diameter), pesticides, ammonia (NH3), 1-nitropyrene (diesel), levoglucosan (wood smoke), pollen, endotoxin, and other potential microbes (animal dander or fungal spores).
Table 3. Sample type and measured flow rates (LPM) for various critical orifices17–36.
| Sample | Critical orifice | Flow rates (LPM) | |||||
|---|---|---|---|---|---|---|---|
|
|
|
|
|||||
| Type | Contaminant | Reference | Media | Needle gauge (G) | Reference range | Measured mean (range) | CV (%) |
| Ammonia | NH3 | Donham and Popendorf, 1985; Donham 1993, Pratt 1993 | Silica | 27 | 0.1–0.5 (OSHA 188; Bishop et al., 1986) | 0.16 (0.10–0.32) | 36.6 |
| OP pesticidesa | Chlorpyrifos | Lu et al., 2000; Li 2007; Armstrong et al., 2013; Fenske et al., 2009 | PUF | 20 | 1.0–5.0 (NIOSH 5600, EPA TO-10A) | 3.37 (2.74–3.91) | 10.6 |
| Malathion Azinphos methyl Parathion | |||||||
| Total dust | Bacterial endotoxin | Clark et al., 1983; Heederik and Sigsgaard, 2005 | PVC (47 mm) | 18 | 2.0–4.0 (Milton et al., 1995, ACGIH 1989) | 4.06 (3.52–4.69) | 10.0 |
| Pollen | Sofiev and Bergmann, 2013 | PVC (47 mm) | 18 | 1.0–3.0 (NIOSH 0500) | 3.96 (3.59–4.52) | 7.48 | |
| Fungal spores | Clark et al., 1983; Pratt et al., 1990 | ||||||
| Animal dander | Kline and Schwartz, 1998; Willams et al., 2011 | ||||||
| Respirable dust | Levoglucosan (wood smoke) | Simpson et al. 2004; Naeher et al., 2007; Allen et al., 2008 | Teflon (37 mm) | 18 | 2.2–2.5; 4 μm dia. (NIOSH 0600), 3.2–3.5; 3.5 μm dia. (NIOSH 0600) | 4.15 (3.70–5.20) | 8.83 |
| 1-Nitropyrene (diesel) | Hoppin et al., 1994 | 4.0–5.0; ≤2.5 μm dia. (Chan and Lippman, 1977) | |||||
OP = Organophosphorus.
All inlets and joints were secured by o-rings, PVC joints, and Teflon tape. The active air sampling devices included two total dust cassettes (47 mm polypropylene SKC# 225-8497) with PVC filters (SKC# 225-809), one respirable dust cyclone (37 mm styrene SKC# 225-3050LF, aluminum cyclone #225-01-02) with PFTE filter (SKC# 225-17-01), a silica bead ammonia sampling tube (SKC#226-10-06), and polyurethane foam (PUF) sampling tube (SKC#226-92).
Sampling media were connected to individual critical orifices with Tygon tubing. Differential sizing of the critical orifices was accomplished by using hypodermic needles (Fisnar Straight Blunt End, 1.5 in, Cat #5601107 and 5601108) to generate varying air flows ranging from 0.1–4.5 liters per minute (LPM) (Table 3). Hypodermic needles have been identified as convenient low-cost critical orifices to control flow rates.7,8 The needles were re-used up to six times and checked for obstructions before being replaced.
The sampler was linked to a modular controller to allow the determination of air concentrations at a weekly temporal scale throughout the 18 months study period. This was done by inserting the sharp ends of the hypodermic needles into rubber padding (2 cm thick) between the control valve opening, which is 2.5 cm in diameter (¾ ″ Orbit Water Master Sprinkler Valve). The control valve is connected to a terminal on an irrigation control timer, Rain Bird ESP-4M Modular, for residential outdoor use with a master valve/pump start relay.9 The controller has an internal transformer that reduces standard voltage to operate the valves. An outdoor power supply was connected to the controller and the rotary vane pump plugged into a properly grounded ground fault interrupter (GFI) into an outdoor 120 VAC outlet. The controller was connected to the exhaust fan, which turned on automatically during pump operation.
