Abstract
Background: Aggressive, or even minimally aggressive, aesthetic interventions are almost inevitably followed by such events as discomfort, erythema, edema and hematoma formation which could lengthen patient downtime and represent a major problem to the surgeon. Recently, low level light therapy with light-emitting diodes (LED-LLLT) at 830 nm has attracted attention in wound healing indications for its anti-inflammatory effects and control of erythema, edema and bruising.
Rationale: The wavelength of 830 nm offers deep penetration into living biological tissue, including bone. A new-generation of 830 nm LEDs, based on those developed in the NASA Space Medicine Laboratory, has enabled the construction of planar array-based LED-LLLT systems with clinically useful irradiances. Irradiation with 830 nm energy has been shown in vitro and in vivo to increase the action potential of epidermal and dermal cells significantly. The response of the inflammatory stage cells is enhanced both in terms of function and trophic factor release, and fibroblasts demonstrate superior collagenesis and elastinogenesis.
Conclusions: A growing body of clinical evidence is showing that applying 830 nm LED-LLLT as soon as possible post-procedure, both invasive and noninvasive, successfully hastens the resolution of sequelae associated with patient downtime in addition to significantly speeding up frank wound healing. This article reviews that evidence, and attempts to show that 830 nm LED-LLLT delivers swift resolution of postoperative sequelae, minimizes downtime and enhances patient satisfaction.
Keywords: Photobiomodulation, low level light therapy, wound healing, collagenesis, angiogenesis, hematoma, edema, erythema
Background & Rationale
Patient downtime after any surgical or nonsurgical aesthetic or other procedure needs to be kept as short as possible to allow patients to return swiftly to their activities of daily living (ADL), and the shorter this downtime can be, the happier and more satisfied are the patients. Satisfied patients reflect positively on the success of the practitioner. In the case of an unhappy and dissatisfied patient, however, the reverse is certainly true. Even when the final results may be good, if the patient has suffered severe or prolonged postoperative sequelae, such as erythema, edema, bruising or a combination of all of these, together with a concomitantly prolonged downtime, she or he will definitely be unhappy at least in the shorter term.
Virtually every interventional approach in the aesthetic field, whether surgical or even nonsurgical with minimally invasive techniques, is associated with some postoperative after-effects as a result of the inevitable epidermal and dermal damage or irritation. The response to this is inflammation as the first, and necessary, phase of the wound healing process. The classic post-wound signs of inflammation are rubor (redness), calor (heat) and dolor (pain) with occasionally tumor (swelling) added to the mix. The redness (erythema) is the result of blood vessel hyperactivity as an early part of the inflammatory process. Increased leukocyte activity to fight any invading pathogens, taken together with the extra volume of blood in the dilated superficial vasculature, leads to the possible rise in temperature in the affected area, or even systemically in the case of a more severe and deeper wound. When sensory nerve endings are damaged, the result is the sensation the cerebral cortex recognizes as pain, and the influx of interstitial serum from damaged blood vessels to the dermal extracellular matrix in the damaged area can result in swelling, or edema. Sometimes this is a protective mechanism, as in a sprained or strained joint, to act as a natural splint immobilizing the affected joint. However, this same edema also causes pressure pain via the pressure-sensing members of the mechanoreceptors. If all of these persist for some time, then the patient is forced to alter or suspend their normal ADL, and that represents undesirable downtime.
The potential of low level light therapy with light-emitting diodes (LED-LLLT) to accelerate wound healing and ameliorate pain has been attracting attention. These findings strongly suggest that LED-LLLT might well have a powerful adjunctive role to play in the clinical practice of the aesthetic or cosmetic surgeon.
Practicalities of LED-LLLT: LEDs have inherent advantages as a phototherapeutic light source compared with lasers or even laser diodes: they are true solid-sate semiconductor chips requiring neither filaments nor flashlamps; they are extremely efficient, needing a small electrical current to produce a lot of light; they can be mounted in large planar arrays, enabling hands-free, clinician non-intensive treatment; finally they are much less expensive than laser diodes, thus offering comparatively inexpensive systems which can still deliver a valid clinical effect, athermally and atraumatically. Although LEDs are not coherent and thus cannot offer a fully collimated monochromatic beam such as that produced by a laser diode, the new generation LEDs are quasimonochromatic, meaning that a very large percentage of the emitted photons is at the rated wavelength, even though the photons are not in phase. This allows LEDs to deliver extremely narrow-band emission of a few nanometers either side of the rated wavelength, which is important when considering wavelength-specific targets.
In a clinical environment, LED LLLT can be administered by a trained nurse or therapist, freeing up the clinician for other duties. Systems can be highly mobile, allowing them to be easily moved between treatment rooms to maximize use. Power consumption is very low, and LED lifetimes are extremely long, with some thousands of hours being the norm. Well-designed treatment heads have adjustable hinged planar arrays to allow even irradiation of all contours found on a patient's body, from fairly flat areas such as the back or décolleté, curved areas such as the face and head, to small diameters such as the extremities.
