Abstract
Resistin, one of the adipokines that has a cycteine-rich C-terminus, is considered to relate to the development of insulin resistance in rats. However, in cats, there is little knowledge regarding resistin. In this study, we cloned the feline resistin cDNA from adipose tissue by RT-PCR. The feline resistin clone contained an entire open reading frame encoding 107 amino acids that had 72.8%, 75.4%, 50.9% and 51.8% homology with bovine, human, mouse and rat homologues, respectively. In both subcutaneous and visceral adipose tissues, the transcription levels of feline resistin mRNA were significantly higher in obese cats than normal cats, and those of feline adiponectin mRNA were significantly lower in obese cats than normal cats. However, there was no difference in the expression of feline leptin between normal and obese cats. On the other hand, in both normal and obese cats, there were no significant differences in resistin, leptin and adiponectin mRNA levels between subcutaneous and visceral adipose tissues. In cats, the altered expression of resistin and adiponectin mRNA with obesity may contribute to the pathogenesis of insulin resistance and subsequent diabetes mellitus. In addition to feline adiponectin, the feline resistin cDNA clone obtained in this study will be useful for further investigation of the pathogenesis of obesity in cats.
Keywords: adipose tissue, feline, obesity, resistin
White adipose tissue is recognized not only as a passive fuel depot but also as an active endocrine organ that communicates with the brain and peripheral tissues by secreting adipokines [34]. Some 100 different adipokines have been characterized in humans and rodents [11]; these factors are involved in many biological systems including glucose homeostasis, inflammation and immunity, hemostasis, fluid balance, vascular biology, hematopoiesis, cell proliferation, angiogenesis and neurotrophic functions [28]. The major adipokines, such as leptin and adiponectin, which have been reported in companion animals, including cats [1, 15, 18, 20, 29], have important roles in the regulation of energy balance and metabolism. Leptin is the best characterized adipokine in domestic animals [28] and has roles in appetite suppression and energy expenditure increase by binding to its receptor in the hypothalamic satiety centers [8, 14]. Adiponectin has a major role in enhancing insulin sensitivity by stimulating the phosphorylation of AMP-activated protein kinase in insulin target organs, such as skeletal muscle and liver [16]. Another adipokine associated with the regulation of energy balance and metabolism, resistin, was relatively recently described in mouse [23, 33]. Resistin contains a C-terminal cysteine-rich sequence that is a common feature of the resistin family [33]. Although an increased blood resistin level is considered to contribute to the development of insulin resistance and metabolic derangements compatible with type 2 diabetes mellitus in rats [28], there is little knowledge about resistin in cats.
Production of adipokines from adipose tissue may be influenced by nutritional status [35]. In particular, obesity dysregulates adipokine secretion, which is generally detrimental to insulin action on peripheral tissues, such as muscle and liver [22]. In humans and rodents, the altered adipokine production with obesity has been implicated in the pathophysiology of diverse diseases, including diabetes mellitus, cardiovascular disease and cancer [28]. Obesity in cats has recently increased in association with changes in diet and breeding environment [31], and has been cited as a cause of developing insulin resistance [27]. Although obesity-related insulin resistance contributes to the pathogenesis of diabetes mellitus in humans, there are few reports describing the change of adipokines with obesity in cats [12]. In particular, to the best of our knowledge, the change in the expression of resistin with obesity has never been examined in cats, although it has been shown that increased resistin with obesity deleteriously affects glucose metabolism and insulin sensitivity in rodents [23].
In the present study, we sought to elucidate the expression of resistin in the cat adipose tissues. However, feline resistin had not been identified. Thus, we report the molecular cloning of the feline resistin gene. In addition, we evaluate the change in the transcription levels of resistin with obesity in subcutaneous and visceral adipose tissues derived from normal and obese cats. Together with the evaluation of feline resistin, we assess the transcription levels of leptin and adiponectin as major adipokines in the adipose tissue of cats.
MATERIALS AND METHODS
Animals and total RNA extraction from tissues: Fifteen mature healthy cats were used in this study. All cats were assessed as healthy based on the results of a physical examination, complete blood count and biochemical profile performed prior to the experiment. All procedures involving the cats were performed at Gifu University and were approved by the Animal Care and Use Committee for Animal Experimentation of Gifu University (approval number: 07050). According to the body condition score (BCS) on a nine-point scale, cats were divided into nine normal (2 males and 7 females, 3.1 ± 1.6 years old, 3.4 ± 0.6 kg body weight and BCS 4.4 ± 1.0) and 6 obese (3 males and 3 females, 5.1 ± 2.6 years old, 4.7 ± 0.5 kg body weight and BCS 7.8 ± 0.4). The subcutaneous and visceral adipose tissues were surgically collected under general anesthesia in 5 male cats kept at Gifu University. In 10 client-owned female cats, the subcutaneous and visceral adipose tissues were collected at the time of spay surgery. These samples were used for the quantitative transcription analysis of feline resistin, leptin and adiponectin mRNA. In addition, a part of the subcutaneous adipose tissues from normal cats was used for the molecular cloning of the feline resistin gene. Collected tissues were immediately submerged in RNAlater (Qiagen, Hilden, Germany) and stored at −30°C until use.
Total RNA samples were extracted from the collected tissues using a commercially available kit (SV Total RNA Isolation System, Promega Corp., Madison, WI, U.S.A.). A cDNA sample was synthesized from the total RNA sample using reverse transcriptase (ReverTra Dash, Toyobo, Osaka, Japan).
Cloning of feline resistin: Oligonucleotide primers to amplify feline resistin cDNA were designed on the basis of the sequences of human and bovine resistin (GenBank/EMBL/DDBJ under accession numbers NM020415 and NM183362; Table 1). The 5′ side of resistin was amplified using a primer pair of RETN-1 and RETN-2, and the 3′ side was amplified using a primer pair of fRES-F2 and RETN-5 for first PCR and RETN-3 and RETN-5 for nested PCR. Using these primer pair, feline resistin cDNA was amplified from the feline subcutaneous adipose tissue with a Taq polymerase (TAKARA Ex Taq, Takara Bio Inc., Otsu, Japan) according to the manufacturer’s instructions. The PCR amplifications consisted of pre-denaturing (94°C for 5 min), 35 cycles of denaturation (94°C for 0.5 min), annealing (64°C for 0.5 min) and extension (72°C for 0.5 min), followed by a final extension (7 min). The PCR products were purified from agarose gel using a commercially available kit (Wizard SV Gel and PCR Clean-Up System, Promega Corp.) and were sequenced using an ABI Prism 3100 Genetic Analyzer (Applied Biosystems, Foster City, CA, U.S.A.). In the present study, three independent clones of PCR products were sequenced to confirm no individual difference.
Table 1. Sequences of the oligonucleotide primers used for molecular cloning of feline resistin cDNA.
| Primers | Sequences (5′-3′) | |
|---|---|---|
| RETN-1 | Forward | TTAGCTGAGCCCACCGAGAGGC |
| RETN-2 | Reverse | GCTCCGGTCCAGTCCATGCCC |
| f RES-F2 | Forward | CGTCACCGCCTGCGCTTGT |
| RETN-3 | Forward | GTCGAGACCACATGCCACTG |
| RETN-5 | Reverse | TCCGGACCTGGAGCCGCCTC |
Quantitative transcription analysis of resistin, leptin and adiponectin mRNA in the visceral and subcutaneous adipose tissues of normal and obese cats: Transcription analyses of feline resistin, leptin and adiponectin mRNA in the adipose tissues of normal and obese cats were performed using quantitative real-time PCR (Thermal Cycler Dice Real Time System, Takara Bio Inc.). These analyses were performed using adipose tissues derived from nine normal and six obese cats, except for the analysis of leptin mRNA in obese cats, which was performed using tissues derived from four of the six obese cats. The transcription level of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA was used as an internal control. For quantitative real-time PCR of feline resistin mRNA, the oligonucleotide primer pair of RETN-R1 and RETN-R2 and the probe RETN-RPr were manufactured by QuantiProbe Design Software (Qiagen) on the basis of the sequences of feline resistin mRNA obtained by molecular cloning (Table 2). The oligonucleotide primers used to amplify feline leptin, adiponectin and GAPDH cDNA were designed on the basis of each feline sequence (GenBank/EMBL/DDBJ under accession numbers NM001009850, AJ639861 and AB038241, respectively). A standard curve-based method was employed for the quantification of resistin, leptin and adiponectin mRNA transcription. The PCR products were quantified by two-step real-time PCR (ABI Prism 7000 Real Time Sequence Detection System, Applied Biosystems) using QuantiTect Prove Master Mix (Qiagen) for resistin or one-step real-time PCR (One Step SYBR PrimeScript RT-PCR Kit II, Takara Bio Inc.) for leptin, adiponectin and GAPDH. Real-time PCR amplifications for resistin, leptin and adiponectin consisted of reverse transcription (95°C for 10 sec), 40 cycles of PCR reaction (95°C for 5 sec and 68°C for 30 sec) and dissociation (95°C for 15 sec, 60°C for 30 sec and 95°C for15 sec). The annealing of GAPDH mRNA was performed at 60°C. All samples were examined in duplicate, and the mean amounts of resistin, leptin and adiponectin mRNA were calculated from the standard curve.
Table 2. Oligonucleotide sequences used as primers and probe for real-time RT-PCR.
| Name | Type | Sequences (5′-3′) | ||
|---|---|---|---|---|
| Resistin | RETN-R1 | Primer | Forward | CAAGTCTCTGTGTCCAGT |
| RETN-R2 | Primer | Reverse | GCCAAAGTTCCTTATTGTCTCC | |
| RETN-RPr | Probe | GCCATCCACGAGAAGA | ||
| Leptin | Lep-F3 | Primer | Forward | GACACCAAAACCCTCATCAAGAC |
| Lep-R3 | Primer | Reverse | ATCTTGGACAAACTCAGGACAGG | |
| Adiponectin | ADP-F3 | Primer | Forward | AAAGGAGAACCTGGAGAAAGTGC |
| ADP-R3 | Primer | Reverse | CATCGTAGTGGTTTTGCTGATTG | |
| GAPDH | GAPDH100-F | Primer | Forward | CTGGAGAAAGCTGCCAAA |
| GAPDH100-Rc | Primer | Reverse | TGTTAAAGTCGCAGGAGA |
Statistical analysis: Data of relative expression are represented as means ± standard deviation (S.D.) The transcription levels of resistin, leptin and adiponectin mRNA in each subcutaneous and visceral adipose tissues were compared between normal and obese cats using Student’s t-test. The transcription level of resistin, leptin and adiponectin mRNA in normal and obese cats was compared between subcutaneous and visceral adipose tissues using a paired t-test. All analyses were performed using Excel 2010 (Microsoft, Redmond, WA, U.S.A.) with the add-in software Statcel 3 (OMS Publishing, Saitama, Japan). Statistical significance was defined as P<0.05.
RESULTS
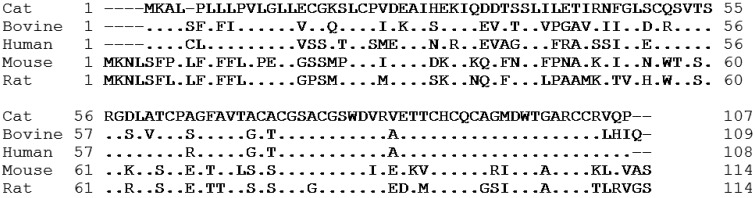
Molecular cloning of feline resistin: The full-length sequence of feline resistin cDNA was determined by combining the sequences of the overlapping 5′, central and 3′ DNA fragments obtained in this study. Feline resistin cDNA was 364 bp long and contained an entire open reading frame of 324 bp encoding 107 amino acid residues (GenBank/EMBL/DDBJ under accession number LC064405; Fig. 1). The deduced amino acid sequence of feline resistin cDNA cloned in this study was shown to have 72.8%, 75.4%, 50.9% and 51.8% similarity with those of its bovine, human, mouse and rat counterparts, respectively (Fig. 2). The C-terminal cysteine-rich sequence, which is a feature of the resistin family, was observed.
Fig. 1.
Nucleotide sequences and deduced amino acid sequences of feline resistin cDNA (GenBank/EMBL/DDBJ accession number LC064405). The feline resistin cDNA was 364 bp long and contained the entire open reading frame (capital letters) which was composed of 324 bp encoding 107 amino acid residues. The square area indicates the position of the initiation codon. The asterisk after the amino acid sequence shows the position of the termination codon.
Fig. 2.
Comparison of the deduced amino acid sequence of feline resistin mRNA with those of bovine, human, mouse and rat homologues. The deduced amino acid sequence of feline resistin mRNA cloned in this study was shown to have 72.8%, 75.4%, 50.9% and 51.8% homology with those of the bovine, human, mouse and rat counterparts, respectively. The dashes and dots indicate gap and homology, respectively. The C-terminal cysteine-rich sequence is a feature of the resistin family.
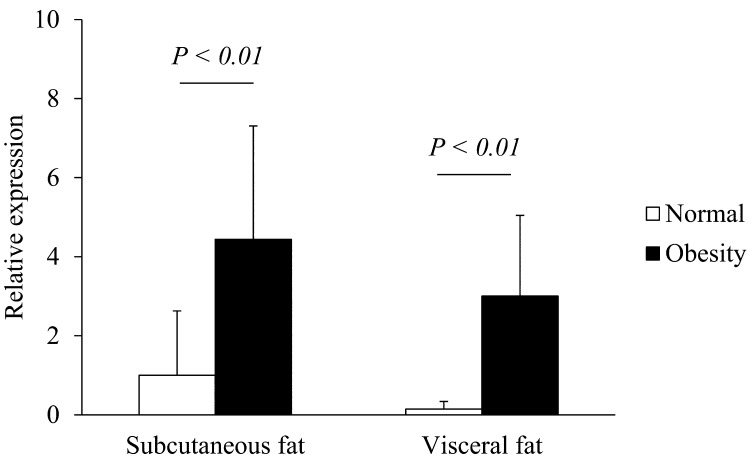
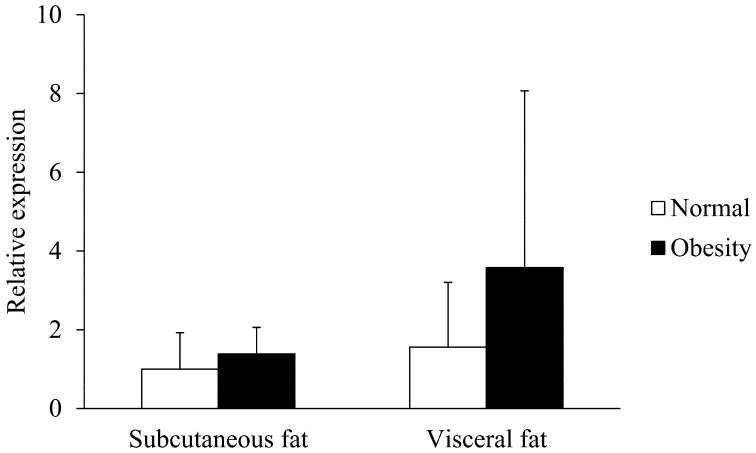
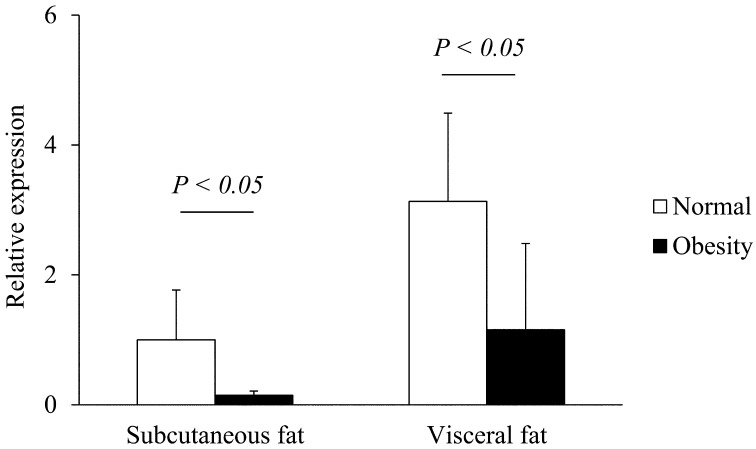
Transcription of resistin, leptin and adiponectin mRNA in subcutaneous and visceral adipose tissues of normal and obese cats: The expressions of feline resistin, leptin and adiponectin mRNA were detected in subcutaneous and visceral adipose tissues of all tested cats. In both subcutaneous and visceral adipose tissues, the resistin mRNA levels were significantly higher in obese cats than normal cats (P<0.01, Fig. 3). In both normal and obese cats, there were no significant differences in resistin mRNA levels between subcutaneous and visceral adipose tissues (Fig. 3). In both subcutaneous and visceral adipose tissues, there were no significant differences in the transcription levels of feline leptin mRNA between normal and obese cats (Fig. 4). In both normal and obese cats, there were no significant differences in leptin mRNA levels between subcutaneous and visceral adipose tissues (Fig. 4). In both subcutaneous and visceral adipose tissues, the adiponectin mRNA levels were significantly lower in obese cats than normal cats (P<0.05, Fig. 5). In both normal and obese cats, there were no significant differences in adiponectin mRNA levels between subcutaneous and visceral adipose tissues (Fig. 5).
Fig. 3.
Transcription levels of resistin mRNA in the subcutaneous and visceral adipose tissues of normal and obese cats. The transcription of GAPDH mRNA was used as an internal control. Data normalized to GAPDH levels are shown. The mean amount of resistin mRNA was calculated from a standard curve. The error bars represent the S.D.
Fig. 4.
Transcription levels of leptin mRNA in the subcutaneous and visceral adipose tissues of normal and obese cats. The transcription of GAPDH mRNA was used as an internal control. Data normalized to GAPDH levels are shown. The mean amount of leptin mRNA was calculated from a standard curve. The error bars represent the S.D.
Fig. 5.
Transcription levels of adiponectin mRNA in the subcutaneous and visceral adipose tissues of normal and obese cats. The transcription of GAPDH mRNA was used as an internal control. Data normalized to GAPDH levels are shown. The mean amount of adiponectin mRNA was calculated from a standard curve. The error bars represent the S.D.
DISCUSSION
The nucleotide sequence reported in this study contained the entire open reading frame of feline resistin cDNA. The cloned sequence of 107 amino acid residues encoded by feline resistin cDNA contained the C-terminal cysteine-rich domain that is a feature of the resistin family [33]. The amino acid sequences of feline resistin were identical to bovine (72.8%) and human (75.4%) resistin, but had a low homology with mouse (50.9%) and rat (51.8%) resistin.
In this study, quantitative real-time PCR showed that resistin mRNA was expressed in the subcutaneous and visceral adipose tissues of cats. In both subcutaneous and visceral adipose tissues, the transcription levels of resistin mRNA in obese cats were significantly higher than those in normal cats. Studies in humans and mouse reported that the expression of resistin mRNA in adipose tissue [30, 33] and the blood resistin concentration [4, 25] increased with obesity. Obesity is characterized by chronic, low-grade systemic inflammation [34] and induces the production of inflammatory cytokines, such as tumor necrosis factor alpha and interleukin-6. Increased inflammatory cytokines with obesity promote an increase in plasma resistin level in humans [37]. A study using skeletal muscle cells from L6 rats [26] demonstrated that resistin inhibited insulin-stimulated glucose uptake, presumably by decreasing the intrinsic activity of the cell membrane glucose transporters. Because the inhibition of glucose uptake implies the failure of insulin signaling, it is believed that hyper-resistinemia contributes to the development of insulin resistance and metabolic derangements compatible with type 2 diabetes mellitus [28]. Therefore, in cats, the obesity-related insulin resistance may be linked to the marked rise of the expression of resistin in adipose tissue. Although we could not analyze the relationship between obesity and resistin blood levels in this study, this relationship needs to be evaluated in cats.
There are reports indicating that the expression of resistin relates with diverse factors, such as the maturity and localization of the adipose tissue [6, 19]. A study in rats demonstrated that the expression of resistin was higher in visceral adipose tissue than in subcutaneous adipose tissue [3]. However, in our cats, there were substantial individual variations in the expression of resistin between subcutaneous and visceral adipose tissues in both normal and obese cats, which resulted in the lack of statistically significant differences.
There were no significant differences in the expression of leptin mRNA between normal and obese cats, in both subcutaneous and visceral adipose tissues. In humans and rodents, the tissue expression and blood level of leptin increased with obesity [21, 36]. Moreover, in cats, circulating leptin primarily reflects body fat mass [1, 5, 32], and weight loss is associated with a fall in peripheral blood leptin levels [15]. However, in the cats used in our study, the transcription level of leptin mRNA in the adipose tissue did not relate with the degree of obesity. In cats, because of considerable individual variability in the expression of leptin mRNA in adipose tissue, the change in mRNA expression may be consistent with the change in blood leptin level in the context of obesity. On the other hand, in both subcutaneous and visceral adipose tissues, the adiponectin mRNA level was significantly lower in obese cats than that in normal cats. One study demonstrated that the serum adiponectin concentration in cats decreases with obesity and increases with weight loss [15]. In humans and rodents, the tissue expression level and blood concentration of adiponectin also decreased with obesity, contrary to leptin [2, 10]. A study in humans had hypothesized that the increased tumor necrosis factor alpha levels, induced by the accumulation of visceral fat, led to decreased expression of adiponectin [7]. In cats, the expression of adiponectin in adipose tissue may be decreased by a mechanism similar to that in humans.
In the present study, in both normal and obese cats, there were no significant differences in the expression of leptin and adiponectin between subcutaneous and visceral adipose tissues. However, studies in humans [13, 17, 24] have demonstrated that the production of leptin and adiponectin was lower in visceral adipose tissue than in subcutaneous adipose tissue. Therefore, it is considered that, in humans, the preferential deposition of fat into visceral rather than subcutaneous deposits increases the risk of insulin resistance, atherosclerosis and diabetes mellitus [9]. However, this interpretation in humans may not apply to the distribution of adipose tissue with obesity in cats.
In conclusion, we cloned and sequenced feline resistin cDNA and investigated its transcription in the subcutaneous and visceral adipose tissues of both normal and obese cats. In cats, the altered transcription levels of resistin and adiponectin mRNA with obesity may contribute to the pathogenesis of insulin resistance and subsequent diabetes mellitus. The present result will provide basic information to clarify the association of resistin, leptin and adiponectin in the pathogenesis of obesity.
REFERENCES
- 1.Appleton D. J., Rand J. S., Sunvold G. D.2000. Plasma leptin concentrations in cats: reference range, effect of weight gain and relationship with adiposity as measured by dual energy X-ray absorptiometry. J. Feline Med. Surg. 2: 191–199. doi: 10.1053/jfms.2000.0103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arita Y., Kihara S., Ouchi N., Takahashi M., Maeda K., Miyagawa J., Hotta K., Shimomura I., Nakamura T., Miyaoka K., Kuriyama H., Nishida M., Yamashita S., Okubo K., Matsubara K., Muraguchi M., Ohmoto Y., Funahashi T., Matsuzawa Y.1999. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem. Biophys. Res. Commun. 257: 79–83. doi: 10.1006/bbrc.1999.0255 [DOI] [PubMed] [Google Scholar]
- 3.Atzmon G., Yang X. M., Muzumdar R., Ma X. H., Gabriely I., Barzilai N.2002. Differential gene expression between visceral and subcutaneous fat depots. Horm. Metab. Res. 34: 622–628. doi: 10.1055/s-2002-38250 [DOI] [PubMed] [Google Scholar]
- 4.Azuma K., Katsukawa F., Oguchi S., Murata M., Yamazaki H., Shimada A., Saruta T.2003. Correlation between serum resistin level and adiposity in obese individuals. Obes. Res. 11: 997–1001. doi: 10.1038/oby.2003.137 [DOI] [PubMed] [Google Scholar]
- 5.Backus R. C., Havel P. J., Gingerich R. L., Rogers Q. R.2000. Relationship between serum leptin immunoreactivity and body fat mass as estimated by use of a novel gas-phase Fourier transform infrared spectroscopy deuterium dilution method in cats. Am. J. Vet. Res. 61: 796–801. doi: 10.2460/ajvr.2000.61.796 [DOI] [PubMed] [Google Scholar]
- 6.Boucher J., Castan-Laurell I., Daviaud D., Guigné C., Buléon M., Carpéné C., Saulnier-Blache J. S., Valet P.2005. Adipokine expression profile in adipocytes of different mouse models of obesity. Horm. Metab. Res. 37: 761–767. doi: 10.1055/s-2005-921098 [DOI] [PubMed] [Google Scholar]
- 7.Bruun J. M., Lihn A. S., Verdich C., Pedersen S. B., Toubro S., Astrup A., Richelsen B.2003. Regulation of adiponectin by adipose tissue-derived cytokines: in vivo and in vitro investigations in humans. Am. J. Physiol. Endocrinol. Metab. 285: E527–E533. doi: 10.1152/ajpendo.00110.2003 [DOI] [PubMed] [Google Scholar]
- 8.Buff P. R., Dodds A. C., Morrison C. D., Whitley N. C., McFadin E. L., Daniel J. A., Djiane J., Keisler D. H.2002. Leptin in horses: tissue localization and relationship between peripheral concentrations of leptin and body condition. J. Anim. Sci. 80: 2942–2948. [DOI] [PubMed] [Google Scholar]
- 9.Cartwright M. J., Tchkonia T., Kirkland J. L.2007. Aging in adipocytes: potential impact of inherent, depot-specific mechanisms. Exp. Gerontol. 42: 463–471. doi: 10.1016/j.exger.2007.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Degawa-Yamauchi M., Moss K. A., Bovenkerk J. E., Shankar S. S., Morrison C. L., Lelliott C. J., Vidal-Puig A., Jones R., Considine R. V.2005. Regulation of adiponectin expression in human adipocytes: effects of adiposity, glucocorticoids, and tumor necrosis factor alpha. Obes. Res. 13: 662–669. doi: 10.1038/oby.2005.74 [DOI] [PubMed] [Google Scholar]
- 11.German A. J., Ryan V. H., German A. C., Wood I. S., Trayhurn P.2010. Obesity, its associated disorders and the role of inflammatory adipokines in companion animals. Vet. J. 185: 4–9. doi: 10.1016/j.tvjl.2010.04.004 [DOI] [PubMed] [Google Scholar]
- 12.Henson M. S., O’Brien T. D.2006. Feline models of type 2 diabetes mellitus. ILAR J. 47: 234–242. doi: 10.1093/ilar.47.3.234 [DOI] [PubMed] [Google Scholar]
- 13.Hernandez-Morante J. J., Milagro F. I., Larque E., Lujan J., Martinez J. A., Zamora S., Garaulet M.2007. Relationship among adiponectin, adiponectin gene expression and fatty acids composition in morbidly obese patients. Obes. Surg. 17: 516–524. doi: 10.1007/s11695-007-9090-6 [DOI] [PubMed] [Google Scholar]
- 14.Heshka J. T., Jones P. J.2001. A role for dietary fat in leptin receptor, OB-Rb, function. Life Sci. 69: 987–1003. doi: 10.1016/S0024-3205(01)01201-2 [DOI] [PubMed] [Google Scholar]
- 15.Hoenig M., Thomaseth K., Waldron M., Ferguson D. C.2007. Insulin sensitivity, fat distribution, and adipocytokine response to different diets in lean and obese cats before and after weight loss. Am. J. Physiol. Regul. Integr. Comp. Physiol. 292: R227–R234. doi: 10.1152/ajpregu.00313.2006 [DOI] [PubMed] [Google Scholar]
- 16.Hopkins T. A., Ouchi N., Shibata R., Walsh K.2007. Adiponectin actions in the cardiovascular system. Cardiovasc. Res. 74: 11–18. doi: 10.1016/j.cardiores.2006.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hube F., Lietz U., Igel M., Jensen P. B., Tornqvist H., Joost H. G., Hauner H.1996. Difference in leptin mRNA levels between omental and subcutaneous abdominal adipose tissue from obese humans. Horm. Metab. Res. 28: 690–693. doi: 10.1055/s-2007-979879 [DOI] [PubMed] [Google Scholar]
- 18.Ishioka K., Omachi A., Sagawa M., Shibata H., Honjoh T., Kimura K., Saito M.2006. Canine adiponectin: cDNA structure, mRNA expression in adipose tissues and reduced plasma levels in obesity. Res. Vet. Sci. 80: 127–132. doi: 10.1016/j.rvsc.2005.05.011 [DOI] [PubMed] [Google Scholar]
- 19.Janke J., Engeli S., Gorzelniak K., Luft F. C., Sharma A. M.2002. Resistin gene expression in human adipocytes is not related to insulin resistance. Obes. Res. 10: 1–5. doi: 10.1038/oby.2002.1 [DOI] [PubMed] [Google Scholar]
- 20.Jeusette I. C., Detilleux J., Shibata H., Saito M., Honjoh T., Delobel A., Istasse L., Diez M.2005. Effects of chronic obesity and weight loss on plasma ghrelin and leptin concentrations in dogs. Res. Vet. Sci. 79: 169–175. doi: 10.1016/j.rvsc.2004.11.012 [DOI] [PubMed] [Google Scholar]
- 21.Klein S., Coppack S. W., Mohamed-Ali V., Landt M.1996. Adipose tissue leptin production and plasma leptin kinetics in humans. Diabetes 45: 984–987. doi: 10.2337/diab.45.7.984 [DOI] [PubMed] [Google Scholar]
- 22.Lazar M. A.2006. The humoral side of insulin resistance. Nat. Med. 12: 43–44. doi: 10.1038/nm0106-43 [DOI] [PubMed] [Google Scholar]
- 23.Lazar M. A.2007. Resistin- and obesity-associated metabolic diseases. Horm. Metab. Res. 39: 710–716. doi: 10.1055/s-2007-985897 [DOI] [PubMed] [Google Scholar]
- 24.Lihn A. S., Bruun J. M., He G., Pedersen S. B., Jensen P. F., Richelsen B.2004. Lower expression of adiponectin mRNA in visceral adipose tissue in lean and obese subjects. Mol. Cell. Endocrinol. 219: 9–15. doi: 10.1016/j.mce.2004.03.002 [DOI] [PubMed] [Google Scholar]
- 25.Lu H. L., Wang H. W., Wen Y., Zhang M. X., Lin H. H.2006. Roles of adipocyte derived hormone adiponectin and resistin in insulin resistance of type 2 diabetes. World J. Gastroenterol. 12: 1747–1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moon B., Kwan J. J., Duddy N., Sweeney G., Begum N.2003. Resistin inhibits glucose uptake in L6 cells independently of changes in insulin signaling and GLUT4 translocation. Am. J. Physiol. Endocrinol. Metab. 285: E106–E115. doi: 10.1152/ajpendo.00457.2002 [DOI] [PubMed] [Google Scholar]
- 27.Prahl A., Guptill L., Glickman N. W., Tetrick M., Glickman L. T.2007. Time trends and risk factors for diabetes mellitus in cats presented to veterinary teaching hospitals. J. Feline Med. Surg. 9: 351–358. doi: 10.1016/j.jfms.2007.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Radin M. J., Sharkey L. C., Holycross B. J.2009. Adipokines: a review of biological and analytical principles and an update in dogs, cats, and horses. Vet. Clin. Pathol. 38: 136–156. doi: 10.1111/j.1939-165X.2009.00133.x [DOI] [PubMed] [Google Scholar]
- 29.Sagawa M. M., Nakadomo F., Honjoh T., Ishioka K., Saito M.2002. Correlation between plasma leptin concentration and body fat content in dogs. Am. J. Vet. Res. 63: 7–10. doi: 10.2460/AJVR.2002.63.7 [DOI] [PubMed] [Google Scholar]
- 30.Savage D. B., Sewter C. P., Klenk E. S., Segal D. G., Vidal-Puig A., Considine R. V., O’Rahilly S.2001. Resistin / Fizz3 expression in relation to obesity and peroxisome proliferator-activated receptor-gamma action in humans. Diabetes 50: 2199–2202. doi: 10.2337/diabetes.50.10.2199 [DOI] [PubMed] [Google Scholar]
- 31.Scarlett J. M., Donoghue S., Saidla J., Wills J.1994. Overweight cats: prevalence and risk factors. Int. J. Obes. Relat. Metab. Disord. 18Suppl 1: S22–S28. [PubMed] [Google Scholar]
- 32.Shibata H., Sasaki N., Honjoh T., Ohishi I., Takiguchi M., Ishioka K., Ahmed M., Soliman M., Kimura K., Saito M.2003. Feline leptin: immunogenic and biological activities of the recombinant protein, and its measurement by ELISA. J. Vet. Med. Sci. 65: 1207–1211. doi: 10.1292/jvms.65.1207 [DOI] [PubMed] [Google Scholar]
- 33.Steppan C. M., Bailey S. T., Bhat S., Brown E. J., Banerjee R. R., Wright C. M., Patel H. R., Ahima R. S., Lazar M. A.2001. The hormone resistin links obesity to diabetes. Nature 409: 307–312. doi: 10.1038/35053000 [DOI] [PubMed] [Google Scholar]
- 34.Trayhurn P.2005. Adipose tissue in obesity–an inflammatory issue. Endocrinology 146: 1003–1005. doi: 10.1210/en.2004-1597 [DOI] [PubMed] [Google Scholar]
- 35.Trujillo M. E., Scherer P. E.2006. Adipose tissue-derived factors: impact on health and disease. Endocr. Rev. 27: 762–778. doi: 10.1210/er.2006-0033 [DOI] [PubMed] [Google Scholar]
- 36.Turban S., Hainault I., Truccolo J., Andre J., Ferre P., Quignard-Boulange A., Guerre-Millo M.2002. Specific increase in leptin production in obese (falfa) rat adipose cells. Biochem. J. 362: 113–118. doi: 10.1042/bj3620113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yaturu S., Daberry R. P., Rains J., Jain S.2006. Resistin and adiponectin levels in subjects with coronary artery disease and type 2 diabetes. Cytokine 34: 219–223. doi: 10.1016/j.cyto.2006.05.005 [DOI] [PubMed] [Google Scholar]