Abstract
Background and Purpose
Endothelial progenitor cells (EPC) are important participants of neovascularization and are mobilized through signaling with stromal-derived factor (SDF-1α), vascular endothelial growth factor (VEGF), granulocyte colony-stimulating factor, and stem cell factor. The association between EPC levels and these growth factors (GF) in acute stroke has not been previously established. We aimed to determine the association between EPC and these GF, and to elucidate a relationship between these GF and stroke severity in acute stroke patients.
Methods
Seventeen patients were selected from 175 patients with imaging-confirmed acute ischemic stroke. EPC were quantified using CD34, CD133, and VEGF-R2 markers. Plasma VEGF, SDF-1α, granulocyte colony-stimulating factor, and stem cell factor were determined by enzyme-linked immunosorbent assay on days 1 and 3, and brain MRI was performed at baseline and on days 1 and 5 after the stroke onset.
Results
Levels of SDF-1α strongly (r=0.6) correlated with the numbers of EPC subsets CD133+VEFG-R2+ (P<0.004), CD34+VEGF-R2+ (P<0.01), and CD34+CD133+VEGF-R2+ (P<0.01) on day 1. Stem cell factor strongly (r=0.5) correlated with CD133+VEGF-R2+ (P<0.05). SDF-1α moderately inversely (r<−0.49) correlated with baseline diffusion-weighted imaging lesion volumes (P<0.04). Median levels of SDF-1α (1561 pg/mL) increased (P<0.04) on day 3 compared to day 1 (1379 pg/mL). Similarly, VEGF at day 3 (95 pg/mL) increased (P<0.03) compared to day 1 (64 pg/mL).
Conclusions
These results indicate that SDF-1α and stem cell factor correlate with an increase in EPC early in ischemic stroke patients.
Keywords: brain ischemia, cerebrovascular disease, endothelium, neuroregeneration
Endothelial progenitor cells (EPC) are immature hematopoietic cells circulating in peripheral blood that are capable of differentiating into mature endothelial cells to aid endothelial recovery.1,2 EPC are mobilized and recruited to the sites of vascular injury after signaling with major growth factors (GF).3 Stromal-derived factor-1α (SDF-1α) is essential for mobilization of EPC;4 vascular endothelial growth factor (VEGF) also influences migration of endothelial and progenitor cells5 and is amplified by SDF-1α release.6 Granulocyte colony-stimulating factor (GCSF) and stem cell factor (SCF) are considered to be important chemoattractants for EPC.7
The capacity of these GF to mobilize EPC was shown in various ischemic models: an injection of SDF-1α to ischemic hind limb along with transplantation of EPC resulted in accumulation of EPC in the ischemic tissue and in increased vasculogenesis;4 also, administration of GCSF in a similar model led to augmentation of angiogenesis.8 Similarly, VEGF contributed to postnatal neovascularization of cornea by mobilizing EPC.9 Finally, administration of SCF in combination with GCSF in experimental stroke increased numbers of CD34+ cells10 and amplified angiogenesis.11
Recently, an association between EPC levels and GF was assessed in patients with coronary artery disease (CAD).12,13 Previous studies implied an association between EPC and GF levels in human stroke: an increase of SDF-1α14 and VEGF15 was demonstrated 7 days after stroke, and circulating CD34+ and CD133+VEGF-R2+ EPC subsets peaked at 7 days after ischemic event.16,17 Nevertheless, a temporal relationship of the GF with EPC in stroke has not yet been fully elucidated.
Acute and final lesion volumes measured on MRI provide an objective quantitative measurement of stroke severity and outcome.18 In addition, lesion volume measurements are widely used as a surrogate of clinical outcome in therapeutic trials.19 We recently demonstrated that the levels of circulating EPC are related to stroke severity.20
The first aim of the current study was to determine the correlation between levels of EPC and the aforementioned GF participating in EPC recruitment. The second aim was to elucidate a relationship between these GF and stroke severity in early stroke patients. Our results show a positive correlation between subsets of EPC and levels of SDF-1α and SCF and demonstrate an inverse association between levels of SDF-1α and acute lesion volume.
Patients and Methods
Study Population
This was a prospective pilot study of patients with imaging-confirmed acute stroke admitted to the NIH Stroke Center at the Washington Hospital Center between October 2008 and May 2009 and whose first MRI was within 24 hours of the time they were last seen normal. The study was approved by the NIH and Washington Hospital Center Institutional Review Board. Written informed consent was obtained from all patients participating in the study according to the institutional guidelines of NIH and Washington Hospital Center. This was a pilot study conducted on a selected population of acute stroke patients admitted to a single center. The preliminary study population consisted of 323 patients referred because of suspicion of an acute cerebrovascular event. Forty (12%) of these patients had hemorrhagic stroke diagnosed, 13 (4%) had TIA diagnosed, 96 (30%) had stroke mimics diagnosed, and 175 (54%) had imaging-confirmed ischemic stroke. Forty-one (23%) of the admitted ischemic stroke patients participated in the natural history protocol. From this subgroup, patients with contraindications to MRI, latency from last seen normal to blood draws >72 hours, known hematologic infectious diseases, severe hepatic infectious diseases, active malignancy, or recent surgery were excluded. This strict selection resulted in the reduction of number of stroke patients eligible for the study.
The final study population included 17 patients (10% of all ischemic stroke patients) and did not differ significantly from the total stroke cohort admitted to the hospital regarding demographics, medication, or stroke subtypes; however, the percentage of patients who received recombinant tissue plasminogen activator was higher in the study group (Table 1).
Table 1.
