Abstract
Background and aims: A 405 nm diode laser is indicated for composite materials polymerizing, thanks to the recent evolution in their compositions, absorbing in blue part of the spectrum. The purpose of this research was to evaluate its performance on two different kinds of composite resins.
Materials and methods: Two different composites were polymerized with a traditional halogen lamp, a LED device and a 405 nm diode laser.
The depth of the cure, the volumetric shrinkage, and the degree of the conversion (DC%) of the double bond during the curing process were measured.
One-way ANOVA test, Kruskal-Wallis tests, and Dunn comparison tests were used for statistic analysis.
Results: Regarding the depth of polymerization, the laser had the worst performance on one composite while on the other, no significant difference with the other devices was observed. The volumetric shrinkage showed that laser produced the lowest change in both of the composites. The DC% measure confirmed these findings.
Conclusions: Based on the results of this preliminary study, it is not possible to recommend the 405 nm diode laser for the polymerization of dental composites.
Keywords: composite, laser, polymerization, LED, Diode, 405 nm
Introduction
In recent years, the interest in dental laser devices has enormously increased. In the past, laser devices were expensive and had selected clinical applications, but they are now considered to be useful in many aspects of operative dentistry, such as surgery, conservative, endodontic, dental bleaching, and photodynamic therapy. Many lasers for dental applications emit in the infrared region of the electromagnetic spectrum (e.g., Nd:YAG, Nd:YAP, Er:YAG, 810 nm and 980 nm diode lasers, CO2, etc.); however, researchers have shown interest in the visible part of the spectrum in which only a few wavelengths are used, such as red diode lasers at 655 nm, green KTP lasers at 532 nm, and blue argon lasers at 488 nm. A diode laser with an emission wavelength of 405 nm has become increasingly interesting to researchers, and the number of studies of the possible applications of this type of device has increased in the literature. Many studies have focused on the antibacterial potentials of this device, effective to destroy many bacterial species that are methicillin resistant, such as P. Aeruginosa, S. Pyogenes and P. Gingivalis 1, 2).
Other studies have highlighted the usefulness of this type of laser as a tool for dental bleaching procedures 3, 4, 5, 6). Again, studies have shown the potential of the use of this laser in Photodynamic Therapy in which a photosensitiser, such as curcumin is used 7). Others have shown promising results in the treatment of the inflammatory acne 8). Normally, halogen lamps are used in conservative dentistry to obtain the polymerization of dental composite materials but in recent years, LED units have become easier to use. Until recently, the main photo-initiator was camphorquinone (CQ), which, due to its outstanding performance, has been adopted in the compositions of the majority of dental composites. Due to its absorption wavelength, CQ has an large b value in the CIELab colour specification system, manifested as a yellow tinge, this being a problem when a clear colour is required in the dental composite 9). To solve this problem, alternative photoinitiators are currently being developed, many of these only experimentally proposed, others used in marketed composites 10).
Many alternative molecules, such phenylpropanedione (PPD) and diphenyl 2,4,6 trimethoxybenzoyl phosphine oxide (Lucirin TPO) have been proposed, and some of these are already commercially and clinically available 11).
The new photo-initiators have absorption spectra shifted toward the violet range, but new problems related to the efficiency of polymerization when LED devices are used arise because their emissions are centred on blue light (480 nm) and CQ absorption. Recently, an interest was focused on the violet laser composite polymerization also thanks to the evolution of composites and the introduction of new photoinitiators 12, 13). The aim of the present research was to test the polymerization performance of a new diode laser prototype emitting on 405 nm compared to traditional Halogen Lamp and LED devices, on two different types of composites, one utilizing CQ, and the other Lucirin TPO as photoinitiators.
Materials and methods
The light sources used in this study were a medical prototype diode laser (405 nm) with Power Density of 100 mW/cm2 (L) (Eufoton, Italy), a traditional Halogen Lamp with a Power Density of 650 mW/cm2 (HL) (Heliolux DLX Vivadent, Austria) and a new generation LED device with a Power Density of 1000 mW/cm2 (DL) (Valo Ultradent, USA).
Fig. 1:
The three devices used for the tests: Halogen Lamp (left), Dual Lamp (centre), Diode laser (right)
All of these devices were tested to evaluate the power emitted before every experimental session, and each of them was used for the time required to produce Fluences of 4, 8, 16, and 32 J/cm2. The emitted power of the laser was checked with a Power-meter (Thorlabs PM 100 Germany), and the powers of the other two devices were examined with a Radiometer (Radiometer LED, Demetron Kerr, USA) even if some studies indicate that, by these devices, the measurements could be not sufficiently accurate 14, 15). Two different flowable, A1 colour dental composites, were used to perform the tests (Tetric EvoFlow® Ivoclar Vivadent, Italy, Lot S 364464 and Filtek™ Supreme XTE flow ESPE, USA, lot N 499628).