830 nm as an ideal wavelength: The early LLLT literature in the late 80s and 90s pointed to the near-infrared wavelength of 830 nm from mostly gallium aluminum arsenide (GaAlAs) laser diode-based low level laser therapy (LLLT) systems as being extremely effective in pain attenuation and wound healing, 1–3) owing to the deep penetration capabilities of that wavelength into soft tissues, and even through bone. 4, 5) The development of the new generation of so-called “NASA LEDs” in the late 1990s added light-emitting diodes as a valid phototherapeutic light source to the clinical armamentarium, 6) and near-infrared LEDs were quickly shown to be effective in wound healing, establishing the efficacy of LED-LLLT in the New Millennium. 7)
The first law of photobiology states that there cannot be any reaction without absorption, so with the comparatively low incident intensities generated by LED-based systems, the wavelength becomes of extreme importance both for dictating the target chromophore, and mediating the depth in living tissue to which the wavelength will penetrate. From these considerations, 830 nm is an ideal wavelength for LLLT, having a number of proven cellular targets and also offering deep tissue penetration.
Representative 830 nm LED-LLLT Systems
Figure 1 shows two examples of well-accepted LED-LLLT systems, both of which were used in examples from the literature referenced herein and both of which have a variety of international regulatory approvals, including US FDA clearance for certain indications in the form of 510(k) approvals. The earlier system (Omnilux™, Radiancy, Israel) is designed to be mounted on a table or trolley, and the later system (HEALITE II™, Lutronic Corporation, South Korea) is a free-standing, mobile lockable castered unit. Both systems can be easily moved from room to room and will operate on standard mains voltage. Both systems offer three wavelengths, namely 415 nm visible blue, 633 nm visible red and 830 nm in the near infrared from interchangeable treatment heads.
Fig. 1:
Two representative high-quality LED-LLLT systems. (Above): The Omnilux™ system (Radiancy, Israel), first developed by Photo Therapeutics, UK, in 2002. This is a table- or trolley mounted system offering 3 wavelengths through interchangeable heads (415 nm, 633 nm & 830 nm). The articulated arm and head panels are held in place with manually unlocked and locked turn buttons. (Below): The HEALITE II™ system, commercially available in 2011 (Lutronic Corporation, South Korea). This is a free-standing mobile unit on lockable casters, offering the same 3 wavelengths as above with 2 monotherapy heads (633 nm and 830 nm) and 3 dual wavelength heads (830 nm/633nm, 830 nm/415 nm and 633 nm/415 nm). The hinges on the articulated arm are of the automatic “place and stay” type, requiring no loosening or tightening of clamps, as is the case with the hinges on the planar LED panels.
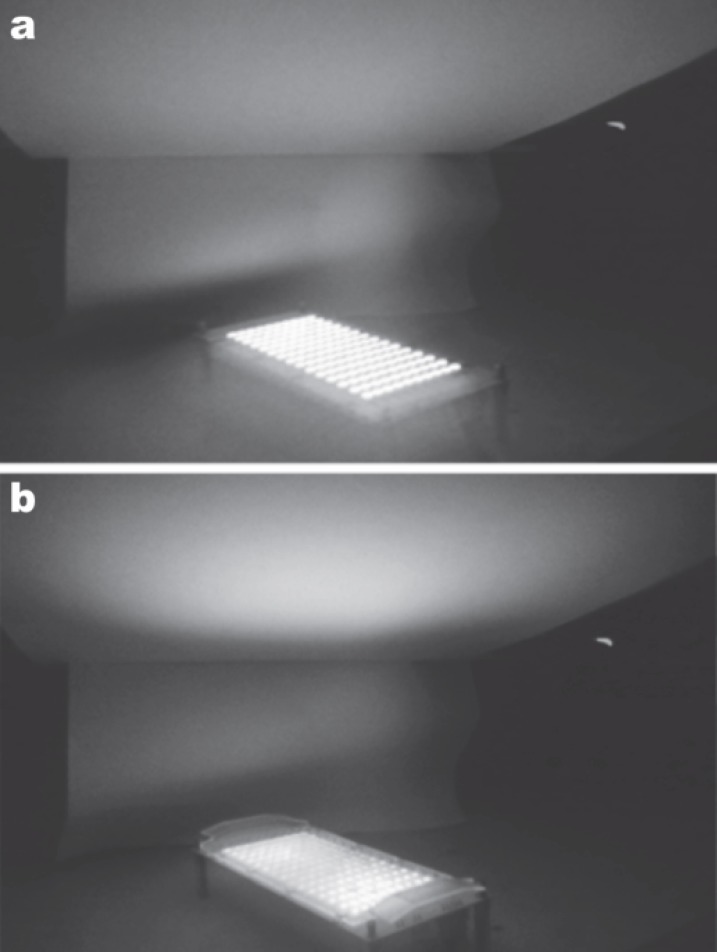
In both systems, the 830 nm head comprises 5 LED planar panels to allow adjustment of the panels to follow any body contour from an almost flat arrangement for treatment of the back to being tightly wrapped around an arm or a leg. The total active area of the head is larger in the later system, meaning that it can treat a larger target area per session. The recommended dosage in the systems for the 830 nm head is 60 J/cm2, delivered in 20 min for the earlier system (50 mW/cm2 irradiance) and in 10 min in the later system (100 mW/cm2). In the later system, the photon intensity at the target is further amplified by patented semi-collimation of each individual LED in an array, thus delivering more clinically efficient intensities for the same LED output power (Figure 2).
