Abstract
Objectives
The purpose of this study was to evaluate the histopathological effects of an antioxidant therapy on the pulp tissue of rat teeth exposed to a bleaching gel with 35% hydrogen peroxide.
Materials and Methods
Forty rats were subjected to oral ingestion by gavage of distilled water (DW) or ascorbic acid (AA) 90 min before the bleaching therapy. For the bleaching treatment, the agent was applied twice for 5 min each to buccal surfaces of the first right mandibular molars. Then, the animals were sacrificed at 6 hr, 24 hr, 3 day, or 7 day post-bleaching, and the teeth were processed for microscopic evaluation of the pulp tissue.
Results
At 6 hr, the pulp tissue showed moderate inflammatory reactions in all teeth of both groups. In the DW and AA groups, 100% and 80% of teeth exhibited pulp tissue with significant necrosis and intense tissue disorganization, respectively. At 24 hr, the AA-treated group demonstrated a greater regenerative capability than the DW group, with less intense inflammatory reaction and new odontoblast layer formation in 60% of the teeth. For up to the 7 day period, the areas of pulpal necrosis were replaced by viable connective tissue, and the dentin was underlined by differentiated odontoblast-like cells in most teeth of both groups.
Conclusions
A slight reduction in initial pulpal damage during post-bleaching was promoted by AA therapy. However, the pulp tissue of AA-treated animals featured faster regenerative potential over time.
Keywords: Ascorbic acid, Dental pulp, Hydrogen peroxide, Tooth bleaching
Introduction
Reports in the literature have already demonstrated that hydrogen peroxide (HP) present in bleaching gels may be toxic to dental pulp cells by inducing oxidative stress, cell membrane damage and up-regulation of inflammatory mediators, culminating in cell death by necrosis.1,2,3 The intensity of these adverse events is directly related to the concentration of HP in the gels and the number of bleaching sessions.1,2,3,4 Moreover, clinical and histopathological studies have shown that the thinner the enamel/dentin thickness, the more intense the tooth sensitivity and the more severe the damage to the pulp tissue.5,6,7
Different therapies have been proposed to prevent or reduce the pulpal damage and the consequent tooth sensitivity caused by professional tooth bleaching. Prebleaching administration of anti-inflammatory drugs and topical application of sodium fluoride and potassium nitrate desensitizing agents as well as the use of antioxidant molecules have been widely assessed.8,9,10,11,12,13,14,15 Since the pathway for pulp tissue damage following tooth bleaching involves oxidative damage to pulp cells, therapies using antioxidant agents have been recognized as interesting alternatives for preventing the occurrence of conditions leading to intense oxidative stress.13,15 Antioxidant agents have been successfully used to restore optimal bonding after bleaching treatment.16,17,18 In addition, previous studies have demonstrated that antioxidant molecules, such as alpha-tocoferol and ascorbic acid (AA), were capable of preventing HP-mediated pulp cell damage.11,12,13,15 According to the authors, these antioxidant agents may inactivate the extracellular reactive oxygen species (ROS) from bleaching gels, as well as the intracellular ROS released by cells undergoing oxidative stress.
It is known that AA therapy has garnered special attention for the treatment of several diseases, such as hypercholesterolemia, atherosclerosis and periodontitis, which are directly related to oxidative cell stress.19,20,21 AA is a hydrosoluble vitamin with intense antioxidant potential and plays an important role in wound healing.22,23,24,25,26 In a recent in vivo clinical trial, Paula et al. observed no reduction in tooth sensitivity in patients subjected to the oral administration of AA prior to bleaching procedures.14 However, the authors did not perform microscopic evaluation of the pulp condition after this esthetic therapy. Conversely, Lima et al. demonstrated that AA can reduce bleaching-induced pulp cell toxicity by 1.5 to 2.4 times.11,12 Therefore, since there are no scientific data available concerning the in vivo protective role of AA against the toxic effects of bleaching agents on pulp tissue, the aim of the present study was to evaluate, under light microscopy, the pulpal responses of bleached teeth of rats subjected or not to prior AA therapy. The null hypothesis was that AA therapy would have no protective effect on the immediate toxicity of bleaching therapy to rat pulp tissue as well as on its regenerative potential over time.