Tab. 1: Main component of the dental composites tested. The photoinitiator of Filtek Supreme Flow 3M™ is CQ (camphoroquinone) for the Tetric Evo Ceram Flow is CQ+ TPO (diphenyl 2,4,6 trimethoxybenzoyl phosphine oxide).
| Tetric® Evo flow main component | Wt% | Filtek™ supreme XTE flow main component | Wt% |
|---|---|---|---|
| Bis-GMA /Urethane dimethacrylate/Decanediol dimethacrylate | 376% | Silane-treated ceramic | 50/60% |
| Barium glass filler, ytterbium trifluoride, silicon dioxide highly dispersed | 41.1% | Bis-GMA | 5/10% |
| Prepolymer | 20.4% | TEDGMA | 5/10% |
| Catalyst, stabilizer, additives | 0.9% | Silane-treated silica | 5/10% |
| Pigment | <0.1% | Ytterbium trifluoride | <5% |
| Dimethacrilate functionalized polimer | <5% |
Determination of the depth of curing
To determine the depth of the polymerization of the composites by the different light sources, we followed a slightly amended ISO 4049 procedure. A stainless steel mould with a 6-mm-long cylindrical hole that was 4 mm in diameter was used to obtain a cylindrical specimen.
Fig. 2:
Stainless steel moulds with cylindrical hole and composite cylinder obtained.
The mould was placed on a piece of transparent polyester film (Hawe Neos Striproll transparent, Kerr, USA), placed on a glass microscope slide. The mould was filled with the test material, caution was exercised to exclude air bubbles, and it was slightly overfilled. A second transparent strip was placed on top, followed by a second microscope slide. The glass was gently pressed to displace the excess material, which was removed. The composite specimens were cured with the three different light sources, and care was taken to completely cover the diameter of the mould with the light and to expose the samples to equal energies of 4, 8, 16, and 32 Joule/cm2. Immediately following irradiation, the specimens were removed from the mould, and the uncured material was eliminated with a plastic spatula. The height of the cylinder of the cured material was measured using an electronic universal micrometer with an accuracy of 0.001 mm (RS component, Italy).
Determination of the volumetric shrinkage
A stainless steel mould with a 6-mm hole with a diameter of 5 mm was used. Two electronic micrometers were used to fix the initial volume of the mould that was used as a control. Ten diameter and length measurements of the cylinder were taken, and the average of these data was used to calculate the volume of the entire mould, which resulted in 116.1878 ± 0.00662 mm3. A procedure similar to that described above was applied to obtain the samples, and the measurement of the depth of curing was subsequently performed. The material was cured first on one side for half of the predetermined time; the sample was then tipped, the first microscope glass was slide removed, and the sample was cured on the other side for the remaining time. The curing times were different for the different light sources, but all of the samples received 16 J/cm2. The cylinder of material was removed from the mould, and its volume was measured with two different methods: first, a micro-meter was used to measure the dimensions of the cylinder of the composite following polymerisation. Each sample was measured three times, and care was taken to perform the measurements at different points on the cylinder. Second, the volume was obtained using the Archimedes principle. A balance with a density kit was used (Pioneer Ohaus Usa, 0.0001g).
The sample was weighed in the air and then in water. The volume was calculated using the following formula: 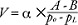 were po = the density of the auxiliary liquid (distilled water, corrected for the temperature as measured with a thermometer), pL = air density (0.0012 g/cm3), A= the weight of the sample in air, B = the weight of the sample in the auxiliary liquid, and α= a balance correction factor (0.99985) that accounts of the buoyancy of the air. The utilized procedures were obtained from the ASTM international D 792 (which is a standard method for the testing of the densities and specific gravities of plastic that utilizes displacement) but were not followed concerning the volumes of the samples. We termed these procedures “gravimetric methods”. Because the two methods did not produce significantly different results, we combined all measurements. The volumes obtained with the two methods were compared to the volume of the mould, which served as a control.
were po = the density of the auxiliary liquid (distilled water, corrected for the temperature as measured with a thermometer), pL = air density (0.0012 g/cm3), A= the weight of the sample in air, B = the weight of the sample in the auxiliary liquid, and α= a balance correction factor (0.99985) that accounts of the buoyancy of the air. The utilized procedures were obtained from the ASTM international D 792 (which is a standard method for the testing of the densities and specific gravities of plastic that utilizes displacement) but were not followed concerning the volumes of the samples. We termed these procedures “gravimetric methods”. Because the two methods did not produce significantly different results, we combined all measurements. The volumes obtained with the two methods were compared to the volume of the mould, which served as a control.
Determination of the Degree of conversion
An FT-IR spectrometer (Biorad FTS 175 C, USA) with an ATR accessory (ATR Quest Specac, USA) was used.
