Abstract
Objectives
Primary carcinoid tumors of the lung are rare tumors which comprise approximately 0.5 to 5% of all lung malignancies in adults and roughly 20 to 30% of all carcinoid tumors. The purpose of this retrospective, descriptive study was to describe the incidence, characteristics, and outcomes of patients treated for primary pulmonary carcinoid tumor at a single institution.
Methods
All patients with a diagnosis of primary pulmonary carcinoid tumor treated from 1989 to 2009 were reviewed. Data collected included demographics, pathology, tobacco use, clinical presentation, tumor location, tumor spread, treatment and survival.
Results
There were 59 cases of pulmonary carcinoid tumors: 47 typical (80%) and 12 atypical (20%). All but 4 patients underwent surgery, including 54 (92%) lung-sparing resections and 1 pneumonectomy. Five out of 55 patients received concurrent adjuvant chemoradiation therapy; 4 patients with atypical and 1 with typical histology. Three additional patients with atypical carcinoid were treated only with adjuvant radiotherapy, palliative radiotherapy, or palliative chemotherapy, respectively. The Kaplan-Meier 5- and 10-year overall survivals were both 80% within the entire population. In the 88% of patients who achieved complete remission, disease-free survival was 98%. A review of a large series from the literature is also presented.
Conclusions
Surgical resection was primary and adequate therapy for most typical carcinoid tumors with high overall survival and disease-free survival. Adjuvant chemotherapy or radiotherapy might be considered for patients with atypical carcinoid tumors who present with adverse pathological findings.
Keywords: carcinoid tumor, pulmonary, lung neoplasm, neuroendocrine tumor
Introduction
Carcinoid tumors are a subset of neuroendocrine tumors and overall incidence of carcinoids in the United States is estimated at 5.25 cases per 100,000 population. The majority of carcinoid tumors arise within the gastrointestinal tract (58%), with the pulmonary system as the next most common site of origin (27%). The remainder (15%) arises from other or unknown locations.1
Pulmonary neuroendocrine tumors comprise a spectrum of tumors, including: low grade neuroendocrine tumors (typical carcinoids), intermediate grade neuroendocrine tumors (atypical carcinoids) and high grade neuroendocrine tumors (large cell neuroendocrine and small cell carcinomas). Carcinoid tumors comprise about 0.5 to 5% of all lung tumors and are malignant tumors with the potential to metastasize.2-4 Typical carcinoids comprise about 90% of all pulmonary carcinoids and show a high degree of differentiation, rare mitoses (<3 mitotic figures/10 hpf), scarce pleomorphism or necrosis. The remaining 10% are atypical carcinoids, which are characterized by increased mitotic activity (>3 but <10 mitoses/10 hpf), cellular atypia and increased pleomorphism or necrosis. Atypical carcinoids tend to have a higher rate of metastasis and are larger at the time of diagnosis.4 Specimens with >10 mitoses/10 hpf are either small cell or large cell neuroendocrine carcinomas. Of these features, it has been shown that only the number of mitoses is a predictor for prognosis.5
There is no known association between typical carcinoid tumors and tobacco use or exposure to other carcinogens. Atypical carcinoid tumors, however, have been reported to have an association with tobacco use.3,6 Typical presenting symptoms reported in the literature include cough, recurrent pulmonary infection, hemoptysis, dyspnea, wheezing or fever. Some patients may be asymptomatic and their tumors are discovered incidentally. Carcinoid syndrome, which is characterized by excessive serotonin release from tumors leading to hot, red flushing of the face; severe and debilitating diarrhea; and asthma attacks, is rarely associated with bronchial carcinoids. Surgical resection is the mainstay of treatment for localized disease.3
To date, there are no randomized trials indicating the most effective management of carcinoid tumors. In patients with advanced pulmonary carcinoids, defined by positive nodal disease, there is a paucity of data regarding management. The most optimal resection strategy and the benefit of adjuvant chemotherapy or radiation therapy is also unknown. Furthermore, the indolent nature of the disease makes interpretation of survival numbers problematic.7 In this study, we report the clinicopathologic features of pulmonary carcinoid tumors from our institution in order to gain a better understanding of prognostic factors related to the management of these rare tumors. We report a single institution experience with pulmonary carcinoids over a 20-year period from a cancer center that serves the majority of the Intermountain West.
Materials and Methods
Following approval by the University of Utah Institutional Review Board (approval #48151), we performed a retrospective search of the medical records to identify patients with primary pulmonary carcinoid tumors diagnosed and treated at the University of Utah Medical Center and Huntsman Cancer Hospital between 1989 and 2009. A cohort of 59 patients with tissue proof of primary pulmonary carcinoid was collected and identified. The data collection included patient demographics, pathology, tobacco use, clinical presentation, tumor location, treatment modalities, surgical margins and pattern of tumor recurrence or metastasis. We excluded lung metastases from primary carcinoid tumors of other organs.