Fig. 2:
Effect of semicollimation on the delivered photon intensity with the same LED output power, visualized with an infrared camera. (a): 830 nm LED array without the optical lens array. Note the irradiation pattern on the target above the array, and the lateral scattering of energy which is not reaching the target. (b): Same 830 nm head with the patented optical lens array in place. There is significantly less lateral spread of the LED energy, and a much higher photon intensity at the target for the same output power, maximizing the clinical effect of the incident beam. (Courtesy of R & D Division, Lutronic Corporation, South Korea)
In both systems, the treatment head is recommended to be set up from 3 cm to 17 cm from the target tissue, and the systems are operated in a hands-free mode, making them easy to use and clinician or therapist non-intensive. Settings for the systems are easily controlled with push buttons and a display screen (earlier system), or an interactive LCD touch screen display (later system). Both systems will automatically recognize the treatment head being used and display that on the screen. The fluence and irradiation time settings are interlinked and microprocessor-controlled: the later system offers a range of intensities, and changing the intensity for any of the preset fluences automatically changes the irradiation time to ensure constant dosage, and vice versa. In the earlier system, the intensity remains constant but fluences can be manually set, automatically altering the treatment time. In both systems, LED emission will automatically shut off when the desired exposure time has been reached.
Indications of LED-LLLT
A large and continuously growing body of evidence now exists pointing to the safety and efficacy of LED-LLLT as a stand-alone modality for a variety of indications, including treatment of inflammatory acne 8, 9) in light-only skin rejuvenation 10, 11) and wound healing in a variety of recalcitrant wounds. 12) In the paper on skin rejuvenation by Lee and colleagues referenced above, 11) the histological, ultrastructural and immuno-histochemical assessments clearly demonstrated the existence of photoenhanced mechanisms associated with the wound healing process which underpinned the successful rejuvenation results. In their paper, Lee et al. compared the effects among LED treatment with 633 nm and 830 nm on their own and the combination of 830 nm and 633 nm with a control group. Although all the LED-treated groups in this study showed statistically significant gross and histological results compared with the controls, the 830 nm group proved superior to the other LED groups in all aspects including collagenesis, skin elasticity, expression of tissue inhibitors of matrix metalloproteinase (TIMP) 1 and subjective patient satisfaction. The importance of photoactivating the remodelling process to show a steady increase in the good results out beyond 12 weeks after the final treatment session was also clearly demonstrated (Figure 3). Based on these and other corroborating data it was thus suggested by Kim and Calderhead that LED-LLLT, especially at 830 nm, was not only effective on its own but would have an even more interesting role as an adjuvant regime to any existing aesthetic intervention. 13)
Fig. 3:
Patient satisfaction curves (“excellent” only) compared for LED-LLLT applied in a blinded, controlled study for skin rejuvenation with 633 nm alone, 633 nm + 830 nm combined and 830 nm on its own, showing the numbers of patients who rated their improvement as excellent on a 5-scale rating. The first set of columns represents the findings immediately after the 8th of 8 sessions, twice per week for 4 weeks. The 2nd, 3rd and 4th sets of columns are the findings at post-treatment weeks 4, 6 and 12 respectively. At all stages, LED phototherapy with 830 nm produced superior satisfaction, and faster. The increase in patient satisfaction over the 12-week post-treatment period is interesting, considering that no other adjunctive skin care was allowed during this follow-up period, suggesting that gradual improvement in the results was achieved through the continued tissue remodeling activity as part of the LED-mediated wound healing process. (Graph based on data adapted from Lee et al., Ref 11).
Basic Mechanism of Phototherapy: Briefly, phototherapy is based on the direct transfer of incident photon energy between the incoming photons and the cellular energy pool resulting in a viable clinical reaction, but without heat or damage. As Karu has stated, the basic mechanism for phototherapy involves absorption of the incident photon energy (photoreception), transduction and amplification of the signal within the target followed by a photoresponse. 14) The main therapeutic target for the visible light wavelengths, 633 nm in particular, is the cytochrome c oxidase enzyme in the mitochondrial respiratory chain in the target cells, resulting in a photochemically-induced cascade leading to the production of adenosine triphosphate (ATP) and eventual cellular photoactivation. 15) Photoactivated cells can repair themselves or be repaired if damaged or compromised; if they have a function to perform, they will do it faster and better; and if there are not enough of them more will be recruited in or they will proliferate. In the case of near-infrared energy such as 830 nm the mechanism is photophysical rather than photochemical and the target is different, the latter being elements within the cellular membrane rather than direct activation of cellular organelles. However the end result is the same, namely photoactivated cells. The mechanism for near-IR energy is schematically summarized in Figure 4, together with a summary of the main skin cells targeted by 830 nm and how they react when photoactivated. 13)
Fig. 4:
Schematic illustration of the processes involved in irradiation of skin with near-infrared light (left column) and a summation of the target cells and their specific photoreactions (boxes on the right). Note the action, interaction/cross-talk between cells and their activities (arrows) [Kim and Calderhead, Ref 13].