Materials and Methods
Experimental design
Eighty teeth from 40 male Wistar rats (less than 300 g) were used in this in vivo study. During the experiment, the animals were housed in plastic cages, with access to food and water ad libitum. All procedures performed were approved by the Institute of Biology's Ethical Committee for Animal Research (CEP/FOAr.-UNESP # 2515-1/2011). The animals were subjected to oral ingestion by gavage of distilled water (DW) or AA (5 mL) 90 minutes before the bleaching therapy. A dose of 200 mg/kg of AA (Sigma-Aldrich Co., St. Louis, MO, USA), diluted in DW, was used in the present investigation since it was previously demonstrated as being capable of preventing oxidative damage mediated by arsenic on blood, liver and kidney cells after oral administration in rats.27 After pretreatment with DW or AA, the animals were subjected to the bleaching procedure, and histopathological analysis was performed at four periods post-bleaching: 6 hours, 24 hours, 3 days, and 7 days. The animals were randomly divided into 8 experimental groups (5 rats / group) such that the 5 right mandibular molars were subjected to bleaching therapy and the 5 left mandibular molars received no treatment (negative control). Therefore, 80 teeth were used in this study according to the established treatments and periods of analysis.
Bleaching procedure
To receive the bleaching treatment, the animals were anesthetized with an intramuscular injection of ketamine (40 mg/kg) (Francotar; Virbac do Brazil Industria e Comércio, Roseira, SP, Brazil) and xylazine (5 mg/mL) (Virbaxil, Virbac do Brazil Industria e Comércio). The buccal surfaces of the teeth were cleaned with a piece of sterile gauze before application of the bleaching agent containing 35% hydrogen peroxide (HP, Whiteness HP Maxx, FGM, Joinville, SC, Brazil). The product was applied to the buccal surfaces of the first right mandibular molars twice for 5 minutes each, to simulate in-office therapy. To prevent any possible damage to oral mucosal tissues caused by the bleaching agent, small cotton rolls were used for relative isolation, and aspiration tips were used to remove the product from tooth surfaces. The non-bleached left mandibular molars were used as negative controls.
In the clinical situation, the 35% HP gel used in the present study is applied for up to three 15 minute periods at each bleaching session. However, in the present study, the application period was shortened to compensate for the differences between enamel/dentin thicknesses in human and rat teeth. In this way, a relationship between the dentin thickness of mandibular incisor human teeth and time of exposure to the bleaching agent was identified, and a similar proportion was used to determine the time of application of the bleaching agent to the rat molars.7,28
Histopathological analysis
At 6 hours, 24 hours, 3 days, and 7 days post-bleaching, the animals were sacrificed, the jaws dissected en bloc, halved, and fixed for 48 hours in 10% formalin solution (Labsynth Produtos para Laboratórios, Diadema, SP, Brazil) at room temperature. After being rinsed for 24 hours at room temperature, the jaws were demineralized in 4.12% ethylenediaminetetraacetic acid (EDTA) for 40 days, and embedded in paraffin. Six-micrometer-thick serial sections were cut (820 Spencer Microtome, Spencer products co., Carson, CA, USA), mounted on glass slides, and stained with hematoxylin and eosin (H&E) and Masson's trichrome. Using a light microscope (62774, Carl Zeiss Inc., Oberköchen, Germany), a calibrated pathologist blinded to the groups evaluated all sections.7,29 The histopathological events were evaluated and classified by a descriptive analysis according to the criteria presented in Table 1.
Table 1. Histopathological events and scores.
| Histopathologic event | Score | |||
|---|---|---|---|---|
| 0 | 1 | 2 | 3 | |
| Inflammatory cell response | None or a few scattered inflammatory cells present in the pulp area corresponding to the buccal surface of the tooth in which the bleaching gel was applied | Slight inflammatory cell infiltrate with polymorphonuclear (PMNs) or mononuclear leukocytes (MNLs) | Moderate inflammatory cell infiltrate | Severe inflammatory cell infiltrate |
| Tissue disorganization | Normal tissue | Odontoblastic layer disorganized but central pulp normal | Partial coronal pulp necrosis associated with discrete disorganization of the reminiscent pulp tissue | Coronal pulp necrosis associated (or not) with dystrophic calcification |
Statistical analysis
Fisher's exact test was applied to evaluate the association between period of evaluation and inflammatory response or tissue disorganization for both treatment groups. Next, treatments were compared within each period of evaluation. Statistical inferences were considered at the 5% level of significance.
