Abstract
Objectives
Fatigue during cancer treatment is associated with depression. Neurotrophic factors play a major role in depression and stress and might provide insight into mechanisms of fatigue. This study investigated the association between plasma concentrations of three neurotrophic factors (BDNF, brain-derived neurotrophic factor; GDNF, glial-derived neurotrophic factor; and SNAPIN, soluble N-ethylmaleimide sensitive fusion attachment receptor-associated protein) and initial fatigue intensification during external beam radiation therapy (EBRT) in euthymic non-metastatic prostate cancer men.
Methods
Fatigue, as measured by the 13-item Functional Assessment of Cancer Therapy-Fatigue (FACT-F), and plasma neurotrophic factors were collected at baseline (prior to EBRT) and mid-EBRT. Subjects were categorized into fatigue and no fatigue groups using a >3-point change in FACT-F scores between the two time points. Multiple linear regressions analysed the associations between fatigue and neurotrophic factors.
Results
FACT-F scores of 47 subjects decreased from baseline (43.95 ± 1.3) to mid-EBRT (38.36 ± 1.5, P < 0.001), indicating worsening fatigue. SNAPIN levels were associated with fatigue scores (rs = 0.43, P = 0.005) at baseline. A significant decrease of BDNF concentration (P = 0.008) was found in fatigued subjects during EBRT (n = 39).
Conclusions
Baseline SNAPIN and decreasing BDNF levels may influence worsening fatigue during EBRT. Further investigations are warranted to confirm their role in the pathophysiology and therapeutics of fatigue.
Keywords: fatigue, depression, brain-derived neurotrophic factor, radiation, prostate neoplasm
Introduction
Fatigue associated with cancer and its therapies is a burdensome syndrome that is commonly experienced by approximately 80% of patients undergoing cancer treatment (Horneber et al. 2012). The National Comprehensive Cancer Network (NCCN) describes fatigue as a persistent, subjective sense of tiredness that interferes with usual functioning (Patrick et al. 2003). Generally, fatigue intensifies a week after cancer treatment initiation, and is unrelieved by rest (Portenoy and Itri 1999). Importantly, fatigue negatively affects patients’adherence to cancer treatment regimens and their overall quality of life (Morrow et al. 2002).
Fatigue related to cancer and its therapy remains poorly managed and defined (Barsevick et al. 2013). Its aetiology is thought to involve diverse physiological mechanisms with links to anaemia, dysregulation of cytokines, adenosine triphosphate, hypothalamic–pituitary–adrenal axis, serotonin, circadian rhythm disruption, or tumuor burden (Glaus et al. 1996; Morrow et al. 2002). Fatigue is often associated with other symptoms such as depression, but empirical evidence suggests that fatigue can be distinguished from depression when persistent fatigue was reported by patients whose depression were in remission (Fava 2003; Ferguson et al. 2014). These studies suggest that fatigue may have mutual psychological and shared neuroanatomical pathways with other symptoms and distinct biological pathways to explain its aetiology.
Neurotrophic factors are classically known as signalling molecules located in the soma of neurons, which play an essential role in brain development, synaptic plasticity, memory, and cognitive performance (Croll et al. 1998; Kaplan and Miller 2000; Tokuyama et al. 2000). Several studies have shown the importance of neurotrophic factors in stress response for neuroprotection and regulation of psychological behaviours (Allen and Dawbarn 2006). The inflammatory response generated during stress stimulates physiological repair mechanisms including activation of neurotrophic factors to enhance neuronal survival and modulate immune function (Vega et al. 2003; Scuri et al. 2010). The role of neurotrophic factors in immune modulation and neuroprotection makes them attractive therapeutic targets for neurodegenerative conditions such as multiple sclerosis (Luhder et al. 2013), as well as for complex syndromes such as chronic fatigue, when both pathways are considered major aetiological players.
The physiologic roles of neurotrophic factors during irradiation have been reported in the past, for example, the brain-derived neurotrophic factor (BDNF) was shown to be protective against neuronal death from irradiation in animal studies (Kim and Zhao 2005). The glial-derived neurotrophic factor (GDNF) has also been reported recently to mitigate the functional damage induced by radiation therapy (Xiao et al. 2014). SNAPIN is reported to maintain neuronal morphology and function (Cai and Sheng 2011), which are important for optimal neurotransmission. Therefore, it is worthwhile to investigate the relationships of these neurotrophic factors in individuals who have clinical fatigue, but are not depressed, while receiving repeated stress.
This study describes the association between changes in the plasma concentrations of BDNF, GDNF, and SNAPIN with the initial intensification of fatigue in euthymic men with non-metastatic prostate cancer (NM-PC) while receiving external beam radiation therapy (EBRT). We hypothesized that declining levels of neurotrophic factors would worsen the fatigue symptoms during EBRT, and that increasing the levels of neurotrophic factors over time would reverse this experience.
Methods
Sample
Study participants were enrolled in an actively-recruiting National Institutes of Health (NIH) Institutional Review Board (IRB)-approved protocol (NCT01143467). Informed written consents were obtained from all study participants. Subjects were recruited from April 2009 to December 2013 at the Mark O. Hatfield Clinical Research Center, NIH, Bethesda, MD, USA. Included in this study were male subjects, who were 18 years or older, with localized prostate cancer with or without prior prostate surgery ± androgen deprivation therapy, and were scheduled to receive EBRT. Subjects with a progressive or unstable medical condition causing clinically significant fatigue, chronic inflammatory disease, infectious disease, depression or other major psychiatric condition within the past 5 years, other types of cancer and receiving chemotherapy, or taking steroids, non-steroid anti-inflammatory medications, or tranquilizers were excluded. Participants received in situ EBRT 5 days a week with an average total dose of 68–75 Gray (Gy).