Post-Procedural Application of 830 nm LED-LLLT: In the paper referenced above, Kim and Calderhead stated in their conclusion that; “… the combination of appropriate LED phototherapy as an adjunct to many other surgical or nonsurgical approaches where the architecture of the patient's skin has been altered will almost certainly provide the clinician with even better results with less patient downtime, in a shorter healing period, and with excellent prophylaxis against obtrusive scar formation.” 13) This statement is based on the two main areas of action of 830 nm LLLT which have been well-demonstrated in the literature: all aspects of wound healing, and pain attenuation. Under the wound healing aspect, 830 nm LED-LLLT can help to control the erythematous and edematous aspects of the all-important inflammatory stage in addition to accelerating regression of any hematoma formation, and post-procedural pain and discomfort are rapidly attenuated.
Why is inflammation so important to the adjunctive application of 830 nm LED-LLLT in all aesthetic procedures? Inflammation is often regarded as an enemy. However, it is only when inflammation is uncontrolled that it becomes a real enemy, otherwise it is a necessary evil because without the effects associated with the inflammatory phase cells, such as fibroblast growth factor synthesized by macrophages,16) the proliferation phase may be delayed or incom plete. It is understood that the inflammatory stage cells must be allowed to complete their cycle, as mast cells, macrophages and neutrophils express growth factors in addition to performing their own functions, and both growth factor release and function performance are enhanced with near-infrared (near-IR) light energy. 17–19) In the case of macrophages irradiated with near-IR energy compared with unirradiated controls, phagocytic activity was significantly enhanced and the expression of fibroblast growth factor (FGF) was greater than 20-fold that of unirradiated controls. 18) A single treat ment of normal skin with 830 nm LED-LLLT compared with unirradiated controls demonstrated rapid photomediated degranulation of mast cells at 48 hr, accompanied by an ultrastructurally-demonstrated inflammatory response with the appearance of significantly greater numbers of mast cells, macrophages and neutrophils even though the skin was uninjured and appeared externally completely normal to the naked eye with no visible inflammation or erythema.17) In other words, the 830 nm LED-LLLT had started off the wound healing process, but without any actual wounding of the target tissue. As for the proliferative phase, 830 nm LED-LLLT has also been shown to stimulate fibroblast proliferation and activity in vivo compared with unirradiated controls (as yet unpublished data, personal communication, Prof. YM Park, Seoul National University, Seoul, South Korea:), and to rejuvenate photodamaged fibroblasts as clearly shown in a split-face ultrastructural assay comparing unirradiated skin with 830 nm LED-LLLT irradiated skin in vivo. 11) For the remodelling phase, near-IR LLLT supported the transformation from fibroblasts to myofibroblasts, resulting in much better remodeling of new tissues following experimental damage which was simulating a surgical procedure. 20) The tensile strength of the well-remodeled irradiated wounded tissues was greater even though the bulk of the unir-radiated tissues was larger, making them appear stronger.
Clinical Experience with 830 nm LED-LLLT
The following is a selection of examples from the peer-reviewed literature of the application of 830 nm LED-LLLT as adjunctive to some clinical procedure; in accelerated wound healing; in control of inflammation, infection and pain as adverse side effects following a procedure; and in prophylaxis against hypertrophic scarring.