A small mould of the PTFE corresponding to the diamond was placed on the attenuated total reflectance (ATR) plate. This mould had a 3-mm-high, 4-mm-diameter hole. The mould was filled with composite resin, and a strip of transparent polyester plastic was placed on top. The strip was pressed with a microscope slide, and the excess composite was removed. Next, the glass microscope slide was removed, and the material was cured with progressive quantities of energy by the three different devices to obtain measures of absorbance of the sample at 0 (i.e., the uncured material that was used a control), 4, 8, 16, and 32 J/cm2. For each quantity of energy, measurements of absorbance at 1640 cm−1 and 1610 cm−1 were taken, and this process was repeated three times for each sample. Note that these measurements recorded the evolution of the polymerization on the surface of the composite that was in contact with the diamond of the ATR, therefore, in this experimental model, we simulated the evolution of the curing in a small cavity and took the measurements from the bottom of the cavity. After the first step of curing, the plastic strip was removed, and the sample was pressed to obtain maximum contact between the sample and the ATR diamond using the same force as the ATR punch. Measures of absorbance were recorded between 2500 cm−1 and 500 cm−1 with a resolution 4 cm−1 and 32 scans. The degree of conversion was obtained using the following equation:
where R= peak height at 1640 cm−1/ peak height at 1610 cm−1.
Statistical calculations regarding the volume and the degree of conversion were performed with Excel software (Microsoft Excel, 2010 USA). The statistical analyses between the different groups were performed with one-way ANOVA test, Kruskal-Wallis tests, and Dunn comparison tests (Graphpad Prism ver. 5.0, Graphpad Software, San Diego, California, USA www.Graphpad.com)
| P value | Wording | Summary |
|---|---|---|
| <0.001 | Extremely significant | *** |
| 0.001 to 0.01 | Very significant | ** |
| 0.01 to 0.05 | Significant | * |
| >0.05 | Not significant | ns |
Results
Depth of polymerization
In the case of the Tetric® composite, comparison of the different levels of polymerization following the application of the different energies (4, 8, 16, 32 J/cm2) with the halogen lamp (HL) revealed that there were no significant differences between 4 and 8, 8 and 16, or 16 and 32 J/cm2. (P<0.05 ns) In contrast, there were significant differences between the 4 and 16 J/cm2 groups (P <0.0001 ***) and between the 8 and 32 J/cm2 (P <0.001 to 0.01 **) groups. When we used the diode LED lamp (DL) to cure the Tetric® composite, we did not observe significant differences following curing with 8, 16, or 32 J/cm2 (P< 0.05 ns); moreover, significant differences were observed between the 4 J/cm2 group and the 16 (P<0.0001 to 0.01 **) and 32 J/cm2 (P<0.0001 ***) groups. When the laser 405 nm (L) was used, results similar to the halogen lamp were recorded. When we compared the depths of the polymerization obtained following the application of the three different devices at the same energy level, no significant differences were observed when applied to the Tetric® composite (P<0.05 ns). When we analysed the behaviour of the Filtek™ supreme composite under the HL, we observed significant differences only between the 4 J/cm2 group and the 16 (P<0.01 to 0.05 *) and 32 J/cm2 (P<0.0001 ***) groups, and the same behaviour was recorded for the DL. Within the different groups of samples cured with the different energy levels applied with the laser device, no significant variances were detected, even between the lowest (4 J/cm2) and highest (32 J/cm2) (P>0.05 ns) levels of energy. Moreover, when we compared the depths of polymerization in the composites cured with the different devices, we observed that, with the exception of the lowest level of energy (4 J/cm2), all the other groups exhibited polymerization depths after the laser irradiation significantly lower than with the other two devices at all energy levels.
Fig. 3:
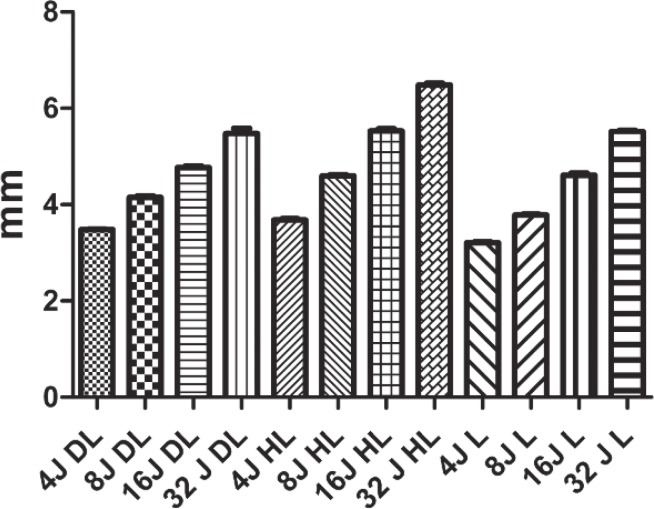
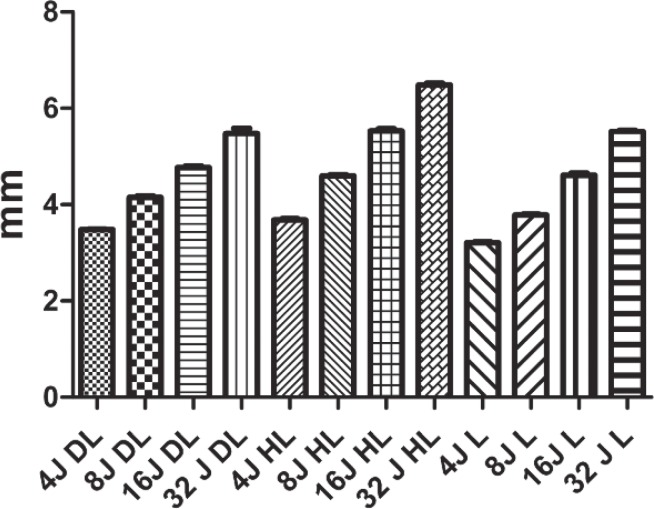
Filtek™ supreme XTE flow polymerization depth test. The laser device produced worse performances at each energy level; moreover, no significant differences were observed between the groups that were polymerized with the laser, including between the 4 Joule and 32 Joule groups (DL, diode LED Valo; HL, halogen lamp; L, 405 nm laser).