Results
The percentages for each histopathological event at each time-point in both experimental groups are presented in Table 2. A significant association was revealed between both treatments and the periods of evaluation, for the inflammatory cell response as well as for tissue disorganization, indicating that a decrease in or absence of inflammatory response and better tissue organization were observed for the longer periods of analysis. In general, similar initial pulp damage was observed in the teeth of animals whether or not subjected to AA administration before bleaching therapy. A tendency toward reduction with time of inflammatory cell response and tissue disorganization was observed in both groups, with the AA-treated groups demonstrating faster recovery capability over time. No statistically significant differences were detected for cellular inflammatory response when both treatments were compared. However, at the 24 hour period, there was significantly better tissue organization when the animals received the administration of AA prior to the bleaching treatment. Detailed information regarding both groups at each period of analysis is presented below.
Table 2. Percentages for each experimental group, according to periods of evaluation, treatment performed and histopathological events.
| Histopathologic event | Treatment | Period | Score (%*) | |||
|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | |||
| Inflammatory cell response | DW + HP | 6 hr | 0 | 0 | 100 | 0 |
| 24 hr | 0 | 40 | 60 | 0 | ||
| 3 day | 0 | 60 | 40 | 0 | ||
| 7 day | 80 | 20 | 0 | 0 | ||
| AA + HP | 6 hr | 0 | 0 | 100 | 0 | |
| 24 hr | 0 | 60 | 40 | 0 | ||
| 3 day | 0 | 80 | 20 | 0 | ||
| 7 day | 100 | 0 | 0 | 0 | ||
| Tissue disorganization | DW + HP | 6 hr | 0 | 0 | 0 | 100 |
| 24 hr | 0 | 0 | 0 | 100 | ||
| 3 day | 0 | 60 | 40 | 0 | ||
| 7 day | 80 | 20 | 0 | 0 | ||
| AA + HP | 6 hr | 0 | 0 | 20 | 80 | |
| 24 hr | 0 | 60 | 40 | 0 | ||
| 3 day | 0 | 80 | 20 | 0 | ||
| 7 day | 100 | 0 | 0 | 0 | ||
DW, distilled water; AA, ascorbic acid.
*Numbers represent the percentage of each score according to the total number of teeth in each group.
6 hour period
Both groups (with or without oral administration of AA) exhibited root pulp preserved with a continuous odontoblast layer. However, areas of root pulp showed hyaline alteration and proliferation of blood vessels. In the coronal pulp tissue, 100% of teeth from the DW-treated group showed pulpal necrosis of the upper part of the pulp horn, associated with intense disorganization of remaining pulp tissue (Figures 1a - 1c). In 20% of those teeth from the AA-treated group, the root pulp tissue was preserved, and a smaller necrotic area was observed in the coronal pulp compared with the DW-treated group (Figures 1d and 1e). Moderate inflammatory reaction was observed in pulp tissue of all teeth (100%) in both the DW- and AA-treated groups.
