Abstract
Purpose
We examined the effects of 3 months of intensive education (IE) after hospital discharge compared to conventional education (CE) on nutritional status and quality of diet and life among South Korean gastrectomy patients.
Methods
The study was conducted among 53 hospitalized gastrectomy in-patients (IE group, n = 28; CE group, n = 25) at Kyung Hee University Hospital at Gangdong. Baseline data were collected from electronic medical records and additional information was gathered via anthropometric measurements, assessment of nutritional status through a patient-generated, subjective global assessment (PG-SGA), diet assessment, and measures of self-efficacy and satisfaction with meals for 3 months following hospital discharge.
Results
Total PG-SGA scores were significantly higher in the CE group than in the IE group at 3-week post-discharge (5.2 in the IE group vs. 10.4 in the CE group, P < 0.001), with higher scores indicating a greater severity of malnutrition. Energy intake over the 3 months increased in both the IE group (from 1,390 to 1,726 kcal/day) and the CE group (from 1,227 to 1,540 kcal/day). At 3-week post-discharge, the IE group had significantly higher daily protein and fat intake (P < 0.05). Self-efficacy improved in each category (P < 0.001), except for 'difficulty eating adequate food'. When assessing satisfaction with meals, there was a difference in the 'satisfaction with the current meal size' (P < 0.001) and 'satisfaction with the menu content' (P < 0.001).
Conclusion
Nutritional status among gastrectomy patients in the IE group improved. Relative to the CE control, the IE group demonstrated improved self-efficacy and meal satisfaction 3-week post-discharge.
Keywords: Gastrectomy, Stomach neoplasms, Nutritional status, Nutrition therapy, Nutrition assessment
INTRODUCTION
According to the World Health Organization, worldwide, there were 14.1 million new cancer cases, 8.2 million cancer deaths and 32.6 million people living with cancer in 2012 [1]. Gastric cancer is the third major cause of cancer death among both sexes (723,000 deaths, 8.8% of the total) and half of these cases occur in Eastern Asia, including China and South Korea [2].
Cancer is the leading cause of death in South Korea [3]. Gastric cancer is the second most common cancer, with a prevalence rate of 15.4% among all cancers in 2011 [3,4]. The etiologic factors of gastric cancer in the Korean population include high Helicobacter pylori seroprevalence (59.6% in 2005) [5], cigarette smoking [6], and dietary factors, such as consumption of salty, spicy, and barbecued (charbroiled) animal products [7,8,9].
The main treatments for gastric cancer are surgery, chemotherapy, targeted therapy, and radiation therapy. Among these methods, surgery, often in combination with other treatments, offers the only real chance to cure gastric cancer, with consideration of the type and stage of disease [10]. However, after gastric resection surgery, the restrictive diet instituted to address postoperation concerns may accelerate malnutrition [11]. Approximately 40% of gastrectomy patients suffer from malnutrition, which acts as a major cause for development of complications and increased length of hospital stay, increasing medical expenses [11,12,13].
In several recent studies, the lack of nutritional education provided to postgastrectomy patients led to increased time required for patients to adjust to normal life, and further reduced patient quality of life, along with increasing the risk of malnutrition [14,15,16].
Previous studies conducted conventional nutrition education interventions for gastrectomy patients for a short period of time, often just the duration of the patient's hospital stay, or just before hospital discharge. Moreover, those interventions included a one-time event rather than continuous management [16].
To improve the nutritional intake among gastrectomy patients after surgery, a balanced diet during the hospitalization is required, as well as further intervention during recuperation at home. Therefore, we evaluated the effectiveness of a multiphase intensive nutrition intervention, implemented for 3 months following hospital discharge, on nutritional status and quality of diet and life among gastrectomy patients in South Korea.
METHODS
Subjects and study design
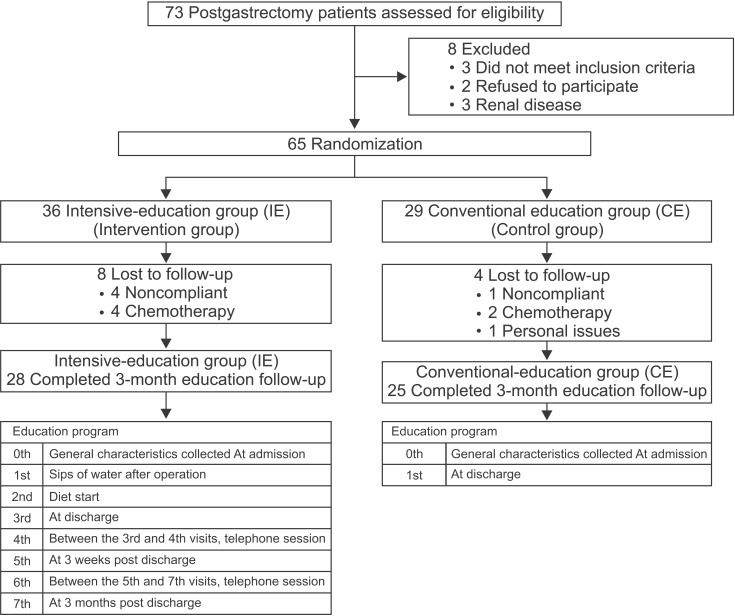
This prospective, controlled clinical trial was conducted at Kyung Hee University Hospital at Gangdong (Seoul, South Korea) from December 2011 to December 2012. Seventy-three inpatients with early gastric cancer underwent gastrectomy surgery (total or partial). Subjects were excluded from the study if they met the following exclusion criteria: diagnosis of any serious disease or condition (e.g., chronic renal failure, dialysis, uncontrolled diabetes mellitus, liver disease, or ascites), currently undergoing chemotherapy, or under 18 years old. Sixty-five eligible subjects were enrolled in this intervention. The subjects were classified into 2 groups: group 1 refers to patients who underwent surgery between December 2011 and May 2012 and received 7 sessions of nutrition education (intensive education group, IE); group 2 refers to patients who underwent surgery between June and December 2012 and received a one-time education session (conventional education group, CE). During the nutrition education period, 12 subjects dropped out for personal issues, such as starting chemotherapy, or were noncompliant during study follow-up. Finally, we analyzed complete data from 53 participants (28 in the IE group and 25 in the CE group). Fig. 1 shows a flowchart of participant enrollment. All participants provided written informed consent and the study was approved by the Institutional Review Board of Kyung Hee University Hospital at Gangdong, Seoul, South Korea (IRB number: KHNMC 2011-071).
Fig. 1. Participant flowchart of the study.
Data collection
General information and blood parameters
We collected information regarding general characteristics (age, length of hospital stay, fasting periods, medical history, type of operation, method of reconstruction, and health-related behaviors, such as smoking status [nonsmoker, ex-smoker, smoker], and alcohol consumption behaviors [nondrinker, social drinker, heavy drinker]) from electronic medical records (EMRs) or face-to-face interviews with participants.
For biochemical parameters, the values of total protein, albumin, hemoglobin, hematocrit, blood urea nitrogen, creatinine, and total lymphocyte count were collected from EMRs at 3-time points (at admission, immediately before discharge, and at 3 months post-discharge).
Anthropometric measurements
Weight (kg) and height (cm) were measured to the nearest 0.1 kg and 0.1 cm, respectively, using a body composition analyzer (Inbody 720, Biospace Company Ltd., Seoul, Korea). Body mass index (BMI) was calculated using this formula: BMI (kg/m2) = body weight (kg)/height (m)2. Triceps skinfold thickness (TSF) and midarm circumference (MAC) were measured. All anthropometry was performed on the nondominant arm. Equipment included calibrated Holtain calipers (Holtain Ltd., Croswell, Crymych, UK) and a fiberglass tape (seca 200, SECA, Hamburg, Germany). The midarm muscle circumference (MAMC) was calculated. Anthropometric data were collected at admission, upon discharge from hospital, and at 3 weeks and 3 months post-discharge in an outpatient setting.
Assessment of nutritional status
Patient-generated subjective global assessment (PG-SGA) is a tool that experienced clinicians use to assess patient nutritional status based on their medical history and physical symptoms [17]. PG-SGA measures includes 7 different categories, including weight change, food intake change, nutrition impact symptoms, activities and function, physical exam, disease and its relation to nutritional requirements and metabolic demand. The total score was computed by adding up the scores obtained for each category, and generally ranged from 0 to 35. A higher score indicates greater severity of malnutrition. The nutritional triage recommendations were as follows: 0–1, no intervention required at this time; 2–3, education required by dietitian; 4–8, intervention required by dietitian; ≥9, intensive intervention required by a dietitian. This measure was administered to patients 5 times over the study period, in face-to-face meetings with a registered dietitian.
Nutrition education process and dietary assessment
Each nutrition education session included 4 steps: assessment, diagnosis, intervention, and monitoring and evaluation. The assessment step began with an evaluation of nutritional status and identification of any problems, using the anthropometric data, blood analysis results and PG-SGA scores for each participant. In the diagnosis step, we identified and described a specific nutrition problem, nutrition etiology, and nutrition symptoms. To resolve or address nutritional problems for each patient, a clinical dietitian carried out the nutrition intervention providing advice, education, or the delivery of the food component of a specific diet or meal plan tailored to the patient's needs. The contents of nutrition education included ways to maintain a balanced diet and recommended foods and dietary habits. Moreover, education explored possible postgastrectomy symptoms, as well as recipes for healthy snacks and recommended menus for a quick surgical recovery. Lastly, the monitoring and evaluation step included nutrition monitoring, nutrition evaluation, nutrition care outcomes, and nutrition care indicators. This step maintains the relationship between the participants and the registered dietitian. Follow-up interviews were used to reinforce the intervention.
The nutritional intervention of the 2 groups varied in both frequency and content. The IE, multiphase nutrition education group had a total of 7 education interventions, including 5 face-to-face meetings with a dietitian (twice during hospitalization, at discharge from hospital, at 3 weeks post-discharge and at 3 months post-discharge) and 2 phone interventions (once between discharge from hospital and 3 weeks post-discharge, and once between 3 weeks and 3 months post-discharge). The clinical registered dietitian followed a standardized protocol for the sessions. Discussion of current weight and oral dietary intake were assessed by patient report and individualized nutritional counseling was conducted to improve dietary intake as required. Gastrointestinal symptoms such as reflux, bloating/wind, anorexia, early satiety, vomiting, and bowel habits were discussed, and advice to alleviate symptoms was provided. In addition to verbal advice, written advice and oral or enteral nutrition supplementation were provided as necessary.
The CE, single-phase nutrition education group underwent only a single education intervention upon discharge from the hospital before randomization. The CE group received education about general care and checkup without nutrition education.
Dietary intake data for both groups were collected using 3-day food records (2 weekdays, 1 weekend day) completed before patients had a scheduled visit to the hospital (the first scheduled visit was 3 weeks after hospital discharge, the second, 3 months after discharge). Patients were instructed to record their usual food intake, all food items and beverages, immediately upon consumption. All of the food items, cooking methods, and amounts consumed were confirmed by the dietitian using food models and measuring tools. Participant nutrient intake was assessed using a computer-aided nutritional analysis program, CAN Pro version 4.0 (The Korean Nutrition Society, 2010; Seoul, Korea).
Self-efficacy and satisfaction with intake
Dietary self-efficacy and satisfaction with intake was assessed in both groups at hospital discharge and 3 months after discharge. This 9-item questionnaire was developed for this study. Each item was ranked on a 5-point Likert scale from 1 (strongly disagree) to 5 (strongly agree). Possible total scores on this measure ranged from 9 to 45, with higher scores indicating greater self-efficacy and satisfaction with intake-related quality of life.
Statistical analysis
Results are expressed as mean ± standard deviation or as percentages. The Mann-Whitney U test was applied to determine differences between the 2 groups; to verify the existence of such differences, the Friedman Test for repeated measures complemented with multiple comparisons tests was computed. Statistical significance was defined as P < 0.05. Statistical analyses were performed using SAS ver. 9.2 (SAS Institute Inc., Cary, NC, USA).
RESULTS
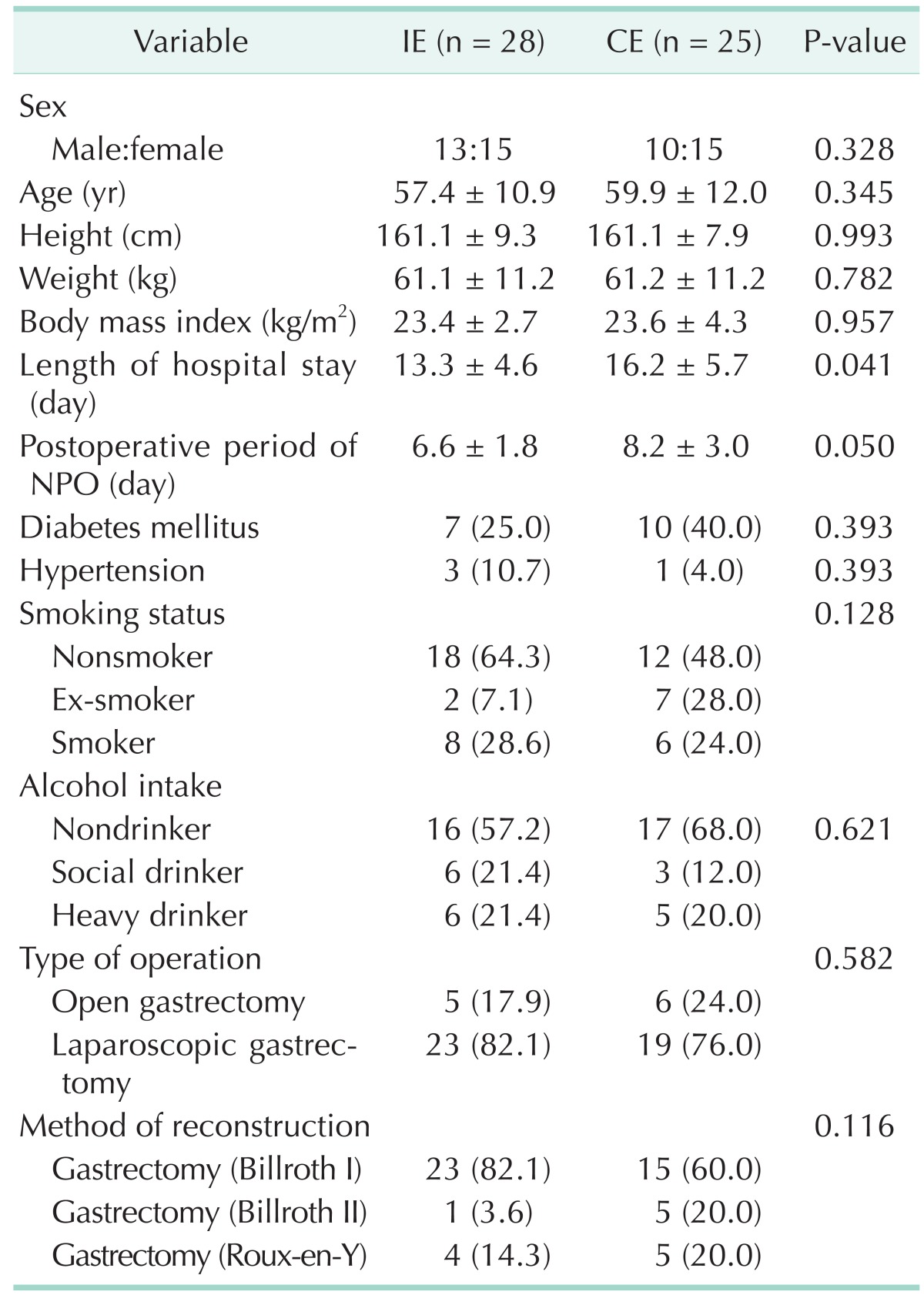
Mean age of the subjects was 57.4 years in the IE group and 59.9 years in the CE group (Table 1). The average hospital length of stay were 13.3 days and 16.2 days, respectively (P = 0.041). The mean length of nothing per oral (NPO) of the IE group was shorter than that of the CE group (6.6 days vs. 8.2 days, P = 0.050). No significant differences were found between the 2 groups regarding BMI, medical history, health-related behaviors, type of operation, or method of reconstruction. Most patients had a laparoscopic gastrectomy (79.2%) and Billroth I operation (71.7%) for reconstruction.
Table 1. General characteristics of gastrectomy patients at baseline.
Values are presented as mean ± standard deviation or number (%). Mann-Whitney test and chi-square test.
CE, conventional education group; IE, intensive education group; NPO, nil per os.
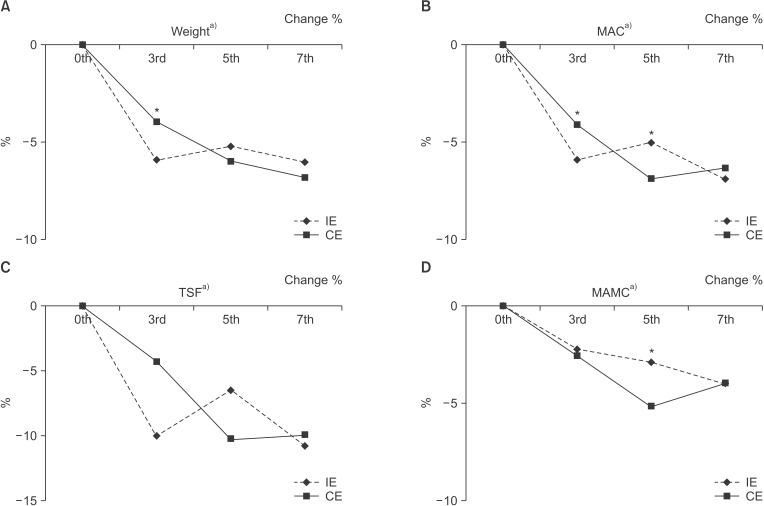
Comparisons of anthropometric variables between the IE and CE groups over time can be seen in Fig. 2. Weight, TSF, MAC, and MAMC significantly decreased over time (P < 0.001) regardless of group membership. At 3-week post-discharge, levels of MAC and MAMC decreased at a significantly higher rate among those in the CE group than among those in the IE group (P < 0.05).
Fig. 2. Comparison of anthropometric parameters between intensive education (IE) and conventional education (CE) groups: (A) weight, (B) midarm circumference (MAC), (C) triceps skinfold thickness (TSF), and (D) midarm muscle circumference (MAMC). *Significantly different at P < 0.05 between group by Mann-Whitney test. a)Significantly different at P < 0.001 within each group using the Friedman test. 0th, at admission (baseline); 3rd, at discharge; 5th, 3 weeks after discharge; 7th, 3 months after discharge.
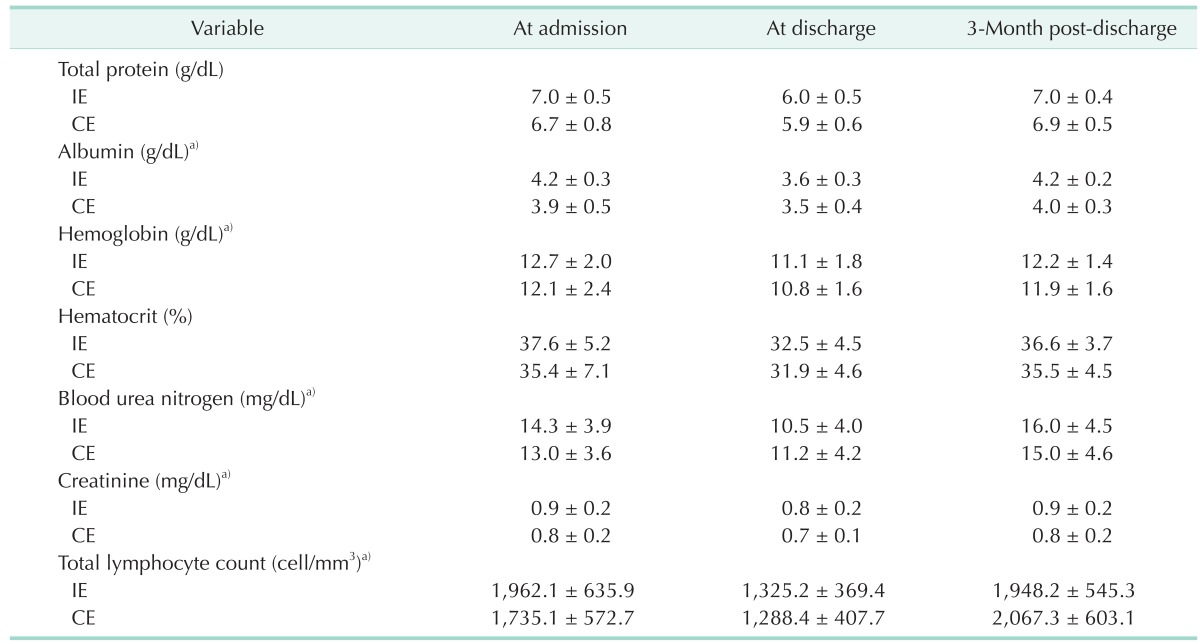
No significant differences were seen in blood parameters between the 2 groups (Table 2). Most blood parameters such as total protein, albumin, hemoglobin, blood urea nitrogen, creatinine, and total lymphocyte count decreased at discharge regardless of the groups. However, by 3-month post-discharge, these parameters returned to their admission levels (P < 0.001).
Table 2. Blood parameters (biochemical) between the intensive and conventional education groups.
Values are presented as mean ± standard deviation.
CE, conventional education each group; IE, intensive education group.
a)Significantly different at P < 0.001 within each group by the Friedman test.
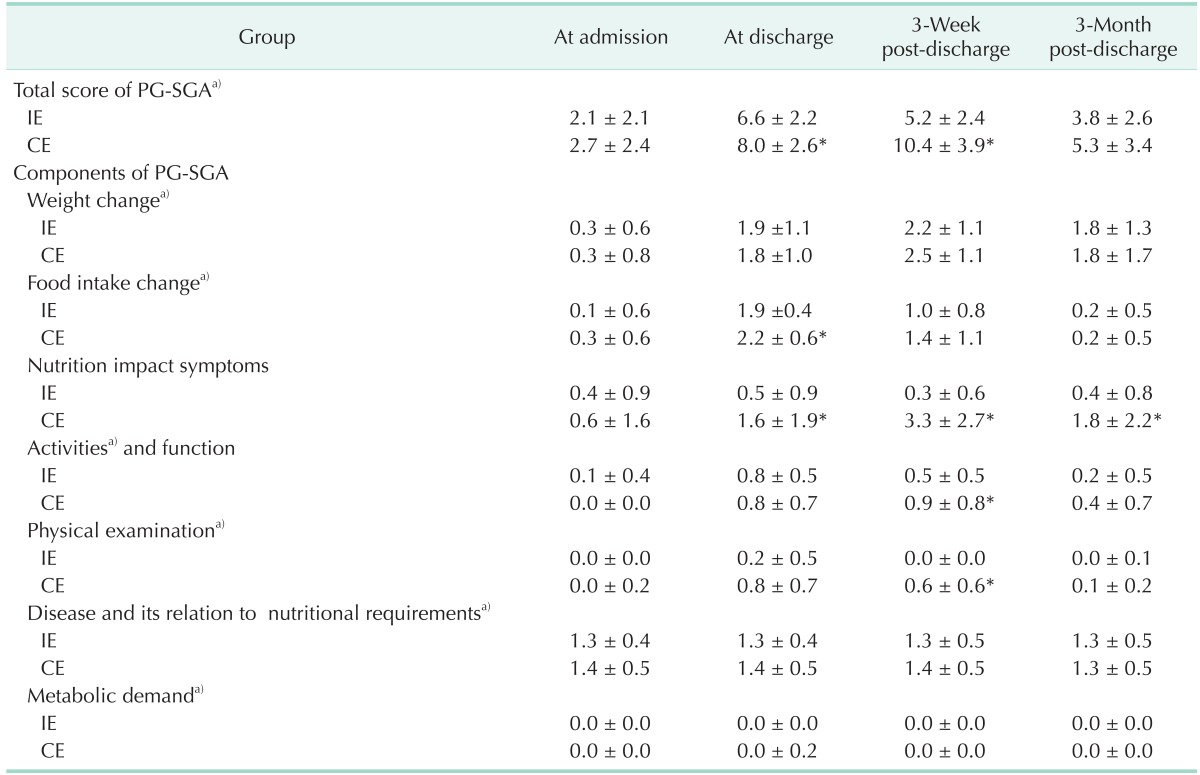
Nutritional statuses of the subjects are summarized in Table 3. According to the PG-SGA criteria, the total SGA scores (based on a 50-point maximum) were significantly higher in the CE group than in the IE group at discharge (6.6 in the IE group vs. 8.0 in the CE group, P < 0.001), and at 3-week post-discharge (5.2 in the IE group vs. 10.4 in the CE group, P < 0.001), with higher scores indicating greater severity of malnutrition. In addition, the IE group scored significantly lower on some PG-SGA subscales (i.e., food intake change at discharge [P < 0.05], activity and function, and physical examination at 3-week post-discharge [P < 0.05]). Of particular note, nutrition impact symptoms of PG-SGA after discharge (at discharge, 3-week and 3-month post-discharge) were significantly better in the IE group than in the CE group (P < 0.05).
Table 3. Total and component scores of PG-SGA between intensive and conventional education groups.
Values are presented as mean ± standard deviation.
CE, conventional education group; IE, intensive education group; PG-SGA, patient-generated subjective global assessment.
*Significantly different at P < 0.05, P < 0.001 between groups by the Mann-Whitney test. a)Significantly different at P < 0.001 within each group by the Friedman test.
PG-SGA is a tool that experienced clinicians use to assess patient nutritional status based on their medical history and physical symptoms [17]. PG-SGA measures includes 7 different categories. A higher score indicates greater severity of malnutrition. The nutritional triage recommendations were as follows: 0–1, no intervention required at this time; 2–3, intervention as indicated by symptom survey and lab values as appropriate; 4–8, intervention required by dietitian; ≥9, indicates a critical need for improved symptom management and/or intensive intervention required by a dietitian.
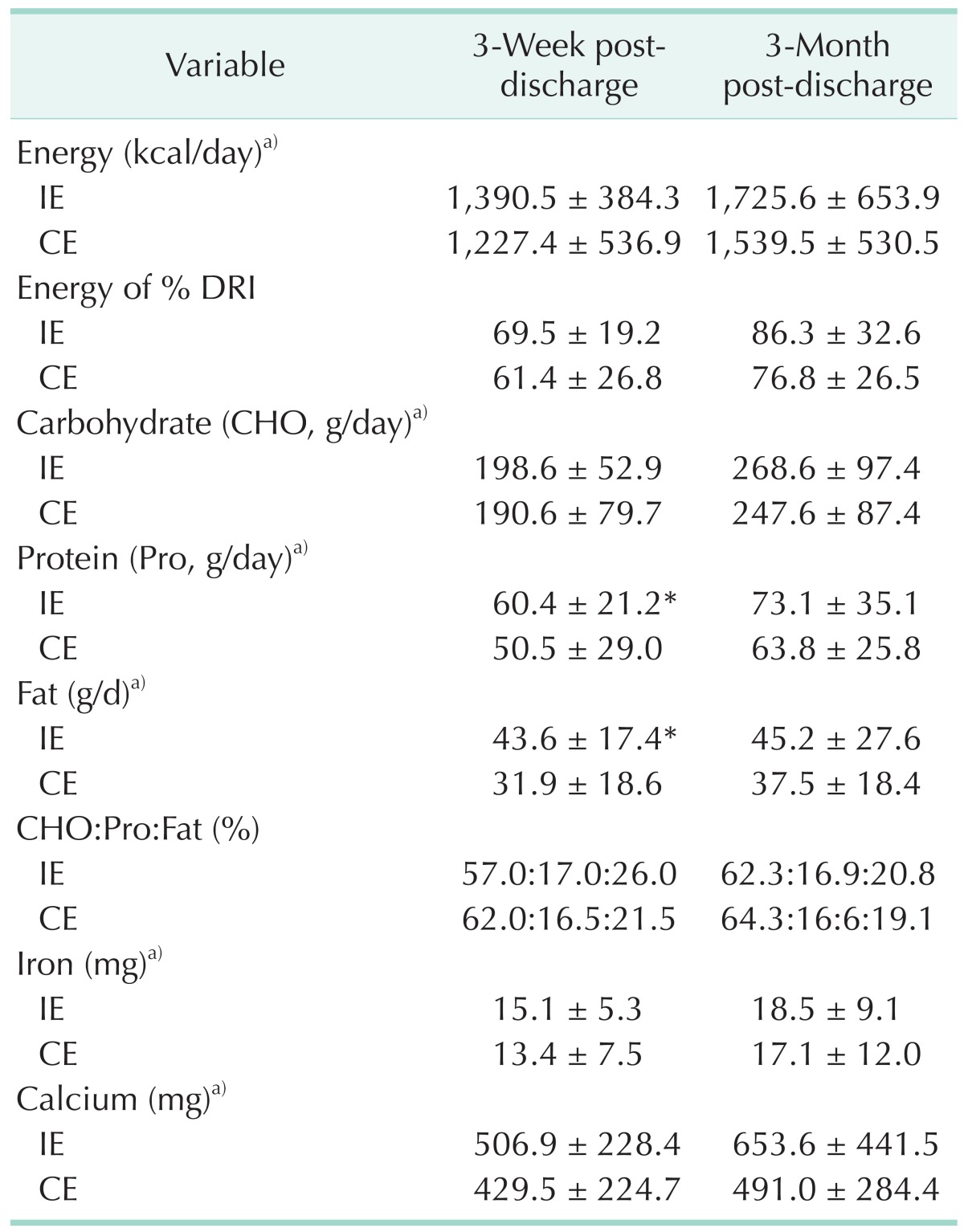
Patient nutrient intake both 3-week and 3-month post-discharge are presented in Table 4. There was a mean increase in energy intake over the 3 months in the IE group (from 1,390 kcal/day at 3-week post-discharge to 1,726 kcal/day at 3-month post-discharge) and in the CE group (from 1,227 kcal/day at 3-week post-discharge to 1,540 kcal/day at 3-month postdischarge). At 3-week post-discharge, the IE group members had significantly higher daily protein and fat intake (P < 0.05) compared with those in the CE group. There were no significant differences in overall nutrient intake (e.g., carbohydrate, protein, fat, iron, calcium) between the 2 groups 3-month postdischarge.
Table 4. Intake of nutrients between intensive and conventional education groups.
Values are presented as mean ± standard deviation unless otherwise indicated.
IE, intensive education group; CE, conventional education group; DRI, dietary reference intakes.