Demographics, Vascular Risk Factors, Stroke Subtypes, Biochemical and Clinical Data, Admission Medication, and Treatment of the Study Population
| Clinical Variables | Study Population | |
|---|---|---|
| Demographics | Age, yr | 62±14 |
| Gender (% female) | 9 (53%) | |
| Race (% white) | 4 (24%) | |
| NIHSS on admission | 10±8 | |
| Pre-admission modified Rankin scale | 1±1 | |
| Stroke subtypes | Large artery atherosclerosis | 3 (18%) |
| Small artery occlusion | 3 (18%) | |
| Cardioembolism | 7 (41%) | |
| Other determined cause | 3 (18%) | |
| Undetermined cause | 1 (6%) | |
| Cardiovascular risk factors | Hypertension | 14 (82%) |
| Diabetes mellitus | 10 (59%) | |
| Hyperlipidemia | 7 (41%) | |
| Coronary artery disease | 4 (24%) | |
| Atrial fibrillation | 6 (35%) | |
| Previous stroke | 2 (12%) | |
| Smoking | 2 (12%) | |
| Ethanol | 3 (18%) | |
| Former cancer | 2 (12%) | |
| Illicit drug | 1 (6%) | |
| Previous intracerebral hemorrhage | 1 (6%) | |
| Biochemical parameters | White blood cells, N (×103/mL) | 8.5±3.9 |
| Red blood cells, N (×109/mL) | 4.56±0.85 | |
| Hemoglobin, mg/dL | 13.2±2.7 | |
| Hematocrit, % | 39.9±7.2 | |
| Systolic blood pressure, mm Hg | 153±33 | |
| Diastolic blood pressure, mm Hg | 88±18 | |
| Glucose level, mg/dL | 179±107 | |
| Platelets, N (×109/mL) | 265±63 | |
| International normalized rate | 1.1±0.3 | |
| Medication on admission | Statins | 5 (29%) |
| Aspirin | 6 (35%) | |
| Coumadin | 2 (12%) | |
| Angiotensin-converting enzyme blockers | 4 (24%) | |
| Angiotensin receptor blockers | 3 (18%) | |
| β-blockers | 5 (30%) | |
| Insulin | 3 (18%) | |
| Oral anti-diabetes mellitus medication | 4 (24%) | |
| Estrogens | 0 (0%) | |
| Intervention/recombinant tissue plasminogen activator (intravenous) | 5/3 (29%/18%) |
Data are represented as mean±SD or %.
Brain MRI and Lesion Volume Measurements
MRI was performed using a 3-T (Philips Medical Systems) clinical scanner.21 Lesion volumes were measured from diffusion-weighted imaging (DWI), mean transit time (MTT), and fluid-attenuated inversion recovery using a semiautomated quantitative method.22 Growth of lesion volume was calculated as a difference between final fluid-attenuated inversion recovery and baseline DWI lesion volumes.
Baseline DWI (baseline lesion volume) and MTT were performed at 9±8 hours after time last seen normal. Day 1 DWI and MTT were performed at 37±19 hours, and fluid-attenuated inversion recovery (final lesion volume) was performed at 10±13 days after time last seen normal. Baseline median DWI lesion volume was 17 mL (first quartile, 3; third quartile, 42) and median MTT was 32 mL (first quartile, 4; third quartile, 136). Day 1 median DWI lesion volume was 20 mL (first quartile, 5; third quartile, 46) and median MTT was 32 mL (first quartile, 0.02; third quartile, 111). Median fluid-attenuated inversion recovery was 27 mL (first quartile, 4; third quartile, 76) and median lesion growth volume was 9 mL (first quartile, 1; third quartile, 39).
Blood Collection
Peripheral blood was collected at 31±13 hours (day 1) and 76±29 hours (day 3) after time last seen normal. Blood was collected by venipuncture in 4 Vacutainer CPT (Cell Preparation Tube; BD) and centrifuged at 2000g for 25 minutes. The mononuclear layer was isolated and resuspended in autologous plasma containing 10% dimethyl sulfoxide and stored at −80°C until analysis. Plasma was separated and stored at −80°C until analysis.23
EPC and GF Measurements
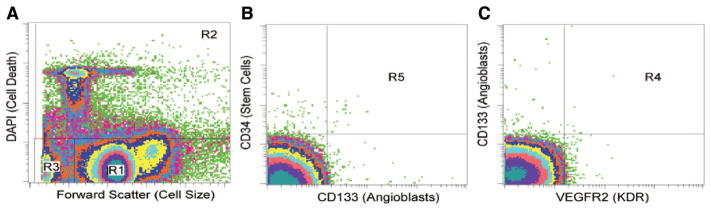
EPC populations were identified using combinations of surface markers (CD34, CD133, VEGF-R2). Briefly, 2×106 cells were incubated in 120 μL buffered saline containing 2% bovine serum albumin with 20 μL Fc-blocking agent (Miltenyi Biotech) for 10 minutes at 25°C to inhibit nonspecific binding of antibodies. Thereafter, the cells were incubated at 4°C for 30 minutes with 20 μL CD133/AC133-PE (Miltenyi Biotech), 20 μL VEGF-R2-FITC (R&D Systems), and 20 μL CD34-ECD (Beckman Coulter) in a total volume of 200 μL. The cells were washed twice before resuspension in 400 μL stain buffer (BD Biosciences). Just before analysis on a FACS Vantage SE (BD Biosciences), DAPI nuclear dye was added to the cell suspension to allow for viability gating, which was consistently 70%±3% across the 2 collection time points for all patients. A minimum of 1×106 live cells were collected, and FACS analysis was performed in triplicate for each sample (Figure 1). Medians of 3 measurements were calculated, and resulting EPC counts were expressed as a percentage of total mononuclear cells in each sample.
Figure 1.
Gating of endothelial progenitor cells in the acute stroke patients. Stained mononuclear cells were assessed as viable by negative staining for DAPI and separated from debris based on size. A, R1=live cells, R2=dead cells, R3=debris. B and C, Viable mononuclear cells in R1 were further gated for costaining of multiple markers. R4=double-positive cells on vascular endothelial growth factor-R2 and CD133. R5=double-positive cells on CD133 and CD34. Gating for triple-positive cells is not shown.
Plasma VEGF, SDF-1α, and SCF levels were measured in duplicate samples using monoclonal antibody-based enzyme-linked immunoassays (Quantikine, R&D Systems). SDF-1α levels were measured in platelet-poor plasma after additional centrifugation at 10 000g for 10 minutes. Plasma GCSF levels were measured using chemiluminescent enzyme-linked immunoassays (R&D Systems).
Statistical Analysis
Correlations were made using the 2-tailed Spearman rank test. Differences between groups were assessed either by the 2-tailed Mann-Whitney test or by the Kruskall-Wallis test. The 95% CI was calculated based on Fisher transformed value of a sample correlation coefficient, and then the confidence limits were back-transformed to derive for the correlation coefficient. The calculation was implemented with R-package (version 2.10.1).