Fig. 4:
Tetric EvoFlow® polymerization depth test. In absolute terms, the halogen Lamp (HL) produced greater polymerization depth values, and the values produced by the laser (L) were lower at every energy level, although some of the differences were not significant.
Fig. 5:
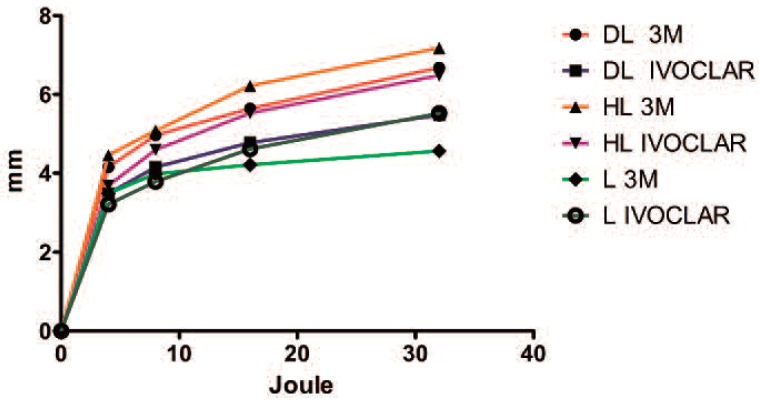
Graphic illustration of the performances of the different devices on the two composites. When the laser (L) was used on the Filtek™ supreme XTE flow (3M), the polymerization depth immediately reached a plateau that was lower than the values achieved with the other devices. When the laser was used on the Tetric® EvoFlow (IVOCLAR), the performance of the laser was similar to that of the diode LED (DL).
Volumetric shrinkage
Measurements of the volumetric shrinkages of the two dental composites were performed with two different methods. In this trial, we decided to use only a single level of energy, considered sufficient for a good polymerization, as reported in the literature. The second reason for this choice was that the two methods tested in advance were not sufficiently sensitive to reveal small volumetric changes with the energy levels used.
Statistical comparison of the two methods did not evidence significant differences in the calculated volumes: indeed the results were the same. Therefore, for the statistical analyses, we decided to unify the data obtained with the two methods, which, in our opinion, was more indicative of the experimental results.
The two composites reacted in different manners to the different light sources. Regardless of the device used, the Tetric® EvoFlow exhibited a lower level of dimensional variation than the Filtek™ Supreme. The DL exhibited the most substantial contractions, while the halogen lamp produced less extensive volumetric changes. The application of the laser for the polymerization of the Tetric® composite produced no significant changes from the initial volume (P>0.05 ns). The Filtek™ composite demonstrated marked dimensional variation when cured with the HL, and minor changes when it was cured with the DL (P<0.0001 ***). Similar to the Tetric®, no significant changes in the volumes of the samples were recorded when the laser was applied (P>0.05 ns). In summary, the laser did not elicit changes in the structures of the double bonds sufficient to provoke significant polymerization and consequent significant and measurable changes in the dimensions of the samples of either composite. Additionally, the HL elicited greater changes in the Filtek ™, while the DL elicited greater changes in the Tetric®.
Fig. 6:
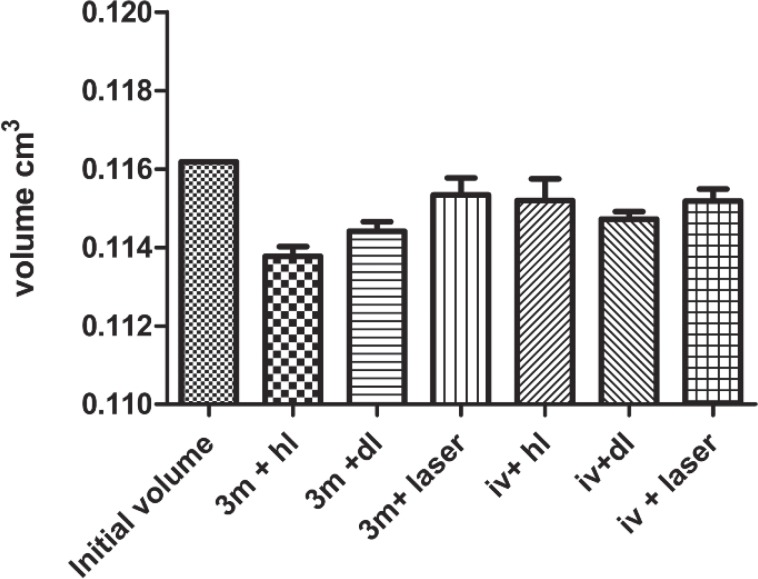
Graphic representation of the volumetric changes (shrinkages) of the two composite when the different devices were applied at the same level of energy (16 Joule /cm2) for polymerization. Note the different behaviours of the two composites; the Tetric® Evo flow exhibited less extensive lower dimensional changes compared to the Filtek™ Supreme XT flow. The laser produced less extensive dimensional changes compared to the other two traditional devices, which indicated the lower efficiency of the laser in the polymerization process.