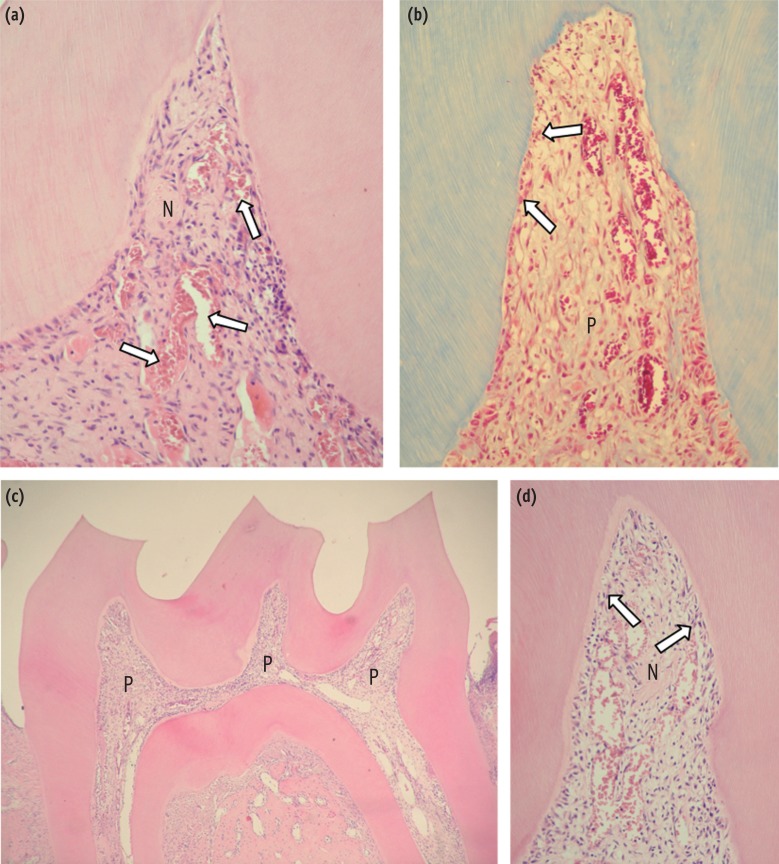
Figure 1. (a) DW-6 hr. Pulp horn presenting partial necrosis (N). The root pulp presents normal histological characteristics (arrows). H&E, ×32; (b) DW-6 hr. High magnification of (a). Observe that adjacent to the necrotic area (N), there is an intense inflammatory infiltrate of macrophages and neutrophils, and an overflow of blood plasma (arrow). H&E, ×250; (c) AA-6 hr. Presence of a large necrotic area (N) in the pulp horn of a DW-treated rat, and adjacent to this area one can observe viable pulp (P) tissue with inflammatory cell response. H&E, ×125; (d) AA-6 hr. Panoramic view of the pulp horn. Note smaller areas of necrosis (N) when compared with the pulp tissue of DW-treated rats in which the molars were bleached in (a). H&E, ×32; (e) AA-6 hr. High magnification of (d), in which one can observe that a broad area of the pulp (P) adjacent to the necrosis (N) is viable. H&E, ×64; (f) AA-6 hr. Viable coronal pulp (P) tissue, albeit with an inflammatory reaction, can be observed adjacent to the necrotic (N) area. H&E, ×125.
24 hour period
Initial fibro-angioblastic proliferation was observed in the pulp tissue of the DW-treated teeth subjected to bleaching treatment. In the upper area of the pulp horn, residual necrotic tissue surrounded by numerous macrophages and neutrophils was still observed in 100% of teeth (Figure 2a). Advanced pulpal regeneration occurred in 60% of those teeth from the AA-treated group, in which some new, differentiated odontoblast-like cells were underlying the predentin (Figure 2b). Necrotic areas were observed in only 40% of AA-treated teeth at this period. This group also featured a higher prevalence of slight inflammatory reaction (60%) in comparison with the DW-treated group, in which 60% of teeth exhibited moderate inflammatory reaction.
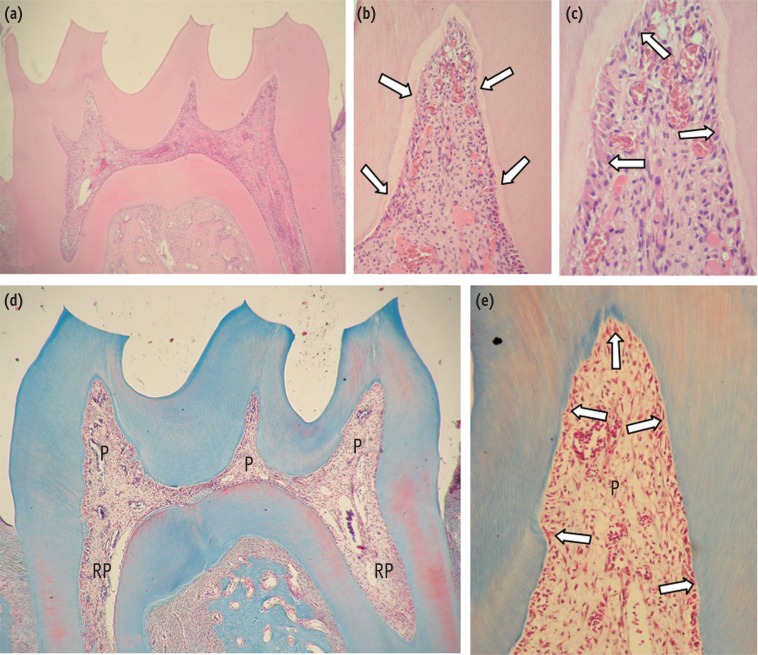
Figure 2. (a) DW-24 hr. Residual necrotic tissue (N) surrounded by macrophages as well as a large area of fibro-angioblastic proliferation can be observed along with numerous dilated and congested blood vessels (arrows). H&E, ×250; (b) AA-24 hr. Intense fibro-angioblastic proliferation in the pulp horn as well as some differentiated odontoblast-like cells underlying the predentin (arrows) can be seen. Only a slight mononuclear inflammatory cell infiltrate in the pulp (P) is observed. Masson's trichrome, ×250; (c) AA-3 day. Panoramic view of the pulp horn in which new sound connective tissue is replacing the previously necrotic areas of the pulp. H&E, ×32; (d) DW-3 day. Detail of Figure 2(c), where a residual necrotic area (N) surrounded by a few macrophages is observed. Note that new viable connective pulp tissue with numerous small blood vessels covers almost the entire pulp horn. A continuous layer of odontoblasts is underlying the predentin (arrows). H&E, ×125.