The main risks of the sampler involved electrical hazards and potential heat generated from the rotary vane vacuum pump. A small amount of noise was present during pump operation. Noise was reduced by housing the sampler in a storage container lined with polyurethane foam and four small vents located 5 cm below the lid.
2.2 Performance
The controller's LCD display was used to program operations at specific time intervals. On every six day interval during the study period, the valve was set to open after receiving the signal from the controller to begin a 24 hour sample. The weekly samples were picked up the following day by research field staff. During that time, the researchers also exchanged sampling media. These samples will provide future information on the changing weekly concentrations of multiple contaminants for the AFARE study.
Once a month, air sampling flow rates were calibrated from each critical orifice while manually operating the pump by attaching a DryCal DC-Lite to the Teflon tubing. Flow rates were calculated separately for each device in order to calculate individual air volumes. During the first 10 months of sampler deployment, we calculated the average flow rate (arithmetic mean) and range of flow rates for all 14 devices(n = 140). The calibrated field flow rates were directly compared with recommended flow rates in other air monitoring studies (see Table 3).
2.3 Deployment
The selection of 14 appropriate air monitoring locations was based on a combination of quantitative and qualitative criteria listed in Table 1. ArcGIS 10.0 software was used to select participants based on their household location near agricultural fields, in towns, proximity to major roadways, and spatial distribution across the agricultural valley. Additionally, a number of participants were chosen primarily because of proximity to both confined animal feeding operations (CAFOs) and agricultural fields commonly requiring pesticide application. The locations were also qualitatively selected based on factors such as outdoor security, power supply, participant willingness, and participant retention in study procedures to date.
Table 1. Quantitative and qualitative selection criteria for AFARE sample site locations.
| Selection criteria | |
|---|---|
|
| |
| Quantitative | Description |
| Source proximity | Distance weighting (km) from CAFOs, major roads (interstate, state highways, county parkways), local roads (gravel), agricultural fields |
| Spatial representation | Sample locations representative of all enrolled participant addresses using Esri ArcGIS |
| Qualitative Stable outdoor power source | 120 VAC |
| Outdoor pets/livestock | No outdoor pets/livestock with complete access to sampler |
| Security | Neighborhood is of low concern for device tampering |
| Weather protection | Wind, rain |
| Household participation | Household participants were recruited based on willingness (questionnaire response), and compliance with AFARE study procedures |
2.3.1 Quantitative selection criteria
The proximity of sampling locations to CAFOs, major roadways, and agricultural fields was determined by combining high resolution satellite imaging (NASATerra, Google Earth Pro 6.2) and geocoding in ArcGIS 10.0. Due to notable differences in agricultural production in the region, the AFARE participant's geocoded addresses was broken into two 20 × 20 kilometer zones (see Fig. 2), referred to as the North and South Zones.
Fig. 2.
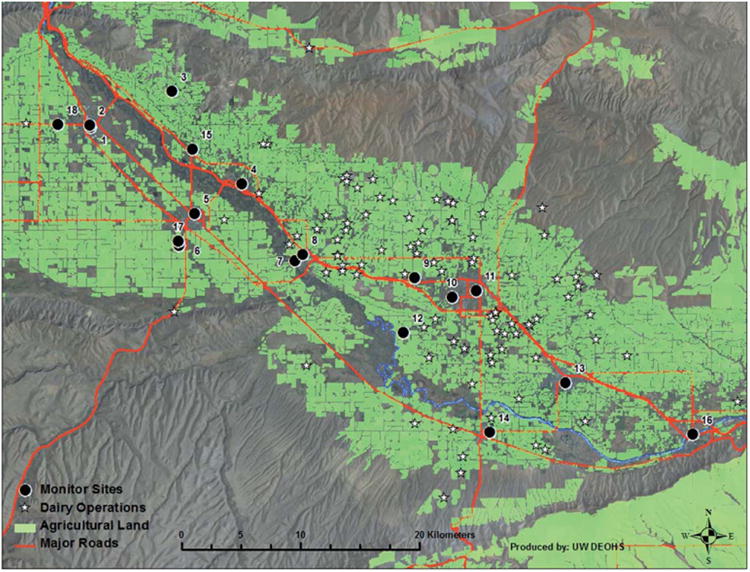
AFARE map of study area. The North Zone includes sites 1–6, 15, 17, and 18. The South Zone includes sites 7–14 and 16. Although 14 monitors were constructed, there are 17 sites on the map to display sites that were relocated.