Measurements
Demographic and clinical characteristics of study participants are presented in Table I. All study participants completed all study measures at baseline (day 0, prior to EBRT) and at midpoint (19–21 sessions after EBRT initiation), which was the period when initial fatigue intensification was found to peak in our previous study (Saligan et al. 2013). All participants were evaluated using the Hamilton Depression Rating Scale (HAM-D; Moberg et al. 2001), and the presence of depressive symptoms was considered an exclusion criterion. Fatigue was measured by the highly reliable and valid Functional Assessment of Cancer Therapy-Fatigue (FACT-F; Yellen et al. 1997). FACT-F scores range from 0 to 52, with higher scores reflecting lower fatigue severity. A ≥ 3-point decrease in FACT-F scores is considered clinically-significant change in fatigue level (Cella et al. 2002); a 3-point change is a minimally important difference in FACT-F score that is large enough to have implications for a patient’s treatment of care (Wyrwich et al. 2005). To optimize the phenotypic characterization of the study participants, subjects were grouped according to changes in FACT-F scores from baseline to midpoint of EBRT: the fatigue group (increasing fatigue severity, > 3-point decrease in FACT-F scores) and the no fatigue group (≤ 3-point decrease in FACT-F scores).
Table I.
Demographics and clinical characteristics of sample.
| EBRT (N = 47) | |||
|---|---|---|---|
| Mean (SE) | Range | n (%) | |
| Age in years | 64.50 (1.11) | 49–81 | |
| Ethnicity | |||
| Caucasian | 30 (63.8) | ||
| African-American | 11 (23.4) | ||
| Other | 6 (12.8) | ||
| Clinical T stage | |||
| T1 (a–c) | 14 (29.7) | ||
| T2 (a–c) | 26 (55.3) | ||
| T3 (a–c) | 7(15.0) | ||
| Gleason score | |||
| 6 | 5 (10.6) | ||
| 7 | 19 (40.4) | ||
| 8 | 12 (25.5) | ||
| 9 | 10 (21.3) | ||
| Others | 1 (2.1) | ||
| BMI | 29.81 (0.61) | 22.9–40.7 | |
| Total Dosage EBRT (Gray) | |||
| 66.0 | 1 (2.1) | ||
| 68.4 | 7 (14.9) | ||
| 75.6 | 39 (83.0) | ||
| PSA Levels (ng/ml) | |||
| Baseline | 7.71 (2.88) | 0.01–104.0 | |
| Completion | 0.34 (0.15) | 0.01–4.84 | |
| Albumin levels (g/dl) | 3.98 (0.07) | 2.7–4.5 | |
| Testosterone (ng/dl) | 252.32 (32.28) | 20–536 | |
| TSH (µIU/ml) | 2.01 (0.21) | 0.24–3.84 | |
| Hemoglobin (g/dl) | 13.69 (0.16) | 10.9–15.8 | |
| FACT-F score | |||
| Baseline | 43.95 (1.28) | 23–52 | |
| Midpoint (day 19–21) | 38.36 (1.52) | 16–52 | |
| Endpoint (day 38–42) | 39.48 (1.45) | 14–52 | |
| HAM-D score | |||
| Baseline | 1.16 (0.24) | 0–8 | |
| Midpoint (day 19–21) | 2.22 (0.49) | 0–13 | |
| Endpoint (day 38–42) | 1.49 (0.20) | 0–8 | |
Demographic and clinical characteristics of sample are presented as mean with standard error (SE), range, number (n) and percentage (%) of subjects.
BMI, body mass index; dl, decilitre; EBRT, external beam radiation therapy; PSA, prostate specific antigen; TSH, thyroid stimulating hormone.; FACT-F, Functional Assessment of Cancer Therapy-Fatigue; HAM-D, Hamilton Depression scale.
Biological samples
Whole blood samples were collected using EDTA tubes. Plasma was centrifuged immediately after collection at 3000 rpm and 4°C for 10 min, divided into 250-µl aliquots, and stored in −80°C freezers until batch analysis. Prior to analysis, frozen plasma samples were thawed on ice and centrifuged at 1000 × g for 10 min at 4°C. Plasma GDNF, BDNF, and SNAPIN concentrations were measured in duplicate from non-diluted plasma samples according to manufacturer’s specifications listed in the Enzyme-linked immunosorbent assay (ELISA) kits (MyBiosource, San Diego, CA). Each ELISA plate was read in a microplate reader VICTOR3 at 450 nm, 0.1 s. Detection limits of the assay were as follows: GDNF (0.05–10 ng/mL), BDNF (0.1–10 ng/ml, and SNAPIN (0.032–1.2 ng/ml). Intra-and inter-assay variations (CVs) were <15%.
Statistical analyses
Descriptive statistics were used to illustrate the demographic and clinical characteristics of the sample. Changes in fatigue and neurotrophic factor levels were calculated by subtracting the baseline from the midpoint levels. General linear models with repeated measures were used to analyse changes in fatigue scores from baseline to midpoint of EBRT. Because data were not normally distributed based on the Kolmogorov–-Smirnov test result, Wilcoxon sign rank tests and Mann–Whitney U-test were used to compare the levels of neurotrophic factors from baseline to day 21. Data are presented as median and mean ± standard deviation. Spearman’s rank correlation for non-normal distributed data was used to examine the correlations of changes in fatigue and neurotrophic factor levels during EBRT in fatigued and non-fatigue groups. All data analyses were conducted using SPSS 21.0 program (SPSS, Chicago, IL, USA).
