Abstract
Frailty is a clinical syndrome initially characterized in geriatric populations with a hallmark of age-related declines in physiologic reserve and function and increased vulnerability to adverse health outcomes. Recently, frailty has increasingly been recognized as a common and important HIV-associated non-AIDS (HANA) condition. This article provides an overview of our current understanding of frailty and its phenotypic characteristics, and evidence that they are related to aging and to chronic inflammation that is associated with aging and also with long-term treated HIV infection. The etiology of this chronic inflammation is unknown but we discuss evidence linking it to persistent infection with cytomegalovirus in both geriatric populations and people living with HIV infection.
Keywords: frailty, inflammation, HIV infection, IL-6, T-cell immunity, CMV infection
INTRODUCTION
The number of older persons living with HIV/AIDS has risen dramatically over the past decade. As a result, chronic conditions commonly encountered in geriatric populations have increasingly become major health concerns for this vulnerable aging population. One example is frailty, an important age-related clinical syndrome characterized by diminished physiologic reserve and increased vulnerability for subsequent morbidity and mortality. A large body of literature suggests that chronic inflammation, an age-related immune dysregulation, may play an important role in the development of frailty in older adults in the general population. More recently, frailty has been described in several cohort studies of HIV-infected persons (1–6). This article provides an overview about our current understanding of frailty, aging, and chronic inflammation in people living with HIV infection.
FRAILTY IN GERIATRIC POPULATIONS
In the geriatrics literature, frailty is defined as a state of increased vulnerability in old age. Frailty is recognized as an important clinical syndrome in old age, which a) results from age-related declines in physiologic reserve and complexity in resting dynamics involving multiple physiologic systems, b) manifests by maladaptive responses to every day or acute stressors, and c) leads to a vicious cycle towards functional decline and other serious adverse health outcomes (7–15). In older adults, this chronic condition is commonly described by two conceptual models: the phenotype model (frailty syndrome(7)) and the cumulative deficit model (frailty index (16)). As the frailty index is defined by counting the number of accumulated deficits identified by a comprehensive geriatrics assessment, which has not been validated in HIV-infected populations (17) and does not easily allow further investigation of underlying mechanisms of frailty, this article will focus on the frailty phenotype model. According to the most widely used model of this type, that described by Fried et al (7), the frailty phenotype (FP) in older adults consists of three or more of the following: weakness (measured by grip strength), low physical activity, slowed motor performance (measured by walking speed), exhaustion, and unintentional weight loss (7). Using this definition, frailty is a distinct clinical entity, differing from disability as measured by impairment in activities of daily living (ADL) and from comorbidity as defined by the presence of ≥ 2 diseases, i.e., two other prevalent conditions in older adults (7;18). Based on this definition and its various modified versions, the estimated prevalence of frailty in the United States is 7–12% among community-dwelling men and women age 65 years and older, and over 25% among those older than 85 years (7;18), with significant geographic variation (19;20). The frailty phenotype independently predicts a number of serious adverse health outcomes in community-dwelling older adults, including acute illness, falls, cognitive decline, hospitalization, disability, dependency, and mortality, adjusting for comorbidities (7;8;21). In addition, substantial evidence suggest that assessment of frailty is useful for pre-operative evaluation for elderly patients who undergo surgery (22;23) as well as risk stratification in patients with cancer (24–26), cardiovascular diseases (27), and end-stage renal disease on chronic hemodialysis or after renal transplantation (28;29). Frailty may also be a clinical marker for overall immune functional decline in older adults, as it has been shown to identify those who fail to mount adequate immune responses to influenza and pneumococcal immunizations and are at high risk for these common infections and their complications (30;31).
AGING AND FRAILTY IN HIV-INFECTED POPULATIONS
Similarities between frailty, aging, and HIV infection were recognized early in the HIV pandemic. For example, weight loss was recognized as a cardinal feature of untreated HIV infection, along with immune activation and high levels of catabolic cytokines and other bioactive molecules (32;33). After the introduction of highly active antiretroviral therapy (HAART) in the mid-1990s, life expectancy for HIV-infected persons increased dramatically (34); in effect, HIV became a treatable disease with long life possible, and the question shifted to the effect of chronic treated HIV infection on health and longevity. It is estimated that about half of the HIV-infected individuals in the United States will be > 50 years of age by 2015 [CDC. HIV/AIDS among persons aged 50 or over. http://www.cdc.gov/hiv/surveillance/resources/reports/2005report/. 2014]. Aging of the HIV-infected population is also evident in Asia and even in sub-Sahara Africa (35;36). As HIV-infected persons live longer, age-related chronic conditions termed HIV-associated non-AIDS (HANA) conditions have increasingly become major health concerns despite suppression of HIV viral load to clinically undetectable levels (37–39). Among HANA conditions, frailty is increasingly being recognized as an important syndrome that identifies HIV-infected people who are at higher risk for adverse health outcomes.
The first studies of frailty in HIV+ men were done in the Multicenter AIDS Cohort Study (MACS) by Desquilbet et al in the late 2000s. The goal of these studies was to take advantage of data already acquired over many years (since 1994) to approximate the prevalence and import of frailty in people with HIV infection, since at this time frailty had never been studied in HIV+ populations. Available data was insufficient to assess the full Fried FP exactly, so it was approximated using available data that incorporated four of the five criteria of the Fried FP: weight loss, exhaustion, slowness, and low physical activity. (The assessment of weakness (i.e., grip strength) was not incorporated into the MACS protocol until October 2005 and therefore could not be used in this study.) The result was a phenotype defined by expression of 3 of the 4 criteria that were addressed. Thus, this phenotype differed slightly from that described by Fried et al; accordingly it was termed the frailty-related phenotype, or FRP. The FRP had a prevalence of 4.4% among HIV-uninfected men aged 65 years and older, which was similar to the prevalence of frailty observed in the Cardiovascular Health Study for men of similar ages (7). Additional analyses showed that the prevalence of the FRP was lower in the HAART era than in the pre-HAART era, and was inversely related to the CD4 T cell count in a similar way in both the pre- and post-HAART eras (3). Finally, presence of the FRP at the time of HAART initiation was an independent predictor of AIDS-free and overall survival after adjusting for other known predictors such as HIV viral load and CD4 T cell count (40). This suggested that the FRP was a valid measure of frailty in HIV+ men, i.e., that it was a predictor of adverse health outcomes, just as the FP has been shown to be in the elderly general population.