The North Zone reports high densities of agricultural tree fruit and row crop production. Proximity of sampling locations to agricultural fields was determined using crop field density maps provided by the Washington State Department of Agriculture and the 2011 crop layer data from the US Department of Agriculture National Agricultural Statics Survey (USDA-NASS) in Esri ArcGIS 10.0 (Table 2).10–12
Table 2.
Source proximity grouping criteria for AFARE sample site locations. There are 14 total sampling locations
| CAFO | Major roads | Agricultural fieldsa | ||||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| Classification | Distance (km) | nb | km | nb | km | nb |
| Proximal | <2.0 | 5 | <0.4 | 5 | <0.4 | 4 |
| Intermediate | 2.1–3.0 | 4 | 0.5–1.0 | 5 | 0.5–1.0 | 5 |
| Distal | >3.1 | 5 | >1.0 | 4 | >1.0 | 5 |
| Total sites | 14 | |||||
Distance from nearest field perimeter.
n = number of sampling locations.
In comparison, the South Zone reports high livestock density (cattle/acre) due to the fact that there are >60 dairies in the region.13 The Washington State Department of Ecology (ECY) geographic metadata provides the location of the milking parlor for dairies in Washington as required by RCW 90.64 and is published online for public use at http://www.ecy.wa.gov/services/gis/data/ag/dairy.htm. The dataset was improved with the use of high resolution 2011 NASATerra satellite imaging in Google Earth Pro 6.2 and ArcGIS to geocode locations of CAFOs with visual indicators. These indicators included the presence of 20 or more cattle, waste treatment lagoons, and feeding facilities. Twenty-seven of the CAFOSs were recoded to their proper center point locations using equidistant measurements from the outer perimeter in meters. Using these techniques, seventeen CAFOs were added in addition to those provided by ECY. These unidentified CAFOs may be non-reporting, newly constructed, or the result of multiple-site locations (e.g. feeding, milking, breeding) for a single reporting operation.
There are two major state highways that run through the North and South Zones with networks of county parkways and unpaved county roads in both areas. Although paved roadways are easily accessed through the Washington State Department of Transportation, there was little data on unpaved road networks in this area.
Air monitoring household locations was randomly selected based on the distance fitting criteria for proximal, intermediate, and non-proximal homes (see Table 2). All distances were fitted to the nearest perimeter. It was less common for households to be located in close proximity (<0.4 km) to CAFOs which are often located in isolate areas; so the fitting criteria was adjusted.
2.3.2 Qualitative selection criteria
In addition, sample site locations were selected according to research participant household retention and reliable participation in the biweekly Asthma Control Questionnaire (ACQ). Field staff technicians consulted with household residences about proper location of the sampler (see qualitative criteria, Table 1) which included areas behind outdoor storage sheds, near decks, and in backyards. The sample location required a stable outdoor electricity source, so apartment housing was excluded. The sampler was situated in areas where it could be protected from additional elements, outdoor animals, or tampering. In this region, it was also common for households to keep poultry and pets outdoors.