All statistical analyses were performed using SPSS Statistics 21 (IBM; Chicago, IL). The Kaplan-Meier method was used for overall survival calculation and the log-rank test for comparisons. A P-value of <0.05 was significant in two tailed analysis. Consistent with prior analyses, all patients who did not achieve complete remission or cure following treatment were censored in disease-free survival calculations.8,9
Results
From 1989 through 2009 there were 59 patients diagnosed with primary pulmonary carcinoid tumor at our institution. There were 36 females and 23 males, with female to male ratio of 1.6:1. The mean age at diagnosis was 54 years (range 24-83). Histopathologically, 47 (80%) were typical carcinoids (18 males, 29 females) with a mean age of 52 (range 24-83), and 12 (20%) atypical carcinoids (5 males, 7 females) with mean age of 59 (range 34-83). The median follow-up time was 4.4 years (Table 1).
Table 1. Patient Population Description.
| Characteristics | No. of Patients (%) | Characteristics | No. of Patients (%) |
|---|---|---|---|
| Typical Carcinoid | 47 (79.7) | ||
| Gender | Tumor Size | ||
| Male | 18 (30.5) | <3 cm | 32 (54.2) |
| Female | 29 (49.2) | >3 cm | 10 (16.9) |
| Clinical Presentation | Unknown | 5 (8.5) | |
| Asymptomatic | 16 (27.2) | Extent Resection | |
| Symptomatic | 31 (52.5) | Lobectomyβ | 44 (74.6) |
| Nodal Involvement | Pneumonectomy | 1 (1.7) | |
| No | 33 (55.9) | No Surgery | 2 (3.4) |
| Yes | 2 (3.4) | Treatment | |
| No Nodes Examinedα | 11 (18.6) | None | 2 (3.4) |
| Unknown | 1 (1.7) | Surg. Only | 44 (74.6) |
| Surg. & ChemoRTδ | 1 (1.7) | ||
|
| |||
| Atypical Carcinoid | 12 (20.3) | ||
| Gender | Tumor Size | ||
| Male | 5 (8.5) | <3 cm | 5 (8.5) |
| Female | 7 (11.9) | >3 cm | 7 (11.9) |
| Clinical Presentation | Extent Resection | ||
| Asymptomatic | 5 (8.5) | Lobectomyβ | 10 (16.9) |
| Symptomatic | 7 (11.9) | No Surgery | 2 (3.4) |
| Nodal Involvement | Treatment | ||
| No | 2 (3.3) | Surg. Only | 5 (8.5) |
| Yes | 9 (15.2) | Surg. & RTδ | 1 (1.7) |
| No Nodes Examinedα | 1 (1.7) | Surg. & ChemoRTδ | 4 (6.8) |
| Chemo Only | 1 (1.7) | ||
| RT Onlyδ | 1 (1.7) | ||
No nodes were resected and submitted for pathologic review;
Lobectomy includes limited resections and bilobectomies;
ChemoRT = chemotherapy and radiotherapy
Tobacco Use
The smoking status was known for 45 of 47 patients with typical carcinoid, of which 22 (47%) had previous or current tobacco use. Of the 12 patients with atypical carcinoid, 4 patients had a history of tobacco use, 4 patients had no prior history and the remaining 4 patients' smoking history was unknown.
Clinical Presentation at Diagnosis
In our series, 39 patients (66%) had clinical symptoms at initial presentation. The most common symptoms were hemoptysis (n=14, 24%), dyspnea (n=13, 22%) and persistent cough (n=9, 15%). Only 5 of 59 (8%) patients presented with carcinoid syndrome including wheezing, diarrhea and flushing. Other symptoms included recurrent pneumonia (n=4, 7%), post-obstructive atelectasis and pneumonitis (n=3, 5%), Cushing's syndrome (n=1, 1.7%), seizures (n=1, 1.7%) and constitutional symptoms (n=4, 7%), which included fever, weight loss and night sweats. Presenting signs and symptoms are summarized in Table 2.
