Abstract
Background
The extent to which economic evaluations have included the healthcare resource and outcome-related implications of information provision in national newborn bloodspot screening programmes (NBSPs) is not currently known.
Objectives
To identify if, and how, information provision has been incorporated into published economic evaluations of NBSPs.
Methods
A systematic review of economic evaluations of NBSPs (up to November 2014) was conducted. Three electronic databases were searched (Ovid: Medline, Embase, CINAHL) using an electronic search strategy combining a published economic search filter with terms related to national NBSPs and screening-related technologies. These electronic searches were supplemented by searching the NHS Economic Evaluations Database (NHS EED) and hand-searching identified study reference lists. The results were tabulated and summarised as part of a narrative synthesis.
Results
A total of 27 economic evaluations [screening-related technologies (n = 11) and NBSPs (n = 16)] were identified. The majority of economic evaluations did not quantify the impact of information provision in terms of healthcare costs or outcomes. Five studies did include an estimate of the time cost associated with information provision. Four studies included a value to reflect the disutility associated with parental anxiety caused by false-positive results, which was used as a proxy for the impact of imperfect information.
Conclusion
A limited evidence base currently quantifies the impact of information provision on the healthcare costs and impact on the users of NBSPs; the parents of newborns. We suggest that economic evaluations of expanded NBSPs need to take account of information provision otherwise the impact on healthcare costs and the outcomes for newborns and their parents may be underestimated.
Electronic supplementary material
The online version of this article (doi:10.1007/s40258-015-0177-2) contains supplementary material, which is available to authorized users.
Key Points for Decision Makers
| The process of information provision as part of a national bloodspot screening programme, with an increasing emphasis on expanding the programme to include more conditions, is likely to have implications in terms of healthcare resource use and also subsequent health and non-health outcomes. |
| Few economic evaluations of NBSPs attempt to quantify the cost of providing information about the programme, or the impact of this information provision on parents and newborns. |
| Research is needed to generate robust data on the actual cost of information provision for healthcare services, the impact of NBSPs on subsequent use of healthcare services and the impact on parents’ health status and ability to make an informed decision in the context of expanded NBSPs. |
Introduction
Newborn bloodspot screening (NBS) is viewed as one of the greatest public health developments of the twentieth century [1]. The procedure involves obtaining a sample of blood from the newborn baby, usually by taking drops of blood from the heel, and using this sample (bloodspots), stored on a collection card, to screen for a number of pre-specified inherited conditions. Screening aims to facilitate the early detection of these inherited conditions and hence allow for the timely initiation of treatment to help prevent or manage the extent of subsequent impact on the health of the newborn baby.
The Guthrie method was developed during the late 1950s by Dr Robert Guthrie, and aims to detect phenylalanine, phenylpyruvic acid, and phenyllactic acid in dried bloodspots stored on a card [2]. The Guthrie method allowed the detection of inborn errors of metabolism, the first being the detection of phenylketonuria (PKU). Tandem mass spectrometry (MS/MS) was developed in the early 1990s to speed up the identification of inborn errors of metabolism and could be adapted to the Guthrie collection protocols [3]. As a result, MS/MS became a more appropriate technology for use in newborn bloodspot screening programmes (NBSPs) provided at a national level [4]. The introduction of MS/MS technology in the UK in 2007 dramatically increased the number of inherited conditions which could potentially be screened for in newborn babies. However, despite the apparently low unit cost of introducing new conditions into existing national screening programmes, there are associated costs in terms of communication, events which are rarely accounted for. Furthermore, the potential scale of population level screening programmes means that small incremental unit costs can add up to large total costs for healthcare providers. For example, in the UK, the NBSP, that screens approximately 800,000 babies a year [5], has a substantial impact on the healthcare budget in terms of the cost of providing the service and the potential to influence long-term use of healthcare resources. The potential impact on scarce healthcare resources and the need to understand the opportunity cost of changing or expanding NBSPs has stimulated the production of a growing literature that applies methods of economic evaluation to help policy makers decide which specific conditions should be included in a NBSP. The ultimate aim of these economic evaluations is to guide whether the proposed NBSP is a cost-effective use of finite healthcare resources.
NBSPs across the world differ in the conditions screened for (see Supplementary Appendix 2) and their requirement of obtaining parental consent. Internationally, there is increasing appreciation of the centrality of communication in ensuring that NBSP benefits are realised whilst reducing potential harms [6–15]. Specifically, there is a recognised need to inform parents prior to screening [16–18], preferably antenatally [16, 19]. In contrast, current NBSPs do not have explicit models of information provision after positive test results beyond setting up appropriate referral to the service to care for the condition diagnosed. A failure to provide adequate information, when parents are deciding whether to take part in the NBSP, and understand the implications of a test result, may result in costs (or dis-benefits) to parents as well as the healthcare provider. For example, there is the suggestion that parents may not make ‘informed decisions’ about screening as although they consent to screening occurring many are not aware that screening has occurred [20–24] or have limited knowledge about it [16, 25–29]. Poor communication of key pieces of information can result in additional anxiety and distress when unexpected results are received [30, 31], which in turn has been shown to impact on health, relationships, ability to work and engagement in society [32]. The cause of anxiety is likely to be multifactorial. However, there may be identifiable and quantifiable consequences of anxiety in terms of the use of healthcare services in terms of additional consultations needed by parents to deal with the sequelae of anxiety resulting from poor information provision [32]. As NBSPs include increasingly rare diseases with less clear treatment benefits, communication will become ever more critical both before and after screening [33]. In summary, it is apparent that the process of information provision as part of a national bloodspot screening programme, with an increasing emphasis on expanding the programme to include more conditions, is likely to have implications in terms of healthcare resource use and also subsequent health and non-health outcomes, such as informed decision making.
Langer et al. [34], produced a set of guidelines to inform the design and conduct of an economic evaluation of an NBSP, which were then applied to published economic evaluations of NBSP for metabolic diseases up to 2011. The authors concluded that the published evaluations were generally poor at measuring and valuing resource data or considering non-health outcomes. This review, however, did not explicitly identify whether the published evaluations included information provision in terms of (1) the impact on resource use, or (2) valuation of the impact on outcomes. The aim of this current study is to identify the extent to which economic evaluations of NBSP take into account the role of information provision and its consequences.
Methods
A systematic review was carried out to identify all published economic evaluations of NBSP provided on a national basis and associated specific screening technologies. The review was conducted according to published methods [35] and followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Checklist.
Literature Search
The electronic databases used to search for relevant published economic evaluations included Ovid Medline, Ovid Embase, Ovid CINAHL and NHS Economic Evaluations Database (NHS EED). The electronic search strategies were specifically designed for each database by combing relevant index and free-text terms for NBS (identified by the literature) with economic evaluation search filters developed by the Centre for Reviews and Dissemination (CRD) [36]. The searches were verified by a researcher who has worked in the field of NBS (FU) and an information specialist (personal communication, Mary Ingram; July 2013). Supplementary Appendix 1 details the electronic search strategies. The electronic searches were supplemented by examining the reference lists of each identified study. All searches were conducted in November 2014.
Inclusion Criteria and Study Selection for Critical Review
The identified economic evaluations, titles and abstracts, were screened by three independent reviewers to assess whether the study satisfied the criteria specified for inclusion in the review (see Table 1). All types of economic evaluations (cost-effectiveness; cost-utility and cost-benefit analysis) and either model-based or retrospective economic analyses based on cohort studies were eligible for inclusion in the review.
Table 1.