3 day period
The coronal pulp tissue of all teeth subjected to the bleaching treatment (DW or AA group) presented intense fibro-angioblastic proliferation, with few macrophages in the pulp horn zone. The necrosis was almost entirely replaced by new, highly cellularized connective pulp tissue, with numerous small blood vessels (Figure 2c). Small necrotic areas in the pulp horn still remained in 40% of teeth of the DW-treated and in 20% of the AA-treated groups (Figure 2d).
7 day period
New connective pulp tissue, highly cellularized and vascularized, was observed, replacing the previously necrotic tissue in 100% of teeth in both groups (Figure 3a). A continuous and homogeneous layer of new odontoblast-like cells underlying the predentin was observed (Figures 3b and 3c). The root pulp tissue presented normal histological features.
Figure 3. (a) AA-7 day. Panoramic view of the coronal pulp horn with viable pulp tissue. H&E, ×32; (b) AA-7 day. A thin layer of collagen-rich dentin matrix (predentin, arrows) is underlying the mineralized dentin. No residual necrotic area can be seen. H&E, ×125; (c) AA-7 day. Upper region of the pulp horn, where the predentin is underlined by a layer of new differentiated odontoblast-like cells (arrows). Note that the pulp tissue regeneration process is basically complete. H&E, ×250; (d) Control group. Coronal (P) and radicular (RP) pulp tissue exhibits normal histologic characteristics. Masson's trichrome, ×32; (e) Control group. Detail of (d). The odontoblast layer underlying the dentin substrate (arrows) and the subjacent pulp (P) tissue present equilibrium between the cells and extracellular matrix. Masson's trichrome, ×125.
Negative control group
In the negative control group (without bleaching treatment), the root and coronal pulp tissue presented normal histological characteristics (Figure 3d). A continuous odontoblastic layer was underlying the entire dentin substrate, and the subjacent pulp tissue presented equilibrium among blood vessels, extracellular matrix components and cells (Figure 3e).
Discussion
The main objective of this study was to evaluate the possible protective effect of oral administration of AA, a well-known and widely used antioxidant agent, on the oxidative pulpal damage caused by a specific bleaching therapy. A previous histopathological study demonstrated that when traditional in-office therapy (35 - 38% HP gels, 30 - 45 minutes) is performed on sound mandibular human incisors, the coronal pulp tissue exhibits superficial necrosis associated with intense tissue disorganization and moderate inflammatory reaction up to 2 days after bleaching.7 In the clinical situation, human mandibular incisors feature the most prevalent and intense tooth sensitivity when compared with that of other groups of teeth.5,6 Therefore, in the present investigation, and taking into account their enamel and dentin thicknesses, rat molars were subjected to an adapted in-office bleaching protocol with a 35% HP gel, to simulate the events observed in mandibular human incisors. We demonstrated that after the application of this bleaching gel for 10 minutes (5 minutes × 2 times) to the buccal surfaces of the rat molars, the pulp tissue featured partial coronal necrosis, intense tissue disorganization and moderate inflammatory reactions, up to 6 hours after bleaching. Therefore, it can be ensured that the methodology used in the present study for evaluation of this clinical procedure in rats was appropriate and valid to mimic in-office bleaching treatment applied to mandibular human incisors. Regardless of the limitations of the present study, the methodology used herein may be useful for further investigations, particularly for the assessment and prediction of the toxicity of different bleaching procedures as well as the comparison of new therapies to prevent pulpal damage caused by this kind of esthetic treatment.