Differences between repeated measurements were calculated by the 2-tailed Wilcoxon rank test. Linear regression was used in adjustments for major stroke risk factors and factors potentially influencing GF and EPC levels. To this end, age and NIHSS score (model 1), glucose and systolic and diastolic blood pressures (model 2), white blood cells and hematocrit (model 3), and significant correlations were entered. SPSS software version 16.0 was used. The level of significance was set at P<0.05.
Results
Temporal Increase of Plasma SDF-1α and SCF in Acute Stroke
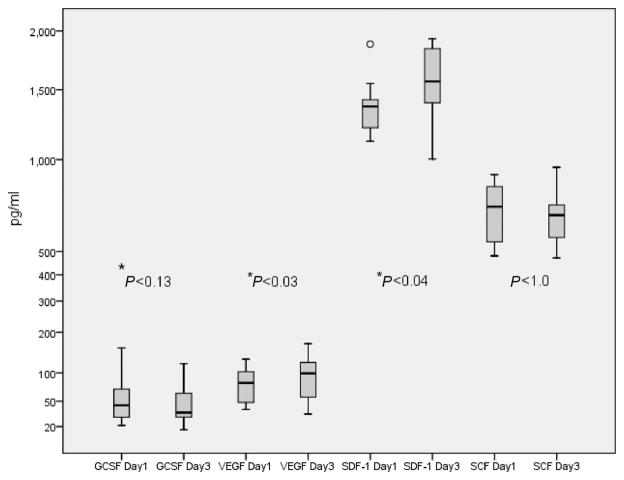
On day 3, SDF-1α increased (P<0.04) compared to day 1, reaching 1561 pg/mL. Median SDF-1α on day 1 was 1379 pg/mL and VEGF levels on day 3 increased (P<0.03) up to 95 pg/mL in comparison to day 1. Median VEGF level on day 1 was 64 pg/mL. There were no significant differences between day 1 and day 3 levels of GCSF (42 vs 34) and SCF (706 vs 654) pg/mL (Figure 2).
Figure 2.
Temporal profiles of plasma growth factor (GF) levels in the stroke patients measured on day 1 and day 3 after the stroke onset. Y-axis is represented as a power scale with exponent 0.5. Box plots show median values (horizontal line inside the plot), quartiles (box boundaries), and the largest and smallest observed values (line drawn from the end on the boxes) of the GF. *P<0.05.
Correlations Between SDF-1α and SCF With EPC Subsets in Stroke Patients
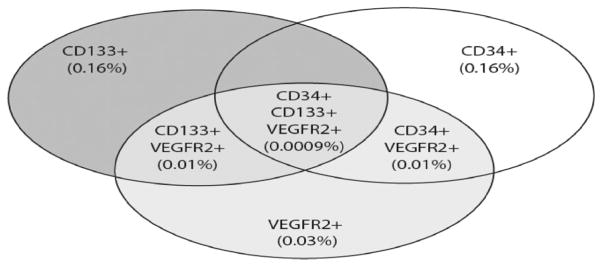
Day 1 SDF-1α levels strongly correlated with single marker EPC CD133+ (r=0.7; P<0.002) and VEGF-R2+ (r=0.7; P<0.002; 95% CI, 0.33–0.883), and with subsets CD133+VEGF-R2+, CD34+VEGF-R2+, and CD34+CD133+VEGF-R2+ (Table 2) on day 1. In addition, day 1 SCF moderately correlated with CD 133+ (r=0.48; P<0.05; 95% CI, −0.0008–0.780) and CD133+VEGF-R2+ (Table 2) populations. Percentages of subsets of EPC on day 1 in the cohort of early stroke patients (medians) are shown in Figure 3. No correlations between day 3 EPC subsets and the GF levels were found.
Table 2.
Spearman Rank Correlation, Significance, and 95% CI of Relationship Between Endothelial Progenitor Cell Subsets Identified by Positive Staining for Double or Triple Markers (Expressed as % of Total Mononuclear Cells) and Plasma Stromal-Derived Factor-1α, Stem Cell Factor, Vascular Endothelial Growth Factor, and Granulocyte Colony-Stimulating Factor Measured at Day 1 in the Cohort of Acute Stroke Patients
| EPC Day 1 | SDF-1α, pg/mL | SCF, pg/mL | VEGF, pg/mL | GCSF, pg/mL |
|---|---|---|---|---|
| CD133+CD34+ | r = − 0.2, P<0.9 (−0.620–0.310) | r = 0.3, P<0.2 (−0.211–0.682) | r = −0.3, P<0.2 (−0.682–0.211) | r = −0.16, P<0.5 (−0.594–0.347) |
| CD133+VEGF-R2+ | r = 0.65, P<0.004* (0.246–0.861) | r = 0.5, P<0.045* (0.025–0.790) | r = −0.07, P<0.9 (−0.532–0.424) | r = −0.16, P<0.5 (0.594–0.347) |
| CD34+VEGF-R2+ | r = 0.6, P<0.01* (0.167–0.838) | r = 0.2, P<0.4 (−0.310–0.620) | r = 0.05, P<0.8 (−0.441–0.518) | r = 0.2, P<0.4 (−0.310–0.620) |
| CD34+CD133+VEGF-R2+ | r = 0.57, P<0.01* (0.123–0.824) | r = 0.1, P<0.6 (−0.399–0.554) | r = 0.07, P<0.8 (−0.424–0.532) | r = 0.09, P<0.7 (−0.408–0.546) |
EPC indicates endothelial progenitor cells; GCSF, granulocyte colony-stimulating factor; SCF, stem cell factor; SDF, stromal-derived factor; VEGF, vascular endothelial growth factor.
P<0.05.
Figure 3.
Circulating endothelial progenitor cells (EPC) in the cohort of stroke patients. Subsets of EPC expressed as a percent of viable mononuclear cells derived from medians of day 1 measurements. Subset of CD133+CD34+ represents 0.02% of total mononuclear cells.