The studies by Desquilbet et al were retrospective, meant to use available data to start to understand the occurrence and meaning of frailty in an HIV+ population. However, it was also important to assess the same FP that had been validated in the elderly general population, i.e., the Fried FP. Therefore, as mentioned above, around the same time as the studies by Desquilbet et al were begun, the MACS began measuring walking speed began and grip strength. Data from 2007 through 2011 were analyzed by Althoff et al (41), who published prevalence, incidence, and reversion statistics for HIV+ men who had received HAART and for HIV− men (Fig. 1). These analyses took advantage of a notable strength of the MACS, namely the ability to compare the HIV+ population to a demographically similar HIV− population rather than to the general aging population. The prevalence of the FP increased with age in both HIV− and HIV+ men, as expected, but there was an excess in the HIV+ men between the ages of 50–69. This excess was at least partially explained by a higher prevalence of several HIV-independent risk factors for the FP in the HIV+ men than in the HIV− men: diabetes mellitus, smoking, kidney disease, and depressive symptoms (i.e., a score of 16 or more on the CES-D questionnaire administered at each semiannual MACS study visit).
Fig. 1.
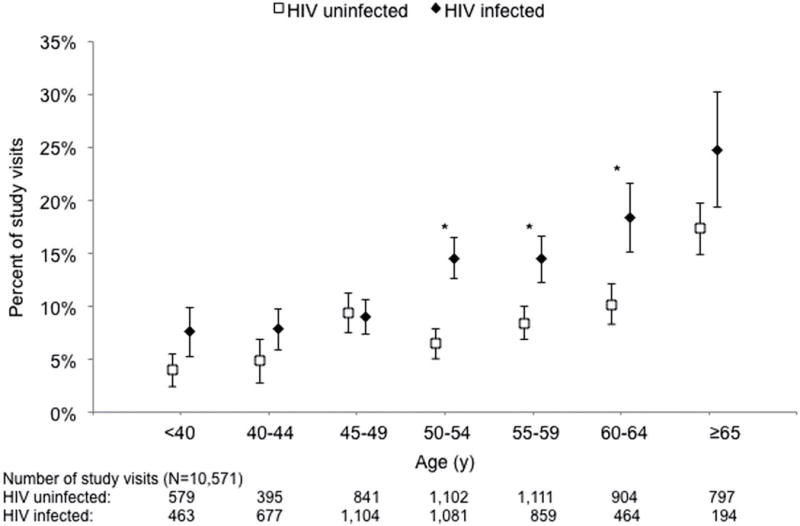
The FP has not yet been validated in this cohort, which is younger than the HIV− cohorts in which it was initially validated and also differs in the inclusion of HIV+ people. Analyses to validate it by linking it to clinically meaningful outcomes are underway in the MACS. However, data from other studies have supported the clinical validity of the FP in HIV+ populations. For example, the Fried FP was measured cross-sectionally in the AIDS Linked to the Intravenous Experience (ALIVE) cohort of people with a history of injection drug use (IDU) in Baltimore, MD (2). This study found that expression of the Fried FP did indeed predict mortality in this cohort of IDUs. Recently, another study from the ALIVE cohort found that poor physical function, as assessed by the short physical performance battery (SPPB) was also an independent predictor of mortality (42). In addition, data from the Veterans Aging Cohort Study (VACS) showed that an adapted frailty-related phenotype was predictive for hospitalization and mortality in aging HIV-infected veterans (6). Other studies of the clinical aspects of frailty in HIV+ populations, as well as the definitions of frailty that have been used in these studies, have recently been reviewed in detail (17).
In these studies, factors that have been associated with frailty in HIV+ populations include age, lower current or nadir CD4 T-cell counts and other HIV-infection related measures, inflammation, hepatitis C co-infection and other comorbidities, depressive symptoms, and certain social factors (e.g., lower education, unemployment) (1;2;4;38;39;41;43).
CHRONIC INFLAMMATION AND FRAILTY
The aging immune system is characterized by a low level chronic systemic inflammatory state, termed “InflammAging” (44). This inflammatory phenotype is marked by elevated circulating levels of markers of inflammation (e.g., C-reactive protein (CRP) and the pro-inflammatory cytokines interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α)), and is associated with increased morbidity and mortality in older adults (44–46). Clinically, an increase in the while blood cell (WBC) count is recognized as an important cellular marker of systemic inflammation. Studies over the past decade, including some from our group, have provided a large body of evidence supporting the role of heightened chronic inflammation in contributing to frailty in the geriatric populations above and beyond the age-related inflammatory state. For example, several studies demonstrated significant associations between frailty and elevated serum levels of IL-6, CRP, and TNF-α in community-dwelling older adults (47–52). Studies also showed direct associations between frailty and increased total WBC count, albeit still under the upper limit of the normal range, and counts of specific subpopulations including neutrophils and monocytes (50;53). With respect to T lymphocyte subpopulations, frailty is associated with increased counts of CD8+CD28− T cells and CCR5+ T cells, the latter of which has a type-1 pro-inflammatory phenotype (54–56). Ex vivo studies showed not only a frailty-associated increase in LPS-stimulated IL-6 production by peripheral blood mononuclear cells (PBMCs), but also upregulation in expression of inflammatory pathway-specific genes by purified peripheral monocytes (57;58). Moreover, constitutive upregulation in monocytic expression of CXCL-10, a potent pro-inflammatory chemokine, was highly correlated with elevation in serum IL-6 levels in frailty (59). Elevated serum levels of neopterin, a well-known molecular marker for immune activation mediated by monocytes and macrophages, were associated with frailty in community-dwelling older adults, independent of IL-6 levels (60).
Individual inflammatory molecules, such as IL-6, may directly contribute to the frailty syndrome or its cardinal components (such as decreased muscle strength/power and slowed motor performance) (47;49;50;61;62). As frailty involves dysregulation in multiple physiologic systems (15), chronic inflammation may contribute to frailty through its detrimental effects on diverse physiologic organ systems. For example, studies have shown that circulating IL-6 levels have inverse associations with hemoglobin concentration and serum insulin-like growth factor-1 (IGF-1) levels in frail older adults, but not in non-frail controls; low hemoglobin and IGF-1 levels were each independently associated with frailty, as well (47;63;64). In addition, WBC counts were inversely associated with IGF-levels (65). Taken together, it is suggested that chronic inflammation plays a key role in the pathogenesis of frailty, directly or through other intermediate pathophysiologic processes.