Post-surgery: Upper eyelid blepharoplasty, with or without CO2 laser assistance, results in edema and erythema caused by the inflammatory process, and hematoma formation. In a split-face study, Trelles and colleagues demonstrated that the application of LED-LLLT significantly accelerated resolution of these postoperative sequelae in the early stages after the operation, although the long-term result was not significantly better (Figure 5). 21) Although red LED-LLLT at 633 nm was applied in this study because at this time the 830 nm head had not been developed, from more recent results the 830 nm wavelength is even more effective. 11, 13)
Fig. 5:
Upper and lower blepharoplasty and periocular resurfacing in a 48-year-old female. a: Baseline findings. Surgery and laser treatment (combined Er:YAG/CO2 laser) was performed bilaterally, but only the right side was treated postoperatively with LED-LLLT. b: 72 hr post-operative findings (2 LED sessions. Omnilux system, 633 nm, 126 J/cm2). The LED-treated side at first glance appears worse than the untreated side, but if the position of the upper eyelids is noted vis-à-vis the pupils, the left eye is more swollen than the right: this is LED-LLLT speeding up and then peaking inflammation on the irradiated side. c: Two weeks postop, and after a further 3 LED sessions the right side is looking much better than the left, more or less normal. d: At 2 weeks after the 8th and final treatment, 6 weeks post-baseline, there is not much difference between the two sides although slight erythema still exists on the left (LED unirradiated) eyelid. (Photography and data courtesy of Mario A Trelles MD PhD, Cambrils, Spain)
Post-ablative resurfacing: Trelles subsequently tri-alled 830 nm LED-LLLT in a split-face pilot study on LED phototherapy adjunctive to full-face ablative laser resurfacing with the combined Er:YAG and CO2 lasers (Unpublished data, personal communication from MA Trelles MD PhD). Figure 6 shows a patient at 72 hr post-procedure with one side of her face which had been treated with 830 nm LED-LLLT and the other covered. The LED-treated side showed reepithelization had already started compared with the total covering of crusting and oozing on the non-LED treated side (Figure 5a): at that stage the patient had received 2 LED-LLLT sessions (830 nm, 60 J/cm2, immediately and 24 hr post-treatment) and was preparing for her 3rd session. At 6 weeks after the procedure, 3 weeks after the final and 7th LED session, the LED untreated side was still erythematous and small areas of crusting had persisted, whereas the LED-treated side was more or less normal (Figure 5b). Based on the good results from the pilot study, Trelles and colleagues designed a controlled study in 60 patients on the use of 830 nm LED-LLLT adjunctive to full-face ablative laser resurfacing. 22) Thirty patients received a course of LED-LLLT immediately after, 24 hr and 72 hr after treatment (60 J/cm2), and then twice weekly for another 2 weeks with the remaining 30 patients as unirradiated controls. Groups were compared for healing time and postoperative sequelae including erythema, pain, bruising and edema. In all aspects the LED-treated group was significantly superior to the controls group (Figure 7), and at 6 months after treatment, although a significant improvement compared with baseline was seen in both groups, an independent panel of assessors judged that the overall skin condition in the LED group was better than the control group based on the clinical photography. Figure 8 illustrates the speedy recovery from full-face Er:YAG/CO2 aggressive ablative resurfacing assisted by 7 sessions of adjunctive 830 nm LED-LLLT in a case of very severe photoaging brought on by long-term exposure to the sun on the ski slopes (Figure 8a). Full healing with no erythema and an excellent result was seen only 6 weeks post-procedure, showing the potential for LED-LLLT to accelerate resolution of the postoperative sequelae, speed up the healing and to enhance the final result (Figure 8b).
Fig. 6:
Full face ablative resurfacing in a 68-year-old female with a split-face comparison of LED-treated and untreated sides. Following full face laser ablation, the left side of the face was treated with 830 nm LED-LLLT (Omnilux, 60 J/cm2) and the right side was covered as an unirradiated control. a: 72 hours after treatment, crusting and oozing are seen on both sides, but on the left side a clear area of reepithelization can be seen on the check with some islands on the forehead (2 LED sessions delivered). None is seen on the LED-unirradiated side. b: At 6 weeks postirradiation and 3 weeks after the 7th and final LED session, the right side is still erythematous and some crusting is still seen on the forehead, whereas the LED-irradiated side is fully healed with normal skin texture. (Photography and data courtesy of Mario A Trelles MD PhD, Cambrils, Spain)
Fig. 7:
Graphical comparison of speed of wound healing and extent of the post-ablative sequelae at 72 hr postirradiation compared between the LED-irradiated and unirradiated groups, 30 patients per group. The LED group was significantly better in all aspects. The LED-LLLT system used was the Omnilux system, 830 nm, 60 J/cm2. (Based on data from Trelles et al., Reference 22)
Fig. 8:
Full-face ablative laser resurfacing in a 58-year old female plus post-procedural LED-LLLT. a: Baseline findings showing severe photoageing with dyschromia, and deep lines and wrinkles due to excessive solar exposure during skiing. b: Result only 6 weeks after Er:YAG/CO2 laser ablation followed by 7 sessions of LED-LLLT Omnilux, 830 nm, 60 J/cm2). No erythema or edema can be seen with an excellent overall result. (Photography and data courtesy of Mario A Trelles MD PhD, Cambrils, Spain.)