Degree of conversion
The measurements of the degrees of conversion of the Filtek™ Supreme composite did not reveal any significant differences between the different groups (P>0.05 ns). The high standard deviation observed during the tests did not allow us to observe small differences in the degree of conversion. When the composite was cured with the halogen lamp, the recorded averages ranged from 16.33 to 21.37%; these ranges were 24.89 and 31.2% for the dual lamp and 9.5 and 14.48 % for the laser. Regarding the Tetric® EvoFlow, the standard deviation was slightly lower, but generally, even for the measurements of this composite, it was not possible to identify any significant differences (P>0.05 ns). Only two groups exhibited differences from the other groups: the composite cured with 4 J of laser energy exhibited a minimal degree of conversion (P<0.0001 ***), and the composite cured with laser 8 J was significantly different from many of the other groups (P 0.001 to 0.01 **), particularly those cured with the halogen lamp; even if with small differences. The averages of the degrees of conversion ranged from 22.24 to 35.64% for the HL, 20.16 to 28.89% for the DL, and −2.2 to 30.57 % for the laser.
Fig. 7:
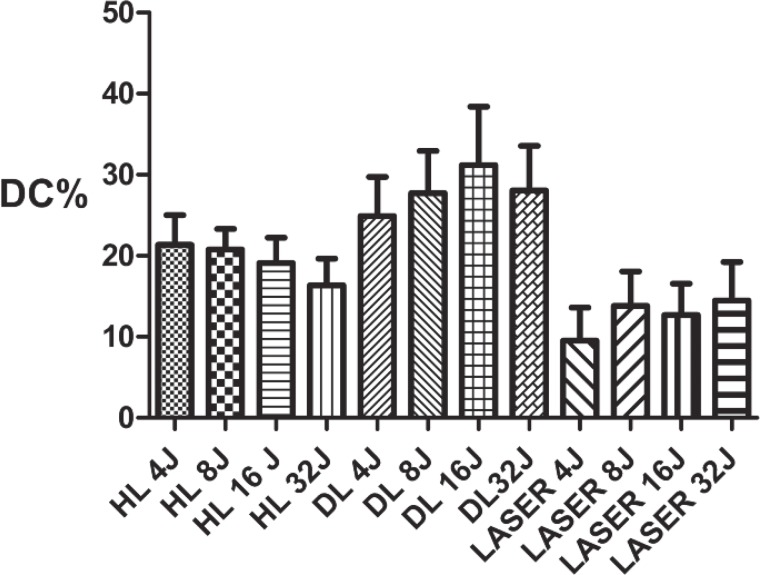
Graphic statistical overview of the degrees of conversion during the polymerizations of the Filtek™ Supreme XTE 3M flow. Although significant differences between the groups were not observed at any energy level, the DC%s was lower compared with those of the other two devices.
Fig. 8:
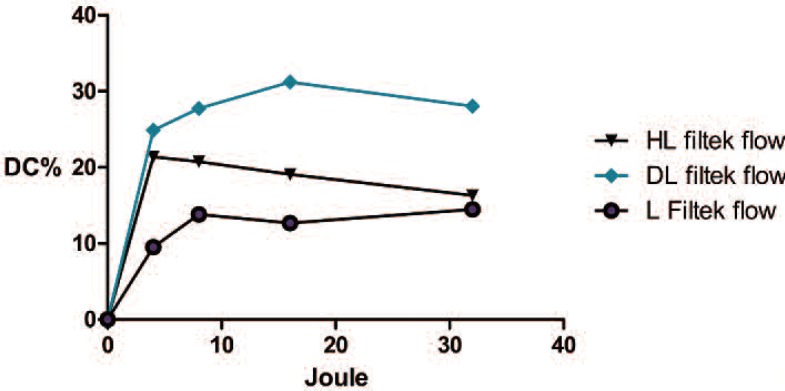
Graphical representation of the evolutions of the DC%s of the Filtek™ Supreme XTE 3M with increasing energy. The laser device exhibited a lower degree of conversion compared to the other devices. The group of the composites cured with the halogen lamp (HL) exhibited decreasing DC% levels, but this was an artefact that was due to volumetric shrinkage during the measurements.