Summary of study inclusion criteria
| Aspect of study | Inclusion criteria |
|---|---|
| Study Design | Full economic evaluation in accordance with definition by Drummond et al. [78]: cost-effectiveness analysis (CEA); cost-utility analysis (CUA); cost-benefit analysis (CBA) |
| Population | Neonates, infants, children |
| Intervention | National newborn screening programme for inherited diseases or a specific screening technology |
| Type | Model-based or prospective/retrospective (RCT or cohort) evaluation |
| Outcomes | Costs (health and/or patient) Health and non-health patient benefits |
| Availability | English; full text |
Data Extraction and Synthesis
Two reviewers (CJ/SW) extracted the data from each identified study using a structured data collection form. The data extraction form was based on a checklist developed by the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) [37]. The data extracted incorporated the (1) author, year of publication and country in which the study was conducted, (2) viewpoint and population, (3) type of evaluation vehicle used, (4) resources used, (5) valuation of the cost of information provision, (6) valuation of benefits, including health and non-health outcomes, and finally (7) key results. The results were tabulated and summarised as part of a narrative synthesis.
Results
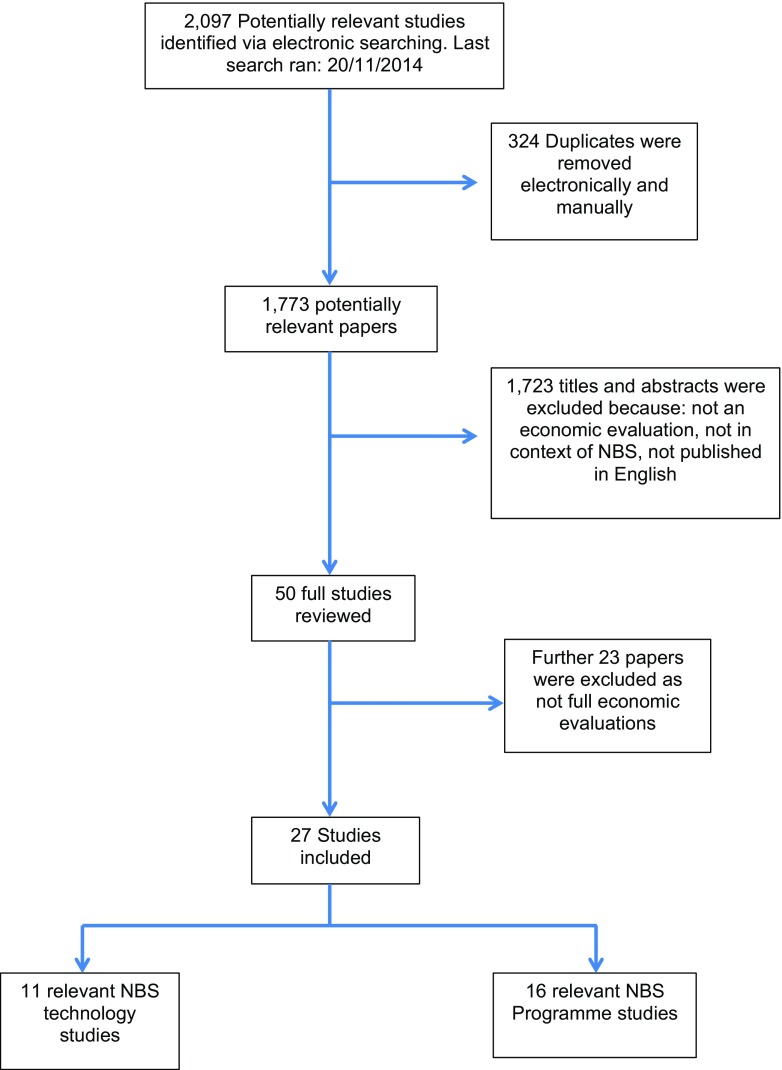
Figure 1 summarises the study identification and inclusion process. A total of 27 economic evaluations were identified and included in this review. Of these studies, 11 evaluated screening technologies used in NBSPs [17, 38–47] (see Supplementary Appendix 3) and 16 evaluated NBSPs [6, 48–62] (see Supplementary Appendix 4). A summary of the conditions presently screened for in each country is provided in Supplementary Appendix 2 to place the evaluations into context.
Fig. 1.
PRISMA flow diagram of search results
Types of NBSP
Newborn screening programmes were defined as a service which includes the provision of information, collection of informed consent (where appropriate to the jurisdiction), collection of samples, use of technology to analyse the sample, provision of test results and an appropriate referral mechanism to subsequent services if a positive test result is confirmed. It is important to distinguish between evaluations that focus on the technology used to run the analysis compared with those that evaluate a NBSP as a whole service because the appropriate study perspective and time horizon for the analysis is likely to be different.
Sixteen studies were identified as economic evaluations of NBSPs and clearly described the programmes included in the analysis. The studies identified that evaluated NBSPs were based in a number of different countries including: the USA (n = 8); The Netherlands (n = 4); Australia (n = 1), Libya (n = 1), France (n = 1) and the UK (n = 1). Not all countries providing NBSPs have the same policies regarding informed consent, the country of origin for the evaluation is key to understanding if, and how, informed consent has been considered. The North American studies included are based in the States of Indiana, Alaska, Texas and Kentucky. All four of these states allow parents to refuse a screening test based on religious grounds [63]. Four studies are based in Australia, France, The Netherlands and the UK, all of which require parental consent prior to performing a screening test. It was not made clear what level of informed consent is required in Libya.
Seven studies discussed the implications of using different screening strategies, for example, universal or targeted strategies [48, 50, 51, 53, 55, 59, 61]. Three studies focused on expanded screening programmes to include additional screening for medium-chain acyl-coenzyme A dehydrogenase deficiency (MCADD) [6, 52, 58], three studies reported screening strategies specifically for cystic fibrosis (CF) [57, 62] and MCADD [60], respectively, and two studies focussed on screening for severe combined immunodeficiency (SCID) [49, 54].
Eight of the studies compared the NBSP with no screening [48, 49, 51, 54–56, 60, 62]. The remaining eight studies compared screening with existing strategies [6, 50, 52, 53, 57–59, 61].
Types of Screening Technologies
In the eleven economic evaluations of screening technologies, two types of technology were evaluated: the Guthrie method [45] and tandem mass spectrometry [17, 38–44, 46–48].
The studies identified for this section of the review were based in countries including: USA (n = 4); Canada (n = 2); UK (n = 2); Iran (n = 1); Finland (n = 1); and Australia (n = 1). The NBS technology studies carried out in North America were based in the States of California, Wisconsin, and Pennsylvania, all of which allow the rejection of the NBS test by parents on religious grounds only [63]. The Canadian studies included in this review were based in Ontario and Nova Scotia. Ontario largely operates its NBS testing on an opt-out basis; however, when new technologies are introduced formal consent is required [64]. In contrast, in Nova Scotia the requirement for consent varies from hospital to hospital [64]. Australia, Finland and the UK all require informed consent before the test can be carried out. In Iran, NBS screening is only mandatory for one specific condition; congenital hypothyroidism (CH).
Shamshiri et al. [45] evaluated the cost effectiveness of the Guthrie method compared with no screening, assuming the perspective of the Iranian health service that, apart from screening for CH, has a voluntary NBSP. Ten studies conducted an economic evaluation of MS/MS. Four of these studies reported using no screening as the relevant comparator [38, 40, 41, 47] but did not justify why no screening was the relevant comparator of choice. Four studies compared new MS/MS with existing technologies [17, 39, 42, 43] and supported their choice of comparator by stating that it allowed for a direct comparison between the cost effectiveness of new and existing screening technologies. Tran et al. [46] compared MS/MS screening with clinical diagnosis. The study by Schoen et al. [44] did not clearly specify a comparator but it can be inferred that no screening was the alternative intervention.