Hematoma control: Unsightly hematoma formation, or bruising, frequently occurs after any procedure where tissues are incised and manipulated, and purpura is another form of bruising associated with pulsed dye laser treatment of blood vessels. LED-LLLT has been demonstrated to resolve bruising rapidly. Figure 9a shows a severe and extensive hematoma on the right leg of a 58-year-old male persisting at 2 weeks following full hip replacement surgery. Three treatment sessions with LED-LLLT were given and Figure 9b shows the very good result one week after Figure 9a. One of the putative mechanisms behind the efficacy of 830 nm LLLT in the resolution of hematoma is the upregulation of prostaglandin (PG)-I2, or prostacyclin, a very powerful platelet anti-aggregant. A study in the Keio Journal of Medicine used an immunoassay to detect levels of PG-I2 in 830 nm-irradiated endothelial cells, which showed significantly high levels compared with unirradiated controls. 23) In another study on human hematoma in vivo, significantly higher levels of PG-I2 were detected following LLLT with 830 nm. 24) It has also been shown that 830 nm LLLT had a direct fibrolytic action on the fibrin mesh associated with hematoma formation and erythrocyte retention, coupled with increased blood flow and enhanced macrophage activity. 25)
Fig. 9:
Large hematoma 2 weeks post-hip replacement before (a) and 1 week with 3 830 nm LED-LLLT sessions (Omnilux, 60 J/cm2) (b). Clinical photography courtesy of RG Calderhead PhD FRSM, South Korea
Post-Peel Dermatitis: When adverse side effects occur after any aesthetic procedure, the use of 830 nm LED-LLLT becomes even more important to control the adverse events and ensure the best possible result. Figure 10 shows the course of irritant contact dermatitis (ICD) on the face of a 26-year-old female, following application of an alpha-hydroxy acid peel. She had been treated with corticosteroids for 2 weeks but no effect was seen. Her quality of life was very poor with pruritis and pain, and she was unwilling to go to work or even to go shopping. She received 3 sessions of 830 nm LED-LLLT, 3 days apart, and the ICD and associated inflammation and irritation resolved. 13)
Fig. 10:
a: Treatment-resistant irritant contact dermatitis in a 26-year-old female following an alpha hydroxy acid peel for inflammatory acne. She was in pain with a very poor quality of life and did not want to go to work. b: She was treated 3 times with 830 nm HEALITE (60 J/cm2), 3 days apart, and this is the result 10 days after baseline. (From Reference 13, used with permission of the publishers. Courtesy of WS Kim MD PhD, South Korea)
In an animal model for LED-LLLT control of inflammation, carrageenan was used to induce inflammation in rats with the pre-treatment histological findings seen in Figure 11a. A control group was handled identically to the treated group, with the LED system turned on but not activated. After 2 sessions of 830 nm LED-LLLT, 2 days apart, all signs of inflammatory infiltration had disappeared leaving completely normal epidermal and dermal architecture (Figure 11b), compared with the sham-irradiated control animals in whom no change was seen in the inflammatory reaction. (As yet unpublished data, courtesy of Professor Won-Serk Kim MD PhD, Department of Dermatology, Sungkyunkwan University, Seoul, South Korea). These findings corroborate earlier reports in the literature. In a series of 112 patients with atopic dermatitis reported by Morita et al. treated with near-IR LLLT, 26) significant improvement in both pruritus and eruption was seen in a large percentage of the patients. The immunohistochemical findings revealed that intercellular adhesion molecule (ICAM)-1 expression and major histocompatibility complex (MHC) class II antigen decreased in dermal and epidermal cells after the treatment. Barolet reported similar findings pointing to the usefulness of LED-LLLT in dermatological conditions. 27)
Fig. 11:
Effect of 830 nm LED-LLLT on carrageenan-induced inflammation in the rat model. (a): After induction, before LED irradiation. A severe inflammatory response has been induced with strong neutrophil infiltration seen in the dermis and inflammation-mediated disruption of the epidermal skin barrier function. (b): Immediately after 2 treatments with HEALITE 830 nm LED LLLT (60 J/cm2, 2 days apart). The inflammatory response has completely resolved, with completely normal-appearing epidermal and dermal architecture. (Hematoxylin and eosin staining, original magnification X20; scale bars as shown. Photomicrographs courtesy of WS Kim MD PhD, Seoul, South Korea)
Ischemic ulcer post-filler: The use of fillers to remodel atrophic scars or depressed areas of the face has become very popular, but is not without some potential adverse side effects, such as tissue ischemia caused by excessive or poorly-placed filler product. A post-filler ischemic ulcer with severe inflammation, pain and infection is seen in Figure 12a on the forehead of a 53-year-old male. He was treated with 12 sessions of 830 nm LED-LLLT over 5 weeks, and Figure 12b shows the excellent result 1 week after the final treatment session. The pain was attenuated completely after the third session, and after the complete treatment protocol, all signs of necrotic ulceration, infection and inflammation had resolved with excellent reepithelialization. 12)
Fig. 12:
a: Ischemic ulcerative necrosis following filler placement in a 52-year-old male with infection, pain and severe inflammation. He was treated with 12 sessions of HEALITE 830 nm LLLT (60 J/cm2/session) over 5 weeks, and the figure shows the result at 1 week after the final treatment session. There is still an area of atrophy, but reepithelialization is excellent with good skin texture. (From Reference 12, used with permission of the publishers. Courtesy of PK Min MD)
Severe sequelae post-lip tattoo: Lip tattoos have become very popular, but there can be major problems when they are performed in an illegal tattoo parlor, as was the case in the 54-year-old Korean lady seen in Figure 13a. 12) She ended up with severe pain, bacterial and viral infection with herpes simplex, severe inflammation and edema so that her lower face was badly swollen, and she had developed a mild case of Bell's palsy. Eleven 830 nm LED-LLLT sessions were given over 3 weeks, without any antiherpetic or antibiotic medical intervention, and the excellent result is seen in Figure 13b, showing that 830 nm LED-LLLT can not only control inflammation, edema and bacterial infection, but can also inactivate the viral component in herpes simplex and help redress the effects of mild Bell's palsy, although further confirmatory controlled studies are needed in appropriately-sized patient populations for the latter two indications. In this patient's case, no recurrence of either the herpes simplex or the neurogenic condition was seen in an 8-month follow-up.