Day 1 SDF-1α strongly correlated with SCF levels (r=0.694; P<0.02; 95% CI, 0.320–0.881). Correlation between SDF-1α and SCF remained significant after adjustments for age and NIHSS score (standardized β, 0.939; unstandardized coefficient B, 1.581; 95% CI, 1.257–1.90; P<0.0001), glucose and systolic and diastolic blood pressures (β, 0.974; coefficient B, 1.63; 95% CI, 1.32–1.95; P<0.0001), white blood cells and hematocrit (β, 0.97; coefficient B, 1.65; 95% CI, 1.293–1.977; P<0.0001). In contrast, there was no correlation between these GF on day 3.
Correlation Between SDF-1α and Acute Lesion Volume
Day 1 SDF-1α levels moderately inversely correlated with acute lesion volumes (r=−0.49; P<0.04; 95% CI, −0.786–0.012). No correlation between other GF and day 1 lesion volume (DWI), MTT, final lesion volume (fluid-attenuated inversion recovery), or lesion growth volume was found.
Association of GF With Patient Demographics, Risk Factors, and Medication
Presence of hyperlipidemia (n=7) was associated with significant (P<0.025) increase of SCF levels on day 1. In patients with hyperlipidemia, median SCF was 841 pg/mL (first quartile, 742; third quartile, 905) vs median 537 pg/mL (first quartile, 473; third quartile, 739) in patients without hyperlipidemia. Other risk factors (Table 1) were not correlated with the GF levels. Six patients used aspirin on admission. Use of aspirin was linked with a significant (P<0.015) decrease in GCSF level on day 1 (median, 28 pg/mL; first quartile, 20 pg/mL; third quartile, 41 pg/mL with aspirin vs median, 50 pg/mL; first quartile, 40 pg/mL; third quartile, 76 pg/mL without aspirin). Three patients had insulin medication on admission. Insulin treatment was associated with significant (P<0.023) decrease in GCSF on day 1 (median, 28 pg/mL; first quartile, 20 pg/mL; third quartile, 29 pg/mL vs median, 50 pg/mL; first quartile, 36 pg/mL; third quartile, 71 pg/mL). No relation was found between levels of these GF and angiotensin convertase enzyme inhibitors, β-blockers, angiotensin receptor blockers, diuretics, coumadin, oral antidiabetics, statins, recombinant tissue plasminogen activators, or endovascular intervention. Similarly, there were no correlations between age, gender, admission NIHSS score, and levels of GF on day 1. There was no difference between these GF levels according to the TOAST stroke criteria.
Association of GF With Blood Biochemical Parameters
White blood cells strongly correlated with day 1 VEGF (r=0.624; P<0.007; 95% CI, 0.205–0.85); however, no correlation between VEGF and any other biochemical parameters was detected. There were no correlations between admission red blood cells, hemoglobin, hematocrit, glucose, platelets, international normalized ratio, systolic and diastolic blood pressures, and GCSF, SDF-1α, and SCF levels.
Subgroup Analysis
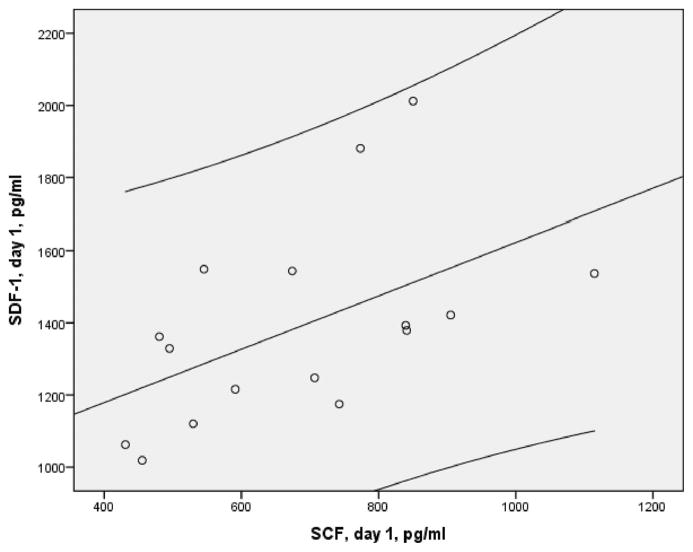
We repeated statistics in 16 stroke patients, excluding 1 patient with high GF levels (Figure 1; SDF-1α and GCSF on day 1). Temporal increases of SDF-1α (P<0.04) and VEGF P<0.03) levels between days 1 and 3 were of the same significance. SDF-1α strongly correlated with CD133+ (r=0.68; P<0.003; 95% CI, 0.296–0.870), VEGF-R2+ (r=0.63; P<0.008; 95% CI, 0.214–0.852), CD34+VEGF-R2+ (r=0.51; P<0.040; 95% CI, 0.039–0.795), and SCF (r=0.53; P<0.04; 95% CI, 0.066–0.805; Figure 4). The correlations between SDF-1α and DWI lesion volume, SCF and CD133+, and CD133+VEGF-R2+were moderate (r=0.4) but no longer significant.
Figure 4.
Scatter plot of day 1 plasma stromal-derived factor-1α and stem cell factor (Spearman r=0.53; P<0.04; n=16) and 95% CI in the early stroke patients.
Discussion
This prospective study shows a relationship between plasma SDF-1α and SCF levels and between stroke severity and brain recovery after early ischemic stroke. The first finding is that high SDF-1α levels were associated with small acute lesion volumes, and that low SDF-1α levels were linked with large acute ischemic strokes and, concomitantly, with worse outcomes. This is in line with data showing that SDF-1α plays a central role in brain regeneration through activation of its receptor CXCR4 in various neuronal cells.24 SDF-1α and its receptor CXCR4 are expressed in neurons, microglia, endothelium, and astrocytes, predominantly in ependyma, cortex, hippocampus, cerebellum, and brain stem of the central nervous system.24 Accordingly, we hypothesized that a temporary decline in production of SDF-1α in early stroke may be related to neuronal necrosis and death of astrocytes, microglia, and vascular endothelium through dysfunction of the neurovascular unit.25 Furthermore, we found strong correlations between SDF-1α and the subsets of EPC that participate in neovascularization,26 which is consistent with reported expression of CXCR4 in newly formed vessels in regions of brain angiogenesis.27 Therefore, our findings suggest that low levels of SDF-1α found in large early strokes may reflect a relative decline in brain neuroregeneration attributable to extensive tissue damage.