Types of Economic Evaluation
Cost-effectiveness analysis (CEA) was the most common type of economic evaluation (n = 8), followed by cost-utility analysis (CUA) (n = 7) for studies evaluating NBSPs. One study reported their findings using cost-benefit analysis (CBA) [50]. CUA and CEA were similarly popular methods used to evaluate screening technologies (CUA n = 7 and CEA n = 6) and one study [40] reported a CBA using hedonic pricing methods to attach a monetary value to the benefits of screening.
There were 24 model-based economic evaluations (14 NBSPs; 10 screening technologies). Of the 24 model-based studies, 12 used a decision tree (7 NBSPs; 5 screening technologies). Two studies used a Markov model (NBSPs) and three studies combined a decision tree with a Markov model (two NBSP; one screening technology). Two studies (NBSPs) used patient level simulation models. Five studies (one NBSPs; four screening technology) used a model-based analysis but did not explicitly report the type of model used. Three studies (two NBSPs; one screening technology) used data collected as part of cohort studies to inform the analysis.
Key Results of Evaluations
All of relevant studies concluded that MS/MS was a cost-effective use of healthcare resources. Two papers [39, 43] went further and stated that the relative cost effectiveness of MS/MS was dependent on the number of conditions being screened for and the results indicated that the combined screening for PKU and MCADD was cost effective. In total, 15 of the evaluations of NBSPs concluded the programmes were a cost-effective use of resources. Panepinto et al. [55] and Gessner et al. [51] both reported that targeted screening with follow up was more cost effective than universal screening. Tiwana et al. [58], Hamers and Rumeau-Pichon [52], Prosser et al. [6], Chan et al. [49], McGhee et al. [54], Simpson et al. [57], Sladkevicius et al. [56] and van der Hilst et al. [60] all reported that introducing an expanded NBSP was cost effective. Van den Akker-van Marle et al. [59], Wildhagen et al. [62] and Wells et al. [61] reported the most cost-effective diagnosis strategies.
Valuation of Benefits
All of the 27 studies focussed on the valuation of health benefits alone. None of the studies included a measure of non-health benefits, for example, the impact on the ability to make an informed decision, which is synonymous with ‘cognitive capability’ (or empowerment [65]). A total of 11 cost-effectiveness analyses (five NBSPs; six screening technologies) used life-years gained (LYG), or saved, to value health benefits. Norman et al. [42] justified their use of LYG together with ‘death-years averted’ by stating that there was a lack of published credible utility weights for newborns with the relevant conditions to ‘quality adjust’ the additional years of life from the screening intervention. Pandor et al. [43] acknowledged the use of LYG may have resulted in serious limitations in their study because it might have underestimated the potential benefits to be gained from the NBSP. There was significant variation in the way each study reported the estimation of LYG. Some authors used multiple life expectancy (LE) estimates to reflect the severity of the condition when calculating LYG [39, 42–44, 52, 60].
Thirteen studies (seven NBSPs; six screening technologies) estimated quality-adjusted life years (QALYs) to value the health benefits. Just six studies explicitly reported the utility estimates and LE figures used to generate the QALYs [41, 44, 48, 49, 52, 57]. Four of the studies [6, 40, 47, 58] did not explicitly report either the assumed impact on LE or utility estimates used in the analyses. Feuchtbaum and Cunningham [40] and Geelheod et al. [50] reported their findings using monetary health benefits. Feuchtbaum and Cunningham [40] valued the health benefits of NBS by calculating the number of lives saved through MS/MS screening and assigned a monetary value of each life saved as reported from the US Environment Protection Agency. Geelheod et al. [50] chose to value benefits in terms of the expected costs of disability avoided through NBS.
Cost of Providing Information
Five studies [38, 43, 50, 57, 62] identified in this review (three NBSPs; two screening technologies), included an estimate of the cost of information provision in their analyses. None of the identified studies captured the impact of information provision after a screening test had been completed. Four of the five studies, which included a cost of information provision, were model-based evaluations while the other was a based on a retrospective cohort of newborns. Costs were developed from a range of sources including: cost data from existing programmes; the cost of information provision in similar healthcare programmes (for example information provision for prenatal or antenatal screening); the estimated time for a consultation with a midwife; patient surveys; and general assumptions made by the authors.
All five studies used different assumptions and methods to estimate the cost of information provision. In the UK-based analysis, Pandor et al. [4] included a unit cost of GBP0.30 (2001 prices) cost per baby screened to account for the extra (incremental) time taken by a midwife to explain the test and gain consent, although the actual length of additional time was not specified. This cost was identified through a consultation with a midwife. Simpson et al. [57] provided a similar incremental cost of “counselling time required to gain consent” for one extra condition. The value of 2.1 min was obtained through a survey conducted by the lead author. This gave an additional cost of GBP0.40 (1998 prices) per child screened.
The evaluation conducted by Geelhoed et al. [50], based in Western Australia, included a cost for the 15 min per child that a nurse would spend providing information about the test. An individual figure for the cost of providing information was not given but it can be calculated by taking the total cost of nursing input in sample collection (AUS $326,875), dividing it by the number of babies seen (25,000) and multiplying this by the proportion of nurse time spent giving information (0.5), giving a figure of AUS $6.54 (2001 prices) per child screened. This study clearly assumed that a greater amount of time was needed to explain carrier status in the context of cystic fibrosis. As Australia operates an informed refusal system of informed consent, this cost is unlikely to account for time taken to receive consent.
Wildhagen et al. [62], based in The Netherlands, also evaluated a cystic fibrosis NBSP. This study calculated the cost of information provision by using the mass media costs of information provision for a breast cancer screening programme. The analysis assumed that the relative cost of providing an NBSP compared with a breast cancer screening programme would be reduced by a figure of 40 % to account for the greater ease of introducing newborn screening into clinical practice. The final value included in the analysis was GBP 136,956.60 (1996 prices), which was then combined with the cost of providing information at the individual level (assumed to be GBP 1.19). This resulted in a cost of information of GBP 2.13 (1996 prices) per child screened. As this paper was investigating the introduction of a screening programme, no particular consent model was assumed and no cost of obtaining consent was included.
Finally, Autti-Rämö et al. [38] included a cost of EUR 303,000 (2002 prices) for information provided in pregnancy about newborn screening. Given a cohort of 56,000 newborns, this would mean a cost of EUR 5.41 per newborn screened. This cost accounts for the resources needed for a nurse to give the information along with a leaflet and for ten percent of parents having an additional consultation with a physician. Unit costs were not provided for each of these components. The authors also account for the cost of gaining consent before the test but as this is combined with the cost for taking the sample and sending it to the laboratory, a unit cost cannot be given for consent alone. No source was given for these costs and they are based on general assumptions that have been made by the authors. The lead author later highlights this in a conference abstract describing the difficulties of evaluating newborn screening interventions stating that “The original assumptions on costs were fictional” [66].
A further two studies explicitly mentioned the omission of the cost of information provision in their evaluation of a NBSP. Hamers and Remeau-Pichon’s [52] economic evaluation of universal screening for MCADD in France, included the start-up costs of the programme but excluded the costs of “producing information and education materials”. No justification for this omission was given. Van der Akker-van Marle et al. [59] explained that they did not include information provision in their model of the cost effectiveness of differing methods of screening for cystic fibrosis because the assumed cost of updating the information leaflet would be insignificant.