Fig. 13:
a: Baseline findings in a 54-year-old lady who attended an illegal lip tattoo parlor. She has bacterial infection, severe edema affecting almost the entire lower third of the face, pain, herpes simplex infection and slight Bell's palsy on the right side of her face. b: The excellent result after 11 HEALITE sessions over 3 weeks (60 J/cm2/session). All problems have been completely resolved, including the edema so that the lower third of the face has returned to its normal size. (From reference 12, used with permission of the publishers. Courtesy of PK Min MD)
Prophylaxis against hypertrophic scarring: Thyroidectomies are comparatively more frequent in Korea than elsewhere due to an aggressive screening programme for thyroid cancer. The site of the thyroidectomy is highly prone to hypertrophic scar formation owing to the anatomical structure of the neck in that zone. A very recent controlled study showed that 830 nm LED LLLT at 60 J/cm2 (HEALITE, Lutronic, Goyang South Korea) significantly suppressed the formation of post-thyroidectomy hypertrophic scars in the treated group of 35 patients compared with an untreated group of 15 patients assessed with the Vancouver Scar Scale (p=0.007). 28) In addition, erythema and pigmentation scores assessed with a tristimulus color analyzer were significantly lower in the LED-LLLT-treated group (p=0.014, p=0.042, respectively).
Conclusions
Even in cases of unexpected and severe adverse events following any aesthetic or cosmetic procedure, swift application of 830 nm LED-LLLT at an energy density of around 60 J/cm2 per session, as seen in most of the case reports and clinical trials referenced above, can rescue the situation and produce excellent results, very quickly. In the case of normal levels of sequelae, the results are even faster. The aesthetic and cosmetic surgeon should therefore consider adding an 830 nm LED-LLLT system to his or her office armamentarium, and using the system routinely after any procedure which has altered the epidermal or dermal architecture in any way, from mild noninvasive procedures, through IPL and ablative fractional laser rejuvenation, to frankly invasive surgical interventions. Erythema and edema as the result of inflammation are swiftly controlled, postoperative pain and discomfort are eliminated, infection both bacterial and viral is controlled and bruising is swiftly resolved. Patients in whom 830 nm LED-LLLT has been applied after any procedure will see better results, sooner, with extremely high satisfaction levels. However, not all LED systems are created equal, and potential users should ascertain that, in addition to having the requisite regulatory approvals for their region, the system of their choice has the correct 830 nm wavelength at an appropriately narrow band, and an appropriate irradiance to deliver the recommended dose of 60 J/cm2 in a reasonably short time.
Acknowledgements
Potential conflicts of interest:
RGC: Vice President, Medicoscientific Affairs, Lutronic Corporation
DBV: Member, Lutronic Corporation Scientific Board
WSK, TO & MAT: None to declare
References
- 1: Ohshiro T, Calderhead RG: Low Level Laser Therapy: A Practical Introduction. 1988. John Wiley & Sons, Chichester, UK. [Google Scholar]
- 2: Baxter G D: Therapeutic lasers. Theory and practice. 1994. Churchill Livingstone, Glasgow & London, UK [Google Scholar]
- 3: Tunér J, Hode L: Laser Therapy in Dentistry and Medicine. 1996. Prima Books, Grängesberg, Sweden. [Google Scholar]
- 4: Ohshiro T, Ogata H, Yoshida M, Tanaka Y, et al. : Penetration depths of 830 nm diode laser irradiation in the head and neck assessed using a radiographic phantom model and wavelength-specific imaging film. Laser Therapy, 1996; 8: 197-204. [Google Scholar]
- 5: Kim MN, Durduran T, Frangos S, Edlow BL, et al. : Noninvasive Measurement of Cerebral Blood Flow and Blood Oxygenation Using Near-Infrared and Diffuse Correlation Spectroscopies in Critically Brain-Injured Adults. Neurocrit Care, 2010; 12: 173-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6: Whelan HT, Houle JM, Whelan NT, Donohoe DL, et al. : The NASA Light-Emitting Diode Medical Program- Progress in Space Flight and Terrestrial Applications. Space Tech. & App. Int'l. Forum, 2000. 504: 37-43. [Google Scholar]
- 7: Whelan HT, Smits RL, Jr, Buchman EV, Whelan NT, et al. : Effect of NASA Light-Emitting Diode (LED) Irradiation on Wound Healing. Journal of Clinical Laser Medicine and Surgery, 2001. 19: 305-314. [DOI] [PubMed] [Google Scholar]