The second important finding is the correlation between circulating EPC and plasma levels of SDF-1α and SCF. SDF-1α is essential for mobilization and trafficking of EPC from bone marrow to the ischemic tissue.4 It binds to CXCR4 located on EPC, mediating their recruitment toward hypoxic gradients and to sites of arterial injury.24 Our data are in line with correlations between EPC subsets and SDF-1α in early ischemia. A correlation between SDF-1α and CD34+ and CD34+CD133+ has been demonstrated in acute coronary syndrome,13 and a correlation between CD34+CD133+and CXCR4+CD34+ and SDF-1α was shown in stroke.14 Importantly, we found correlations between SDF-1α and different distinct subsets of EPC (CD133+VEFGR2+, CD34+VEGF-R2+, CD34+CD133+VEGF-R2+), among which the latter represents a population with proliferative potential capable of generating mature endothelial cells.26
Similarly, SCF is a potent activator of EPC mobilization and acts as a ligand for the c-Kit receptor expressed on endothelial cells.7 We found a correlation between levels of SCF and CD133+ and CD133+VEGF-R2+ subsets in early stroke. Importantly, CD133+ is a marker of nonmature angioblast-type cells,3 and administration of CD133+ bone marrow stromal cells has a neuroprotecitve effect in stroke.28
The third finding is the striking correlation between levels of SDF-1α and SCF, which are 2 major GF participating in EPC recruitment (Figure 4). Our data are in line with experimental findings showing that SDF-1α and SCF share signaling pathways,29 and SCF regulates activity of the SDF-1α/CXCR4 axis. However, no association between SDF-1α and SCF in early stroke patients has been reported previously. Importantly, this correlation remained significant after adjustments for the well-recognized prognostic factors of stroke, including age, NIHSS score, hyperglycemia, hypertension, inflammation, and dehydration.
Plasma levels of SDF-1α and VEGF significantly increased on day 3 compared to day 1. However, no increase in any GF between days 1 and 3 was found in patients with acute myocardial ischemia.12,13 Nevertheless, our data are in accordance with the persistent increase in VEGF levels, peaking at 7 days in stroke patients.15 Notably, we demonstrated a significant increase in VEGF levels between days 1 and 3. Furthermore, a recent study showed an increase in SDF-1α between days 1 and 7 after stroke onset.14 We subsequently found a sharper increase in SDF-1α level between days 1 and 3 after stoke onset. Therefore, our results might reflect a robust activation of signaling between SDF-1α and VEGF and EPC, leading to increased recruitment of EPC and neovascularization in subacute stroke. No difference between day 1 and day 3 GCSF was detected in our study, which is in agreement with previous reports.30 In addition, no significant changes in SCF levels were found during the follow-up and, to the best of our knowledge, no studies of SCF levels in plasma of early ischemic stroke patients have been performed before.
Our data (Figure 3) are comparable to published numbers of EPC subsets in healthy volunteers, with the exception of the CD34+VEGF-R+CD133+ subset,31 which is lower. The decrease in subsets of circulating EPC is in agreement with reported temporary decrease of EPC in acute ischemic stroke.16 Post hoc analysis revealed that use of aspirin on admission was linked to a decrease in GCSF level. The indication for aspirin in the cohort of stroke patients was mainly CAD or previous stroke. Capacity for neovascularization in patients with CAD is profoundly reduced.32 In addition, an association between levels of GCSF and capacity for neovascularization and for spontaneous mobilization of CD34+ was recently shown in CAD.12 Therefore, we suggest that that the association between aspirin use and low GCSF in this stroke cohort can be explained by underlying ischemic vascular disease (CAD or previous stroke) and, accordingly, by reduced capacity of bone marrow to neovascularize.
Post hoc analysis showed that insulin treatment was associated with a decrease in GCSF levels. The indication for insulin administration in stroke patients was a presence of underlying diabetes. GCSF administration prevented development of type 1 diabetes and destructive insulinitis in nonobese diabetic mice.33 We hypothesized that diabetes may be linked with low levels of GCSF in the stroke cohort.
In our cohort of stroke patients, high white blood cell counts were associated with higher VEGF levels. This is in line with a recent volunteer study demonstrating that induction of systemic inflammation by Salmonella typhus vaccination is associated with increase of both white blood cells (P<0.0001) and VEGF (P<0.009).34
Day 1 SDF-1α levels inversely correlated with acute lesion volumes evaluated on DWI. DWI is sensitive for ischemia detection during the acute period.35 Ischemic lesion volumes in acute stroke by DWI correlate with clinical outcome.18 Therefore, we used lesion volume measurements as a variable related to outcome. Accordingly, our data show that large ischemic lesions are linked to low SDF-1α levels. We hypothesize that low SDF-1α may be related to decreased mobilization of EPC to the injured vessels and to diminished neovasularization, possibly leading to worse functional outcomes.
Measurements of EPC and these GF may have a prognostic value for assessment of stroke recovery. EPC represent a surrogate biological marker for vascular function and cumulative cardiovascular risk, and patients with high Framingham scores have low EPC levels.2 In addition, metabolic syndrome,36 diabetes,37 and atherosclerosis38 are associated with decreased numbers of EPC. However, the increase in EPC levels 7 days after the stroke is associated with better outcome at 3 months.39 Likewise, levels of SDF-1α have prognostic value in patients with recurrent stroke14 and concomitant CAD.13 Finally, increased VEGF levels at 24 hours after acute stroke predict better functional outcome.40
The nature of interactions between EPC and the measured GF is a complex process involving multiple mechanisms that are not fully elucidated. Other tissue-specific chemoattractants and GF, such as erythropoietin, angiopoietin-1, estrogens, platelet-derived growth factor, and others, also may be involved in mobilization of EPC in ischemic stroke.41 However, the biological significance of the link between EPC subsets and SDF-1α is emphasized by the broad involvement of SDF-1α in the central nervous system homeostasis, because SDF-1α has been found in the developing brain and in the adult brain.24 In addition, SDF-1α mediates the migration of neuronal progenitors and EPC to participate in neovascularization.40 Moreover, SDF-1α is upregulated under hypoxic conditions24 and in stroke.14 These findings all support the biological significance of associations between EPC and SDF-1α levels in stroke patients. Other explanations for increases in SDF-1α and VEGF may be that SDF-1α is increased in hypercholesterolemia42 and in metastatic cancer progression,11 whereas VEGF is implicated in skeletal growth, pregnancy, and spreading of cancer.42 However, because of our strict inclusion criteria, patients with these underlying disorders and conditions were excluded. Because cholesterol levels were not simultaneously evaluated in the stroke cohort, the influence of cholesterol on increased SDF-1α remains to be established in future clinical studies.