Results
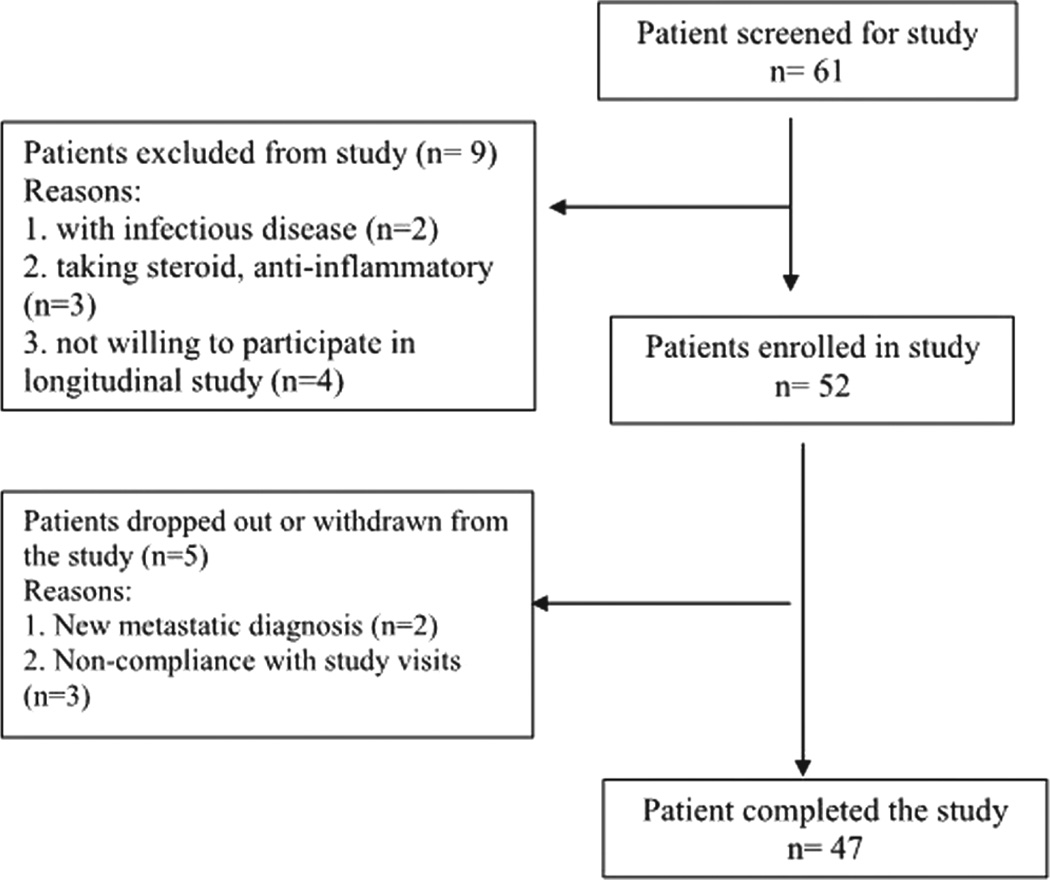
A total of 47 men, ages 49 to 81 years (63.09 ± 12), with NM-PC, and scheduled to receive EBRT were enrolled in the study. Figure 1 describes the flow diagram of patient enrollment for this study. Nearly half (N = 23/47) had T2 (a–c) clinical stage of disease, with Gleason scores ranging from 6 to 9, indicating that most participants had disease that had not spread outside the prostate gland. Karnofsky performance scores of all participants indicate that they are active and able to carry on all pre-disease activities without restrictions (Johnson et al. 2014).
Figure 1.
Flow diagram of patient enrollment. Patients screened for the study were scheduled to be treated with external beam radiation therapy for localized prostate cancer at a radiation oncology clinic at the Clinical Center of the National Institutes of Health, Bethesda, Maryland. Study eligibility were determined using inclusion and exclusion criteria.