- 8: Goldberg DG, Russell B: Combination blue (415 nm) and red (633 nm) LED phototherapy in the treatment of mild to severe acne vulgaris. J Cos Laser Therapy, 2004. 8: 71-75. [DOI] [PubMed] [Google Scholar]
- 9: Lee SY, You CE, Park MY: Blue and red light combination LED phototherapy for acne vulgaris in patients with skin phototype IV. Lasers Surg Med, 2007. 39: 180-188. [DOI] [PubMed] [Google Scholar]
- 10: Goldberg DJ, Amin S, Russell BA, Phelps R, et al. : Combined 633-nm and 830-nm led treatment of photoaging skin. J Drugs Dermatol, 2006. 5: 748-753. [PubMed] [Google Scholar]
- 11: Lee SY, Park KH, Choi JW, Kwon JK, et al. : A prospective, randomized, placebo-controlled, double-blinded, and split-face clinical study on LED phototherapy for skin rejuvenation: Clinical, profilometric, histologic, ultrastructural, and biochemical evaluations and comparison of three different treatment settings. J Photochem Photobiol (B), 2007. 88: 51-67. [DOI] [PubMed] [Google Scholar]
- 12: Min PK, Goo BCL: 830 nm light-emitting diode low level light therapy (LED-LLLT) enhances wound healing: a preliminary study. Laser Ther, 2013; 22: 43-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13: Kim WS, Calderhead RG: Is light-emitting diode low level light therapy (LED-LLLT) really effective? Laser Therapy, 2011; 20: 205-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14: Karu T: Primary and secondary mechanisms of action of visible to near-IR radiation on cells. J Photochem Photobiol B, 1999. 49: 1-17. [DOI] [PubMed] [Google Scholar]
- 15: Karu T: Identification of the photoreceptors. In: Ten Lectures on Basic Science of Laser Phototherapy. 2007, Prima Books AB, Grangesberg, Sweden. [Google Scholar]
- 16: Koh TJ, DiPietro LA: Inflammation and wound healing: the role of the macrophage. Expert Rev Mol Med, 2011. 11:13 e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17: Calderhead RG, Kubota J, Trelles MA, Ohshiro T: One mechanism behind LED phototherapy for wound healing and skin rejuvenation: key role of the mast cell. Laser Therapy, 2008;17: 141-148. [Google Scholar]
- 18: Young S, Bolton P, Dyson M, Harvey W, Diamantopoulos C: Macrophage responsiveness to light therapy. Lasers Surg Med, 1989; 9: 497-505. [DOI] [PubMed] [Google Scholar]
- 19: Osanai T, Shiroto C, Mikami Y, Kudou E, et al. : Measurement of GaAlAs diode laser action on phagocytic activity of human neutrophils as a possible therapeutic dosimetry determinant. Laser Therapy, 1990. 2: 123-134. [Google Scholar]
- 20: Enwemeka CS, Cohen-Kornberg E, Duswalt EP, Weber DM, Rodriguez IM: Biomechanical effects of three different periods of GaAs laser photostimulation on tenotomized tendons. Laser Therapy, 1994; 6: 181-188. [Google Scholar]
- 21: Trelles MA, Allones I: Red light-emitting diode (LED) therapy accelerates wound healing post-blepharoplasty and periocular laser ablative resurfacing. J Cosmet Laser Ther, 2006; 8: 39-42. [DOI] [PubMed] [Google Scholar]
- 22: Trelles MA, Allones I, Mayo E: Combined visible light and infrared light-emitting diode (LED) therapy enhances wound healing after laser ablative resurfacing of photodamaged facial skin. Med Laser App, 2006; 28: 165-175. [Google Scholar]
- 23: Fujino T: Plastic and Reconstructive Surgical Aspects of Low-reactive Level Laser Therapy (LLLT). In Ohshiro T, Calderhead RG. (Eds) Progress in Laser Therapy. 1991, John Wiley & Sons, Chichester, UK: pp 143-149. [Google Scholar]
- 24: Kiyoizumi T: Low level diode laser treatment for haematomata under grafted skin and its photobiological mechanism. Leio J Med, 1988; 37: 415-428. [DOI] [PubMed] [Google Scholar]
- 25: Fujino T, Kiyoizumi T, Kubota J, Ohshiro T: Clinical effect of the diode laser to improve the fair take of the grafted skin. Keio J Med, 1986: 35: 28-35. [DOI] [PubMed] [Google Scholar]
- 26: Morita H, Kohno J, Hori M, Kitano Y. (1993): Clinical application of low reactive level laser therapy (LLLT) for atopic dermatitis. Keio J Med, 42: 174-176. [DOI] [PubMed] [Google Scholar]
- 27: Barolet D. (2008): Light-emitting diodes (LEDs) in dermatology. Semin Cutan Med Surg, 27: 227-238. [DOI] [PubMed] [Google Scholar]
- 28: Park YJ, Kim SJ, Song HS, Kim SK, Lee JH, et al. : Prevention of thyroidectomy scars in Asian adults with low-level light therapy. Dermatol Surg, 2015. (Accepted, In press) [DOI] [PubMed] [Google Scholar]