Table 2. Tumor Location and Mode of Presentation.
| Site | Central | Peripheralα | Total |
|---|---|---|---|
| Right lung | 21 | 18 | 39 |
| Main bronchus | 6 | 0 | 6 |
| Upper lobe | 4 | 3 | 7 |
| Middle lobe | 6 | 4 | 10 |
| Lower lobe | 5 | 9 | 14 |
| Overlapping Lesionβ | 0 | 2 | 2 |
| Left lung | 13 | 7 | 20 |
| Main bronchus | 4 | 0 | 4 |
| Upper lobe | 6 | 4 | 10 |
| Lower lobe | 3 | 3 | 6 |
|
| |||
| Presenting symptom/signδ | Patients (no.) | (%) | |
|
| |||
| Hemoptysis | 14 | 24 | |
| Cough | 9 | 15 | |
| Dyspnea | 13 | 22 | |
| Evidence of bronchial obstruction | |||
| Pneumonia | 4 | 7 | |
| Post-obstructive atelectasis and pneumonitis | 3 | 5 | |
| Other | |||
| Carcinoid syndrome | 5 | 8 | |
| Constitutional symptoms | 4 | 7 | |
| Cushing's syndrome | 1 | 2 | |
| Seizures | 1 | 2 | |
| Incidental findings | 20 | 34 | |
Peripheral in this study indicates at segmental bronchus or beyond;
Two patients had overlapping lesions involving two lobes with one patient's tumor involving both the right upper and middle lobes and another patient's involving the right middle and lower lobes;
Majority of patients presented with more than one symptom
Tumor Location
Most frequently, the patients' tumors in our study arose from the lobar bronchi (n=24, 41%). Ten patients had tumors which arose from the major/main-stem bronchi (17%). Forty-two percent of patients' tumors arose peripherally, i.e, at the segmental bronchi or beyond. The most common site was the right lower lobe. The tumor was located in the right lung in 39 (66%) patients, and left lung in 20 (34%) patients. The presenting locations are summarized in Table 2.
Tumor Spread
At initial presentation, 1 out of 47 patients (2%) with primary pulmonary typical carcinoid tumors had positive mediastinal nodes while 9 of 12 patients (75%) with atypical carcinoid tumors had positive mediastinal nodes at diagnosis. One typical carcinoid patient (2%) later developed recurrent disease in mediastinal nodes. Three patients with atypical carcinoid tumors also presented with distant metastatic disease at diagnosis. Three additional patients (25%) with atypical carcinoid progressed to Stage IV disease during the follow-up period.
Treatment Modalities
Surgery was the primary treatment in 55 of 59 patients (93%), including lobectomy (n=35), wedge resection/segmentectomy or sub-lobectomy (n=19) and pneumonectomy (n=1) (See table 1 for demographic description and clinical summary). The only patient who underwent pneumonectomy had a 6 cm typical carcinoid tumor involving the orifice of the right bronchus intermedius and to achieve adequate margins a pneumonectomy was required. Most patients diagnosed with atypical carcinoid (n=9) underwent mediastinal lymph node dissection. Surgical margins were negative in 87% and 83% of patients with typical and atypical carciniods, respectively. Data was not available for review in 2 patients with typical carcinoid and surgical margins were not applicable in 4 and 2 patients with typical and atypical carcinoid, respectively. These 4 typical carcinoid patients underwent endobronchial resection and the 2 atypical carcinoid patients never underwent surgical resection of the primary site due to metastatic disease present at diagnosis. Surgery was the only treatment in 49 patients while 6 other patients also received adjuvant therapy. Adjuvant chemoradiation was given in 4 patients with atypical carcinoid and 1 with typical carcinoid. A fifth patient with atypical carcinoid and positive nodal metastasis received only adjuvant radiotherapy (Table 3). Palliative chemotherapy or radiotherapy were each used in 2 additional patients with atypical carcinoid who presented with metastatic disease at diagnosis. Finally, 2 more patients with typical carcinoid received no treatment and were followed clinically. The indications for adjuvant radiotherapy or chemoradiation were positive microscopic margins, or positive mediastinal node(s) for atypical carcinoid tumor and positive node(s) or other adverse pathologic features for typical carcinoid tumor.
Table 3. All patients treated with adjuvant therapy with curative intent.