Fig. 9:
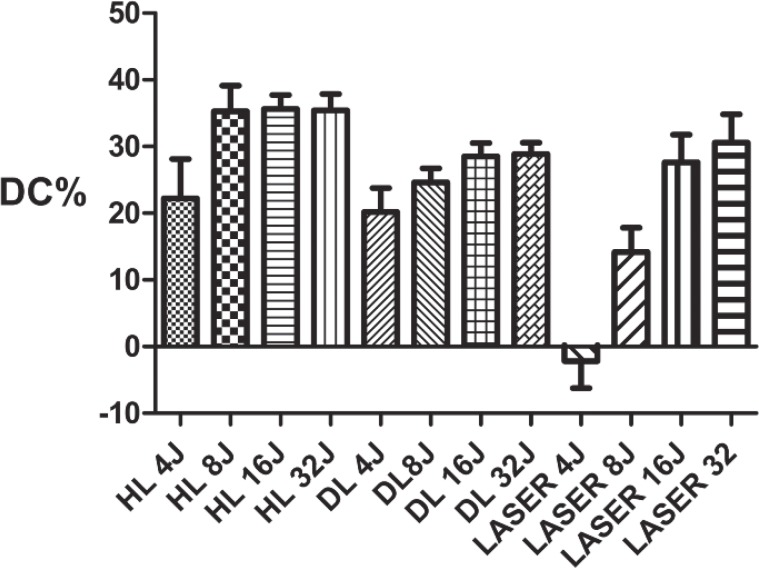
Degree of conversion of the Tetric® Evo Flow (Ivoclar). The laser was not able to initiate the polymerization of the composite when a very low level of energy was applied. At higher energy levels, the degree of conversion became similar to those of the other devices.
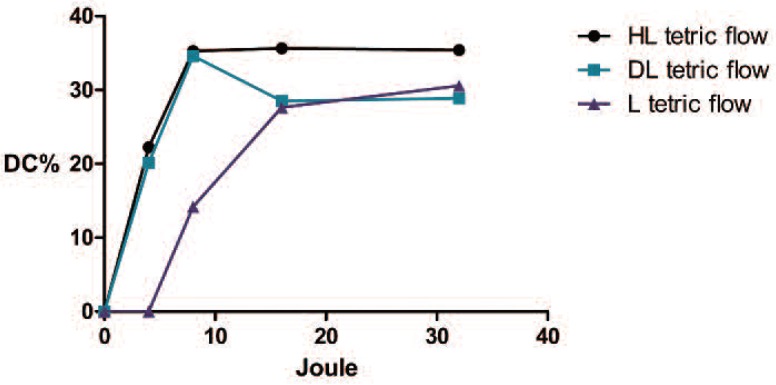
Fig. 10:
Graphic representation of the evolution of the DC%s of the Tetric ®Evo flow ivoclar with increasing the energy. The curve began with a shift compared with those of the other device, but later reached a level of polymerization that was comparable to those of the other two devices
Discussion
To evaluate the ability of the 405-nm diode laser device to activate the curing process, we selected two different dental composites. Among other aspects, these two composites differ in terms of the photoactivation system utilized. The Filtek™ Supreme XTE flow utilises camphoroquinone (CQ) as a photoactivator, whereas the Tetric® Evo flow use CQ plus Lucirin TPO (diphenyl 2,4,6 trimethoxybenzoyl phosphine oxide). This choice was made with the goal of testing the laser on commercial composites that mimicked the two different situations currently encountered in the clinic: traditional composites with CQ photoactivation, and composites that are, at least partially, based on new photoactivated molecules requiring need of shorter wavelengths.
Furthermore, we elected to compare the performance of the laser device with two other devices typically used in clinical practice: a halogen lamp (HL), and a diode LED device (DL). Both devices are able to correctly cure all of the composites independently of the type of photo-activating system for reasons related to the emission spectra 16). The emission of the HL ranged from 380 nm to 500 nm with a peak emission of 480 nm, which matched the absorption of CQ but only marginally aligned with the absorption peaks of the PPD and Lucirin TPO. The LED used in our study was particular if compared to other LED devices having not one but three distinct LED each with a different emission (i.e., 410, 439, and 460 nm), this resulting in better overall coverage of the peaks of all of the photoinitiators 17). Lasers are coherent and monochromatic beams and their emissions are centred on a single wavelength, which, in our case, was 405 nm. This wavelength intercepts the absorption spectrum of CQ at the beginning of its rise when it is still weak, and intercepts the absorption spectra of TPO and PPD when the absorptions are still high 16). The polymerization performances were evaluated by comparing the three devices with three different tests. The first test was related to the depth of curing. Our first observation was focused on the depth of polymerization growth with the energy level increase. This gain was not linear but rather followed a curve that exhibited a fast climb ensued by moderate growth until a plateau was reached. We applied 4 J/cm2 of energy to the composite and measured the cured composite heights, which ranged from 3.21 mm to 4.45 mm. The lowest measures satisfied the minimum requirements of the ISO 4049 rule even when low energies were used. Regarding the highest level of energy (32 J/cm2), the maximum depths of polymerization ranged from 4.56 mm to 7.81 mm. This test allowed use to understand to extent to which each composite was prone to curing as an indication (even if overestimated) of the limits of the thickness of a material that can be correctly cured in clinical practice 18, 19). With the inverted reasoning and under identical conditions, the procedure became a test to verify the ability of the light cure unit (LCU) to correctly polymerize a composite 20, 21). The ISO 4049 standards required for restorative class 2 materials are cure depths of 0.5 mm (1 mm) for more opaque composites and 1.5 mm (3 mm) for others. These values were always exceeded in our tests, but when the 405-nm laser was used on the composite photoactivated by CQ, lower curing depths were recorded at every energy level, suggesting that the activation of the CQ was slow and difficult. In contrast, no differences were observed when the laser was used on the composites activated by different photoinitiators.