We measured plasma GF levels in acute stroke patients but we were not able to perform immunohistochemistry to demonstrate a local increase of these GF in the brain. An increased expression of SDF-1α is shown in ischemic penumbra.43 Likewise, ischemia stimulates VEGF expression in brain macrophages, neurons, and glia,44 and increased expression of GCSF in neuronal and endothelial cells has been shown in stroke patients.45 Thus, the experimental and clinical evidence supports the hypothesis that the GF levels originate from damaged brain tissue.
After ischemic stroke, newly formed capillars are not always filled with red blood cells;46 therefore, angiogenesis may not be explicitly related to augmentation of cerebral flow. Newly formed microvessels increase migration of macrophages to the ischemic area for rapid removal of necrotic debris,47 which is beneficial for survival, and, consequently, the number of newly formed vessels correlates with longer survival of acute stroke patients.46
The major limitation of this study is a small sample size and an absence of baseline data with patients’ premorbid characteristics. The outlier fulfilled the inclusion and the exclusion criteria and represented a biological variation in EPC and GF levels.13,48 However, the subgroup analysis supported the observations of the total cohort of patients. A rigorous understanding of the interactions between EPC and GF at the molecular and cellular level is further warranted.
Conclusions
SDF-1α and SCF strongly correlate with circulating EPC levels and with each other in early ischemic stroke. In addition, SDF-1α inversely correlates with acute ischemic lesion volumes. Finally, SDF-1α and VEGF increased during the follow-up after early ischemic stroke.
Acknowledgments
The authors thank the members of the NIH Stroke Program at the Washington Hospital Center who assisted with data collection and patient care.
Source of Funding
This research was supported by the Division of Intramural Research of the National Institute of Neurological Disorders and Stroke, National Institutes of Health, and by research grants from the Finnish Medical Foundation and the Biomedicum Foundation.
Footnotes
Disclosure
None.
References
- 1.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 2.Hill JM, Zalos G, Halcox JP, Schenke WH, Waclawiw MA, Quyyumi AA, Finkel T. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med. 2003;348:593–600. doi: 10.1056/NEJMoa022287. [DOI] [PubMed] [Google Scholar]
- 3.Leone AM, Valgimigli M, Giannico MB, Zaccone V, Perfetti M, D’Amario D, Rebuzzi AG, Crea F. From bone marrow to the arterial wall: the ongoing tale of endothelial progenitor cells. Eur Heart J. 2009;30:890–899. doi: 10.1093/eurheartj/ehp078. [DOI] [PubMed] [Google Scholar]
- 4.Yamaguchi J, Kusano KF, Masuo O, Kawamoto A, Silver M, Murasawa S, Bosch-Marce M, Masuda H, Losordo DW, Isner JM, Asahara T. Stromal cell-derived factor-1 effects on ex vivo expanded endothelial progenitor cell recruitment for ischemic neovascularization. Circulation. 2003;107:1322–1328. doi: 10.1161/01.cir.0000055313.77510.22. [DOI] [PubMed] [Google Scholar]
- 5.Zhang H, Vutskits L, Pepper MS, Kiss JZ. VEGF is a chemoattractant for FGF-2-stimulated neural progenitors. J Cell Biol. 2003;163:1375–1384. doi: 10.1083/jcb.200308040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Salcedo R, Wasserman K, Young HA, Grimm MC, Howard OM, Anver MR, Kleinman HK, Murphy WJ, Oppenheim JJ. Vascular endothelial growth factor and basic fibroblast growth factor induce expression of CXCR4 on human endothelial cells: In vivo neovascularization induced by stromal-derived factor-1alpha. Am J Pathol. 1999;154:1125–1135. doi: 10.1016/s0002-9440(10)65365-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Broudy VC. Stem cell factor and hematopoiesis. Blood. 1997;90:1345–1364. [PubMed] [Google Scholar]
- 8.Capoccia BJ, Shepherd RM, Link DC. G-CSF and AMD3100 mobilize monocytes into the blood that stimulate angiogenesis in vivo through a paracrine mechanism. Blood. 2006;108:2438–2445. doi: 10.1182/blood-2006-04-013755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Asahara T, Takahashi T, Masuda H, Kalka C, Chen D, Iwaguro H, Inai Y, Silver M, Isner JM. VEGF contributes to postnatal neovascularization by mobilizing bone marrow-derived endothelial progenitor cells. EMBO J. 1999;18:3964–3972. doi: 10.1093/emboj/18.14.3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tong J, Gordon MS, Srour EF, Cooper RJ, Orazi A, McNiece I, Hoffman R. In vivo administration of recombinant methionyl human stem cell factor expands the number of human marrow hematopoietic stem cells. Blood. 1993;82:784–791. [PubMed] [Google Scholar]
- 11.Toth ZE, Leker RR, Shahar T, Pastorino S, Szalayova I, Asemenew B, Key S, Parmelee A, Mayer B, Nemeth K, Bratincsák A, Mezey E. The combination of granulocyte colony-stimulating factor and stem cell factor significantly increases the number of bone marrow-derived endothelial cells in brains of mice following cerebral ischemia. Blood. 2008;111:5544–5552. doi: 10.1182/blood-2007-10-119073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leone AM, Rutella S, Bonanno G, Contemi AM, de Ritis DG, Giannico MB, Rebuzzi AG, Leone G, Crea F. Endogenous G-CSF and CD34+ cell mobilization after acute myocardial infarction. Int J Cardiol. 2006;111:202–208. doi: 10.1016/j.ijcard.2005.06.043. [DOI] [PubMed] [Google Scholar]