| Case | Carcinoid Type | Adverse Pathologic Features | Initial TNM Stage | Surgical Treatmentα | Adjuvant Treatment | Outcome | |
|---|---|---|---|---|---|---|---|
| Chemotherapy | Radiotherapy | ||||||
| 1 | Typical | ACTH Secreting | T1aN2M0-IIIA | Lobectomy and repeat MNDβ for recurrence seen on PETδ | 4 cycles etoposide+carboplatin | 50 Gy/28 fractions AP:PAγ fields | Disease free |
| 2 | Atypical | Extensive necrosis | T2bN2M0 - IIIA | Lobectomy/MND | 6 cycles etoposide+carboplatin | 50 Gy/25 fractions 3D conformal | Progression to stage IV |
| 3 | Atypical | Lymphovascular and visceral pleura invasion, | T1bN1M0 - IIA | Lobectomy/MND | 4 cycles etoposide+carboplatin | 41 Gy/23 fractions AP:PA fields | Disease free |
| 4 | Atypical | Unknown | T1aN1M0 - IIA | Lobectomy/MND | None | External beam | Progression to stage IV |
| 5 | Atypical | Unknown | T2aN2M0 - IIIA | Lobectomy/MND | 3 cycles etoposide+carboplatin | 50 Gy/25 fractions AP:PA fields | Progression to indolent stage IV |
| 6 | Atypical | None | T1aN2M0 - IIIA | Lobectomy/MND | 4 cycles etoposide+carboplatin | 44 Gy/25 fractions AP:PA fields | Disease free |
All surgical margins were negative;
MND=mediastinal node dissection;
PET=positron emission tomography;
AP:PA=anteriorposterior:posterioranterior
Survival
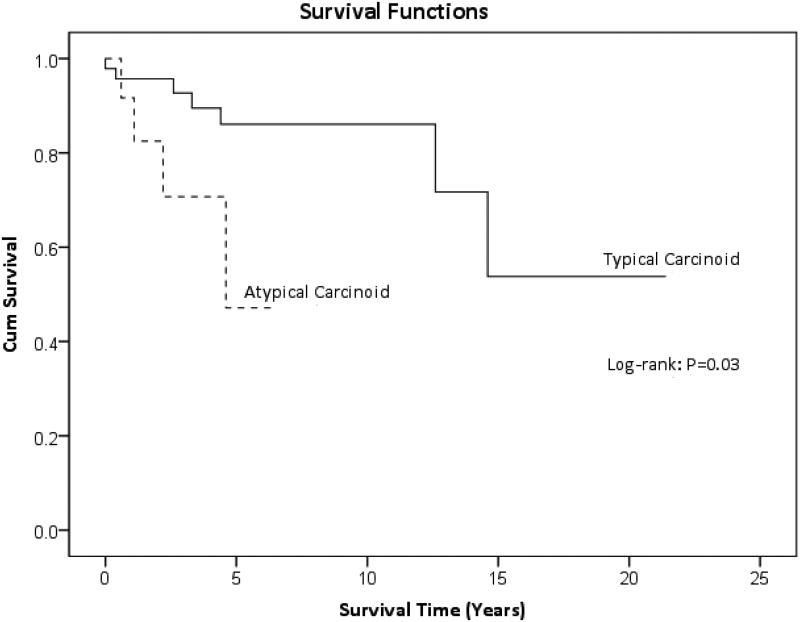
The Kaplan-Meier 2-, 5- and 10-year overall survivals for the entire group were 93, 80 and 80%, respectively. Disease free survival in the population who achieved complete remission following treatment was 98%. Disease recurred in only one patient in our study who had an adrenocorticotropic hormone (ACTH) secreting typical carcinoid tumor. Seven patients in the study (12%) never achieved complete cure and 3 of the 7 (43%) died with evidence of disease. Two of the 7 (29%) patients with persistent disease have typical carcinoids, which remained clinically stable since diagnosis. Figure 1 displays the overall survival curves for typical and atypical carcinoids separately, which were 96, 86 and 86% at 2, 5 and 10 years, respectively, for typical carcinoids and 83 and 47% at 2 and 5 years, respectively, for atypical carcinoid tumors.
Figure 1.
Overall survival in patients with typical and atypical carcinoid tumors.
Discussion
Pulmonary carcinoid tumors are rare tumors with an incidence of approximately 0.5-5% of all diagnosed lung cancers as reported in the literature.1,4,5,10,11 The incidence at our institution was approximately 1.9% (59/3,034). Due to their low incidence, there is a lack of large phase II and III trials regarding the treatment and management of pulmonary carcinoid tumors. Furthermore, the results typically reported in the literature are heterogeneous series, or involve case reports and small sample sizes. The main flaw of many of these studies is that they include carcinoids from a number of different sites, which demonstrate different clinical behaviors.7
In the literature reviewed, we found that atypical carcinoid tumors comprise from 1012 to 35%13 of all pulmonary carcinoid tumors. The prevalence of atypical carcinoid tumors in our group was 20%. In addition, the mean age at diagnosis in the entire group was 54 years (range 24-88). This finding is consistent with the range found in the literature of 43 to 60,3,10,12-20 but is younger than the mean age of incidence for other pulmonary tumors.3,11 The atypical carcinoid patients tended to be older (mean 59) than those with typical carcinoids (mean 52), a finding opposite that reported by other studies.12,21 Our study was not designed to evaluate the relationship between tobacco use and development of typical versus atypical tumors. A few studies, however, have shown an association but not a definitive relationship with tobacco use and the development of atypical pathology more frequently than typical.3
Most patients (53-100%) with carcinoids were symptomatic at presentation across the literature series (Table 4).22 In our study, the most common symptom was hemoptysis followed by dyspnea and cough (Table 2). Hemoptysis and cough are the most common presenting symptoms in some studies;3,13 others have reported recurrent bronchopulmonary infections as the most common presenting symptom.12,23
Table 4. Data Collected from Literature Series.
| Source | Year | No. | Mean Age, yr | Histology (%) | Extent Resection (%) | Overall Survivalα | |||
|---|---|---|---|---|---|---|---|---|---|
| Typ. | Atyp. | Lob. | Pneum. | 5-yr | 10-yr | ||||
| Hurt et al. | 1984 | 79 | 47 | 99 | 1 | 99 | 1 | - | 94 |
| Bertelsen et al. | 1985 | 82 | 45 | 79 | 21 | 54 | 6 | 88 | 85 |
| Harpole et al. | 1992 | 126 | 53 | 66 | 34 | 50 | 12 | 97/40 | 90/30 |
| Gould et al.β | 1998 | 87 | 55 | 74 | 26 | 69 | 7 | 95/70 | - |
| Descovich | 2000 | 35 | 43 | 86 | 14 | 57 | 17 | 96/80 | - |
| Fink et al. | 2001 | 142 | 52 | 90 | 10 | 56 | 16 | 89/75 | 82/56 |
| Filosso et al. | 2001 | 126 | 47 | 65 | 35 | 87 | 12 | 97/77 | 93/52 |
| Thomas et al.δ | 2001 | 36 | 56 | 23 | 11 | - | - | 95/54 | 95/54 |
| Schrevens et al. | 2004 | 67 | 44 | 88 | 12 | 85 | 13 | 92/67 | 89/50 |
| Thomas et al. | 2008 | 25 | 38.3 | 88 | 12 | 16 | 52 | - | - |
| Tsuta et al. | 2011 | 80 | 54.3 | 85 | 15 | - | - | - | - |
| Madrid-Carbajal et al. | 2013 | 60 | 60 | 87 | 13 | - | - | 90/86 | 85/57 |
| Our study | 2013 | 59 | 54 | 80 | 20 | 90 | 2 | 86/47 | 86/- |
xx/xx indicates typical/atypical histology survival rates;
4-year survival;
Study only included patients with node positive disease;
(-) data not available; typ – typical; atyp – atypical; lob – lobectomy (includes limited resections & bilobectomies); pneum – pneumonectomy
Several studies have shown a higher incidence of centrally located tumors3,12,13,24 than peripheral, though there is some disagreement in the literature.18,23 In our data, 42% of tumors were localized peripherally. We assigned tumors a peripheral location if they were located at the segmental bronchus or distal as others have done. There is no universally accepted definition for where a tumor becomes peripheral versus central which can explain some of the disagreement in the literature. Centrally located tumors are more often symptomatic.3,19 Eleven (92%) of the atypical carcinoid tumors were located peripherally, which is consistent with other reports in the literature.7,25
Atypical pathology was the most important prognostic factor common to all studies reviewed with nodal involvement significant in most studies as well (Table 5).24 Tsuta, et al. also reported additional adverse pathologic features including, active fibroblast proliferation (p=0.041) and comedo-like necrosis (p=0.001), that were significant in their study. Two factors determined survival in our series: atypical histology (p=0.03) and nodal involvement (p<0.001). Other factors evaluated included tumor size (<3 vs. ≥3 cm), symptomatic presentation and older age (<60 vs. ≥60), which were not of prognostic value in our experience, though others have found these factors significant (Table 5).
Table 5. Survival and Prognostic Factors in Literature Series.
| Negative Prognostic Factorsα | Schrevens et al. | Fink et al. | Harpole et al. | Filosso et al. | Garcia-Yuste et al. | Tsuta et al. | Our Study |
|---|---|---|---|---|---|---|---|
| Atypical Histology | Yes P=0.02 HR=0.22β | Yes P<0.05 | Yes P<0.001 | Yes P=0.0004 | Yes P=0.0001 | Yes P<0.0001 | Yes P=0.031 |
| Tumor Size | No P=0.57 | - | Yes P<0.001 | - | - | - | No P=0.97 |
| LN Involvement | Yes P=0.0009 HR=0.13 | - | Yes P<0.001 | Yes P=0.21 | - | - | Yes P<0.001 |
| Pathologic Stage | - | - | Yes P<0.01 | - | - | Yes P=0.04 | - |
| Symptomatic Presentation | - | - | Yes P<0.01 | - | - | - | No P=0.36 |
| Older Ageδ | - | - | No P>0.1 | - | - | Yes P=0.002 | No P>0.05 |
P-value: Comparison between subgroups according to log-rank statistic;
Only study to report hazard ratio;
<60 vs. ≥60 years
The average 5- and 10-year overall survival rates reported in the reviewed literature for typical carcinoids were 93% and 89% (range 88-97% and 82-95%), respectively. The average 5- and 10-year overall survival rates reported in the literature for atypical carcinoids were 69% and 50% (range 40-86% and 30-57%), respectively. In our series, the 5- and 10-year survival rates were both 86% for typical carcinoids, and the 5-year survival rate for atypical carcinoids was 47% (Table 4). The follow-up period was not long enough to calculate the 10-year survival rate in the atypical carcinoid group. Disease-free survival in this population was 98% with 7 patients who were never disease free and 3 of whom died with evidence of disease. Surgery is currently the mainstay of treatment for pulmonary carcinoids with lung sparing resection used as frequently as possible. Surgery has been reported to provide a 5- and 10-year survival rate of >90% for typical carcinoid, while 5- and 10-year survival is 70 and 50%, respectively, for atypical carcinoid.7 A large retrospective study showed equivalent 5-, 10- and 15-year survival rates for limited resection versus lobectomy/pneumonectomy.26 In our study, only one patient in the group received a pneumonectomy for a large tumor with all others undergoing lobectomy, bilobectomy, or limited resections. Fifty-nine percent of patients also underwent mediastinal lymph node dissection. The benefit of a mediastinal lymph node dissection is unclear, although a systematic sampling is recommended to define the pathologic stage.7,27 Significantly more patients underwent pneumonectomy in the literature reviewed with a range from 1 to 52% (Table 4). The overall survival rate from our study was very favorable for typical carcinoid tumors, indicating lung sparing resection strategies are often adequate and excellent treatment for most patients whose tumors show typical histology.
Adjuvant treatment with either radiotherapy or chemotherapy has not been well studied. Some reports have shown that patients with atypical carcinoids and regional lymph node metastases have a high likelihood of developing recurrent disease if treated with surgical resection alone.22,28 The role of radiotherapy in patients with typical carcinoids with positive nodal disease is controversial and felt by some authors to be unnecessary.28 In our study, adjuvant chemotherapy and radiotherapy were reserved for cases of atypical carcinoid or a case of typical carcinoid with adverse pathologic findings.
The one patient with typical carcinoid and positive mediastinal nodes to receive adjuvant chemoradiation and repeat resection did so for recurrent disease within the mediastinum. This patient initially underwent only lobectomy and ACTH levels were followed clinically. This patient was subsequently disease free. There was one other patient in our study that also had an ACTH positive tumor and had a single positive mediastinal node that resulted from focal extension of the tumor. This patient received only surgical treatment with sleeve resection, lobectomy and mediastinal node dissection. Surgical margins were negative so the patient was followed without disease recurrence. ACTH secreting tumors, have been associated with more aggressive typical carcinoid tumors in several studies.29,30
Reserving adjuvant chemotherapy and radiation for cases of atypical carcinoid or typical carcinoid with adverse pathologic features was also an effective treatment strategy for our group of patients. There was only one recurrence as mentioned including among the 5 patients with atypical carcinoid who presented with non-metastatic disease atdiagnosis and were managed with adjuvant treatment. Three of these 5 (60%) patients did go on to develop metastatic disease despite treatment. All patients in our study with positive lymph nodes did receive adjuvant treatment except 1 of 3 patients with metastatic disease at diagnosis whose disease seemed to remain indolent and another patient with locally advanced disease who deferred further treatment. Typical carcinoid and atypical carcinoid have a recurrence rate of approximately 3-5 and 25%, respectively.7 Thus, adjuvant therapy should be considered in completely resected typical and atypical carcinoids with mediastinal lymph node involvement and/or adverse pathologic features.22,28
Conclusions
In summary, carcinoid tumors are a relatively rare subset of pulmonary tumors that most often present with non-specific pulmonary symptoms (e.g. cough, hemoptysis or recurrent pneumonia), and rarely present with classic carcinoid syndrome. Atypical histology and nodal involvement are the most important adverse prognostic factors as these were reported almost universally to be statistically significant among the literature and in our study. Lung sparing resection as a primary therapy seems to be adequate for most carcinoid tumors, with complete resection yielding excellent local control and long-term survival. Adjuvant radiotherapy or chemoradiation have not been well studied, but should be considered for atypical carcinoids with concerning pathologic features until data is available indicating otherwise. Larger and randomized studies in the future should be conducted to determine the most optimal management of patients with primary pulmonary carcinoid tumor.
Acknowledgments
Funding: Short-Term Training: Students in Health Professional Schools. Source: NIH/NHLBI; Grant #: T35 HL007744
Footnotes
Conflict of interest: No authors listed in this manuscript have a conflict of interest to disclose.
Part of this article has been presented in abstract form at the Radiological Society of North America Meeting, Nov. 25-30, 2012, Chicago, IL.
References
- 1.Yao JC, Hassan M, Phan A, et al. One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26:3063–3072. doi: 10.1200/JCO.2007.15.4377. [DOI] [PubMed] [Google Scholar]
- 2.Jensen RT. Endocrine tumors of the gastrointestinal tract and pancreas. In: Longo D, Fauci A, Kasper D, et al., editors. Harrison's Principles of Internal Medicine. New York, NY: McGraw-Hill; 2012. [Google Scholar]
- 3.Fink G, Krelbaum T, Yellin A, et al. Pulmonary carcinoid: presentation, diagnosis, and outcome in 142 cases in Israel and review of 640 cases from the literature. Chest. 2001;119:1647–1651. doi: 10.1378/chest.119.6.1647. [DOI] [PubMed] [Google Scholar]
- 4.Langfort R, Rudzinski P, Burakowska B. Pulmonary neuroendocrine tumors. The spectrum of histologic subtypes and current concept on diagnosis and treatment. Pneumonol Alergol Pol. 2010;78:33–46. [PubMed] [Google Scholar]
- 5.Travis WD. Lung tumours with neuroendocrine differentiation. Eur J Cancer. 2009;45(Suppl 1):251–266. doi: 10.1016/S0959-8049(09)70040-1. [DOI] [PubMed] [Google Scholar]
- 6.Corrin B. Neuroendocrine neoplasms of the lung. Curr Diagn Pathol. 1997;4:239–250. [Google Scholar]
- 7.Gridelli C, Rossi A, Airoma G, et al. Treatment of pulmonary neuroendocrine tumours: State of the art and future developments. Cancer Treat Rev. 2012 doi: 10.1016/j.ctrv.2012.06.012. [DOI] [PubMed] [Google Scholar]
- 8.Sargent DJ, Wieand HS, Haller DG, et al. Disease-free survival versus overall survival as a primary end point for adjuvant colon cancer studies: individual patient data from 20,898 patients on 18 randomized trials. J Clin Oncol. 2005;23:8664–8670. doi: 10.1200/JCO.2005.01.6071. [DOI] [PubMed] [Google Scholar]
- 9.Oba K, Paoletti X, Alberts S, et al. Disease-free survival as a surrogate for overall survival in adjuvant trials of gastric cancer: a meta-analysis. J Natl Cancer Inst. 2013;105:1600–1607. doi: 10.1093/jnci/djt270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsuta K, Raso MG, Kalhor N, et al. Histologic features of low- and intermediate-grade neuroendocrine carcinoma (typical and atypical carcinoid tumors) of the lung. Lung Cancer. 2011;71:34–41. doi: 10.1016/j.lungcan.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 11.Kantarjian HM, Wolff RA, Koller CA. Neuroendocrine tumors. In: Strauss M, Loeb M, Edmonson K, editors. The MD Anderson Manual of Medical Oncology. New York, NY: McGraw-Hill Education; 2011. [Google Scholar]
- 12.Schrevens L, Vansteenkiste J, Deneffe G, et al. Clinical-radiological presentation and outcome of surgically treated pulmonary carcinoid tumours: a long-term single institution experience. Lung Cancer. 2004;43:39–45. doi: 10.1016/j.lungcan.2003.08.017. [DOI] [PubMed] [Google Scholar]
- 13.Filosso PL, Rena O, Donati G, et al. Bronchial carcinoid tumors: surgical management and long-term outcome. J Thorac Cardiovasc Surg. 2002;123:303–309. doi: 10.1067/mtc.2002.119886. [DOI] [PubMed] [Google Scholar]
- 14.Hurt R, Bates M. Carcinoid tumours of the bronchus: a 33 year experience. Thorax. 1984;39:617–623. doi: 10.1136/thx.39.8.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bertelsen S, Aasted A, Lund C, et al. Bronchial carcinoid tumours. A clinicopathologic study of 82 cases. Scand J Thorac Cardiovasc Surg. 1985;19:105–111. doi: 10.3109/14017438509102831. [DOI] [PubMed] [Google Scholar]