Although immunological changes associated with frailty have been investigated in many studies in the general elderly population, few studies have been done in the HIV+ population. These immunological changes are very pertinent to studies of aging and frailty in HIV+ people, because the huge immune activation that is characteristic of untreated HIV infection, though dramatically improved by HAART, is not completely resolved even with achievement of clinically undetectable HIV viral load and the most complete viral suppression possible (reviewed in (66). In particular, many immune activation markers that were associated with aging remain elevated, including IL-6 and TNF-α (67). This residual immune activation, along with the occurrence of many age-related diseases at younger ages in people living with HIV than in the general population, led to the hypothesis that people living with HIV experienced premature or accelerated aging (39). This hypothesis has been controversial, partly because of fundamental gaps in our knowledge of the biological basis and metrics of aging (68) and partly because the HIV-infected population is younger than the general population, thus predisposing to a younger age distribution of any aging-related disease in the HIV+ population than in the general population (69).
With these considerations in mind, recent studies have begun to address this question in HIV+ populations. Erlandson et al (70) analyzed a panel of cellular and serologic immune activation markers in HIV+ people with high (n=49) or low (n=31) physical functioning, matched by age, sex, and date of HIV diagnosis. Frailty was assessed (as defined by the Fried phenotype), but frailty per se was not an outcome in this analysis, but was one of the criteria for low functioning, along with performance on the Short Physical Performance Battery. They found that the low-functioning group had higher serum IL-6 and T-cell activation (expression of CD38 and HLA-DR); the latter was statistically significant for CD8 T-cells. Significance persisted after adjustment for most recent CD4 T-cell count, tobacco use, and hepatitis B or C. Markers were not analyzed explicitly in relation to frailty, and there was no HIV− comparison population. In the VACS, the VACS risk index, which includes age, clinical biomarkers including CD4 T-cell count and plasma HIV RNA and predicts hospitalization and mortality, was also found to be related to markers of inflammation (71) and with fragility fractures (72).
In the MACS, we have analyzed the relationships between a panel of 24 serologic immune activation markers, including pro-inflammatory cytokines, chemokines, and CRP (73), and frailty. For this study, we defined frailty (or non-frailty) as expression (or non-expression) of the Fried FP at two consecutive semiannual study visits. This definition was implemented because MACS studies indicated that nearly half of men who expressed the FP at one semiannual study visit did not express it at the next visit, and we wanted to minimize misclassification of frailty (41). The study was nested within a group of men in whom these markers had been measured over many years, which included a preponderance of HIV+ men. We found that many markers were significantly higher in the frail HIV+ men (n=109) than in the non-frail HIV+ (n=605) men, including IL-6, C-reactive protein (CRP), TNF-α, soluble CD14, and soluble TNF receptor II ((74) and manuscript submitted). The higher CRP remained significant after adjustment for demographics and comorbidities. It is noteworthy that most of these markers are similar to the markers that have been associated with frailty in the elderly HIV− population. These studies establish an association of immune activation, particularly of monocyte-macrophages (as evidenced by higher serum levels of IL-6, CRP, TNF-α, and related molecules), and frailty in HIV+ populations. Studies to assess whether this activation precedes or predicts the onset of frailty are now underway.
CHRONIC CMV INFECTION, FRAILTY, AND HIV INFECTION
Because of the profound medical, functional, and socioeconomic consequences of frailty for the vulnerable aging HIV-infected population, it is imperative to advance our understanding of the pathogenesis of this syndrome and use this information to develop interventional strategies. However, despite the apparent role of chronic inflammation in the pathogenesis of frailty, etiologies and mechanisms that underlie the immune and inflammatory pathway activation leading to chronic inflammation remain to be elucidated (see (66) for review). One important contributing factor may be chronic cytomegalovirus (CMV) infection. This virus, which is highly prevalent in both the geriatric population and HIV+ persons, may cause clonal T-cell expansion, leading to immune activation and chronic inflammation with their associated adverse health outcomes (75–79). The potential importance of CMV in the pathogenesis of systemic inflammation is supported by the fact that it is the target for a very large proportion of circulating T cells in both HIV− and HIV+ people, which is often greater than 10% and can be 30–40% (for example,(80)(81;82)).Some studies have found a direct association between positive CMV serology and frailty and functional decline in the elderly (49;83), but data are conflicting (84;85).
A possible explanation for this conflict is that anti-CMV IgG serology is a crude measure that merely indicates prior exposure to the virus, not necessarily a chronic (persistent) infection. We recently showed that defining chronic CMV infection by detection of CMV DNA in peripheral blood monocytes, rather than by positive CMV serology, led to a much stronger association of CMV infection with increased frequencies of CMV pp65 (NLV)-specific CD8+ T cells and immune activation (elevated serum neopterin levels) in an elderly population (86;87). In addition, we demonstrated higher serum IL-6 levels and increased CMV pp65 (NLV)-specific CD8+ T cells in older women with chronic CMV infection as assessed by CMV DNA in monocytes.(88). These findings support the hypothesis that chronic CMV infection is correlated with immune activation. Although CMV infection is common in people infected with HIV, and a very large proportion of the T-cell repertoire is devoted to CMV in HIV-infected persons, even those on long term HAART, (39;81;82), it remains to be determined if CMV-induced immune responses are quantitatively related to systemic levels of inflammation or to frailty in either HIV+ or HIV− populations.
CONCLUDING REMARKS
Whether HIV infection is a good model for accelerated aging is debatable (89). It is clear that as HIV-infected individuals age, frailty, initially described as a geriatric syndrome, has increasingly been recognized as a common HANA condition in this population. Mounting evidence supports the role of chronic inflammation as a contributing factor to frailty in older adults, and this is likely to be true in people with chronic, well-treated HIV infection, who have higher levels of systemic inflammation than demographically similar HIV− people, some of which persist even after taking co-morbidities into account. The precise mechanisms of this residual immune activation are poorly understood, and it remains to be determined if the mechanisms of inflammation in normal aging and in treated HIV infection are similar or different. Chronic CMV infection, a common persistent viral co-infection in both older HIV− people and people living with HIV, appears likely to be an important etiology and underlying mechanism for activation of immune and inflammatory pathways that contribute to the development of frailty and other HANA conditions in this population (39;90). Trials of anti-CMV pharmacotherapy could help address this question (91). An attractive long-term perspective is the potential development of effective vaccine-based preventative strategy for CMV infection, based on promising results from recent studies on CMV immunization in other patient populations (92–95). However, much remains to be learned about this complex viral infection, particularly its diagnostic evaluation as well as its impact on T-cell immunity, and a more exciting era of this active research is coming.