- 13.Stellos K, Bigalke B, Langer H, Geisler T, Schad A, Kögel A, Pfaff F, Stakos D, Seizer P, Müller I, Htun P, Lindemann S, Gawaz M. Expression of stromal-cell-derived factor-1 on circulating platelets is increased in patients with acute coronary syndrome and correlates with the number of CD34+ progenitor cells. Eur Heart J. 2009;30:584–593. doi: 10.1093/eurheartj/ehn566. [DOI] [PubMed] [Google Scholar]
- 14.Paczkowska E, Kucia M, Koziarska D, Halasa M, Safranow K, Masiuk M, Karbicka A, Nowik M, Nowacki P, Ratajczak MZ, Machalinski B. Clinical evidence that very small embryonic-like stem cells are mobilized into peripheral blood in patients after stroke. Stroke. 2009;40:1237–1244. doi: 10.1161/STROKEAHA.108.535062. [DOI] [PubMed] [Google Scholar]
- 15.Slevin M, Krupinski J, Slowik A, Kumar P, Szczudlik A, Gaffney J. Serial measurement of vascular endothelial growth factor and transforming growth factor-beta1 in serum of patients with acute ischemic stroke. Stroke. 2000;31:1863–1870. doi: 10.1161/01.str.31.8.1863. [DOI] [PubMed] [Google Scholar]
- 16.Taguchi A, Matsuyama T, Moriwaki H, Hayashi T, Hayashida K, Nagatsuka K, Todo K, Mori K, Stern DM, Soma T, Naritomi H. Circulating CD34-positive cells provide an index of cerebrovascular function. Circulation. 2004;109:2972–2975. doi: 10.1161/01.CIR.0000133311.25587.DE. [DOI] [PubMed] [Google Scholar]
- 17.Zhou WJ, Zhu DL, Yang GY, Zhang Y, Wang HY, Ji KD, Lu YM, Gao PJ. Circulating endothelial progenitor cells in Chinese patients with acute stroke. Hypertens Res. 2009;32:306–310. doi: 10.1038/hr.2009.16. [DOI] [PubMed] [Google Scholar]
- 18.Lövblad KO, Baird AE, Schlaug G, Benfield A, Siewert B, Voetsch B, Connor A, Burzynski C, Edelman RR, Warach S. Ischemic lesion volumes in acute stroke by diffusion-weighted magnetic resonance imaging correlate with clinical outcome. Ann Neurol. 1997;42:164–170. doi: 10.1002/ana.410420206. [DOI] [PubMed] [Google Scholar]
- 19.Warach S, Kaufman D, Chiu D, Devlin T, Luby M, Rashid A, Clayton L, Kaste M, Lees KR, Sacco R, Fisher M. Effect of the glycine antagonist gavestinel on cerebral infarcts in acute stroke patients, a randomized placebo-controlled trial: The gain MRI substudy. Cerebrovasc Dis. 2006;21:106–111. doi: 10.1159/000090208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bogoslovsky Endothelial progenitor cells correlate with lesion volume and growth in acute stroke. Neurology. 2010;75:2059–2062. doi: 10.1212/WNL.0b013e318200d741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Latour LL, Kang DW, Ezzeddine MA, Chalela JA, Warach S. Early blood-brain barrier disruption in human focal brain ischemia. Ann Neurol. 2004;56:468–477. doi: 10.1002/ana.20199. [DOI] [PubMed] [Google Scholar]
- 22.Luby M, Bykowski JL, Schellinger PD, Merino JG, Warach S. Intra- and interrater reliability of ischemic lesion volume measurements on diffusion-weighted, mean transit time and fluid-attenuated inversion recovery MRI. Stroke. 2006;37:2951–2956. doi: 10.1161/01.STR.0000249416.77132.1a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ruitenberg JJ, Mulder CB, Maino VC, Landay AL, Ghanekar SA. Vacutainer cpt and ficoll density gradient separation perform equivalently in maintaining the quality and function of PBMC from HIV seropositive blood samples. BMC Immunol. 2006;7:11. doi: 10.1186/1471-2172-7-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lazarini F, Tham TN, Casanova P, Arenzana-Seisdedos F, Dubois-Dalcq M. Role of the alpha-chemokine stromal cell-derived factor (SDF-1) in the developing and mature central nervous system. Glia. 2003;42:139–148. doi: 10.1002/glia.10139. [DOI] [PubMed] [Google Scholar]
- 25.del Zoppo GJ. Virchow’s triad: The vascular basis of cerebral injury. Rev Neurol Dis. 2008;5(Suppl 1):S12–S21. [PMC free article] [PubMed] [Google Scholar]
- 26.Peichev M, Naiyer AJ, Pereira D, Zhu Z, Lane WJ, Williams M, Oz MC, Hicklin DJ, Witte L, Moore MA, Rafii S. Expression of VEGFR-2 and AC133 by circulating human CD34(+) cells identifies a population of functional endothelial precursors. Blood. 2000;95:952–958. [PubMed] [Google Scholar]
- 27.Rempel SA, Dudas S, Ge S, Gutiérrez JA. Identification and localization of the cytokine SDF1 and its receptor, CXC chemokine receptor 4, to regions of necrosis and angiogenesis in human glioblastoma. Clin Cancer Res. 2000;6:102–111. [PubMed] [Google Scholar]
- 28.Bakondi B, Shimada IS, Perry A, Munoz JR, Ylostalo J, Howard AB, Gregory CA, Spees JL. CD133 identifies a human bone marrow stem/progenitor cell sub-population with a repertoire of secreted factors that protect against stroke. Mol Ther. 2009;17:1938–1947. doi: 10.1038/mt.2009.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dutt P, Wang JF, Groopman JE. Stromal cell-derived factor-1 alpha and stem cell factor/kit ligand share signaling pathways in hemopoietic progenitors: A potential mechanism for cooperative induction of chemotaxis. J Immunol. 1998;161:3652–3658. [PubMed] [Google Scholar]