Costs of Imperfect Information Provision
Inappropriate, or imperfect, information provision may lead to further costs to the health service and parents as a ‘knock on’ consequence (or sequelae) of anxiety induced by poor information [16, 17, 32]. For example, parents who do not have a clear understanding about their child’s condition, or are overly anxious about their child’s health, may, as a consequence, subsequently require healthcare resources and seek frequent consultations with clinicians to allay their fears [16]. At times specialist consultations are required as trust has been broken between parents and health professionals [32]. Only one study [44] mentioned costs related to the impact of imperfect information, which was specifically made in reference to false-positive results, and where a part of the resulting anxiety may possibly be attributed to a lack of understanding. Schoen et al. [44] (p. 785) identified that whilst false-positive results could cause increased parental anxiety, the results may also lead to “a cascade of costly clinical events, including emergency department visits, hospital admissions, additional definitive laboratory studies, and use of on-call medical personnel”.
Chan et al. [49] also highlighted that children with false-positive results would face additional costs from misdiagnosis and subsequent induced anxiety in parents. They assumed that “this cost is transient and can be minimised by educating providers and parents”. Despite this, the costs of providing this information were omitted from their model of the cost effectiveness of newborn screening for SCID.
(Dis)-Benefits of Imperfect Information Provision
A lack of good understanding of NBS amongst parents may also lead to quantifiable dis-benefits, or negative outcomes, of screening. These negative outcomes should be described, identified and valued appropriately and distinguished from the impact on healthcare resource use to avoid ‘double-counting’. One theme which appeared in the identified literature was that false-positive results could be a potential source of such dis-benefits in that they cause anxiety for the parents of children receiving them that may impact on health gain and be captured as a dis-utility. Whilst receiving such results may naturally cause a certain degree of stress, anxiety may be exacerbated by a lack of information provision about the meaning of a false-positive result. Two of the studies in this review highlighted a link between the level of information a parent had received about newborn screening and the magnitude of the anxiety they would experience on receipt of a false-positive result [17, 46]. These studies argued that the more informed a parent was about screening, the lower the anxiety they would experience upon receipt of a false-positive result.
The remaining studies (n = 25) did not explicitly state the link between false-positive results and anxiety but some did include valuations of parental anxiety or made reference to its potential impact. If improved parental understanding does mitigate some of the stress (induced anxiety) caused by false-positive results, potential reductions in anxiety, captured in the ‘quality-adjustment (utility)’ component of QALYs should be quantified in the outcome side of the calculation of relative cost effectiveness of the screening programme.
One example of a valuation of parental disutility was identified in a previous study by Prosser et al. [67]. These authors used the time trade-off approach and showed that on average, parents were willing to give up 1 week of their life in order to not receive false-positive results, which yielded an estimate of a quality of life loss of 0.003. Prosser et al. [6] included this estimate in a model of newborn screening for MCADD but found that the resulting 0.0005 QALYs lost from false-positive results made no significant impact on the cost-effectiveness conclusion. This QALY value would indicate that disutility of false-positive results is experienced for 2 months, although this was not clearly stated in the paper. In absolute terms, including this dis-benefit raised the incremental cost-effectiveness ratio by GBP50 per QALY.
Venditti et al. [47] conducted a cost-effectiveness analysis of screening for MCADD, and reported that they couldn’t find a published value for anxiety caused by false-positive results in newborn screening. Therefore, they used a value based on the anxiety that oncology patients experience when receiving false-positive results experienced over 3 months. This value was varied between 0.01 and 0.03 in the sensitivity analysis, which resulted in a QALY loss of between 0.0025 and 0.0075. Introducing this disutility into the model did not significantly affect the results.
The 0.03 value for disutility used in Venditti et al. [47] was also used in two more recent evaluations of newborn screening in Texas [58] and Canada [39]. Both studies used the three month time horizon which was used in the source paper to calculate the QALY loss from false-positive results. In the 2012 study, the authors allowed the effect of parental anxiety to vary from a disutility of 0.01 to a value of 0.05 (a QALY loss of 0.0125). Although the dis-benefit of parental anxiety was included, the authors do not explain the implications of including it with regards to the cost-effectiveness conclusions of the analysis. In the Canadian-based study, the output from the analysis is reported as a cost per life-year saved. However, the authors do report that the analysis included a disutility to account for parental anxiety but did not report this as a quality adjustment to the stated cost per life-year saved. The analysis appeared to have a significant impact on the cost per life-year saved when screening was for 15 conditions, making the result appear less cost effective, but this was probably due the incorrect application of utility decrements to life years. A decrement of 0.01–0.03 was applied to the life-years accrued by parents rather than using a utility decrement of 0.01–0.03. The duration of 3 months for the disutility did not appear to have been accounted for and this would have reduced the impact of false-positive results. In this way the paper may be overstating the negative impact of receiving false-positive results.
Despite directly highlighting the need to provide information to reduce the anxiety parents feel on receipt of false-positive results, Pandor et al. [43], did not include an explicit value to quantify the impact of this ‘harm’ in their model. The authors justified this omission by stating that any psychological dis-benefits caused by screening would be far outweighed by the improvement in quality of life for children that a screening programme would bring. In a later paper by the same authors, Pandor et al. [4] used a similar argument for the omission of a quantified harm. However, they further developed the argument by stating that the ratio of false-positives to true-positives is 3:1 and hence the psychological harm from a false-positive result would have to be at least one-third of the psychological benefit from a true-positive to make a difference on the results of the analysis.
Two studies assumed that the disutility associated with parental anxiety would be negligible and so omitted the parameter from their model. In Geelhoed et al. [50] no cited evidence was given to support the omission, but they did suggest that only five children had received false-positive results in Western Australia in 2001 and this would mean the total “negative benefit” of the screening programme would be small. Hamers and Rumeau-Pichon [52] also excluded anxiety because of its negligible potential impact and cited papers from the USA which suggested that parents had a high tolerance for false-positive results and that including them in a model made no significant difference to the outcome.
Discussion
This review identified a relatively low number of published economic evaluations of NBSP and screening technologies given the extent of national screening programmes on a global basis. Around half of the identified studies focussed on understanding the economic impact of newborn screening as a national programme. Of these studies, half were based in countries (Australia, France and The Netherlands) that all require parental consent prior to performing a newborn screening test. The need for parental consent infers an explicit need for a clear mechanism of information provision to be built into a national NBSP and has associated resource use implications and a potential to impact on the overall effectiveness of the programme and that parents should be able to make a choice about whether to participate in the programme. Even in countries where screening is mandatory, there is evidence of a clear demand for parental information [23, 68].
The advent of new technologies has meant it is now possible to expand the number of conditions included in an NBSP. The UK has recently almost doubled the number of conditions screened for [69] with more potential additions being debated. Internationally, there is continuous pressure to increase the number of conditions included in programmes. This expansion in the scope and scale of an NBSP involving informed consent will have direct resource use implications in terms of the required use of healthcare staff to provide information to parents, affect the potential use of subsequent healthcare resources, and have an impact on parents. Robust methods of economic evaluation are needed to quantify the impact of expanding newborn screening programmes in terms of the health gain to neonates and also the impact on parents and wider families. Furthermore, the impact on healthcare resources associated with information provision and subsequent use of follow-up services needs to be robustly identified and quantified to understand the full opportunity cost of expanding a newborn screening programme. Especially considering suboptimal communication does appear to have an impact on the overall cost of a NBSP, even if this does not necessarily mean that they are not cost effective.