Analysis of the data obtained in the present investigation demonstrated that when bleaching was performed in teeth of rats previously subjected to AA administration, a more rapid pulp tissue recovery capability over time (24 hours) was observed. After 24 hours, the regenerative potential in the DW- and AA-treated groups could be clearly observed. In the DW-treated group, 100% of teeth featured necrosis, while the AA-treated animals showed tissue regeneration, with only 40% of teeth exhibiting small areas of necrosis associated with discrete disorganization of remaining pulp tissue. Therefore, we determined the tendency toward more rapid pulp tissue regeneration in rats subjected to oral administration of AA, compared with that in the control group (DW-treated). One can conclude that the oral administration of AA enhances pulpal healing over time. Therefore, the null hypothesis of this study was partially rejected since AA treatment did not significantly influence the initial bleaching-treatment-induced pulpal damage; however, it enhanced the regenerative potential of this specialized connective tissue 24 hours after exposure to the bleaching agent.
At 3 days, the pulpal regeneration process was evident for both groups, since large necrotic areas were replaced by new connective pulp tissue. The pulpal regeneration was almost complete at 7 days after the bleaching procedure. According to the histological data obtained in the present study, the sequence of pulpal regeneration events differed from that observed in human teeth. In rats, the initial necrotic areas that occurred in the coronal pulp tissue were continuously replaced over time by new connective tissue, which was highly cellularized and vascularized. The pulp tissue present on the root canals of these animals presented normal histological characteristics. In contrast, after being bleached, the mandibular human incisors exhibited extensive pulpal necrosis, which was partially replaced by dystrophic calcification.7,30 The authors also demonstrated that the root pulp tissue exhibited intense deposition of tertiary dentin and inflammatory infiltrate.30 Despite the fact that the methodology used in this study simulated the immediate pulp damage caused by the in-office bleaching therapy commonly applied to human incisors, the results indicated that the mechanism and sequence of pulpal healing are probably different when rat and human teeth are compared.7 However, considering the limitations of the protocol used in the present study, the results of this in vivo investigation corroborate those of previous studies in which the authors stated that scientific data obtained from animal teeth cannot be directly extrapolated to human teeth.31,32,33
In a recent randomized clinical trial, oral administration of 500 mg AA was carried out in human subjects one hour prior to in-office bleaching (35% HP, 15 minutes × 3 times) and up to 48 hours post-bleaching (8 hours each). No statistically significant difference was found for the risk and intensity of tooth sensitivity in patients with or without AA administration.14 In the present investigation, a similar prevalence of inflammatory cell response scores was observed in rat teeth pre-treated or not with AA up to 24 hours after being bleached. Since the most acceptable pathway for bleaching-induced tooth sensitivity involves the development of pulpal inflammation triggered by toxic bleaching gel by-products, the lack of reduction in inflammatory reaction by AA administration at initial periods may explain, at least in part, the post-bleaching sensitivity reported by Paula et al.14 Another current clinical trial demonstrated that the use of an anti-inflammatory drug (400 mg ibuprofen) before tooth bleaching reduced the sensitivity experienced by the patients compared with the control group up to one hour post-bleaching.8 Reduced tooth sensitivity was also found when a desensitizing agent (potassium nitrate), which acts on pain transmission capability, was used prior to tooth bleaching.10 However, both anti-inflammatory drugs and desensitizing agents are considered as palliative alternatives to minimize tooth sensitivity symptoms and do not prevent the pulpal damage caused by ROS-mediated oxidative stress.
The antioxidant agents may act to limit the extent of oxidative damage by donating an electron to the arriving free radicals. Previous studies carried out in rats showed the direct relationship between high AA serum plasma levels and low rates of lipid peroxidation, oxidized proteins and DNA damage to liver, kidney and blood cells, demonstrating its action in reducing oxidative stress mediated by toxic substances.22,24,34 Also, increased concentrations of AA in rat serum plasma enhanced the activity of several antioxidant enzymes, such as catalase, glutathione peroxidase and superoxide dismutase.19,20,23 Associated with this antioxidant power, it has been well-demonstrated that AA plays an important role in wound healing, by minimizing the quantity of inflammatory cells and contributing to the synthesis, maturation and secretion of collagen.25,26,35 Therefore, the more rapid healing capability observed in the first 24 hours in the pulp of the AA-treated animals subjected to tooth bleaching probably occurred due to the locally increased plasma levels of antioxidizing agents, which can positively modulate tissue responses by more effective inactivation of the ROS that diffuses to the dental pulp tissue. However, in this in vivo study, the oral administration of AA was incapable of completely preventing pulpal damage immediately after the external bleaching therapy. A possible explanation for that, especially at initial periods (up to 6 hours), is that a very high amount of ROS released from the bleaching gel diffused quickly through enamel and dentin to reach the pulpal space. Consequently, the contact of high concentrations of ROS with a small connective pulp tissue surrounded by hard structures (enamel, dentin, and cement) caused immediate cell death, which made it impossible for AA to have efficient pulp-protective action.