Supplementary Material
Reference List
- 1.Desquilbet L, Jacobson L, Fried LP, Phair JP, Williams CM, Jamieson BD, et al. HIV-1 infection is associated with an earlier occurrence of a phenotype related to frailty. J Gerontol A Biol Sci Med Sci. 2007;62:1279–1286. doi: 10.1093/gerona/62.11.1279. [DOI] [PubMed] [Google Scholar]
- 2•.Piggott DA, Muzaale AD, Mehta SH, Brown TT, Patel KV, Leng SX, et al. Frailty, HIV infection, and mortality in an aging cohort of injection drug users. PLoS ONE. 2013;8(1):e54910. doi: 10.1371/journal.pone.0054910. This study demonstrated the prognostic import of frailty in a cohort of injection drug users with HIV infcction. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3•.Desquilbet L, Margolick JB, Fried LP, Phair JP, Jamieson BD, Holloway M, et al. Relationship between a frailty-related phenotype and progressive deterioration of the immune system in HIV-1 infected men. J Acquir Immun Defic Syndr. 2009;50:299–306. doi: 10.1097/QAI.0b013e3181945eb0. This study illustrated the relationship between the frailty-related phenotype studied and the CD4 cell count in HIV-infected and -uninfected men who have sex with men. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Terzian AS, Holman S, Nathwani N, Robison E, Weber K, Young M, et al. Factors associated with preclinical disability and frailty among HIV-infected and HIV-uninfected women in the era of cART. J Womens Health (Larchmt) 2009;18(12):1965–1974. doi: 10.1089/jwh.2008.1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pathai S, Gilbert C, Weiss HA, Cook C, Wood R, Bekker LG, et al. Frailty in HIV-infected adults in South Africa. J Acquir Immune Defic Syndr. 2013;62(1):43–51. doi: 10.1097/QAI.0b013e318273b631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6•.Akgün KM, Tate JP, Crothers K, Crystal S, Leaf DA, Womack J, Brown TT, Justice AC, Oursler KK. An adapted frailty-related phenotype and the VACS index as predictors of hospitalization and mortality in HIV-infected and uninfected individuals. JAIDS. 2014;67:397–404. doi: 10.1097/QAI.0000000000000341. Using a modified frailty phenotype, this study demonstrated the clinical import of frailty in HIV-infected and -unifected veterans. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7••.Fried L, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults: evidence for a phenotype. Journal of Gerontology: Medical Sciences J Gerontol Med Sci. 2001;56A:M146–M156. doi: 10.1093/gerona/56.3.m146. This paper described the frailty phenotype that has been most widely used and is focused on in the present review. [DOI] [PubMed] [Google Scholar]
- 8.Bandeen-Roche K, Xue QL, Ferrucci L, Walston J, Guralnik JM, Chaves P, et al. Phenotype of frailty: characterization in the women’s health and aging studies. J Gerontol A Biol Sci Med Sci. 2006;61(3):262–266. doi: 10.1093/gerona/61.3.262. [DOI] [PubMed] [Google Scholar]
- 9.Lipsitz LA. Dynamics of stability: the physiologic basis of functional health and frailty. J Gerontol A Biol Sci Med Sci. 2002;57(3):B115–B125. doi: 10.1093/gerona/57.3.b115. [DOI] [PubMed] [Google Scholar]
- 10.Fried LP, Hadley EC, Walston JD, Newman AB, Guralnik JM, Studenski S, et al. From bedside to bench: research agenda for frailty. Sci Aging Knowledge Environ. 2005;2005(31):e24. doi: 10.1126/sageke.2005.31.pe24. [DOI] [PubMed] [Google Scholar]
- 11.Walston J, Hadley EC, Ferrucci L, Guralnik JM, Newman AB, Studenski SA, et al. Research agenda for frailty in older adults: toward a better understanding of physiology and etiology: summary from the American Geriatrics Society/National Institute on Aging Research Conference on Frailty in Older Adults. J Am Geriatr Soc. 2006;54(6):991–1001. doi: 10.1111/j.1532-5415.2006.00745.x. [DOI] [PubMed] [Google Scholar]
- 12.Yao X, Li H, Leng SX. Inflammation and immune system alterations in frailty. Clin Geriatr Med. 2011;27(1):79–87. doi: 10.1016/j.cger.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen X, Mao G, Leng SX. Frailty syndrome: an overview. Clin Interv Aging. 2014;9:433–441. doi: 10.2147/CIA.S45300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14•.Morley JE, Vellas B, van Kan GA, Anker SD, Bauer JM, Bernabei R, et al. Frailty consensus: a call to action. J Am Med Dir Assoc. 2013;14(6):392–397. doi: 10.1016/j.jamda.2013.03.022. This paper reviews the basis for considering frailty as a clnical syndrome that merits attention in clinical care. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15•.Fried LP, Xue QL, Cappola AR, Ferrucci L, Chaves P, Varadhan R, et al. Nonlinear multisystem physiological dysregulation associated with frailty in older women: implications for etiology and treatment. J Gerontol A Biol Sci Med Sci. 2009;64(10):1049–1057. doi: 10.1093/gerona/glp076. This paper explores the multisystem nature of frailty. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jones DM, Song X, Rockwood K. Operationalizing a frailty index from a standardized comprehensive geriatric assessment. J Am Geriatr Soc. 2004;52(11):1929–1933. doi: 10.1111/j.1532-5415.2004.52521.x. [DOI] [PubMed] [Google Scholar]
- 17•.Brothers TD, Kirkland S, Guaraldi G, Falutz J, Theou O, Johnston BL, et al. Frailty in people aging with human immunodeficiency virus (HIV) infection. J Infect Dis. 2014;210(8):1170–1179. doi: 10.1093/infdis/jiu258. This paper reviews the clinical aspects of frailty and studies of frailty in people living with HIV infection. [DOI] [PubMed] [Google Scholar]
- 18.Fried LP, Ferrucci L, Darer J, Williamson JD, Anderson G. Untangling the concepts of disability, frailty, and comorbidity: implications for improved targeting and care. J Gerontol A Biol Sci Med Sci. 2004;59(3):255–263. doi: 10.1093/gerona/59.3.m255. [DOI] [PubMed] [Google Scholar]
- 19.Santos-Eggimann B, Cuenoud P, Spagnoli J, Junod J. Prevalence of frailty in middle-aged and older community-dwelling Europeans living in 10 countries. J Gerontol A Biol Sci Med Sci. 2009;64(6):675–681. doi: 10.1093/gerona/glp012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alvarado BE, Zunzunegui MV, Beland F, Bamvita JM. Life course social and health conditions linked to frailty in Latin American older men and women. J Gerontol A Biol Sci Med Sci. 2008;63(12):1399–1406. doi: 10.1093/gerona/63.12.1399. [DOI] [PubMed] [Google Scholar]
- 21.Buchman AS, Boyle PA, Wilson RS, Tang Y, Bennett DA. Frailty is associated with incident Alzheimer’s disease and cognitive decline in the elderly. Psychosom Med. 2007;69(5):483–489. doi: 10.1097/psy.0b013e318068de1d. [DOI] [PubMed] [Google Scholar]
- 22.Makary MA, Segev DL, Pronovost PJ, Syin D, Bandeen-Roche K, Patel P, et al. Frailty as a predictor of surgical outcomes in older patients. J Am Coll Surg. 2010;210(6):901–908. doi: 10.1016/j.jamcollsurg.2010.01.028. [DOI] [PubMed] [Google Scholar]
- 23.Ronning B, Wyller TB, Seljeflot I, Jordhoy MS, Skovlund E, Nesbakken A, et al. Frailty measures, inflammatory biomarkers and post-operative complications in older surgical patients. Age Ageing. 2010;39(6):758–761. doi: 10.1093/ageing/afq123. [DOI] [PubMed] [Google Scholar]
- 24.Audisio RA, van LB. When reporting on older patients with cancer, frailty information is needed. Ann Surg Oncol. 2011;18(1):4–5. doi: 10.1245/s10434-010-1327-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Courtney-Brooks M, Tellawi AR, Scalici J, Duska LR, Jazaeri AA, Modesitt SC, et al. Frailty: an outcome predictor for elderly gynecologic oncology patients. Gynecol Oncol. 2012;126(1):20–24. doi: 10.1016/j.ygyno.2012.04.019. [DOI] [PubMed] [Google Scholar]
- 26.Aaldriks AA, van der Geest LG, Giltay EJ, le CS, Portielje JE, Tanis BC, et al. Frailty and malnutrition predictive of mortality risk in older patients with advanced colorectal cancer receiving chemotherapy. J Geriatr Oncol. 2013;4(3):218–226. doi: 10.1016/j.jgo.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 27.Iqbal J, Denvir M, Gunn J. Frailty assessment in elderly people. Lancet. 2013;381(9882):1985–1986. doi: 10.1016/S0140-6736(13)61203-9. [DOI] [PubMed] [Google Scholar]
- 28.McAdams-DeMarco MA, Suresh S, Law A, Salter ML, Gimenez LF, Jaar BG, et al. Frailty and falls among adult patients undergoing chronic hemodialysis: a prospective cohort study. BMC Nephrol. 2013;14:224. doi: 10.1186/1471-2369-14-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McAdams-DeMarco MA, Law A, Tan J, Delp C, King EA, Orandi B, et al. Mycophenolate Reduction, and Graft Loss in Kidney Transplant Recipients. Transplantation. 2014 doi: 10.1097/TP.0000000000000444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yao X, Hamilton RG, Weng NP, Xue QL, Bream JH, Li H, et al. Frailty is associated with impairment of vaccine-induced antibody response and increase in post-vaccination influenza infection in community-dwelling older adults. Vaccine. 2011;29(31):5015–5021. doi: 10.1016/j.vaccine.2011.04.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ridda I, Macintyre CR, Lindley R, Gao Z, Sullivan JS, Yuan FF, et al. Immunological responses to pneumococcal vaccine in frail older people. Vaccine. 2009;27(10):1628–1636. doi: 10.1016/j.vaccine.2008.11.098. [DOI] [PubMed] [Google Scholar]
- 32.Fahey JL, Taylor JMG, Detels R, Hofmann B, Melmed R, Nishanian P, et al. The prognostic value of cellular and serologic markers in infection with human immunodeficiency virus type 1. N Engl J Med. 1990;322:166–172. doi: 10.1056/NEJM199001183220305. [DOI] [PubMed] [Google Scholar]
- 33.Lahdevirta J, Maury CPJ, Teppo A-M, Repo H. Elevated levels of circulating cachectin/tumor necrosis factor in patients with acquired immunodeficiency syndrome. Am J Med. 1988;85:289–291. doi: 10.1016/0002-9343(88)90576-1. [DOI] [PubMed] [Google Scholar]
- 34.Palella FJ, Delaney KM, Moorman AC, Loveless MO, Fuhrer J, Satten GA, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. NEJM. 1998;338:853–860. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 35.Liu H, Lin X, Xu Y, Chen S, Shi J, Morisky D. Emerging HIV epidemic among older adults in Nanning China. AIDS Patient Care STDS. 2012;26:565–567. doi: 10.1089/apc.2012.0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hontelez JA, Lurie MN, Newell ML, Bakker R, Tanser F, Barnighausen T, et al. Ageing with HIV in South Africa. AIDS. 2011;25(13):1665–1667. doi: 10.1097/QAD.0b013e32834982ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37•.High KP, Brennan-Ing M, Clifford DB, Cohen MH, Currier J, Deeks SG, et al. HIV and aging: state of knowledge and areas of critical need for research. A report to the NIH Office of AIDS Research by the HIV and Aging Working Group. J Acquir Immune Defic Syndr. 2012;60(Suppl 1):S1–18. doi: 10.1097/QAI.0b013e31825a3668. This paper provides a consensus view of the research agenda on frailty and other aspects of aging in people living with HIV infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guaraldi G, Orlando G, Zona S, Menozzi M, Carli F, Garlassi E, et al. Premature age-related comorbidities among HIV-infected persons compared with the general population. Clin Infect Dis. 2011;53(11):1120–1126. doi: 10.1093/cid/cir627. [DOI] [PubMed] [Google Scholar]
- 39•.Deeks SG. HIV infection, inflammation, immunosenescence, and aging. Annu Rev Med. 2011;62:141–155. doi: 10.1146/annurev-med-042909-093756. This is a thorough review of evidence linking inflammation with aging in people with and without HIV infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40•.Desquilbet L, Jacobson L, Fried LP, Phair JP, Jamieson BD, Holloway M, et al. A frailty-related phenotype before HAART initiation as an independent risk factor for AIDS or death after HAART among HIV-infected men. J Gerontol A Biol Sci Med Sci. 2011;66A:1030–1038. doi: 10.1093/gerona/glr097. This paper provides evidene that a frailty-related phenotype independent predicted onset of AIDS or death in a cohort of men who have sex with men. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41•.Althoff KN, Jacobson LP, Cranston RD, Detels R, Phair JP, Li X, et al. Age, comorbidities and AIDS predict the frailty phenotype in men who have sex with men. J Gerontol A Biol Sci Med Sci. 2014;69A:189–198. doi: 10.1093/gerona/glt148. This paper reports data on the prevalence of the frailty phenotype in men who have sex with men, as well as factors associated with the incidence of this phenotype. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Green M, Covinsky K, Astemborski J, Piggot D, Brown T, Leng S, et al. The relationship of physical performance with HIV disease and mortality. AIDS. 2014;28 doi: 10.1097/QAD.0000000000000507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Onen NF, Agbebi A, Shacham E, Stamm KE, Onen AR, Overton ET. Frailty among HIV-infected persons in an urban outpatient care setting. J Infect. 2009;59(5):346–352. doi: 10.1016/j.jinf.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 44.Franceschi C, Bonafe M, Valensin S, Olivieri F, De LM, Ottaviani E, et al. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci. 2000;908:244–254. doi: 10.1111/j.1749-6632.2000.tb06651.x. [DOI] [PubMed] [Google Scholar]
- 45.De Martinis M, Franceschi C, Monti D, Ginaldi L. Inflammation markers predicting frailty and mortality in the elderly. Exp Mol Pathol. 2006;80(3):219–227. doi: 10.1016/j.yexmp.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 46.Roubenoff R, Parise H, Payette HA, Abad LW, D’Agostino R, Jacques PF, et al. Cytokines, insulin-like growth factor 1, sarcopenia, and mortality in very old community-dwelling men and women: the Framingham Heart Study. Am J Med. 2003;115(6):429–435. doi: 10.1016/j.amjmed.2003.05.001. [DOI] [PubMed] [Google Scholar]
- 47•.Leng S, Chaves P, Koenig K, Walston J. Serum interleukin-6 and hemoglobin as physiological correlates in the geriatric syndrome of frailty: a pilot study. J Am Geriatr Soc. 2002;50(7):1268–1271. doi: 10.1046/j.1532-5415.2002.50315.x. This paper was the first reporting supportive evidence for chronic inflammation as indicated by elevated IL-6 levels in frailty in the geriatric population. [DOI] [PubMed] [Google Scholar]
- 48.Walston J, McBurnie MA, Newman A, Tracy RP, Kop WJ, Hirsch CH, et al. Frailty and activation of the inflammation and coagulation systems with and without clinical comorbidities: results from the Cardiovascular Health Study. Arch Intern Med. 2002;162(20):2333–2341. doi: 10.1001/archinte.162.20.2333. [DOI] [PubMed] [Google Scholar]
- 49.Schmaltz HN, Fried LP, Xue QL, Walston J, Leng SX, Semba RD. Chronic cytomegalovirus infection and inflammation are associated with prevalent frailty in community-dwelling older women. J Am Geriatr Soc. 2005;53(5):747–754. doi: 10.1111/j.1532-5415.2005.53250.x. [DOI] [PubMed] [Google Scholar]
- 50•.Leng SX, Xue QL, Tian J, Walston JD, Fried LP. Inflammation and frailty in older women. J Am Geriatr Soc. 2007;55(6):864–871. doi: 10.1111/j.1532-5415.2007.01186.x. This paper further describes chronic inflammation as indicated by both higher IL-6 levels and white blood cell counts in frailty in the geriatric population. [DOI] [PubMed] [Google Scholar]
- 51.Hubbard RE, O’Mahony MS, Savva GM, Calver BL, Woodhouse KW. Inflammation and frailty measures in older people. J Cell Mol Med. 2009;13(9B):3103–3109. doi: 10.1111/j.1582-4934.2009.00733.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Collerton J, Martin-Ruiz C, Davies K, Hilkens CM, Isaacs J, Kolenda C, et al. Frailty and the role of inflammation, immunosenescence and cellular ageing in the very old: cross-sectional findings from the Newcastle 85+ Study. Mech Ageing Dev. 2012;133(6):456–466. doi: 10.1016/j.mad.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 53.Leng SX, Xue QL, Tian J, Huang Y, Yeh SH, Fried LP. Associations of neutrophil and monocyte counts with frailty in community-dwelling disabled older women: results from the Women’s Health and Aging Studies I. Exp Gerontol. 2009;44(8):511–516. doi: 10.1016/j.exger.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 54.Semba RD, Margolick JB, Leng S, Walston J, Ricks MO, Fried LP. T cell subsets and mortality in older community-dwelling women. Experimental Gerontology. 2005;40:81–87. doi: 10.1016/j.exger.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 55.De Fanis U, Wang GC, Fedarko NS, Walston JD, Casolaro V, Leng SX. T-lymphocytes expressing CC chemokine receptor-5 are increased in frail older adults. J Am Geriatr Soc. 2008;56(5):904–908. doi: 10.1111/j.1532-5415.2008.01673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Loetscher P, Uguccioni M, Bordoli L, Baggiolini M, Moser B, Chizzolini C, et al. CCR5 is characteristic of Th1 lymphocytes. Nature. 1998;391(6665):344–345. doi: 10.1038/34814. [DOI] [PubMed] [Google Scholar]
- 57.Leng SX, Yang H, Walston JD. Decreased cell proliferation and altered cytokine production in frail older adults. Aging Clin Exp Res. 2004;16(3):249–252. doi: 10.1007/BF03327392. [DOI] [PubMed] [Google Scholar]