- 30.Hennemann B, Ickenstein G, Sauerbruch S, Luecke K, Haas S, Horn M, Andreesen R, Bogdahn U, Winkler J. Mobilization of CD34+ hematopoietic cells, colony-forming cells and long-term culture-initiating cells into the peripheral blood of patients with an acute cerebral ischemic insult. Cytotherapy. 2008;10:303–311. doi: 10.1080/14653240801949994. [DOI] [PubMed] [Google Scholar]
- 31.George J, Shmilovich H, Deutsch V, Miller H, Keren G, Roth A. Comparative analysis of methods for assessment of circulating endothelial progenitor cells. Tissue Eng. 2006;12:331–335. doi: 10.1089/ten.2006.12.331. [DOI] [PubMed] [Google Scholar]
- 32.Heeschen C, Lehmann R, Honold J, Assmus B, Aicher A, Walter DH, Martin H, Zeiher AM, Dimmeler S. Profoundly reduced neovascularization capacity of bone marrow mononuclear cells derived from patients with chronic ischemic heart disease. Circulation. 2004;109:1615–1622. doi: 10.1161/01.CIR.0000124476.32871.E3. [DOI] [PubMed] [Google Scholar]
- 33.Kared H, Masson A, Adle-Biassette H, Bach JF, Chatenoud L, Zavala F. Treatment with granulocyte colony-stimulating factor prevents diabetes in nod mice by recruiting plasmacytoid dendritic cells and functional CD4(+)CD25(+) regulatory T-cells. Diabetes. 2005;54:78–84. doi: 10.2337/diabetes.54.1.78. [DOI] [PubMed] [Google Scholar]
- 34.Padfield GJ, Tura O, Haeck ML, Short A, Freyer E, Barclay GR, Newby DE, Mills NL. Circulating endothelial progenitor cells are not affected by acute systemic inflammation. Am J Physiol Heart Circ Physiol. 298:H2054–H2061. doi: 10.1152/ajpheart.00921.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Warach S, Gaa J, Siewert B, Wielopolski P, Edelman RR. Acute human stroke studied by whole brain echo planar diffusion-weighted magnetic resonance imaging. Ann Neurol. 1995;37:231–241. doi: 10.1002/ana.410370214. [DOI] [PubMed] [Google Scholar]
- 36.Jialal I, Devaraj S, Singh U, Huet BA. Decreased number and impaired functionality of endothelial progenitor cells in subjects with metabolic syndrome: Implications for increased cardiovascular risk. Atherosclerosis. 2010;211:297–302. doi: 10.1016/j.atherosclerosis.2010.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tepper OM, Galiano RD, Capla JM, Kalka C, Gagne PJ, Jacobowitz GR, Levine JP, Gurtner GC. Human endothelial progenitor cells from type II diabetics exhibit impaired proliferation, adhesion, and incorporation into vascular structures. Circulation. 2002;106:2781–2786. doi: 10.1161/01.cir.0000039526.42991.93. [DOI] [PubMed] [Google Scholar]
- 38.Chen JZ, Zhang FR, Tao QM, Wang XX, Zhu JH. Number and activity of endothelial progenitor cells from peripheral blood in patients with hypercholesterolaemia. Clin Sci (Lond) 2004;107:273–280. doi: 10.1042/CS20030389. [DOI] [PubMed] [Google Scholar]
- 39.Sobrino T, Hurtado O, Moro MA, Rodríguez-Yáñez M, Castellanos M, Brea D, Moldes O, Blanco M, Arenillas JF, Leira R, Dávalos A, Lizasoain I, Castillo J. The increase of circulating endothelial progenitor cells after acute ischemic stroke is associated with good outcome. Stroke. 2007;38:2759–2764. doi: 10.1161/STROKEAHA.107.484386. [DOI] [PubMed] [Google Scholar]
- 40.Lee SC, Lee KY, Kim YJ, Kim SH, Koh SH, Lee YJ. Serum vegf levels in acute ischaemic strokes are correlated with long-term prognosis. Eur J Neurol. 17:45–51. doi: 10.1111/j.1468-1331.2009.02731.x. [DOI] [PubMed] [Google Scholar]
- 41.Smart N, Riley PR. The stem cell movement. Circ Res. 2008;102:1155–1168. doi: 10.1161/CIRCRESAHA.108.175158. [DOI] [PubMed] [Google Scholar]
- 42.Gomes AL, Carvalho T, Serpa J, Torre C, Dias S. Hypercholesterolemia promotes bone marrow cell mobilization by perturbing the sdf-1:Cxcr4 axis. Blood. 115:3886–3894. doi: 10.1182/blood-2009-08-240580. [DOI] [PubMed] [Google Scholar]
- 43.Wang Y, Deng Y, Zhou GQ. SDF-1alpha/CXCR4-mediated migration of systemically transplanted bone marrow stromal cells towards ischemic brain lesion in a rat model. Brain Res. 2008;1195:104–112. doi: 10.1016/j.brainres.2007.11.068. [DOI] [PubMed] [Google Scholar]
- 44.Ning M, Furie KL, Koroshetz WJ, Lee H, Barron M, Lederer M, Wang X, Zhu M, Sorensen AG, Lo EH, Kelly PJ. Association between tPA therapy and raised early matrix metalloproteinase-9 in acute stroke. Neurology. 2006;66:1550–1555. doi: 10.1212/01.wnl.0000216133.98416.b4. [DOI] [PubMed] [Google Scholar]
- 45.Hasselblatt M, Jeibmann A, Riesmeier B, Maintz D, Schäbitz WR. Granulocyte-colony stimulating factor (G-CSF) and G-CSF receptor expression in human ischemic stroke. Acta Neuropathol. 2007;113:45–51. doi: 10.1007/s00401-006-0152-y. [DOI] [PubMed] [Google Scholar]
- 46.Krupinski J, Kaluza J, Kumar P, Kumar S, Wang JM. Role of angiogenesis in patients with cerebral ischemic stroke. Stroke. 1994;25:1794–1798. doi: 10.1161/01.str.25.9.1794. [DOI] [PubMed] [Google Scholar]
- 47.Yu SW, Friedman B, Cheng Q, Lyden PD. Stroke-evoked angiogenesis results in a transient population of microvessels. J Cereb Blood Flow Metab. 2007;27:755–763. doi: 10.1038/sj.jcbfm.9600378. [DOI] [PubMed] [Google Scholar]
- 48.Wojakowski W, Tendera M, Zebzda A, Michalowska A, Majka M, Kucia M, Maslankiewicz K, Wyderka R, Król M, Ochala A, Kozakiewicz K, Ratajczak MZ. Mobilization of CD34(+), CD117(+), CXCR4(+), c-met(+) stem cells is correlated with left ventricular ejection fraction and plasma NT-proBNP levels in patients with acute myocardial infarction. Eur Heart J. 2006;27:283–289. doi: 10.1093/eurheartj/ehi628. [DOI] [PubMed] [Google Scholar]