Existing economic evaluations have not generally considered the healthcare costs or impact on parents of information provision as part of NBSP. Just five studies included a cost of information provision in their evaluation. A further three papers accounted for the potential effect of imperfect information provision and the impact on total health gain by capturing the disutility attached to parental anxiety. If the relevant parameters to quantify the impact of information provision in an economic evaluation of NBS are omitted, an implicit assumption is being made that parents, acting as advocates for their newborn, fully understand the benefits and costs of the NBSP, will always make informed decisions about whether or not to consent to screening and subsequently use healthcare resources appropriately. Yet, this does not fit with the evidence [70]. A lack of information and knowledge about the informed consent process has the potential to create additional subsequent costs which will not be quantified when economic evaluations assume that information provision is perfect and results in behaviours such as use of healthcare services. Importantly, when expanding a programme to include more conditions, the potential for increased costs associated with the time element of information provision should also be taken into account. On a population level, information provision costs are likely to be substantial and conclusions regarding the relative cost effectiveness of a introducing an expanded NBSP are likely to be influenced by this additional cost.
Expanding a newborn screening programme introduces a key element of uncertainty for parents. Parents have to make a decision about whether to participate in the newly expanded screening programme and also whether to screen for all the conditions now included. The process of expanding the NBSP has introduced the need for parents to understand additional, potentially complex, detail on the relative harms and benefits of screening for each condition. This information must be explained sufficiently by the person taking informed consent and must be read, digested and understood by the parent making the informed consent on behalf of the newborn baby. If sufficient information, or the process of providing the information, is not available then parents could be seen as making an uninformed choice. An informed choice would be made if more appropriate and tailored information were made available as part of the NBSP. Indeed there are repeated findings that people fail to appreciate the personal relevance of NBSP information and may opt out of screening [16, 71]. Alternatively, parents may consent to screening, but then experience high levels of anxiety and seek further services when they receive results which do not fit with their expectations (i.e. positive, false-positive or carrier) [32]. There is evidence, therefore, that insufficient information provision does have an impact on parents but this review has shown that there are few attempts to quantify the impact of information provision in published economic evaluations of expanded NBSP.
Inaccurate test results are likely to yield some anxiety in parents. Some degree of anxiety is appropriate in these circumstances but the impact of poor understanding of the implications of the test result may cause inappropriately sustained levels of anxiety leading to a measurable impact on health status of the parent [32]. Not only parents of newborns with an equivocal first result (commonly, referred to as a false-positive result) will experience momentary phases of anxiety. The true incidence of false-positive results, including the initial screening test and follow-up confirmatory test used in an NBSP, is likely to be extremely low. A more relevant concern will be any situation where the health professional must return to the parents be it for an initial equivocal test result that requires a follow-up test, or a new sample, to be carried out that also implies the need for repeat communication with the parents of the newborn. There is an increasing suggestion that whenever there is a need for a health professional to return to families for further samples (such as when the sample has been taken incorrectly) this can trigger parents to become quantifiably anxious and potentially reassess their engagement with screening. It has also been shown that parents receiving carrier results go through a similar phase of anxiety when waiting for confirmatory results of carrier status of cystic fibrosis [32]. There is, therefore, an argument that balancing the benefits compared with the cost of ‘false-positive’ test results is not a useful analytic approach. Rather it is necessary to identify and quantify the opportunity cost of repeat communication cases. This fits with an ongoing debate and the increasing call to stop using the term “false-positive” in the context of NBSPs as the terminology is of limited utility in this clinical context.
Anxiety and service use when waiting for test results regarding carrier results have been shown to be lower in parents who are adequately prepared and when communication is effective [32]. Whilst the studies [6, 39, 47, 58] in this review suggested that the impact of such anxiety may be relatively small when quantified in terms of relative health loss (as a utility value), there may still be an effect on the cost-effectiveness estimate and particular care should be made to take this into account when evaluating expanded programmes or technologies which may increase not only the false-positive rate, but the number of “repeat communication” cases. Furthermore, there are concerns that this is not adequately being accounted for in terms of resource use as there are numerous papers examining the impact of communication which suggest that there are real impacts on wider families [32].
We argue that the costs and dis-benefits from information provision should be included in economic evaluations of expanded NBS. However, we also recognise the methodological and practical challenges that this requirement to capture the costs and benefits of information provision introduces. There is limited evidence on the actual resource implications associated with information provision as part of NBSP and the potential impact on resource use if a programme was expanded. An ongoing study, funded as part of the UK National Institute for Health Research Health Technology Assessment programme [72] is currently generating these data for England, which would be needed to appropriately populate an economic evaluation. However, the requirements for non-English healthcare systems are likely to be different and country-specific data will be needed. There are no current data on the on the impact of poor information and false-positive results on the subsequent use of healthcare resources as a result of expanded NBSP, which is a substantial topic for further research.
Traditional units of measurements in economic evaluations assess the impact on health status using life-years saved or QALYs, which do include the impact on anxiety. The impact of anxiety in terms of QALYs is generally built up from a direct assessment of the utility associated anxiety, such as the time trade-off method used by Prosser et al. or by using an indirect measure of anxiety such as the health status measure the EQ-5D [73]. However, this focus on health status does not capture the impact on informed decision making per se and some commentators have suggested the need to extend beyond capturing the impact on health status alone by using measures of capability (or empowerment) to capture the impact of complex interventions, such as genetic testing or screening, on the ability to make an informed decision [65]. Using a measure that values the ability to make an informed decision as cognitive capability allows the analyst to capture non-health aspects of effective information provision. Measures of capability and associated population tariffs are available, such as the ICECAP-A [74]. Further methodological work is needed to understand if, and how, such measures can be used in the context of economic evaluations used to inform resource allocation in national healthcare systems. Specifically, it is necessary to understand how society values the relative benefits attached to health and non-health aspects of interventions [65]. Other evaluative approaches, such as discrete choice experiments and contingent valuation methods, have been suggested as a means of valuing the impact of informed decision making by estimating willingness to pay [75]. Using these methods to elicit willingness to pay could capture aspects such as the process utility [76] (value of how and by whom information is provided) in addition to the outcome [value of the (dis) benefit from the information]. Discrete choice experiments and contingent valuation have limited current practical application as they introduce a new evaluative framework of cost-benefit analysis, which is not consistent with the evaluation methods used in most national jurisdictions to inform resource allocation decisions [77].
Conclusion
This review has systematically identified if, and how, published economic evaluations of NBSP and screening-related technologies have taken account of the information provision as part of the informed consent process. There is a limited evidence base that quantifies the impact of information provision on the healthcare costs and impact on users of NBSP—the parents of newborns. We suggest that economic evaluations of expanded NBSP need to take account of information provision otherwise the impact on healthcare costs, newborns and their parents will be underestimated. To take this forward, however, research is needed to generate robust data on the actual cost of information provision for healthcare services, the impact of NBSP on subsequent use of healthcare services, particularly when a repeat communication event occurs, and also the impact on parents’ health caused by increased anxiety levels and ability to make an informed decision in the context of expanded NBSP.
Electronic supplementary material
Acknowledgments
Thanks go to Mary Ingram for her guidance on the electronic search strategy. We also want to thank Eleanor Heather for her assistance in screening the abstracts.
Compliance with ethical standards
The manuscript does not report new clinical studies or patient data and it was therefore not necessary to seek ethical approval for this study.
This project was funded by the National Institute for Health Research Health Technology Assessment (HTA) Programme (project number 11/62/02). The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the HTA, NIHR, NHS or the Department of Health.
Stuart Wright is employed on the NIHR HTA programme study (project number 11/62/02) “Providing Information about Newborn Screening Antenatally”. Otherwise he has no conflict of interest.