Fatigue assessment
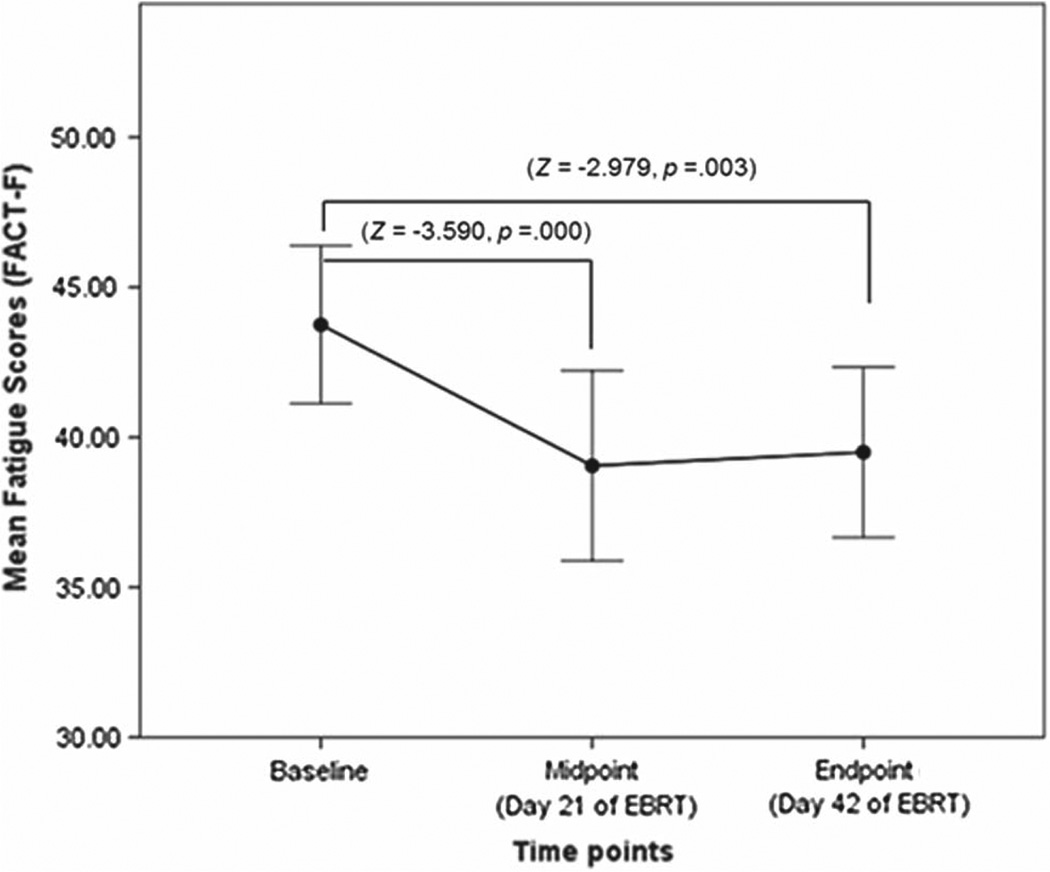
Mean FACT-F scores at baseline (43.95 ± 1.3) were similar to fatigue levels found in the general population (Cella et al. 2002). FACT-F scores of the EBRT subjects significantly decreased at midpoint of EBRT (38.36 ± 1.5, P = 0.001), reflecting worsening of fatigue after 19–21 sessions (midpoint) of EBRT. FACT-F scores stayed lower than baseline at completion of EBRT (39.48 ± 1.4, P = 0.003), but there was no significant difference in FACT-F scores from midpoint to EBRT completion. To address the study objective, we focused our investigation on the associations of the levels of neurotrophic factors and fatigue at baseline and midpoint of EBRT study time points (Figure 2).
Figure 2.
Fatigue scores during external beam radiation therapy. There is significant decrease in fatigue scores (indicated by the standard scores or z scores) as measured by the Functional Assessment of Cancer Therapy-Fatigue (FACT-F) scale from baseline to midpoint (day 21) of external beam radiation therapy (EBRT, P = 0.000) and from baseline to completion (day 42) of EBRT (P = 0.003).
Neurotrophic factors during EBRT
GDNF and SNAPIN showed similar levels at baseline (GDNF = 2.01 ± 1.22, SNAPIN = 34.95 ± 7.33) and at midpoint (GDNF = 1.49 ± 0.8, SNAPIN = 34.95 ± 8.72) of EBRT. However, BDNF levels significantly decreased from baseline to midpoint of EBRT (Z =−2.30, P = 0.02) with moderate effect size (r = 0.33) (Table II).
Table II.
Comparing fatigue and neurotrophic factors during EBRT.
| EBRT patients | |||||||
|---|---|---|---|---|---|---|---|
| Baseline (N = 47) | Midpoint of EBRT (N = 47) |
Wilcoxon Sign Rank Test | |||||
| Proteins | Mean ± SE | Median | Mean ± SE | Median | Z | P value | Effect size (r) |
| Fatigue score (FACT-F) | 43.95 ± 1.28 | 47.00 | 38.36 ± 1.52 | 39.50 | −3.590 | 0.000 | 0.37 |
| Glial cell derived neurotrophic factor (GDNF) (ng/mL) | 2.01 ± 1.22 | 0.13 | 1.49 ± 0.80 | 0.13 | −1.18 | 0.24 | 0.12 |
| Brain derived neurotrophic factor (BDNF) (ng/mL) | 1.10 ± 0.12 | 0.86 | 0.84 ± 0.10 | 0.82 | −2.30 | 0.02* | 0.24 |
| SNARE-associated protein (SNAPIN) (pg/mL) | 34.95 ± 7.33 | 20.29 | 34.95 ± 8.72 | 20.13 | −0.01 | 0.99 | 0.00 |
Levels of fatigue as measured by the Functional Assessment of Cancer Therapy-Fatigue (FACT-F) scale and plasma concentrations of neurotrophic factors measured in ng/mL during external beam radiation therapy (EBRT) are presented as mean with standard error (SE) and median.
Changes in fatigue and neurotrophic factors are indicated by standard scores or z scores, and significant difference between time points indicated by * P < 0.05 and effect size (r).
Correlations between fatigue and neurotropic factors
At baseline, SNAPIN levels were correlated with FACT-F scores (high SNAPIN levels were associated with low fatigue symptoms, rs = 0.434, P = 0.005). However, this association was not observed at midpoint of EBRT (P = 0.13). GDNF and BDNF concentrations were not associated with fatigue scores at any time point (Table III). For participants with a clinically-significant change in fatigue (n = 39), there was a significant decrease in BDNF levels (P = 0.008) after 19–21 sessions of EBRT. This was not observed for subjects in the no fatigue group (n = 8). No other significant change in plasma concentrations of the other neurotrophic factors during EBRT was observed using the fatigue grouping (Table IV).