- 58.Qu T, Walston JD, Yang H, Fedarko NS, Xue QL, Beamer BA, et al. Upregulated ex vivo expression of stress-responsive inflammatory pathway genes by LPS-challenged CD14(+) monocytes in frail older adults. Mech Ageing Dev. 2009;130(3):161–166. doi: 10.1016/j.mad.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Qu T, Yang H, Walston JD, Fedarko NS, Leng SX. Upregulated monocytic expression of CXC chemokine ligand 10 (CXCL-10) and its relationship with serum interleukin-6 levels in the syndrome of frailty. Cytokine. 2009;46(3):319–324. doi: 10.1016/j.cyto.2009.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Leng SX, Tian X, Matteini A, Li H, Hughes J, Jain A, et al. IL-6-independent association of elevated serum neopterin levels with prevalent frailty in community-dwelling older adults. Age Ageing. 2011;40(4):475–481. doi: 10.1093/ageing/afr047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ferrucci L, Penninx BW, Volpato S, Harris TB, Bandeen-Roche K, Balfour J, et al. Change in muscle strength explains accelerated decline of physical function in older women with high interleukin-6 serum levels. J Am Geriatr Soc. 2002;50(12):1947–1954. doi: 10.1046/j.1532-5415.2002.50605.x. [DOI] [PubMed] [Google Scholar]
- 62.Maggio M, Guralnik JM, Longo DL, Ferrucci L. Interleukin-6 in aging and chronic disease: a magnificent pathway. J Gerontol A Biol Sci Med Sci. 2006;61(6):575–584. doi: 10.1093/gerona/61.6.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chaves PH, Semba RD, Leng SX, Woodman RC, Ferrucci L, Guralnik JM, et al. Impact of anemia and cardiovascular disease on frailty status of community-dwelling older women: the Women’s Health and Aging Studies I and II. J Gerontol A Biol Sci Med Sci. 2005;60(6):729–735. doi: 10.1093/gerona/60.6.729. [DOI] [PubMed] [Google Scholar]
- 64.Leng SX, Cappola AR, Andersen RE, Blackman MR, Koenig K, Blair M, et al. Serum levels of insulin-like growth factor-I (IGF-I) and dehydroepiandrosterone sulfate (DHEA-S), and their relationships with serum interleukin-6, in the geriatric syndrome of frailty. Aging Clin Exp Res. 2004;16(2):153–157. doi: 10.1007/BF03324545. [DOI] [PubMed] [Google Scholar]
- 65.Leng SX, Hung W, Cappola AR, Yu Q, Xue QL, Fried LP. White blood cell counts, insulin-like growth factor-1 levels, and frailty in community-dwelling older women. J Gerontol A Biol Sci Med Sci. 2009;64(4):499–502. doi: 10.1093/gerona/gln047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66•.Lederman MM, Funderburg NT, Sekaly RP, Klatt NR, Hunt PW. Residual immune dysregulation syndrome in treated HIV infection. Adv Immunol. 2013;119:51–83. doi: 10.1016/B978-0-12-407707-2.00002-3. This paper reviews the mechanisms of immune activation that persists in people with well-treated HIV infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Regidor DL, Detels R, Breen EC, Widney DP, Jacobson LP, Palella F, et al. Effect of highly active antiretroviral therapy on biomarkers of B-lymphocyte activation and inflammation. AIDS. 2011;25(3):303–314. doi: 10.1097/QAD.0b013e32834273ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Martin J, Volberding P. HIV and premature aging: A field still in its infancy. Ann Intern Med. 2010;153(7):477–479. doi: 10.7326/0003-4819-153-7-201010050-00013. [DOI] [PubMed] [Google Scholar]
- 69•.Shiels MS, Pfeiffer RM, Engels EA. Age at cancer diagnosis among persons with AIDS in the United States. Ann Intern Med. 2010;153(7):452–460. doi: 10.1059/0003-4819-153-7-201010050-00008. This paper shows the effect of underlying age distributions in estimating relative rates of cancer (and by implication any disease) in groups that are not the same age. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70•.Erlandson KM, Allshouse AA, Jankowski CM, Lee EJ, Rufner KM, Palmer BE, et al. Association of functional impairment with inflammation and immune activation in HIV type 1-infected adults receiving effective antiretroviral therapy. J Infect Dis. 2013;208(2):249–259. doi: 10.1093/infdis/jit147. This is one of the few studies to examine inflammation and frailty in people with HIV infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Justice AC, Freiberg ms, Tracy R, Kuller L, Tate JP, Goetz MB, et al. Does an index composed of clinical data reflect effects of inflammation, coagulation, and monocyte activation on mortality among those aging with HIV? Clin Infect Dis. 2012;54:984–994. doi: 10.1093/cid/cir989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Womack JA, Goulet JL, Gibert C, Brandt CA, Skanderson M, Gulanski B, et al. Physiologic frailty and fragility fracture in HIV-infected male veterans. Clin Infect Dis. 2013;56(10):1498–1504. doi: 10.1093/cid/cit056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73•.Wada NI, Jacobson LP, Margolick JB, Breen EB, Macatangay B, Penugonda S, et al. The effect of HAART-induced HIV suppression on circulating markers of inflammation and immune activation. AIDS. doi: 10.1097/QAD.0000000000000545. In press. This paper shows the magnitude and time course of effects of HIV suppression on systems inflammation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Margolick JB, Martinez-Maza O, Jacobson L, Lopez J, Li X, Phair J, et al. Frailty and circulating markers of inflammation in HIV-infected and -uninfected men in the Multicenter AIDS Cohort Study (MACS). Presented at the 20th Conference on Retroviruses and Opportunistic Infections; Atlanta, GA. March 2013; Abstract #800. 2013. [Google Scholar]
- 75.Looney RJ, Falsey A, CAMPBELL D, Torres A, Kolassa J, Brower C, et al. Role of cytomegalovirus in the T cell changes seen in elderly individuals. Clin Immunol. 1999;90(2):213–219. doi: 10.1006/clim.1998.4638. [DOI] [PubMed] [Google Scholar]
- 76.Olsson J, Wikby A, Johansson B, Lofgren S, Nilsson BO, Ferguson FG. Age-related change in peripheral blood T-lymphocyte subpopulations and cytomegalovirus infection in the very old: the Swedish longitudinal OCTO immune study. Mech Ageing Dev. 2000;121(1–3):187–201. doi: 10.1016/s0047-6374(00)00210-4. [DOI] [PubMed] [Google Scholar]
- 77.Pawelec G, Akbar A, Caruso C, Solana R, Grubeck-Loebenstein B, Wikby A. Human immunosenescence: is it infectious? Immunol Rev. 2005;205:257–268. doi: 10.1111/j.0105-2896.2005.00271.x. [DOI] [PubMed] [Google Scholar]
- 78.Khan N, Shariff N, Cobbold M, Bruton R, Ainsworth JA, Sinclair AJ, et al. Cytomegalovirus seropositivity drives the CD8 T cell repertoire toward greater clonality in healthy elderly individuals. J Immunol. 2002;169(4):1984–1992. doi: 10.4049/jimmunol.169.4.1984. [DOI] [PubMed] [Google Scholar]
- 79.Koch S, Larbi A, Ozcelik D, Solana R, Gouttefangeas C, Attig S, et al. Cytomegalovirus infection: a driving force in human T cell immunosenescence. Ann N Y Acad Sci. 2007;1114:23–35. doi: 10.1196/annals.1396.043. [DOI] [PubMed] [Google Scholar]
- 80•.Sylwester AW, Mitchell BL, Edgar JB, Taormina C, Pelte C, Ruchti F, et al. Broadly targeted human cytomegalovirus-specific CD4+ and CD8+ T cells dominate the memory compartments of exposed subjects. J Exp Med. 2005;202(5):673–685. doi: 10.1084/jem.20050882. This is a study of the immune response to the entire CMV genome (!) in healthy CMV-seropositive donors. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Naeger DM, Martin JN, Sinclair E, Hunt PW, Bangsberg DR, Hecht F, et al. Cytomegalovirus-specific T cells persist at very high levels during long-term antiretroviral treatment of HIV disease. PLoS One. 2010;5(1):e8886. doi: 10.1371/journal.pone.0008886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Li H, Margolick JB, Bream JH, Nilles TL, Langan S, Bui HT, et al. Heterogeneity of CD4+ and CD8+ T-cell responses of cytomegalovirus in HIV-infected and -uninfected men who have sex with men. J Infect Dis. 2014;210:400–404. doi: 10.1093/infdis/jiu093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang GC, Kao WH, Murakami P, Xue QL, Chiou RB, Detrick B, et al. Cytomegalovirus infection and the risk of mortality and frailty in older women: a prospective observational cohort study. Am J Epidemiol. 2010;171(10):1144–1152. doi: 10.1093/aje/kwq062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mathei C, Vaes B, Wallemacq P, Degryse J. Associations between cytomegalovirus infection and functional impairment and frailty in the BELFRAIL Cohort. J Am Geriatr Soc. 2011;59(12):2201–2208. doi: 10.1111/j.1532-5415.2011.03719.x. [DOI] [PubMed] [Google Scholar]
- 85.Vallejo AN, Hamel DL, Jr, Mueller RG, Ives DG, Michel JJ, Boudreau RM, et al. NK-like T cells and plasma cytokines, but not anti-viral serology, define immune fingerprints of resilience and mild disability in exceptional aging. PLoS ONE. 2011;6(10):e26558. doi: 10.1371/journal.pone.0026558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86•.Leng SX, Qu T, Semba RD, Yao X, Nilles T, Yang X, et al. Relationship between cytomegalovirus (CMV) IgG seropositivity, detectable CMV DNA peripheral monocytes, and CMV-specific CD8+ T-cells in older adults. AGE (Dordr) 2011;33:607–614. doi: 10.1007/s11357-011-9205-9. This paper provides evidence hat presence of detectable CMV DNA in monocytes is a better predictor of a sizable CMV response than presence of antibody to CMV. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Leng SX, Li H, Xue QL, Tian J, Yang X, Ferrucci L, et al. Association of detectable cytomegalovirus (CMV) DNA in monocytes rather than positive CMV IgG serology with elevated neopterin levels in community-dwelling older adults. Exp Gerontol. 2011;46(8):679–684. doi: 10.1016/j.exger.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88•.Li H, Weng P, Najarro K, Xue Q-L, Semba RD, Margolick JB, et al. Chronic CMV infection in older women: longitudinal comparisons of CMV DNA in peripheral monocytes, anti-CMV IgG titers, serum IL-6 levels, and CMV pp65 (NLV)-specific CD8+ T-cell frequencies with twelve year follow-up. Exp Gerontol. 2013;54:84–89. doi: 10.1016/j.exger.2014.01.010. In this study presence of CMV DNA in monocytes was associated with higher levels of serum IL-6, a marker that is one of the most closely associated with frailty and aging. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pathai S, Bajillan H, Landay AL, High KP. Is HIV a Model of Accelerated or Accentuated Aging? J Gerontol A Biol Sci Med Sci. 2013 doi: 10.1093/gerona/glt168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Barrett L, Fowke KR, Grant MD. Cytomegalovirus, aging, and HIV: a perfect storm. AIDS Rev. 2012;14(3):159–167. [PubMed] [Google Scholar]
- 91•.Hunt PW, Martin JN, Sinclair E, Epling L, Teague J, Jacobson MA, et al. Valganciclovir reduces T cell activation in HIV-infected individuals with incomplete CD4+ T cell recovery on antiretroviral therapy. J Infect Dis. 2011;203(10):1474–1483. doi: 10.1093/infdis/jir060. This is a treatment trial in which an anti-CMV agent, valganciclovir, reduces CMV expression and some markers of cellular immune activation, particularly on CD8 T-cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pass RF, Zhang C, Evans A, Simpson T, Andrews W, Huang ML, et al. Vaccine prevention of maternal cytomegalovirus infection. N Engl J Med. 2009;360(12):1191–1199. doi: 10.1056/NEJMoa0804749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Griffiths PD, Stanton A, McCarrell E, Smith C, Osman M, Harber M, et al. Cytomegalovirus glycoprotein-B vaccine with MF59 adjuvant in transplant recipients: a phase 2 randomised placebo-controlled trial. Lancet. 2011;377(9773):1256–1263. doi: 10.1016/S0140-6736(11)60136-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sabbaj S, Pass RF, Goepfert PA, Pichon S. Glycoprotein B vaccine is capable of boosting both antibody and CD4 T-cell responses to cytomegalovirus in chronically infected women. J Infect Dis. 2011;203(11):1534–1541. doi: 10.1093/infdis/jir138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kharfan-Dabaja MA, Boeckh M, Wilck MB, Langston AA, Chu AH, Wloch MK, et al. A novel therapeutic cytomegalovirus DNA vaccine in allogeneic haemopoietic stem-cell transplantation: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Infect Dis. 2012;12(4):290–299. doi: 10.1016/S1473-3099(11)70344-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