Cheryl Jones has no conflict of interest.
Katherine Payne is a co-applicant on the NIHR HTA programme study (project number 11/62/02) “Providing Information about Newborn Screening Antenatally”. Otherwise she has no conflict of interest.
Nimarta Dharni is employed on the NIHR HTA programme study (project number 11/62/02) “Providing Information about Newborn Screening Antenatally”. Otherwise she has no conflict of interest.
Fiona Ulph is lead-applicant on the NIHR HTA programme study (project number 11/62/02) “Providing Information about Newborn Screening Antenatally”. Otherwise she has no conflict of interest.
Author contributions
Stuart Wright was involved in the study conceptualisation, screening of abstracts in the review, data extraction, narrative synthesis and drafting of the manuscript.
Cheryl Jones was involved in the study conceptualisation, construction of the literature search, screening of abstracts and data extraction.
Katherine Payne was involved in the study conceptualisation, screening of abstracts, data extraction, drafting and editing of the manuscript.
Nimarta Dharni was involved in the study conceptualisation and editing of the manuscript.
Fiona Ulph was involved in the study conceptualisation and editing of the manuscript.
Katherine Payne is the guarantor for the content of the paper.
References
- 1.Centre for Disease Control Ten great public health achievements—United States, 2001–2010. Morb Mortal Wkl Rep. 2011;60:619–623. [PubMed] [Google Scholar]
- 2.Guthrie R, Susi A. A simple phenylalanine method for detecting phenylketonuria in large populations of newborn infants. Pediatrics. 1963;32(3):338–343. [PubMed] [Google Scholar]
- 3.Sweetman L. Newborn screening by tandem mass spectrometry (MS-MS) Clin Chem. 1996;42:345–346. [PubMed] [Google Scholar]
- 4.Pandor A, Eastham J, Chilcott J, Paisley S, Beverley C. Economics of tandem mass spectrometry screening of neonatal inherited disorders. Int J Technol Assess. 2006;3:321–326. doi: 10.1017/s026646230605121x. [DOI] [PubMed] [Google Scholar]
- 5.Public Health England. Introducing newborn blood spot screening for parents. 2013. http://newbornbloodspot.screening.nhs.uk/public. Accessed 5 Nov 2014.
- 6.Prosser LA, Kong CY, Rusinak D, Waisbren SL. Projected costs, risks, and benefits of expanded newborn screening for MCADD. Pediatrics. 2010;125(2):E286–E294. doi: 10.1542/peds.2009-0605. [DOI] [PubMed] [Google Scholar]
- 7.La Pean A, Farrell MH. Initially misleading communication of carrier results after newborn genetic screening. Pediatrics. 2005;116(6):1499–1505. doi: 10.1542/peds.2005-0449. [DOI] [PubMed] [Google Scholar]
- 8.Farrell MH, Certain LK, Farrell PM. Genetic counselling and risk communication services of newborn screening programmes. Arch Pediat Adol Med. 2001;155:120–126. doi: 10.1001/archpedi.155.2.120. [DOI] [PubMed] [Google Scholar]
- 9.Farrell MH, Farrell PM. Newborn screening for cystic fibrosis: ensuring more good than harm. J Pediatr. 2003;143:707–712. doi: 10.1016/j.jpeds.2003.09.022. [DOI] [PubMed] [Google Scholar]
- 10.Southern KW. Newborn screening for cystic fibrosis: the practical implications. J R Soc Med. 2004;97(44):57–59. [PMC free article] [PubMed] [Google Scholar]
- 11.Dillard JP, Tluczek A. Information flow after a positive newborn screening for cystic fibrosis. J Pediatr. 2005;147:s94–s97. doi: 10.1016/j.jpeds.2005.08.021. [DOI] [PubMed] [Google Scholar]
- 12.Burgard P, Cornel M, Di Filippo F, Haege G, Hoffmann GF, Lindner M, Loeber, JG, Rigter, T, Rupp, K, Taruscio, D, Weinreich, S, Vittozzi, L. Report on the practices of newborn screening for rare disorders implemented in Member States of the European Union, Candidate, Potential Candidate and EFTA Countries. 2012. http://ec.europa.eu/chafea/documents/news/Report_NBS_Current_Practices_20120108_FINAL.pdf. Accessed 5 Nov 2014.
- 13.Waisbren SE. Newborn screening for metabolic disorders. JAMA. 2006;296(8):993–995. doi: 10.1001/jama.296.8.993. [DOI] [PubMed] [Google Scholar]
- 14.Tluczek A, Orland KM, Nick SW, Brown RL. Newborn screening an appeal for improved parent education. J Perinat Neonatal Nurs. 2009;23(4):326–334. doi: 10.1097/JPN.0b013e3181a1bc1f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wilcken B. Expanded newborn screening: reducing harm, assessing benefit. J Inherit Metab Dis. 2010;33:205–210. doi: 10.1007/s10545-010-9106-6. [DOI] [PubMed] [Google Scholar]
- 16.Kai J, Ulph F, Cullinan T, Qureshi N. Communication of carrier status information following universal newborn screening for sickle cell disorders and cystic fibrosis: qualitative study of experience and practice. Health Technol Assess. 2009;13(57):1–106. doi: 10.3310/hta13570. [DOI] [PubMed] [Google Scholar]
- 17.Pollitt RJ, Green A, McCabe CJ, Booth A, Cooper NJ, Leonard JV, Nicholl J, Nicholson P, Tunaley JR, Virdi NK. Neonatal screening for inborn errors of metabolism: cost, yield and outcome. Health Technol Assess. 1997;1(7):i–iv, 1–202. [PubMed]
- 18.Morrison DR, Clayton EW. False positive newborn screening results are not always benign. Public Health Genomics. 2011;14(3):173–177. doi: 10.1159/000322527. [DOI] [PubMed] [Google Scholar]
- 19.Nicholls S, Southern KW. Parental information use in the context of newborn bloodspot screening. An exploratory mixed methods study. J Community Genet. 2012;3(4):251–257. doi: 10.1007/s12687-012-0082-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Campbell E, Ross LF. Incorporating newborn screening into prenatal care. Am J Obstet Gynecol. 2004;190:876–877. doi: 10.1016/j.ajog.2003.12.026. [DOI] [PubMed] [Google Scholar]
- 21.Smith R, Williams D, Sibert J, Harper P. Attitudes of mothers to neonatal screening for Duchenne muscular dystrophy. BMJ. 1990;300:1112. doi: 10.1136/bmj.300.6732.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tluczek A, Mischler E, Farrell PM. Parents’ knowledge of neonatal screening and response to false-positive cystic fibrosis testing. J Dev Behav Pedaitr. 1992;13:181–186. [PubMed] [Google Scholar]
- 23.Campbell E, Ross LF. Parental attitudes regarding newborn screening of PKU and DMD. Am J Med Genet. 2003;120A:209–214. doi: 10.1002/ajmg.a.20031. [DOI] [PubMed] [Google Scholar]
- 24.Suriadi C, Jovanovska M, Quinlivian J. Factors affecting mothers’ knowledge of genetic screening. Aust NZ J Obstet Gynecol. 2004;44:30–34. doi: 10.1111/j.1479-828X.2004.00171.x. [DOI] [PubMed] [Google Scholar]