Previous studies have shown that rats subjected to oral ingestion of AA presented concentrations of this antioxidizing agent in the plasma blood high enough to cause significant antioxidative action in the liver, brain, kidney, blood vessels and periodontal tissues.19,22,34 It is known that the availability of AA differs for tissues in the body after oral administration. Therefore, the amount of AA that reaches the plasma and extracellular fluid around the pulp cells after oral ingestion is unknown.36 Thus, it may be speculated that the use of a higher concentration of AA than used in this study could be more effective against the toxicity of the bleaching agent. Conversely, it is known that the degree of AA absorption decreases as intake increases. At high intake (1.25 g), fractional human absorption of AA may be as low as 33%; at low intake (< 200 mg), the absorption rate can reach 98%.37 AA is accumulated in the blood until the plasma levels reach the renal resorption limit, with excess being quickly excreted in the urine (half-life of about 30 minutes).36 Thus, the administration dosages of AA higher than those used in this study may not provide additional benefits. Despite the increased healing capability of AA-treated pulp tissue observed in the present investigation, more studies are necessary to identify the ideal concentration of AA able to prevent the initial oxidative damage caused by toxic bleaching components to pulp cells.
Soares et al. demonstrated that human pulp cells are highly sensitive to bleaching gel by-products able to diffuse through enamel and dentin, with remaining cells featuring slight proliferative potential over time.2 It was also demonstrated that the odontoblastic differentiation capability of human dental pulp cells exposed to toxic concentrations of HP is significantly impaired up to 7 days after bleaching and that a delay in mineralized matrix deposition takes place when odontoblast-like cells are exposed to such bleaching gel by-products.3,4 Since impairment of the odontoblastic differentiation and maturation process may interfere with the homeostasis of the pulp-dentin complex, and pre-treatment of pulp cells with AA may increase the pulp healing capability after bleaching, we consider that this antioxidizing agent has great potential as a co-adjutant during tooth bleaching, to increase the healing potential over time.38
The histological assessment of the responses of the pulpdentin complex to dental materials and different clinical procedures can provide reliable information regarding the efficacy and safety of new therapies used in dentistry. The interesting methodology used in this in vivo study in rat teeth, as well as analysis of the original data obtained concerning the protective effect of the oral administration of an ascorbic agent before tooth bleaching with an antioxidant agent, will certainly drive further studies to identify external bleaching techniques that are not only clinically effective but also safe and painless for patients.
Conclusions
The application of a bleaching gel with 35% hydrogen peroxide for 10 minutes to the teeth of rats causes partial necrosis of coronal pulp tissue. The oral administration of ascorbic acid prior to external tooth bleaching does not prevent the immediate toxic effects caused by the product to the connective pulp tissue, but this therapy enhances pulpal regeneration after 24 hours.
Acknowledgement
This work is part of a thesis submitted to the Piracicaba Dental School in partial fulfillment of the requirements for the PhD degree. This study was supported by the Fundação de Amparo à Pesquisa do Estado de São Paulo – FAPESP (Grants: 2009/08992-7-8 and 2010/00645-3), and Conselho Nacional de Desenvolvimento Científico e Tecnológico - CNPq (Grants: 301291/2010-1).
Footnotes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
References
- 1.Cintra LT, Benetti F, da Silva Facundo AC, Ferreira LL, Gomes-Filho JE, Ervolino E, Rahal V, Briso AL. The number of bleaching sessions influences pulp tissue damage in rat teeth. J Endod. 2013;39:1576–1580. doi: 10.1016/j.joen.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 2.Soares DG, Basso FG, Hebling J, de Souza Costa CA. Concentrations of and application protocols for hydrogen peroxide bleaching gels: effects on pulp cell viability and whitening efficacy. J Dent. 2014;42:185–198. doi: 10.1016/j.jdent.2013.10.021. [DOI] [PubMed] [Google Scholar]
- 3.Soares DG, