Alemtuzumab has recently been approved for the treatment of relapsing-remitting multiple sclerosis (MS) in Europe and North America. With its broad mechanism of action on the cellular immune system and high efficacy within the approved indication, alemtuzumab may also be considered as a rescue therapy in other autoimmune conditions, like neuromyelitis optica spectrum diseases (NMOSD). However, data on its potency and safety in NMOSD are largely lacking.
We report on a patient with highly active anti-aquaporin-4 (anti-AQP4) antibody-positive NMOSD who was unstable on first-line treatment with rituximab and did not benefit from 3 cycles of alemtuzumab as an add-on therapy.
Case report.
A 43-year-old woman was seen in our hospital for the first time in 2009 when AQP4-antibody-positive NMOSD had been initially diagnosed. Her disease was already longstanding, starting with her first optic neuritis episode in 1998, her second in 2005, and her first transverse myelitis episode in 2005. Between 2005 and 2009, she had had another 6 relapses, resulting in an Expanded Disability Status Scale (EDSS) score of 3.0.
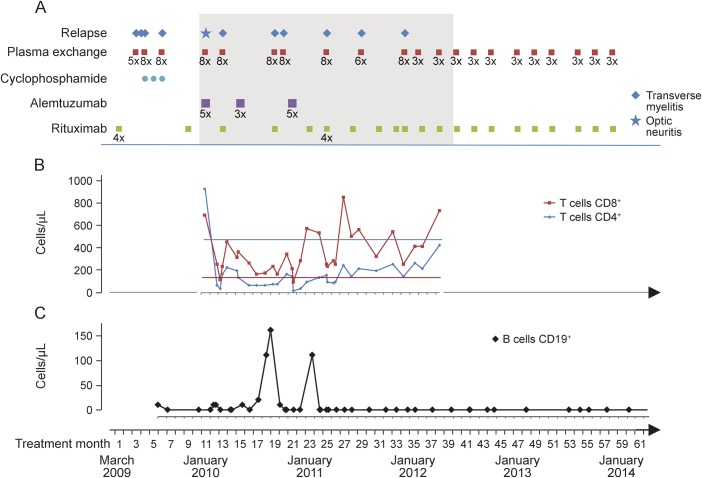
Following current guidelines,1 the patient was started on rituximab (4 × 375 mg/m2 weekly), but she relapsed 3 times in the following 2 months and needed plasma exchange therapy to stabilize. Three cycles of cyclophosphamide (first cycle 500 mg/m2 IV, 2 cycles 750 mg/m2 IV each) over 3 months were added. Two months later, CD19+ B cells returned, and she had a myelitic flare; thus rituximab (375 mg/m2 IV once) was regiven. Two months later, with CD20+ B cells still depleted, severe optic neuritis developed. Thus, we decided to administer alemtuzumab (12 mg IV daily, 5 times) as an off-label therapy. Another 2 months later, we observed an early repopulation of lymphocytes in conjunction with the patient's next myelitis episode. Rituximab was redosed, and 2 months later, a second cycle of alemtuzumab (3 infusions) was applied. Four and 5 months later, the patient had further myelitic relapses. In November 2010, she received another 5 alemtuzumab infusions, but still relapsed 3 months later. Despite continuing rituximab in high frequency (375 mg/m2 IV every 2 months), she had another 2 relapses in the remaining first year after the last alemtuzumab treatment. Her EDSS score was 7.0. Thus, alemtuzumab was considered to be ineffective, and rituximab in combination with scheduled plasma exchanges (3 plasma exchanges each) every 2 to 3 months were started in January 2012. Since then, the patient has remained clinically stable (figure), and the annual relapse rate declined from 3.3 (before alemtuzumab) and 3.5 (during alemtuzumab) to 0.
Figure. Clinical course, treatment, and lymphocyte populations.
(A) Clinical course and treatment between March 2009 and January 2014. The period of alemtuzumab treatment is shaded. (B) CD4+ and CD8+ T cells in the peripheral blood during and after alemtuzumab treatment between January 2010 and April 2012. Flat lines indicate the lower limit of normal. (C) CD19+ B cells in peripheral blood.
Discussion.
There are anecdotal data in the literature that alemtuzumab might be ineffective in the treatment of NMOSD. To our knowledge, 4 cases with a negative outcome have been published so far.2–5 Several findings in our case are noteworthy. First, with rituximab being insufficient as a monotherapy, we decided to add alemtuzumab because it depletes a wider spectrum of B cells and T cells, NK cells, and monocytes. However, repopulation of lymphocytes, especially CD8+ T cells, occurred much earlier than expected from MS cohort studies6 and were accompanied by relapses. Although it is even in MS a matter of debate whether the repopulation kinetics are relevant for the efficacy of alemtuzumab, this early rebound of lymphocytes might eventually be responsible for ongoing disease activity, and tempted us twice to redose alemtuzumab early, with no clinical effect. Second, some of the relapses were associated with the recurrence of B cells, which has already been described by others,7 but happened although the B-cell-depleting agent rituximab was applied in high frequency. Interestingly, in MS, alemtuzumab itself may trigger antibody-driven autoimmune reactions such as Graves disease or idiopathic thrombocytopenic purpura, and reconstitution autoimmunity with a possible imbalance of T regulatory cells and IL-21 receptor-positive effector T cells and their effects on B cells during repopulation of secondary lymphoid organs have recently been discussed as underlying mechanisms.8 Although our patient tolerated alemtuzumab well and did not develop any other antibody-driven autoimmune disease in 5 years of follow-up, the development of new anti-aquaporin-4-reactive B cells during repopulation or alemtuzumab-specific permissive effects on T- and B-cell crosstalk could have contributed to ongoing disease activity. Third, after alemtuzumab, the patient only stabilized when rituximab was combined with scheduled plasma exchanges. This supports the assumption that NMOSD therapy is more efficacious when it targets humoral responses of the immune system. In line with this, there are data that autologous hematopoietic stem cell transplantation is likely ineffective in NMOSD,2 and the most promising drugs currently developed in NMOSD treatment inhibit complement activation and the proinflammatory cytokine interleukin-6.
The presented case adds to the impression of previously published cases that alemtuzumab cannot be recommended for the treatment of NMOSD. With β-interferons and natalizumab also being ineffective or even harmful in the treatment of NMOSD, the failure of alemtuzumab further strengthens the notion that NMOSD and MS are separate diseases necessitating evaluated rather than adopted therapeutic regimens for NMOSD.
Footnotes
Author contributions: Dr. Kowarik analyzed the data and drafted the manuscript. Dr. Hoshi analyzed the data and revised the manuscript for intellectual content. Dr. Hemmer analyzed the data and revised the manuscript for intellectual content. Dr. Berthele analyzed the data and drafted the manuscript.
Study funding: No targeted funding.
Disclosure: M.C. Kowarik received travel funding from Merck-Serono, Bayer Health Care, and Novartis, and received research support from Novartis. M. Hoshi received travel funding from Bayer Health Care, Biogen Idec, and Merck Serono. M. Hemmer served on the scientific advisory board for Bayer, Biogen Idec, Roche, Novartis, Merck Serono, Chugai, GSK, and Genentech; received travel funding and/or speaker honoraria from Bayer, Biogen Idec, Roche, Novartis, and Merck Serono; is on the editorial board for Archives of Neurologie, Experimental Neurology, and MS Journal; holds a patent for anti-KIR4.1 antibody testing in MS, Genetic factors influencing the development of NABs; has consulted for Gerson Lehrman Group; and received research support from Bayer, Biogen Idec, Roche, Novartis, Merck Serono, Metanomics, Chigai, Deutsche Forschungsgemeinschaft, Bundesministerium fur Bildung und Forschung, European Community, Competence Network for Multiple Sclerosis Synergy Excellence Cluster, and German Research Foundation. A. Berthele received travel funding and/or speaker honoraria from Biogen Idec, Merck Serono, Novartis, Teva, and Bayer; and received research support from Bayer. Go to Neurology.org/nn for full disclosure forms. The Article Processing Charge was paid by the authors.
References
- 1.Trebst C, Jarius S, Berthele A, et al. Update on the diagnosis and treatment of neuromyelitis optica: recommendations of the Neuromyelitis Optica Study Group (NEMOS). J Neurol 2014;261:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Matiello M, Pittock SJ, Porrata L, Weinshenker BG. Failure of autologous hematopoietic stem cell transplantation to prevent relapse of neuromyelitis optica. Arch Neurol 2011;68:953–955. [DOI] [PubMed] [Google Scholar]
- 3.Qian PQ, Cross AH, Naismith RT. Neuromyelitis optica unresponsive to monoclonal antibody therapy. Arch Neurol 2011;68:250–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kieseier BC, Stüve O, Dehmel T, et al. Disease amelioration with tocilizumab in a treatment-resistant patient with neuromyelitis optica: implication for cellular immune responses. JAMA Neurol 2013;70:390–393. [DOI] [PubMed] [Google Scholar]
- 5.Gelfand JM, Cotter J, Klingman J, et al. Massive CNS monocytic infiltration at autopsy in an alemtuzumab-treated patient with NMO. Neurol Neuroimmunol Neuroinflamm 2014;1:e34 doi: 10.1212/NXI.0000000000000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cossburn MD, Harding K, Ingram G, et al. Clinical relevance of differential lymphocyte recovery after alemtuzumab therapy for multiple sclerosis. Neurology 2013;80:55–61. [DOI] [PubMed] [Google Scholar]
- 7.Pellkofer HL, Krumbholz M, Berthele A, et al. Long-term follow-up of patients with neuromyelitis optica after repeated therapy with rituximab. Neurology 2011;76:1310–1315. [DOI] [PubMed] [Google Scholar]
- 8.Tsourdi E, Gruber M, Rauner M, Blankenburg J, Ziemssen T, Hofbauer LC. Graves' disease after treatment with Alemtuzumab for multiple sclerosis. Hormones 2015;14:148–153. [DOI] [PubMed] [Google Scholar]