*Significantly different at P < 0.05 between the two groups by the Mann-Whitney test. a)Significantly different at P < 0.001 within each group by the Friedman test
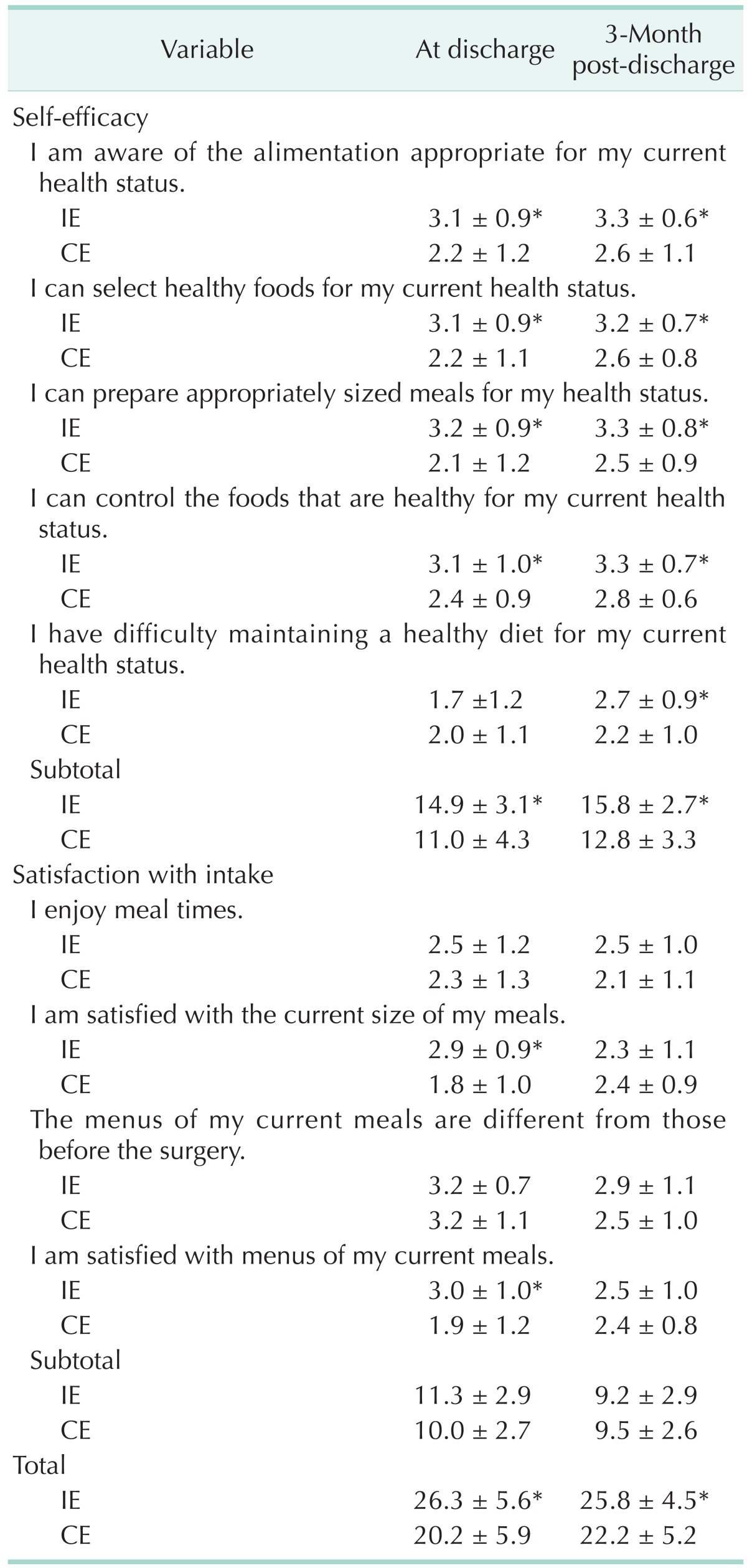
The overall value of self-efficacy and satisfaction with intake differed on both depending on the state of being hospitalized (Table 5). The value of self-efficacy while being hospitalized differed in each category and subtotal (P < 0.001) except on the item stating, "I am having difficulty eating adequate food for my health status." Moreover, the value of satisfaction with meals significantly varied on the item, "I am satisfied with the current meal size (portion)" (P < 0.001) and "I am satisfied with the menu content of my meal" (P < 0.001). While the value of self-efficacy varied significantly in all categories, satisfaction with intake did not. The value of dietary intake did not show any statistically significant differences at 3-month postdischarge.
Table 5. Self-efficacy and satisfaction with intake-related quality of life between intensive and conventional education groups.
Values are presented as mean ± standard deviation.
CE, conventional education group; IE, intensive education group.
Subjects were asked to respond on a 5-point scale ranging from 1 (strongly disagree) to 5 (strongly agree).
*Significantly different at P < 0.05, P < 0.001 between group by the Mann-Whitney test.
DISCUSSION
This study compared and evaluated the effects of an intensive multiphase nutrition intervention relative to a conventional single-phase nutrition education on nutritional status and quality of diet and life among gastrectomy patients in South Korea.
Weight loss after gastrectomy results from insufficient intake, and malabsorption is a common symptom of malnutrition right after surgery [18,19]. Yu et al. [20] suggested that the cause of weight loss and continued malnutrition among gastrectomy patients, even after being discharged, is a poor supply of nutrients. We found that over 90% of subjects lost about 5% (~3 kg) of their body weight after surgery. Other studies reported rates of weight loss after gastrectomy ranging from 4% to 72.8% [20,21,22]. For example, Jeong et al. [22] reported that 72.8% of subjects suffered from severe weight loss (more than 5% weight loss) and suggested the importance of nutrition intervention on nutritional status of patients undergoing gastrectomy. In addition, some previous studies defined nutritional problems appearing after surgery as malnutrition caused by severe weight loss, and highlighted the need for a nutritional intervention with patients after hospital discharge [18,23]. In accordance with weight loss, other anthropometric parameters (e.g., MAC, TSF, and MAMC) also decreased after surgery in our study. These results are similar to those of previous studies, and these aspects changed with time and intervention type [24].
For an accurate assessment of nutritional status, a validated and appropriate method of assessment is necessary. Some studies emphasized the need for both a subjective and objective nutritional evaluation for patients with gastric cancer [11]. There are several comprehensive nutrition assessment tools such as the PG-SGA, nutritional risk screening (NRS-2002), nutritional risk index (NRI), and mini nutritional assessment. Like other studies [16], we used the PG-SGA as a nutritional assessment tool for gastrectomy patients, and examined the significance of this intervention. The IE group, having received nutritional education multiple times, showed significant improvement in their nutritional status in terms of changes in intake, symptoms, physical activity and somatic signs of PG-SGA when compared to the CE group, with a one-time education. We could confirm the progress of nutritional status among patients who had undergone gastrectomy with limitrophic care using the PG-SGA, even after they were discharged. Therefore, the PG-SGA is a useful nutritional assessment tool for gastrectomy patients both pre and postsurgery, even during the convalescence period.
In another study, 84.6% of South Korean gastrectomy patients demonstrated symptoms of malnutrition 5 days postsurgery, as measured using the NRI; among them, 10.9% of patients developed complications. Therefore, a comprehensive screening tool was able to detect the development of malnutrition as a complication after surgery [12]. While still assessing the nutritional status with one or two indicators (e.g., serum albumin, protein, etc.), using comprehensive and multilateral tools to assess nutritional status rather than just these single indicators is useful for gastrectomy patients.
In terms of the effect of nutrition education, there are several studies specific to the effects of gastrectomy among gastric cancer patients [21,25]. For example, in Carey et al. [21]'s recent study, a prospective randomized controlled trial, 27 gastrointestinal surgery patients were randomly assigned to have dietetic follow-up fortnightly for 6 months and compared to a control group. More recently, a study found that health-related quality of life concerns are associated with dietary management. After surgery, nutritional intervention is needed to improve the patient's eating, to prevent malnutrition and excessive weight loss [26]. In addition, a study suggested that eating strategies for gastrectomy patients were still maladaptive. Therefore, health professionals should support nutrition education for appropriate eating after gastric surgery [27]. Our study is far from being complete but significant enough to recognize the importance of intensive nutrition education for gastrectomy patients.
There are very few well-designed studies in this area, particularly Korean studies demonstrating the benefits of intensive and multiphase nutrition counseling. Our results showed that intensive nutrition education was more effective in improving nutritional status, intake, and quality of life than a single session control. Regarding change in diet, the intake of gastrectomy patients is known to be very poor immediately after surgery [28]. We found that energy intake increased by approximately 300 kcal and daily protein and fat intakes significantly increased after the 3-month multiphase education intervention.
Gastrectomy patients generally lack energy and nutrients resulting from poor food intake after surgery. To improve patient nutritional status and to prevent weight loss, the efforts of a clinical dietitian providing repetitive nutrition education and management are necessary.
In addition, evidence suggests that nutritional status and food intake are strongly linked to quality of life [22,29]. Based on the study of Song et al. [30] stating that quality of life for long-term cancer survivors is higher than that of short-term survivors, we can draw the following conclusions: follow-up management on nutritional intervention for patients undergoing gastrectomy will have a positive impact on their quality of life, as well as improving their nutritional status.
Self-efficacy and satisfaction with intake in our study varied by the frequency and type of nutritional intervention. Reported self-efficacy improved after intensive nutritional education. Our subjects who received repeated education at discharge were satisfied with their current meal size and the menu contents of their meals. The results suggested that the clinical dietitian gave patients the courage to overcome their fear of food intake and achieve better quality of life by improving their confidence. Although there has been little to no research regarding self-efficacy and satisfaction with meals among gastrectomy patients, these factors related to quality of life will affect patient prognosis. This study has several strengths. Specifically, this is one of few attempts to examine nutritional status, diet quality, and quality of life between single- and multi-phase nutrition interventions among Korean gastric cancer patients who recently underwent gastrectomy.
Despite these strengths, this study has several limitations. First, we were not able to control all environmental factors, severity of the disease, even all early gastric cancer patients, or underlying medical conditions for each subject. Moreover, the characteristics of the subjects are different. Despite of the nonsignificance of the type of operation and method of reconstruction, the study group's baseline characteristics might be different in operative methods (open vs. laparoscopic gastrectomy, Billroth I/ Billroth II vs. Roux-en-Y reconstruction). In addition, the difference of the postoperative period of NPO might be related to the type of operation method. Second, the sample size of our study is relatively small and the observation periods are short. Although nutritional intervention is too complicated to perform for the enlarged population, some variables may be neglected due to small case numbers. Third, as the study subjects were operation patients, the level of nutrition education given to participants may be higher than that of the general public. The same may be true regarding participants' level of interest and willingness to attend the nutrition education sessions, and this could affect the outcomes of the study. In addition, nutrition education intervention is very hard to control the educator's effect generally. In order to reduce the influence, the division of the groups was selected based on duration. During each period, the patients were enrolled in this intervention randomly. Although there was an inevitable selection bias, the clinical characteristics of patients by the diagnosis of doctor in charge were of no significant differences between the 2 groups.
In conclusion, nutritional status as measured with the PG-SGA improved following an intensive nutritional intervention compared to single-phase nutrition education among gastrectomy in Korean patients. The IE group also demonstrated significantly greater self-efficacy and meal satisfaction, especially at 3-week post-discharge. Moreover, we believe that IE will help to build up eating habits that reduce the probability of complications, and contribute to the early detection of malnutrition, as well as reduce psychological stress and improve quality of life.
ACKNOWLEDGEMENTS
This work was supported by a 2013 grant from Kyung Hee University (KHU-20131806). We thank Han Na Kwon for aiding in the development of this manuscript.
Footnotes
CONFLICTS OF INTEREST: No potential conflict of interest relevant to this article was reported.
References
- 1.International Agency for Research on Cancer. Globocan 2012: estimated cancer incidence, mortality and prevalence worldwide in 2012 – all cancers (excluding non-melanoma skin cancer) [Internet] Lyon: International Agency for Research on Cancer; c2015. [cited 2015 May 12]. Available from: http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx. [Google Scholar]
- 2.International Agency for Research on Cancer. Globocan 2012: Estimated cancer incidence, mortality and prevalence worldwide in 2012: Stomach cancer [Internet] Lyon: International Agency for Research on Cancer; c2015. [cited 2015 May 12]. Available from: http://globocan.iarc.fr/old/FactSheets/cancers/stomachnew.asp. [Google Scholar]
- 3.Statistics Korea [Internet] Daejeon: Statistics Korea; c1996. [cited 2015 May 12]. Available from: http://kostat.go.kr/portal/korea/index.action. [Google Scholar]
- 4.National Cancer Information Center [Internet] Korea: National Cancer Information Center; c2005. [cited 2015 May 12]. Available from: http://www.cancer.go.kr/mbs/cancer/subview.jsp?id=cancer_040102000000. [Google Scholar]
- 5.Yim JY, Kim N, Choi SH, Kim YS, Cho KR, Kim SS, et al. Seroprevalence of Helicobacter pylori in South Korea. Helicobacter. 2007;12:333–340. doi: 10.1111/j.1523-5378.2007.00504.x. [DOI] [PubMed] [Google Scholar]
- 6.Jee SH, Samet JM, Ohrr H, Kim JH, Kim IS. Smoking and cancer risk in Korean men and women. Cancer Causes Control. 2004;15:341–348. doi: 10.1023/B:CACO.0000027481.48153.97. [DOI] [PubMed] [Google Scholar]
- 7.Shin A, Kim J, Park S. Gastric cancer epidemiology in Korea. J Gastric Cancer. 2011;11:135–140. doi: 10.5230/jgc.2011.11.3.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim HJ, Chang WK, Kim MK, Lee SS, Choi BY. Dietary factors and gastric cancer in Korea: a case-control study. Int J Cancer. 2002;97:531–535. doi: 10.1002/ijc.10111. [DOI] [PubMed] [Google Scholar]
- 9.Lee JK, Park BJ, Yoo KY, Ahn YO. Dietary factors and stomach cancer: a case-control study in Korea. Int J Epidemiol. 1995;24:33–41. doi: 10.1093/ije/24.1.33. [DOI] [PubMed] [Google Scholar]
- 10.American Cancer Society [Internet] Atlanta (GA): American Cancer Society; c2009. [cited 2015 May 12]. Available from: http://www.cancer.org/cancer/stomachcancer/detailedguide/stomachcancer-treating-general-info. [Google Scholar]
- 11.Ryu SW, Kim IH. Comparison of different nutritional assessments in detecting malnutrition among gastric cancer patients. World J Gastroenterol. 2010;16:3310–3317. doi: 10.3748/wjg.v16.i26.3310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oh CA, Kim DH, Oh SJ, Choi MG, Noh JH, Sohn TS, et al. Nutritional risk index as a predictor of postoperative wound complications after gastrectomy. World J Gastroenterol. 2012;18:673–678. doi: 10.3748/wjg.v18.i7.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carey S, Storey D, Biankin AV, Martin D, Young J, Allman-Farinelli M. Long term nutritional status and quality of life following major upper gastrointestinal surgery: a cross-sectional study. Clin Nutr. 2011;30:774–779. doi: 10.1016/j.clnu.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 14.Hirao M, Tsujinaka T, Takeno A, Fujitani K, Kurata M. Patient-controlled dietary schedule improves clinical outcome after gastrectomy for gastric cancer. World J Surg. 2005;29:853–857. doi: 10.1007/s00268-005-7760-x. [DOI] [PubMed] [Google Scholar]
- 15.Liu H, Ling W, Shen ZY, Jin X, Cao H. Clinical application of immune-enhanced enteral nutrition in patients with advanced gastric cancer after total gastrectomy. J Dig Dis. 2012;13:401–406. doi: 10.1111/j.1751-2980.2012.00596.x. [DOI] [PubMed] [Google Scholar]
- 16.Kim H, Suh EE, Lee HJ, Yang HK. The effects of patient participation-based dietary intervention on nutritional and functional status for patients with gastrectomy: a randomized controlled trial. Cancer Nurs. 2014;37:E10–E20. doi: 10.1097/NCC.0b013e31829193c8. [DOI] [PubMed] [Google Scholar]
- 17.Ottery FD. Definition of standardized nutritional assessment and interventional pathways in oncology. Nutrition. 1996;12:S15–S19. doi: 10.1016/0899-9007(96)90011-8. [DOI] [PubMed] [Google Scholar]
- 18.Ryan AM, Healy LA, Power DG, Rowley SP, Reynolds JV. Short-term nutritional implications of total gastrectomy for malignancy, and the impact of parenteral nutritional support. Clin Nutr. 2007;26:718–727. doi: 10.1016/j.clnu.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 19.Park JY, Kim YJ. Successful laparoscopic reversal of gastric bypass in a patient with malnutrition. Ann Surg Treat Res. 2014;87:217–221. doi: 10.4174/astr.2014.87.4.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu EJ, Kang JH, Yoon S, Chung HK. Changes in nutritional status according to biochemical assay, body weight, and nutrient intake levels in gastrectomy patients. J Korean Diet Assoc. 2012;18:16–29. [Google Scholar]
- 21.Carey S, Ferrie S, Ryan R, Beaton J, Young J, Allman-Farinelli M. Long-term nutrition intervention following major upper gastrointestinal surgery: a prospective randomized controlled trial. Eur J Clin Nutr. 2013;67:324–329. doi: 10.1038/ejcn.2013.17. [DOI] [PubMed] [Google Scholar]
- 22.Jeong MJ, Kim CY, Kim SB. A study on nutritional status after gastrectomy of gastric cancer patients in Jeonbuk province. Korean J Community Nutr. 2006;11:785–792. [Google Scholar]
- 23.Beattie AH, Prach AT, Baxter JP, Pennington CR. A randomised controlled trial evaluating the use of enteral nutritional supplements postoperatively in malnourished surgical patients. Gut. 2000;46:813–818. doi: 10.1136/gut.46.6.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Na JR, Suh YS, Kong SH, Lim JH, Ju DL, Yang HK, et al. A prospective observational study evaluating the change of nutritional status and the incidence of dumping syndrome after gastrectomy. J Clin Nutr. 2014;6:59–70. [Google Scholar]
- 25.Chen W, Zhang Z, Xiong M, Meng X, Dai F, Fang J, et al. Early enteral nutrition after total gastrectomy for gastric cancer. Asia Pac J Clin Nutr. 2014;23:607–611. doi: 10.6133/apjcn.2014.23.4.15. [DOI] [PubMed] [Google Scholar]
- 26.Sun V, Kim J, Kim JY, Raz DJ, Merchant S, Chao J, et al. Dietary alterations and restrictions following surgery for upper gastrointestinal cancers: key components of a health-related quality of life intervention. Eur J Oncol Nurs. 2015;19:343–348. doi: 10.1016/j.ejon.2015.01.008. [DOI] [PubMed] [Google Scholar]
- 27.Carey S, Laws R, Ferrie S, Young J, Allman-Farinelli M. Struggling with food and eating--life after major upper gastrointestinal surgery. Support Care Cancer. 2013;21:2749–2757. doi: 10.1007/s00520-013-1858-8. [DOI] [PubMed] [Google Scholar]
- 28.Copland L, Liedman B, Rothenberg E, Bosaeus I. Effects of nutritional support long time after total gastrectomy. Clin Nutr. 2007;26:605–613. doi: 10.1016/j.clnu.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 29.Tian J, Chen JS. Nutritional status and quality of life of the gastric cancer patients in Changle County of China. World J Gastroenterol. 2005;11:1582–1586. doi: 10.3748/wjg.v11.i11.1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Song WJ, Kang KC, Heo YS, Shin SH. Comparison of short-term and long-term qualities of life after curative open gastrectomy in patients with gastric cancer. Korean J Clin Oncol. 2010;6:12–19. [Google Scholar]