2.4 Sampler acceptability
One main component of sampler development was to assess acceptability by participant families. The study subjects were children with asthma participating in an asthma outreach program operated by the Yakima Valley Farmworker Clinic. Mean and standard deviation of age was 10.4 ± 2.6. There were roughly equal numbers of boys and girls. Representative of this region with a high proportion of Mexican American immigrants most self-identified as Hispanic (93%) although most were U.S. born (78%). The subjects came from households with limited socioeconomic resources with the majority (73%) reporting household incomes below $30 000 and average household size 5.3 ± 1.6. Subject surveys of residential proximity to sources while the study region is a major rural agricultural center, many of the children lived in a residence within one of the small town areas (59%).
Four questions were added to the routine AFARE cohort final health status survey administered within three months of the end of the research project. Trained research staff administered the surveys in the family's preferred language (Spanish or English.) The parent/guardian of the child subject was asked 1. Was it bothersome to have the air monitor at your home?” Answer selections included “not bothersome at all”; “yes, a little”; or “yes, a lot”). If there were inconveniences, further inquiries were made to pinpoint specific causes. Since the sampler included a rotary vane pump, households were also asked if the noise generated was an issue “How disturbing was the noise from the air monitor?” Answer selections included “not,” “a little,” or “very”). Similar responses were recorded for the question “How bothersome was it to have the study staff visit every 6 days to collect samples?” Finally, caregivers responded whether given the opportunity, they would elect to have a monitor in their yard again.
Responses were collected from fourteen participant families, which included all thirteen who had a monitor at their home at the time of the final survey and one subject who discontinued participation.
3 Results
This paper reports primarily on the findings regarding the performance of the air sampler and participant acceptability. Although preliminary analysis of air monitoring results show spatial and seasonal trends in airborne concentrations, these will be discussed elsewhere.
3.1 Sampler design and performance in the field
Based on individual component costs, the approximate cost of constructing the sampler was $700 USD. This was considered an economical improvement when compared to more complex active air systems requiring multiple pumps. Labor costs were reduced by requiring field staff to visit the samplers only once per week.
During the entire 18 months period of outdoor sampling, three samplers moved with the subjects during change of residence, and one family discontinued the study (n = 1) when a large amount of their household moved out of the area. Since the sampler was non-stationary and readily portable, it could be moved with the family or to nearby participant households. Table 3 reports on mean measured flow rates in the field for various critical orifices from all 14 air monitoring site locations. The silica tubes were connected to a 27 G critical orifice, resulting in an average flow rate of 0.16 LPM. The PUF tubes were connected to a 20 G critical orifice, resulting in average flow rates of 3.37 LPM. These were within the range of recommended sampling rates. The PVC cassettes were connected to 18 G critical orifices, resulting in 4.06 and 3.96 LPM, respectively. Although total dust sampling rates were higher than recommended, they were intentionally set at higher rates (i.e., 3–6 LPM) to achieve larger sampling volumes for lower limits of detection. This was particularly important for attaining pollen counts and conducting endotoxin analysis from polypropylene filters. Since the average respirable dust flow rate was 4.15 LPM, these samplers were more likely capturing particle sizes ≤2.5 μm in diameter.14
3.2 Sampler acceptability
Overall, subjects and their families were overwhelmingly positive in descriptions of their study experience at the time of the final annual survey. Thirteen subjects (93%) noted it was not bothersome while one (7%) responded “yes, a little” and explained that the pump was “a little noisy”. However, when directly asked the second question specifically about noise, all subjects reported “not at all disturbing”.
Thirteen subjects felt that the visits by study staff every six days were “not at all bothersome” while one subject said that the field research staff talked during their visits a lot and woke up the family early in the morning (i.e. “a little bothersome”). When asked whether they would choose to host an air sampler in their yard again if given the opportunity, thirteen subjects replied “yes” and one answered equivocally, saying it would depend on the results of the study.