Table III.
Correlations between GDNF, BDNF, SNAPIN, and fatigue (N = 47).
| Variables | Fatigue (rs) | |
|---|---|---|
| Baseline | Glial cell derived neurotrophic factor (GDNF) | −0.097 |
| Brain derived neurotrophic factor (BDNF) | −0.197 | |
| SNARE-associated protein (SNAPIN) | 0.434** | |
| Midpoint | Glial cell derived neurotrophic factor (GDNF) | −0.263 |
| Brain derived neurotrophic factor (BDNF) | −0.158 | |
| SNARE-associated protein (SNAPIN) | 0.120 |
Significant correlations (rs) in concentrations of neurotrophic factors and fatigue scores at each time point are indicated by P value (**P < 0.01).
Table IV.
Comparing fatigue scores and levels of neurotrophic factors between fatigue groups.
| No Fatigue (n = 8) |
Fatigue (n = 39) |
||||
|---|---|---|---|---|---|
| Mean rank | Median | Mean rank | Median | ||
| Fatigue score | Baseline | 2.00 | 42.00 | 18.12 | 47.00 |
| Midpoint | 2.00 | 36.00 | 10.71 | 40.00 | |
| Wilcoxon signed rank test | 0.564 | 0.000** | |||
| GDNF concentration | Baseline | 3.50 | 0.13 | 20.66 | 0.14 |
| Midpoint | 3.50 | 0.12 | 16.09 | 0.14 | |
| Wilcoxon signed rank test | 0.463 | 0.350 | |||
| BDNF concentration | Baseline | 7.50 | 0.94 | 22.35 | 0.84 |
| Midpoint | 3.50 | 0.73 | 15.31 | 0.82 | |
| Wilcoxon signed rank test | 0.674 | 0.008** | |||
| SNAPIN concentration | Baseline | 5.50 | 19.72 | 20.35 | 20.29 |
| Midpoint | 4.17 | 21.91 | 18.56 | 10.66 | |
| Wilcoxon signed rank test | 0.327 | 0.597 | |||
Significant difference in fatigue scores and concentrations of glial cell-derived neurotrophic factor (GDNF), brain-derived neurotrophic factor (BDNF), and SNARE-associated protein (SNAPIN) between study time points are indicated by p values using Wilcoxon signed rank test (**P < 0.01).
Discussion
We observed declining BDNF levels in euthymic subjects who initially developed a clinically significant change in fatigue symptoms during EBRT. This study showed the potential role of BDNF in worsening fatigue, without the influence of depression, during localized radiation therapy (RT) for non-metastatic cancer. It also highlights the relationship of SNAPIN and fatigue before cancer therapy. The physiological implications of our findings are discussed below. The novelty of this study is critical to understanding the aetiology of fatigue related to RT.
Our findings highlight the role of neurotrophic factors (NTs) in worsening fatigue during cancer therapy. NTs support neuronal survival and growth (Tyler et al. 2002). Moreover, NTs strongly influence morphogenetic and chemotrophic effects on neurons and are key modulators of synapses and synaptic structure and function (McAllister et al. 1995; Song et al. 1997; Schuman 1999).
BDNF, which is one of the best-characterized NTs, plays a significant role in synaptic plasticity and prevention of neuronal apoptosis (Autry and Monteggia 2012). Both of these BDNF roles trigger a cascade of intercellular mechanisms that are components of adaptive mechanisms that are critical to re-establish homeostasis in response to stress (Depperman et al. 2014). Hence, BDNF is an attractive molecular mediator for the experience of symptoms such as pain and depression, because these symptoms are thought to be contributed by alterations in homeostasis (Coenen et al. 2011). For example, increasing low peripheral and brain BDNF levels in depressed patients are often the preferred outcome for antidepressant therapies (Sen et al. 2008; Guilloux et al. 2012). In pain studies, deleting BDNF or inhibiting its actions reduces morphine-induced analgesia in an animal study (Sarhan et al. 2013). Our findings suggest that BDNF may also play a role in the fatigue experience, because we observed decreasing BDNF concentrations with worsening fatigue during EBRT. This association may be related to the body’s physiological response to repeated stress delivered through incremental RT.
Stress regulates BDNF expression in the brain (Duman and Monteggia 2006). There is rapid consumption of BDNF by the central nervous system during stressful situations (Chen et al. 2013), where it is seen as a neuroprotective response (Azoulay et al. 2005). BDNF is involved with cellular growth and synaptic changes after stress exposure, which contributes to long-lasting memory formation that is necessary to adapt and react to stressors (Depperman et al. 2014). BDNF is particularly expressed in the hippocampus (Kuczewski et al. 2009). Recent studies have shown that chronic stress leads to structural and functional alterations in the hippocampus (Depperman et al. 2014), causing a decrease in BDNF hippocampal levels (Lakshminarasimhan and Chattarji 2012). Reduced BDNF signalling results in lowered synaptic plasticity and subsequent impairment in cognitive function (Suri and Vaidya 2013). The results of our study suggest that low plasma levels of BDNF from repeated stress delivered by daily doses of EBRT may influence the BDNF expression in the hippocampus reducing hippocampal neurogenesis and subsequent hippocampal-dependent performance, which may contribute to the experience of fatigue.