- 25.Faden R, Chwalow AJ, Holtzman NA, Horn SD. A survey to evaluate parental consent as public policy for neonatal screening. Am J Public Health. 1982;72:1347–1352. doi: 10.2105/AJPH.72.12.1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dankert-Roelse J, Knol K, ten Kate L. Effects of neonatal screening for cystic fibrosis on reproduction, attitudes toward reproductive behaviour and genetic knowledge. Acta Univ Carol. 1990;36:99–101. [PubMed] [Google Scholar]
- 27.Statham H, Green J, Snowdon C. Mothers’ consent to screening newborn babies for disease. BMJ. 1993;306:858–859. doi: 10.1136/bmj.306.6881.858-c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Davis TC, Humiston SG, Arnold CL, Bocchini JA, Bass PF, Kennen EM, Bocchini A, Williams D, Kyler P, Lloyds-Puryear M. Recommendations for effective newborn screening communication: results of focus groups with parents, providers, and experts. Pediatrics. 2006;117:S326–S340. doi: 10.1542/peds.2005-2633M. [DOI] [PubMed] [Google Scholar]
- 29.Locock L, Kai J. Parents’ experiences of universal screening for haemoglobin disorders: implications for practice in a new genetics era. Br J Gen Pract. 2008;58:161–168. doi: 10.3399/bjgp08X277276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schmidt JL, Castellanos-Brown K, Childress S, Bonhomme N, Oktay JS, Terry SF, Kyler P, Davidoff A, Greene C. The impact of false-positive newborn screening results on families: a qualitative study. Genet Med. 2012;14:76–80. doi: 10.1038/gim.2011.5. [DOI] [PubMed] [Google Scholar]
- 31.Waisbren SE, Albers S, Amato S, Ampola M, Brewster TG, Demmer L, Eaton RB, Greenstein R, Korson M, Larson C, Marsden D, Msall M, Naylor EW, Pueschel S, Seashore M, Shi VE, Levy HL. Effect of expanded newborn screening for biochemical genetic disorders on child outcomes and parental stress. JAMA. 2003;290(19):2564–2572. doi: 10.1001/jama.290.19.2564. [DOI] [PubMed] [Google Scholar]
- 32.Ulph F, Cullinan T, Qureshi N, Kai J. Parents’ responses to receiving sickle cell or cystic fibrosis carrier results for their child following newborn screening. Eur J Hum Genet. 2015;23:459–465. doi: 10.1038/ejhg.2014.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Burton H, Moorthie S. Expanded newborn screening: a review of the evidence. Cambridge PHG Foundation. 2010. http://www.phgfoundation.org/reports/5504/. Accessed 6 Nov 2014.
- 34.Langer A, Holle R, John J. Specific guidelines for assessing and improving the methodological quality of economic evaluations of newborn screening. BMC Health Serv. 2012;12:300. doi: 10.1186/1472-6963-12-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Centre for Reviews and Dissemination. Systematic reviews: CRD’s guidance for undertaking reviews in health care. 2009. http://www.york.ac.uk/inst/crd/SysRev/!SSL!/WebHelp/SysRev3.htm. Accessed 6 Nov 2014.
- 36.Centre for Reviews and Dissemination. NHS economic evaluation database handbook. 2007. http://www.york.ac.uk/inst/crd/pdf/nhseed-handbook2007.pdf. Accessed 6 Nov 2014.
- 37.Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, Augustovski F, Briggs AH, Mauskopf J, Loder E. Consolidated health economic evaluation reporting standards (CHEERS) statement. BMJ. 2013;346:f1049. doi: 10.1136/bmj.f1049. [DOI] [PubMed] [Google Scholar]
- 38.Autti-Rämö I, Mäkelä M, Sintonen H, Koskinen H, Laajalahti L, Halila R, Kääriäinen H, Lapatto R, Näntö-Salonen K, Pulkki K, Renlund M, Salo M, Tyni T. Expanding screening for rare metabolic disease in the newborn: an analysis of costs, effect and ethical consequences for decision-making in Finland. Acta Paediatr. 2005;94(8):1126–1136. doi: 10.1080/08035250510029497. [DOI] [PubMed] [Google Scholar]
- 39.Cipriano LE, Rupar CA, Zaric GS. The cost-effectiveness of expanding newborn screening for up to 21 inherited metabolic disorders using tandem mass spectrometry: results from a decision-analytic model. Value Health. 2007;10(2):8397. doi: 10.1111/j.1524-4733.2006.00156.x. [DOI] [PubMed] [Google Scholar]
- 40.Feuchtbaum L, Cunningham G. Economic evaluation of tandem mass spectrometry screening in California. Pediatrics. 2006;117(5 Pt 2):S280–S286. doi: 10.1542/peds.2005-2633G. [DOI] [PubMed] [Google Scholar]
- 41.Insinga RP, Laessig RH, Hoffman GL. Newborn screening with tandem mass spectrometry: examining its cost-effectiveness in the Wisconsin Newborn Screening Panel. J Pediatr. 2002;141(4):524–531. doi: 10.1067/mpd.2002.128116. [DOI] [PubMed] [Google Scholar]
- 42.Norman R, Hall J, Street D, Viney R. Economic evaluation of tandem mass spectrometry newborn screening in Australia. Pediatrics. 2009;123(2):451–457. doi: 10.1542/peds.2008-0911. [DOI] [PubMed] [Google Scholar]
- 43.Pandor A, Eastham J, Beverley C, Chilcott J, Paisley S. Neonatal screening for inborn errors of metabolism using tandem mass spectrometry. Health Technol Assess. 2004;8:12. doi: 10.3310/hta8120. [DOI] [PubMed] [Google Scholar]
- 44.Schoen EJ, Baker JC, Colby CJ, To TT. Cost-benefit analysis of universal tandem mass spectrometry for newborn screening. Pediatrics. 2002;110(4):781–786. doi: 10.1542/peds.110.4.781. [DOI] [PubMed] [Google Scholar]
- 45.Shamshiri AR, Yarahmadi S, Forouzanfar MH, Haghdoost AA, Hamzehloo G, Naieni KH. Evaluation of current guthrie TSH cut-off point in Iran congenital hypothyroidism screening program: a cost-effectiveness analysis. Arch Iran Med. 2012;15(3):136–141. [PubMed] [Google Scholar]
- 46.Tran K, Banerjee S, Li H, Noorani HZ, Mensinkai S, Dooley K. Clinical efficacy and cost-effectiveness of newborn screening for medium chain acyl-CoA dehydrogenase deficiency using tandem mass spectrometry. Clin Biochem. 2007;40(3–4):235–241. doi: 10.1016/j.clinbiochem.2006.10.022. [DOI] [PubMed] [Google Scholar]
- 47.Venditti LN, Venditti CP, Berry GT, Kaplan PB, Kaye EM, Glick H, Stanley CA. Newborn Screening by tandem mass spectrometry for medium-chain acyl-CoA dehydrogenase deficiency: a cost-effectiveness analysis. Pediatrics. 2003;112(5):1005–1015. doi: 10.1542/peds.112.5.1005. [DOI] [PubMed] [Google Scholar]
- 48.Carroll AE, Downs SM. Comprehensive cost-utility analysis of newborn screening strategies. Pediatrics. 2006;117(5 Pt 2):S287–S295. doi: 10.1542/peds.2005-2633H. [DOI] [PubMed] [Google Scholar]
- 49.Chan K, Davis J, Pai S, Bonilla FA, Puck JM, Apkon M. A Markov model to analyze cost-effectiveness of screening for severe combined immunodeficiency (SCID) Mol Genet Metab. 2011;104(3):383–389. doi: 10.1016/j.ymgme.2011.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Geelhoed E, Lewis B, Hounsome D, O’Leary P. Economic evaluation of neonatal screening for phenylketonuria and congenital hypothyroidism. J Paediatr Child Health. 2005;41(11):575–579. doi: 10.1111/j.1440-1754.2005.00725.x. [DOI] [PubMed] [Google Scholar]