One family elected to complete their participation in the study procedures (surveys and monitoring) after five months of involvement and was unable to participate in the study final survey. They did report that in addition to not wanting to participate in the ongoing surveys, their property owner had expressed concerns about the air sampler. While they were invited to continue to participate without an air sampler, they elected to leave the study. Records of the biweekly phone interviews which included a question about “problems with the air monitor” revealed no prior reports of problems or concerns with the air samplers during the first five months of study involvement. The property owner did not respond to outreach by the principal investigator to answer any questions or respond to any concerns (phone message).
4 Discussion
We developed a relatively low cost novel active air sampling device for measuring ambient air contaminant concentrations of multiple contaminants of concern in an area of intense agricultural production. Using a spatially representative approach, we placed a network of 14 devices at subject residences in our 20 × 60 km study region to collect 24 hours samples every six days. This will provide a uniquely rich sample databank (∼300 samples) of temporal-spatial exposure data which can be linked to our longitudinal asthma data on a cohort of 50 children with asthma who reside in this area.
A limitation of the study was that some locations had monitoring devices ≤3 meters of a building or tree. The inlet of the sampling device was repeatedly checked for obstructions such as brush, vegetation, dirt, or dust. According to guidelines for sampling provided for EPA National Air Monitoring Stations (NAMS) and a number of the State and Local Air Monitoring Stations (SLAMS), outdoor ambient air monitoring devices should be placed 8−10 meters from potential obstructions such as buildings and trees.15,16 However, the aim of the primary study was to design a sampling device to generate an exposure metric at the household level. Researchers should be cautious if applying these results for air quality classifications on a regional scale. Another limitation was that although the air sample results will be used to provide information on the changing weekly concentrations of multiple contaminants, they were deployed for 24 hours because the cost of running daily samples was prohibitive. Therefore, day-to-day variations should be considered when interpreting this “snap shot” of time over the course of longer time periods. Finally, there may have been bias during the qualitative selection process (Table 1). For example, AFARE households in rental apartments were less likely to fulfill qualitative criteria outlined in Table 1. Therefore, the households situated on secluded private land may have been more likely to qualify for the study. There is no clear data to support this assumption.
Although the air sampling device was almost uniformly acceptable, one case underscores importance of sensitivity of potential housing-landowner conflict in conducting residentially-related environmental research in a population which may be disempowered (e.g. Hispanic immigrant farmworking households) and particularly in communities where land owners may also be employers. This is a common challenge for many environmental health studies that take place in home environments. It highlights the need for the development of non-invasive sampling devices (e.g., passive devices) that are simple to explain to all invested parties. In addition, our experience was positive that the sampler was easy to relocate when three subject households changed residences.
If future studies are to use multi-contaminant sampling devices to examine potential asthmatic triggers in rural areas stemming from CAFOs or unpaved roads, we have demonstrated some geocoding challenges. It is difficult to locate all CAFO locations due to frequent construction of new operations and multiple animal confinement sites part of one larger reporting operation. In this study, >38% of CAFO locations were not reported and had to be identified visually with a satellite mapping tool. This task was tedious because it involved visually scanning the entire research area at low resolution. A single address or reporting location may be insufficient if the aim is to identify all potential sources of contaminants. There was little data on unpaved road networks in this rural area, and we experienced difficulty in using satellite imaging to ascertain a nearest distance to gravel or dirt roads. This was a limiting factor, as unpaved roads are a major source of re-suspended particulates associated with agriculture. Nevertheless in the past it has been difficult for researchers in rural communities to allocate potential sources of agricultural emissions. New technologies such as 2011 NASATerra satellite imaging in Google Earth Pro 6.2 may allow the collection of information at lower cost than previously recognized geographical software, and the applications may be easily accessible to local agencies.