In addition, as an anti-apoptotic regulator, BDNF also has the ability to modify respiration and increase respiratory control index to support metabolic changes and monitor overall energy balance (Markham et al. 2012). Alterations in apoptotic regulation, such as reduction in BDNF levels, may directly influence how the body perceives its overall energy balance and fatigability, which may explain the fatigue intensification we observed in our subjects. This fatigue is often described by oncology patients as a lack of energy, the need for exaggerated effort to complete a task, and the need for greater rest periods (Hofman et al. 2007; Cheville et al. 2009).
We also found a significant correlation between SNAPIN concentration and fatigue before EBRT, indicating that high SNAPIN levels are associated with reduced fatigue severity. SNAPIN is a regulatory protein that is associated with the SNARE complexes (Ilardi et al. 1999) and is critical for efficient lysosomal function to maintain neuronal morphology and function (Cai and Sheng 2011). High SNAPIN level translates into an effective neuroprotection and synapse transmission thereby reducing severity of symptoms such as fatigue. The loss of association between SNAPIN and fatigue at midpoint of EBRT may be related to other mechanisms related to the radiation therapy-directed killing of prostate cancer cells, where SNAPIN plays a major role in disturbing vesicular traffic, which is a hallmark of prostate adenocarcinoma (Quintero et al. 2013).
Our population was enrolled from a tertiary research hospital and may not be generalizable to the appropriate clinical population. Neurotrophic factors, including BDNF are secreted in several tissues in the body. Our findings observed changes in the plasma concentrations of neurotrophic factors, which may be limited to provide a more appropriate picture of the biology behind a specific behaviour. However, further investigation of neurotrophic factors including BDNF as potential biomarkers of CRF would be worthwhile to better inform clinical management of this disabling symptom.
Conclusion
Our findings suggest that BDNF may have a role in fatigue intensification during repeated stress in nondepressed cancer patients. BDNF has been associated with other neuroprotection and immune modulation, which are known pathways associated with cancer-related fatigue. Understanding the physiological pathways of BDNF during chronic stress may be informative to understand the aetiology of fatigue, especially related to cancer therapy.
Acknowledgments
This study was supported by the Division of Intramural Research of the National Institute of Nursing Research of the National Institutes of Health, Bethesda, Maryland.
Footnotes
This work was authored as part of the Contributor’s official duties as an Employee of the United States Government and is therefore a work of the United States Government. In accordance with 17 U.S.C. 105, no copyright protection is available for such works under U.S. Law.
Statement of Interest
None to declare.
References
- Allen SJ, Dawbarn D. Clinical relevance of the neurotrophins and their receptors. Clin Sci. 2006;110:175–191. doi: 10.1042/CS20050161. [DOI] [PubMed] [Google Scholar]
- Autry AE, Monteggia LM. Brain-derived neurotrophic factor and neuropsychiatric disorders. Pharmacol Rev. 2012;64:238–258. doi: 10.1124/pr.111.005108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azoulay D, Vachapova V, Shihman B, Miler A, Karni A. Lower brain-derived neurotrophic factor in serum of relapsing remitting MS: Reversal by glatiramer acetate. J Neuroimmunol. 2005;167:215–218. doi: 10.1016/j.jneuroim.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Barsevick AM, Irwin MR, Hinds P, Miller A, Berger A, Jacobsen P, et al. Recommendations for high-priority research on cancer-related fatigue in children and adults. J Natl Cancer Inst. 2013;105:1432–1440. doi: 10.1093/jnci/djt242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Q, Sheng ZH. Uncovering the role of Snapin in regulating autophagy-lysosomal function. Autophagy. 2011;7:445–447. doi: 10.4161/auto.7.4.14682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cella D, Eton DT, Lai JS, Peterman AH, Merkel DE. Combining anchor and distribution-based methods to derive minimal clinically important differences on the Functional Assessment of Cancer Therapy (FACT) anemia and fatigue scales. J Pain Symptom Manage. 2002;24:547–561. doi: 10.1016/s0885-3924(02)00529-8. [DOI] [PubMed] [Google Scholar]
- Chen A, Xiong LJ, Tong Y, Mao M. The neuroprotective roles of BDNF in hypoxic ischemic brain injury. Biomed Rep. 2013;1:167–176. doi: 10.3892/br.2012.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheville AL, Basford JR, Troxel AB, Kornblith AB. Performance of common clinician- and self-report measures in assessing the function of community-dwelling people with metastatic breast cancer. Arch Phys Med Rehabil. 2009;90:2116–2124. doi: 10.1016/j.apmr.2009.06.020. [DOI] [PubMed] [Google Scholar]