The second test measured the volumetric shrinkages of the two materials following polymerization with the different devices. During polymerization process, the mass of the composite is subjected to transformation due to the growth of the complex network that originates in the links between the monomer molecules. At the end of the process, the volume of the composite is diminished to an extent that depends on many factor, such as the amount of inorganic filler, the type and size of the composite structure, and the monomer (higher molecular weights lead to less shrinkage) 22). When the composite it is well polymerized, the maximum volumetric shrinkage occurs; in contrast, a composite that it is not well polymerized will have a higher volume than a well-polymerized composite. The Source 3M ESPE reported the volumetric shrinkage %s for Filtek™ supreme XTE flow and Tetric® Evo flow as tested with the Watts and Cash methods as 3.5 and 3.9%, respectively.(brochure scientific documentation) The downloadable Ivoclar scientific documentation indicate that the volumetric shrinkage of the Tetric Evo flow is 1.5%, and that of the Filtek™ supreme flow is 2.2% (method mercury dilatometer, Investigation R&D Ivoclar Vivadent) In our research, we recorded different values, but this is not unusual based on the reports of different methods in the literature (e.g., dilatometer, microscope, densitometry, linometer, and laser beam scanning), which can produce different results 22). The average value recorded for the Filtek™ Supreme XTE polymerized with the HL was 2.07±0.42%, and the value for the Tetric® Evo flow polymerized with HL was 0.78±0.4%. However, the most important aspect for the purposes of our study was that, when the laser was used either composite, no significant differences were found between the initial volumes and the final volumes after polymerization. In contrast, significant differences were observed when the other two devices were used. The lack of significant volume changes provided evidence of the minimal ability of the laser device to initiate and advance the polymerization process. The third test we applied examined the degree of conversion, which was determined by the proportion of the remaining concentration of the aliphatic C=C double bonds in the cured sample relative to the total number of C=C bonds in the uncured material. Fourier transform infrared spectroscopy (FT-IR) combined with another technique called FT Raman spectroscopy, which is a method that is widely used to determination of the degree of conversion have been 23, 11). In the present study, the test performed was not fully satisfactory, and the information that we obtained was not as informative as we had hoped due to the high standard deviations that were recorded during the tests. Nevertheless, we did obtain some similarities with the results of the previous test. The DC%s obtained from the composites that were cured with the laser were lower than those of the composites cured with the other two devices. In the case of the Filtek ™supreme XTE, the curing process begin at low energy and increased slowly with increasing energies, but did not reach the level achieved by the other device. Regarding the Tetric® evo flow, we observed that low energy levels were insufficient to initiate the polymerization process, but when the right energy level was provided, the degree of polymerization was ultimately comparable to that achieved with other devices.
Conclusions
The performed tests showed a great difference in the behaviour of the laser on the two dental composites in a way depending on the photoactivating system. When a co-activating substance was present (e.g., Lucirin, TPO, etc.), the performance of the laser was sufficient and comparable to the conventional device, at least at higher energy levels. This device might have potential for use in the polymerization of dental composite if, in the future, new photo-activators operating in the UV portion of the spectrum will be developed. Currently, because most dental composites use CQ, the use of this device is not recommended even at high levels of energy due to the risk of not achieving sufficient polymerization.
Acknowledgements
The Authors indicate no potential conflicts of interest.
References
- 1: Kawano T, Prananingrum W, Ishida Y, Goto T, Naito Y, Watanabe M, Tomotake Y, Ichikawa T. (2013). Blue violet laser modification of titania treated titanium: antibacterial and osteo-inductive effects. PLoS One, 17; 8(12):e84327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2: Kotoku Y, Kato J, Akashi G, Hirai Y, Ishihara K. (2009). Bactericidal effect of a 405 nm diode laser on a Porphiromonas gingivalis. Laser Physics Letters. Vol. 6, p. 388-392. [Google Scholar]
- 3: Sakai K, Kato J, Nakazawa Y, Hirai Y. (2007). The amounts of Hydroxyl radicals generated by titanium dioxide and 3.5% hydrogen peroxide under 405 nm diode laser irradiation. Laser Physics. Vol. 17, p.1062-1066. [Google Scholar]
- 4: Suemori T, Kato J, Nakazawa T, Akashi G, Hirai Y. (2008). A new non vital tooth bleaching method using titanium dioxide and 3.5% hydrogen peroxide with a 405 nm diode laser or halogen lamp. Laser Physics Letters. Vol. 5, p. 454-459. [Google Scholar]
- 5: Suyama Y, Otsuki M, Ogisu S, Kishikawa R, Tagami J, Ikeda M, Kurata H, Cho T. (2009). Effects of light sources and visible light activated titanium dioxide photocatalyst on bleaching. Dental Materials Journal. Vol. 28, p. 693-699. [DOI] [PubMed] [Google Scholar]
- 6: Lagori G, Rocca JP, Brulat N, Merigo E, Vescovi P, Fornaini C. (2015). Comparison of two different laser wavelengths dental bleaching results by photo fenton reaction: in vitro study. Lasers in Medical Science Vol. 30(3), 1001-1006. [DOI] [PubMed] [Google Scholar]
- 7: Rego-Filho FG, De Araujo MT, De Oliveira KT, Bagnato VS. (2014). Validation of photodynamic action via photobleaching of a new curcumin based composite with enhanced water solubility. Journal of Fluorescence, Vol. 24(5), p.1407-1413. [DOI] [PubMed] [Google Scholar]
- 8: Borelli C, Merk K, Plewig G, Degitz K. (2005) Light, laser and PDT therapy for acne. s.l.: Hautarzt, Vol. 56 p. 1027-1032. [DOI] [PubMed] [Google Scholar]
- 9: Ikemura K, Ichizawa K, Jogetsu Y, Endo T. (2010) Synthesis of a novel camphorquinone derivative having acylphosphine oxide group, characterization by UV-VIS spectroscopy and evaluation of photopolymerization performance. Dent Mater J. 29(2):122-131. [DOI] [PubMed] [Google Scholar]
- 10: Ikemura K, Endo T. (2010). A review of development of radical photopolimerization initiators used for designing light curing dental adhesives and resin composites. Dental Materials Journal, Vol. 29 p. 481-501. [DOI] [PubMed] [Google Scholar]
- 11: Moraes Porto IC, Soares LE, Martin AA, Cavalli V, Liporoni PC. (2010). Influence of photoinitiator system and light photoactivation units on the degree of conversion of dental composites. Brazilian Oral Research, Vol. 24 p. 475-481. [DOI] [PubMed] [Google Scholar]
- 12: Kameyama A, Hatayama H, Kato J, Haruyama A, Teraoka H, Takase Y, Yoshinari M, Tsunoda M. (2011). Light Curing of dental resin with GaN violet laser diode: the effect of photoinitiator on mechanical strength. Laser in medical science Vol. 26, p. 279-283. [DOI] [PubMed] [Google Scholar]
- 13: Kameyama A1, Kato J, De Munck J, Hatayama H, Haruyama A, Yoshinari M, Takase Y, Van Meerbeek B, Tsunoda M. (2011). Light cure efficiency of dental adhesives by gallium nitride violet laser diode determined in terms of ultimate micro tensile strength. Biomed Mater Eng. Vol. 21, p. 347-356. [DOI] [PubMed] [Google Scholar]
- 14: Kameyama A, Haruyama A, Asami M, Takahashi T. (2013) Effect of emitted wavelength and light guide type on irradiance discrepancies in hand-held dental curing radiometers. Scientific World Journal; 2013:647941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15: Price RB, Labrie D, Kazmi S, Fahey J, Felix CM. (2012) Intra and inter brand accuracy of four dental radiometers. Clinical Oral Investigation Vol. 16, p. 707-717. [DOI] [PubMed] [Google Scholar]
- 16: Lee D-S, Jeong TS, Kim H, II, Kwon YH. (2012). Effect of dual peak Led unit on the polymerization of coinitiator-containing composite resin. Dental Material Journal. Vol. 31(4), p. 656-661. [DOI] [PubMed] [Google Scholar]
- 17: Price RBT, Labrie D, Rueggeberg FA, Felix CM. (2010). Irradiance differences in the violet (405nm) and blue (460nm) spectral ranges among dental light curing units. J. Esthet. Restor. Dent. Vol. 22(6), p. 363-377. [DOI] [PubMed] [Google Scholar]
- 18: International, Standard. ISO 4049 (2009). Dentistry -polymer based restorative materials. Vol. 4 [Google Scholar]
- 19: Heintze SD, Zimmerli B. (2011). Relevance of in vitro test of Adhesive and composite dental material. Schweiz Monatsschr Zahnmed. Vol. 121, p. 810-816. [PubMed] [Google Scholar]
- 20: Mousavinasab SM, Meyers I. (2009) Curing efficacy of light emitting diodes of dental curing units. Journal of Dental Research and Dental Clinic Prospective. Vol. 3(1); p. 11-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21: Nishimaki M. (2012). Depth of cure and hardness of indirect composite material polymerized with two metal halide laboratory curing units. Journal of Oral Science. Vol. 54(1), p. 121-125. [DOI] [PubMed] [Google Scholar]
- 22: Ersoy M, Civelek A, L'Hotelier E, Say EC, Soyman M. (2004). Phisical properties of different composite. Dental Material Journal. 23(3) p. 278-283. [DOI] [PubMed] [Google Scholar]
- 23: Galvão MR, Caldas SG, Bagnato VS, de Souza Rastelli AN, de Andrade MF. (2013). Evaluation of degree of conversion and hardness of dental composites photo-activated with different light guide tips. European Journal of Dentistry. Vol. 7 p. 86-93. [PMC free article] [PubMed] [Google Scholar]