- 51.Gessner BD, Teutsch SM, Shaffer P. A cost-effectiveness evaluation of newborn hemoglobinopathy screening from the perspective of state health care systems. Early Hum Dev. 1996;45:257–275. doi: 10.1016/0378-3782(96)01761-6. [DOI] [PubMed] [Google Scholar]
- 52.Hamers FF, Rumeau-Pichon C. Cost-effectiveness analysis of universal newborn screening for medium chain acyl-CoA dehydrogenase deficiency in France. BMC Paediatr. 2012;12(1):60. doi: 10.1186/1471-2431-12-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lanting CI, van Tijn DA, Loeber JG, Vulsma T, de Vijlder JJM, Verkerk PH. Clinical effectiveness and cost-effectiveness of the use of the thyroxine/thyroxine-binding globulin ratio to detect congenital hypothyroidism of thyroidal and central origin in a neonatal screening program. Pediatrics. 2005;116(1):168–173. doi: 10.1542/peds.2004-2162. [DOI] [PubMed] [Google Scholar]
- 54.McGhee S, Stiehm E, McCabe E. Potential costs and benefits of newborn screening for severe combined immunodeficiency. J Paediatr. 2005;147(5):603–608. doi: 10.1016/j.jpeds.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 55.Panepinto JA, Magid D, Rewers MJ, Lane PA. Universal versus targeted screening of infants for sickle cell disease: a cost-effectiveness analysis. J Pediatr. 2000;136(2):201–208. doi: 10.1016/S0022-3476(00)70102-8. [DOI] [PubMed] [Google Scholar]
- 56.Sladkevicius E, Pollitt RJ, Mgadmi A, Guest JF. Cost effectiveness of establishing a neonatal screening programme for phenylketonuria in Libya. Appl Health Econ Health Policy. 2010;8(6):407–420. doi: 10.2165/11535530-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 57.Simpson N, Anderson R, Sassi F, Pitman A, Lewis P, Tu K, Lannin H. The cost-effectiveness of neonatal screening for cystic fibrosis: an analysis of alternative scenarios using a decision model. Cost Eff Resour Alloc: C/E. 2005;3:8. doi: 10.1186/1478-7547-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tiwana SK, Rascati KL, Park H. Cost-effectiveness of expanded newborn screening in Texas. Value Health. 2012;15(5):613–621. doi: 10.1016/j.jval.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 59.van den Akker-van Marle ME, Dankert HM, Verkerk PH, Dankert-Roelse J. Cost-effectiveness of 4 neonatal screening strategies for cystic fibrosis. Pediatrics. 2006;118(3):896–905. doi: 10.1542/peds.2005-2782. [DOI] [PubMed] [Google Scholar]
- 60.van der Hilst CS, Derks TGJ, Rejngoud D, Smit PA, TenVergert EM. Cost-effectiveness of neonatal screening for medium chain acyl-CoA dehydrogenase deficiency: the homogeneous population of The Netherlands. J Paediatr. 2007;151(2):115–120. doi: 10.1016/j.jpeds.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 61.Wells J, Rosenberg M, Hoffman G, Anstead M, Farrell PM. A decision-tree approach to cost comparison of newborn screening strategies for cystic fibrosis. Pediatrics. 2012;129(2):e339–e347. doi: 10.1542/peds.2011-0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wildhagen MF, Hilderink HBM, Verzijl JG, Verheij JBGM, Kooij L, Tijmstra T, ten Kate LP, Habbema JDF. Costs, effects, and savings of screening for cystic fibrosis gene carriers. J Epidemiol Commun H. 1998;52:459–467. doi: 10.1136/jech.52.7.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kilakkathi V. Newborn screening in america: problems and policies. Council Responsible for Genetics. 2012. http://www.councilforresponsiblegenetics.org/pageDocuments/WNMAKEPP1P.pdf. Accessed 6 Nov 2014.
- 64.Hanley WB. Newborn screening in Canada-Are we out of step? Paediatr Child Health. 2005;10(4):203–207. doi: 10.1093/pch/10.4.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Payne K, McAllister M, Davies LM. Valuing the economic benefits of complex interventions: when maximising health is not sufficient. Health Econ. 2013;22:258–271. doi: 10.1002/hec.2795. [DOI] [PubMed] [Google Scholar]
- 66.Autti-Rämö I. HTA on neonatal screening for rare metabolic disorders faced misconceptions and blurred objectivity. Orphanet J Rare Dis. 2012;7(Suppl 2):A17. doi: 10.1186/1750-1172-7-S2-A17. [DOI] [Google Scholar]
- 67.Prosser LA, Ladapo JA, Rusinak D, Waisbren SE. Parental tolerance of false-positive newborn screening results. Arch Pediatr Adolesc Med. 2008;162(9):870–876. doi: 10.1001/archpediatrics.2008.1. [DOI] [PubMed] [Google Scholar]
- 68.Lipstein EA, Nabi E, Perrin JM, Luff D, Browning MF, Kuhlthau KA. Parents’ decision-making in newborn screening: opinions, choices, and information needs. Pediatrics. 2010;126:696–704. doi: 10.1542/peds.2010-0217. [DOI] [PubMed] [Google Scholar]
- 69.NHS Choices. Four rare diseases added to newborn screening. 2014. http://www.nhs.uk/news/2014/05May/Pages/Four-rare-diseases-added-to-newborn-screening.aspx. Accessed 6 Nov 2014.
- 70.Moody L, Choudhry K. Parental views on informed consent for expanded newborn screening. Health Expect. 2013;16(3):239–250. doi: 10.1111/j.1369-7625.2011.00710.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Noke M, Ulph F. Adults’ pre-existing knowledge of cystic fibrosis and sickle cell diseases: implications for newborn screening. J Genet Couns. 2014;23(1):121–130. doi: 10.1007/s10897-013-9622-2. [DOI] [PubMed] [Google Scholar]
- 72.National Institute for Health Research. HTA—11/62/02: the provision of antenatal information for the NHS Newborn Bloodspot Screening Programme (NBSP). 2013. http://www.nets.nihr.ac.uk/projects/hta/116202. Accessed 6 Nov 2014.
- 73.EuroQol. What is EQ-5D. 2014. http://www.euroqol.org/. Accessed 6 Nov 2014.
- 74.Flynn TN, Huynh E, Peters TJ, Al-Janabi H, Moody A, Clemens S, Coast J. Scoring the ICECAP—a capability instrument. Estimation of a UK general population tariff. Health Econ. 2015;24(3):258–269. doi: 10.1002/hec.3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Grosse SD, Wordsworth S, Payne K. Economic methods for valuing the outcomes of genetic testing: beyond cost-effectiveness analysis. Genet Med. 2008;10(9):648–654. doi: 10.1097/GIM.0b013e3181837217. [DOI] [PubMed] [Google Scholar]
- 76.Donaldson C, Shackley P. Does, “process utility” exist? A case study of willingness to pay for laparoscopic cholecystectomy. Soc Sci Med. 1997;44(5):699–707. doi: 10.1016/S0277-9536(96)00215-8. [DOI] [PubMed] [Google Scholar]
- 77.International Society for Pharmacoeconomics and Outcomes Research. Pharmacoeconomic guidelines around the world. 2014. http://www.ispor.org/peguidelines/index.asp. Accessed 6 Nov 2014.
- 78.Drummond MF, Sculpher MJ, Torrance GW, O’Brien BJ, Stoddart GL. Methods for the economic evaluation of health care programmes. 3. New York: Oxford University Press; 2005. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.