- Coenen VA, Schlaepfer TE, Maedler B, Panksepp J. Cross-species affective functions of the medial forebrain bundle - Implications for the treatment of affective pain and depression in humans. Neurosci Biobehav Rev. 2011;35:1971–1981. doi: 10.1016/j.neubiorev.2010.12.009. [DOI] [PubMed] [Google Scholar]
- Croll SD, Ip NY, Lindsay RM, Wiegand SJ. Expression of BDNF and trkB as a function of age and cognitive performance. Brain Res. 1998;812:200–208. doi: 10.1016/s0006-8993(98)00993-7. [DOI] [PubMed] [Google Scholar]
- Depperman S, Storchak H, Fallgatter AJ, Ehlis AS. Stress-induced neuroplasticity: maladaptation to adverse life events in patients with PTSD – a critical overview. Neuroscience. 2014;283:166–177. doi: 10.1016/j.neuroscience.2014.08.037. [DOI] [PubMed] [Google Scholar]
- Duman RS, Monteggia LM. A neurotrophic model for stress-related mood disorders. Biol Psychiatry. 2006;59:1116–1127. doi: 10.1016/j.biopsych.2006.02.013. [DOI] [PubMed] [Google Scholar]
- Fava M. Symptoms of fatigue and cognitive/executive dysfunction in major depressive disorder before and after antidepressant treatment. J Clin Psych. 2003;64(Suppl 14):30–34. [PubMed] [Google Scholar]
- Ferguson M, Dennehy EB, Marangell LB, Martinez J, Wisniewski SR. Impact of fatigue on outcome of selective serotonin reuptake inhibitor treatment: Secondary analysis of STAR D. Curr Med Res Opin. 2014;30:2109–2118. doi: 10.1185/03007995.2014.936553. [DOI] [PubMed] [Google Scholar]
- Glaus A, Crow R, Hammond S. A qualitative study to explore the concept of fatigue/tiredness in cancer patients and in healthy individuals. Eur J Cancer Care (Engl) 1996;5(2 Suppl):8–23. doi: 10.1111/j.1365-2354.1996.tb00247.x. [DOI] [PubMed] [Google Scholar]
- Guilloux JP, Douillard-Guilloux G, Kota R, Wang X, Gardier AM, Martinowich K, et al. Molecular evidence for BDNF- and GABA-related dysfunctions in the amygdala of female subjects with major depression. Mol Psychiatry. 2012;17:1130–1142. doi: 10.1038/mp.2011.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofman M, Ryan JL, Figueroa-Moseley CD, Jean-Pierre P, Morrow GR. Cancer-related fatigue: The scale of the problem. Oncologist. 2007;12(Suppl 1):4–10. doi: 10.1634/theoncologist.12-S1-4. [DOI] [PubMed] [Google Scholar]
- Horneber M, Fischer I, Dimeo F, Ruffer JU, Weis J. Cancer-related fatigue: Epidemiology, pathogenesis, diagnosis, and treatment. Dtsch Arztebl Int. 2012;109:161–171. doi: 10.3238/arztebl.2012.0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilardi JM, Mochida S, Sheng ZH. Snapin: A SNARE-associated protein implicated in synaptic transmission. Nat Neurosci. 1999;2:119–124. doi: 10.1038/5673. [DOI] [PubMed] [Google Scholar]
- Johnson MJ, Bland JM, Davidson PM, Newton PJ, Oxberry SG, Abernethy AP, Currow DC. The relationship between two performance scales: New York Heart Association Classification and Karnofsky Performance Status Scale. J Pain Symptom Manage. 2014;47:652–658. doi: 10.1016/j.jpainsymman.2013.05.006. [DOI] [PubMed] [Google Scholar]
- Kaplan DR, Miller FD. Neurotrophin signal transduction in the nervous system. Curr Opin Neurobiol. 2000;10:381–391. doi: 10.1016/s0959-4388(00)00092-1. [DOI] [PubMed] [Google Scholar]
- Kim DH, Zhao X. BDNF protects neurons following injury by modulation of caspase activity. Neurocrit Care. 2005;3:71–76. doi: 10.1385/NCC:3:1:071. [DOI] [PubMed] [Google Scholar]
- Kuczewski N, Porcher C, Lessmann V, Medina I, Gaiarsa J-L. Activity-dependent dendritic release of BDNF and biological consequences. Mol Neurobiol. 2009;39:37–49. doi: 10.1007/s12035-009-8050-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakshminarasimhan H, Chattarji S. Stress leads to contrasting effects on the levels of brain derived neurotrophic factor in the hippocampus and amygdala. PLoS One. 2012;7:e30481. doi: 10.1371/journal.pone.0030481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licinio J, Wong M. Brain-derived neurotrophic factor (BDNF) in stress and affective disorders. Mol Psychiatry. 2002;7:519. doi: 10.1038/sj.mp.4001211. [DOI] [PubMed] [Google Scholar]
- Luhder F, Gold R, Flugel A, Linker RA. Brain-derived neurotrophic factor in neuroimmunology: Lessons learned from multiple sclerosis patients and experimental autoimmune encephalomyelitis models. Arch Immunol Ther Exp (Warsz) 2013;61:95–105. doi: 10.1007/s00005-012-0211-0. [DOI] [PubMed] [Google Scholar]
- Markham A, Cameron I, Bains R, Franklin P, Kiss JP, Schwendimann L, et al. Brain-derived neurotrophic factor-mediated effects on mitochondrial respiratory coupling and neuroprotection share the same molecular signalling pathways. Eur J Neurosci. 2012;35:366–374. doi: 10.1111/j.1460-9568.2011.07965.x. [DOI] [PubMed] [Google Scholar]
- McAllister AK, Lo DC, Katz LC. Neurotrophins regulate dendritic growth in developing visual cortex. Neuron. 1995;15:791–803. doi: 10.1016/0896-6273(95)90171-x. [DOI] [PubMed] [Google Scholar]