- 16.Harpole DH, Jr, Feldman JM, Buchanan S, et al. Bronchial carcinoid tumors: a retrospective analysis of 126 patients. Ann Thorac Surg. 1992;54:50–54. doi: 10.1016/0003-4975(92)91139-z. discussion 54-55. [DOI] [PubMed] [Google Scholar]
- 17.Gould PM, Bonner JA, Sawyer TE, et al. Bronchial carcinoid tumors: importance of prognostic factors that influence patterns of recurrence and overall survival. Radiology. 1998;208:181–185. doi: 10.1148/radiology.208.1.9646811. [DOI] [PubMed] [Google Scholar]
- 18.Descovich P, Ansaloni L, Grazia M, et al. Bronchial carcinoids. Our experience with 35 cases. Minerva Chir. 2000;55:113–119. [PubMed] [Google Scholar]
- 19.Thomas R, Christopher DJ, Balamugesh T, et al. Clinico-pathologic study of pulmonary carcinoid tumours--a retrospective analysis and review of literature. Respir Med. 2008;102:1611–1614. doi: 10.1016/j.rmed.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 20.Madrid-Carbajal C, Garcia-Clemente M, Pando-Sandoval A, et al. Bronchial carcinoid tumor: study of 60 patients. Med Clin (Barc) 2013 doi: 10.1016/j.medcli.2013.02.034. [DOI] [PubMed] [Google Scholar]
- 21.Grote TH, Macon WR, Davis B, et al. Atypical carcinoid of the lung. A distinct clinicopathologic entity. Chest. 1988;93:370–375. doi: 10.1378/chest.93.2.370. [DOI] [PubMed] [Google Scholar]
- 22.Thomas CF, Jr, Tazelaar HD, Jett JR. Typical and atypical pulmonary carcinoids: outcome in patients presenting with regional lymph node involvement. Chest. 2001;119:1143–1150. doi: 10.1378/chest.119.4.1143. [DOI] [PubMed] [Google Scholar]
- 23.Marty-Ane CH, Costes V, Pujol JL, et al. Carcinoid tumors of the lung: do atypical features require aggressive management? Ann Thorac Surg. 1995;59:78–83. doi: 10.1016/0003-4975(94)00630-P. [DOI] [PubMed] [Google Scholar]
- 24.Garcia-Yuste M, Matilla JM, Alvarez-Gago T, et al. Prognostic factors in neuroendocrine lung tumors: a Spanish Multicenter Study. Spanish Multicenter Study of Neuroendocrine Tumors of the Lung of the Spanish Society of Pneumonology and Thoracic Surgery (EMETNE-SEPAR) Ann Thorac Surg. 2000;70:258–263. doi: 10.1016/s0003-4975(00)01369-2. [DOI] [PubMed] [Google Scholar]
- 25.Wilkins EW, Jr, Grillo HC, Moncure AC, et al. Changing times in surgical management of bronchopulmonary carcinoid tumor. Ann Thorac Surg. 1984;38:339–344. doi: 10.1016/s0003-4975(10)62283-7. [DOI] [PubMed] [Google Scholar]
- 26.Ducrocq X, Thomas P, Massard G, et al. Operative risk and prognostic factors of typical bronchial carcinoid tumors. Ann Thorac Surg. 1998;65:1410–1414. doi: 10.1016/s0003-4975(98)00083-6. [DOI] [PubMed] [Google Scholar]
- 27.Shields TW, LoCicero J, Reed CE, et al. General Thoracic Surgery. 7th. Philadelphia, PA: Lippincott Williams & Wilkins; 2009. pp. 1539–1554. [Google Scholar]
- 28.Mackley HB, Videtic GM. Primary carcinoid tumors of the lung: a role for radiotherapy. Oncology (Williston Park) 2006;20:1537–1543. discussion 1544-1535, 1549. [PubMed] [Google Scholar]
- 29.Shrager JB, Wright CD, Wain JC, et al. Bronchopulmonary carcinoid tumors associated with Cushing's syndrome: a more aggressive variant of typical carcinoid. J Thorac Cardiovasc Surg. 1997;114:367–375. doi: 10.1016/S0022-5223(97)70182-X. [DOI] [PubMed] [Google Scholar]
- 30.Deb SJ, Nichols FC, Allen MS, et al. Pulmonary carcinoid tumors with Cushing's syndrome: an aggressive variant or not? Ann Thorac Surg. 2005;79:1132–1136. doi: 10.1016/j.athoracsur.2004.07.021. discussion 1132-1136. [DOI] [PubMed] [Google Scholar]