Environmental factors that may aggravate asthma in this and other rural communities include multiple contaminants derived from different types of agricultural production. In this study, we have designed a sampling approach that allows collection of multiple samples contemporaneously to address airborne contaminants such as fugitive dust, endotoxin, pesticides, respirable dust, and other markers of exposure that can be analyzed from dust samples (e.g., levoglucosan for wood-smoke, 1-nitropyrene for diesel). Depending on study location and study questions, the sampling media and flow rates can be modified to suit research needs depending on the exposure metrics or timing of interest. The modular irrigation control timer may be applied for more complex studies because it can be adjusted to operate at different times automatically. The system is able to run several times within a day or week to acquire air samples in tandem without the need for technicians to be present. This will significantly cut labor costs in the field and be less invasive to research participants. This serves as an example for gaining information on simultaneously collected exposure metrics without the need for multiple pumps or a stationary device.
Environmental impact.
Past methods of evaluating airborne environmental triggers of pediatric asthma have typically relied on individual sampling for a singular component in air and many of these studies have taken place primarily in urban settings. In most cases, children in rural areas may experience very different airborne exposures than their urban counterparts, but it is not feasible to place ambient monitoring devices in remote areas. Therefore, the acquisition of rural air quality data in rural areas on the household level can be a challenge for researchers. In this article, we describe the development and performance of an outdoor household air sampler that was created as a novel analytical tool for examining multiple air contaminants simultaneously at low cost for the Aggravating Factors of Asthma in a Rural Environment project (AFARE). The sampler is unique in that it is one of the first developed to examine multiple contaminants focusing on a rural and agricultural setting with one device. Research participant feedback demonstrated high household acceptability, demonstrating that the sampler may be particularly useful for community-based field studies.
Acknowledgments
This work was supported by the NIEHS/EPA 1R21ES017906-01. Resources were also provided by the NIOSH Agricultural Centers Program (NIOSH-2 U50 OH07544). Stacey Holland and Kris Hartin of the Pacific Northwest Agricultural Safety and Health Center helped develop the graphics and maps for this study. A special thanks to the KDNA Radio field research staff, including Elizabeth Torres, Carmen Mirales, and Amelia Ramon; and Griselda Arias and John Thayer of the Yakima Valley Farmworkers Clinic Asthma Program.
Footnotes
This article is dedicated to Peter S. Thorne PhD, and Kelly Donham PhD at the University of Iowa for their commitment to research in the field of respiratory disease and agricultural health.
References
- 1.Schenker M, Ferguson T, Gamsky T. Respiratory risks associated with agriculture. Occup Med: State of the Art Rev. 1991;6:415–428. [PubMed] [Google Scholar]
- 2.Studnicka M, Hackl E, Pischinger J, et al. Traffic-related NO2 and the prevalence of asthma and respiratory symptoms in seven year olds. Eur Respir J. 1997;10:2275–2278. doi: 10.1183/09031936.97.10102275. [DOI] [PubMed] [Google Scholar]