- Moberg PJ, Lazarus LW, Mesholam RI, Bilker W, Chuy IL, Neyman I, et al. Comparison of the standard and structured interview guide for the Hamilton Depression Rating Scale in depressed geriatric inpatients. Am J Geriatr Psychiatry. 2001;9:35–40. [PubMed] [Google Scholar]
- Morrow GR, Andrews PL, Hickok JT, Roscoe JA, Matteson S. Fatigue associated with cancer and its treatment. Support Care Cancer. 2002;10:389–398. doi: 10.1007/s005200100293. [DOI] [PubMed] [Google Scholar]
- Norden DM, Bicer S, Clark Y, Jing R, Henry CJ, Wold LE, et al. Tumor growth increases neuroinflammation, fatigue and depressive-like behavior prior to alterations in muscle function. Brain Behav Immun. 2014;43:76–85. doi: 10.1016/j.bbi.2014.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick DL, Ferketich SL, Frame PS, Harris JJ, Hendricks CB, Levin B, et al. National Institutes of Health state-of-the-science conference statement: Symptom management in cancer: Pain, depression, and fatigue. J Natl Cancer Inst. 2003;95:1110–1117. doi: 10.1093/jnci/djg014. [DOI] [PubMed] [Google Scholar]
- Portenoy RK, Itri LM. Cancer-related fatigue: Guidelines for evaluation and management. Oncologist. 1999;4:1–10. [PubMed] [Google Scholar]
- Quintero IB, Herrala AM, Araujo CL, Pulkka AE, Hautaniemi S, Ovaska K, et al. Transmembrane prostatic acid phosphatase (TMPAP) interacts with snapin and deficient mice develop prostate adenocarcinoma. PLos One. 2013;8:e73072. doi: 10.1371/journal.pone.0073072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saligan LN, Hsiao CP, Wang D, Wang XM, John LS, Kaushal A, et al. Upregulation of alpha-synuclein during localized radiation therapy signals the association of cancer-related fatigue with the activation of inflammatory and neuroprotective pathways. Brain Behav Immun. 2013;27:63–70. doi: 10.1016/j.bbi.2012.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarhan M, Pawlowski SA, Barthas F, Yalcin I, Kaufling J, Dardente H, et al. BDNF parabrachio-amygdaloid pathway in morphine-induced analgesia. Int J Neuropsychopharmacol. 2013;16:1649–1660. doi: 10.1017/S146114571200168X. [DOI] [PubMed] [Google Scholar]
- Schuman EM. Neurotrophin regulation of synaptic transmission. Curr Opin Neurobiol. 1999;9:105–109. doi: 10.1016/s0959-4388(99)80013-0. [DOI] [PubMed] [Google Scholar]
- Scuri M, Samsell L, Piedimonte G. The role of neurotrophins in inflammation and allergy. Inflamm Allergy Drug Targets. 2010;9:173–180. doi: 10.2174/187152810792231913. [DOI] [PubMed] [Google Scholar]
- Sen S, Duman R, Sanacora G. Serum brain-derived neurotrophic factor, depression, and antidepressant medications: meta-analyses and implications. Biol Psychiatry. 2008;64:527–532. doi: 10.1016/j.biopsych.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song HJ, Ming GL, Poo MM. cAMP-induced switching in turning direction of nerve growth cones. Nature. 1997;388:275–279. doi: 10.1038/40864. [DOI] [PubMed] [Google Scholar]
- Suri D, Vaidya VA. Glucocorticoid regulation of brain-derived neurotrophic factor: Relevance to hippocampal structural and functional plasticity. Neuroscience. 2013;239:196–213. doi: 10.1016/j.neuroscience.2012.08.065. [DOI] [PubMed] [Google Scholar]
- Tokuyama W, Okuno H, Hashimoto T, Xin Li Y, Miyashita Y. BDNF upregulation during declarative memory formation in monkey inferior temporal cortex. Nat Neurosci. 2000;3:1134–1142. doi: 10.1038/80655. [DOI] [PubMed] [Google Scholar]
- Tyler WJ, Perrett SP, Pozzo-Miller LD. The role of neurotrophins in neurotransmitter release. Neuroscientist. 2002;8:524–531. doi: 10.1177/1073858402238511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaynman SS, Ying Z, Yin D, Gomez-Pinilla F. Exercise differentially regulates synaptic proteins associated to the function of BDNF. Brain Res. 2006;1070:124–130. doi: 10.1016/j.brainres.2005.11.062. [DOI] [PubMed] [Google Scholar]
- Vega JA, Garcia-Suarez O, Hannestad J, Pérez-Pérez M, Germanà A. Neurotrophins and the immune system. J Anat. 2003;203:1–19. doi: 10.1046/j.1469-7580.2003.00203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyrwich KW, Bullinger M, Aaronson N, Hays RD, Patrick DL, Symonds T. Estimating clinically significant differences in quality of life outcomes. Qual Life Res. 2005;14:285–295. doi: 10.1007/s11136-004-0705-2. [DOI] [PubMed] [Google Scholar]
- Xiao N, Lin Y, Cao H, Sirjani D, Giaccia AJ, Koong CS, et al. Neurotrophic factor GDNF promotes survival of salivary stem cells. J Clin Invest. 2014;124:3364–3377. doi: 10.1172/JCI74096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yellen SB, Cella DF, Webster K, Blendowski C, Kaplan E. Measuring fatigue and other anemia-related symptoms with the Functional Assessment of Cancer Therapy (FACT) measurement system. J Pain Symptom Manage. 1997;13:63–74. doi: 10.1016/s0885-3924(96)00274-6. [DOI] [PubMed] [Google Scholar]