- 3.Krivoshto IN, Richards JR, Albertson TE, Derlet RW. The toxicity of diesel exhaust: implications for primary care. J Am Board Fam Med. 2008;21:55–62. doi: 10.3122/jabfm.2008.01.070139. [DOI] [PubMed] [Google Scholar]
- 4.Arslan S, Aybek A. Particulate matter exposure in agriculture. In: Haryanto B, editor. Air Pollution—A Comprehensive Perspective. ch. 3 Intech; 2012. [Google Scholar]
- 5.Studnicka M, Hackl E, Pischinger J, et al. Traffic-related NO2 and the prevalence of asthma and respiratory symptoms in seven year olds. Eur Respir J. 1997;10:2275–2278. doi: 10.1183/09031936.97.10102275. [DOI] [PubMed] [Google Scholar]
- 6.GAST Series Oil-less Vacuum Pumps and Compressors Operation and Maintenance Manual. Gast Manufacturing Inc.; Benton Harbor, MI: Aug 7, 2012. http://www.gastmfg.com. [Google Scholar]
- 7.Kotrappa P, Pimpale NS, Subrahmanyam PS, Joshi PP. Evaluation of critical orifices made from sections of hypodermic needles. Ann Occup Hyg. 1977;20:189–194. doi: 10.1093/annhyg/20.2.189. [DOI] [PubMed] [Google Scholar]
- 8.Lodge JP, Pate J, Ammons B, Swarson G. The use of hypodermic needles as critical orifices in air sampling. J Air Pollut Control Assoc. 1966;16:197–200. [Google Scholar]
- 9.Rain Bird ESP-4M Controller Installation, Programming, and Operation Guide 2009. Rain Bird Corporation; Azusa, CA: Aug 7, 2012. http://ww.rainbird.com. [Google Scholar]
- 10.US Department of Agriculture National Agricultural Statistics Survey (USDA NASS), Washington State. Fruit Summary. Washington, DC: U.S. Department of Agriculture; 2001. Agricultural Chemical Usage Program. [Google Scholar]
- 11.US Department of Agriculture National Agricultural Statistics Survey (USDA NASS), Washington State. Fruit Crops. Washington, DC: U.S. Department of Agriculture; 2009. Agricultural Chemical Usage Program. [Google Scholar]
- 12.USDA. National Agricultural Statistic Service, CropScape: Crop Land Layer Data. [accessed June 2012];2011 http://nassgeodata.gmu.edu/CropScape/
- 13.Washington State Department of Ecology. Dairy Farms of Washington, RCW 90.64. 2012 http://www.ecy.wa.gov/services/gis/data/data.htm#d.
- 14.Chan T, Lippmann M. Particle collection efficiencies of air sampling cyclones: an empirical theory. Environ Sci Technol. 1986;11:377–382. [Google Scholar]
- 15.USEPA. Probe and monitoring path siting criteria for ambient air quality monitoring, Appendix E, 40 CFR Part 58, 2011. Aug 31, 2011. [accessed January 2013]. As amended, 76 FR 54342. [Google Scholar]
- 16.USEPA. Atmospheric Sampling. BiblioGov; 2012. APTI Course 435, Chapters 1 and 7. [Google Scholar]
- 17.Armstrong JL, Fenske RA, Yost MG, Tchong-French M, Yu J. Comparison of recommended air sampling matrices to measure airborne exposures to organophosphorus pesticides and oxygen analogs in agricultural communities. Chemosphere. 2013;92:451–457. doi: 10.1016/j.chemosphere.2013.01.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fenske RA, Yost MG, Galvin K, Tchong-French M, Negrete M, Palmendez P, Fitzpatrick C. University of Washington; 2009. Organophosphorus Pesticide Air Monitoring Project, Final Report. Available from the Washington State Department of Health Pesticide Program, http://www.doh.wa.gov/ehp/pest/uwdrift-report.pdf. [Google Scholar]
- 19.Clark CS, Rylander R, Larsson L. Airborne bacteria, endotoxin, and fingi in dust in poultry and swine confinement buildings. Am Ind Hyg Assoc J. 1983;44:537–541. doi: 10.1080/15298668391405265. [DOI] [PubMed] [Google Scholar]
- 20.Donham KJ, Popendorf WJ. Ambient levels of selected gases inside swine confinement buildings. Am Ind Hyg Assoc J. 1985;46:658–661. doi: 10.1080/15298668591395490. [DOI] [PubMed] [Google Scholar]
- 21.Bishop RW, Belkin F, Gaffney R. Evaluation of a new ammonia sampling and analytical procedure. Am Ind Hyg Assoc J. 1986;47:135–137. doi: 10.1080/15298668691389450. [DOI] [PubMed] [Google Scholar]
- 22.American Conference of Governmental Industrial Hygienists. Guidelines for the Assessment of Bioaerosols in the Indoor Environment; American Conference of Governmental Industrial Hygienists; Cinnincati, Ohio. 1989. [Google Scholar]
- 23.Pratt DS. Respiratory hazards in agriculture: beyond dangerous dust. Semin Respir Med. 1993;14:8–14. [Google Scholar]
- 24.Milton DK, Feldman HA, Neuberg DS, Bruckner RJ, Greaves IA. Environmental endotoxin measurement: the kinetic limulus assay with resistant-parallel-line estimation. Environ Res. 1992;57:212–230. doi: 10.1016/s0013-9351(05)80081-7. [DOI] [PubMed] [Google Scholar]
- 25.NIOSH. NIOSH Manual of Analytical Methods (NMAM) Fourth. 2 1994. Method 6015, Ammonia; 5600, Organophosphorus Pesticides; 0500, Total Dust; and 0600, Respirable Dust. [Google Scholar]
- 26.Kline JN, Schwartz DA. Agricultural dust-induced lung disease. In: Rom WN, editor. Environmental and Occupational Medicine. ch. 36. Lippincott-Raven Publishers; Philadelphia: 1998. pp. 565–571. [Google Scholar]
- 27.EPA. Determination of pesticides and PCBs in ambient air using low volume polyurethane foam (PUF) sampling followed by gas chromatographic/multi-detector detection (GC/MD) Center for Environmental Research Information; Cincinnati, OH: 1999. Compendium Method TO-10A. [Google Scholar]
- 28.Lu C, Fenske RA, Simcox NJ, Kalman D. Pesticide exposure of children in an agricultural community: evidence of household proximity to farm land and take home exposure pathways. Environ Res. 2000;84:290–302. doi: 10.1006/enrs.2000.4076. [DOI] [PubMed] [Google Scholar]
- 29.Hoppin JA, Umbach DM, London SJ, et al. Diesel exhaust, solvents, and other occupational exposures as risk factors for wheeze among farmers. Am J Respir Crit Care Med. 2004;169:1308–1313. doi: 10.1164/rccm.200309-1228OC. [DOI] [PubMed] [Google Scholar]
- 30.Simpson CD, Dills RL, Katz BS, Kalman DA. Determination of levoglucosan in atmospheric fine particulate matter. J Air Waste Manage Assoc. 2004;54:689–694. doi: 10.1080/10473289.2004.10470945. [DOI] [PubMed] [Google Scholar]
- 31.Heederik D, Sisgaard T. Respiratory allergy in agricultural workers: recent developments. Curr Opin Allergy Clin Immunol. 2005;5:129–134. doi: 10.1097/01.all.0000162304.66986.7d. [DOI] [PubMed] [Google Scholar]
- 32.Li Q. New mechanism of organophosphate pesticide induced immunotoxicity. J Nippon Med Sch. 2007;741:92–105. doi: 10.1272/jnms.74.92. [DOI] [PubMed] [Google Scholar]
- 33.Naeher LP, Brauer M, Lipsett M, Zelikoff JT, Simpson CD, Koenig JQ, Smith KR. Woodsmoke health effects: a review. Inhalation Toxicol. 2007;19:67–106. doi: 10.1080/08958370600985875. [DOI] [PubMed] [Google Scholar]
- 34.Allen RW, Mar T, Koenig J, Liu S, Gould T, Simpson CD, Larson T. Changes in lung function and airway inflammation among asthmatic children residing in a wood-smoke impacted area. Inhalation Toxicol. 2008;20:423–433. doi: 10.1080/08958370801903826. [DOI] [PubMed] [Google Scholar]
- 35.Williams DL, Breysse PN, McCormack MC, Diette GB, McKenzie S, Geyh AS. Airborne cow allergen, ammonia and particulate matter at homes vary with distance to industrial scale dairy operations: an exposure assessment. Environ Health. 2011;10:72. doi: 10.1186/1476-069X-10-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sofiev M, Bergmann KC. Allergenic Pollen: A Review of the Production, Release, Distribution, and Health Impacts. Springer-Science+Business Media; 2013. [Google Scholar]


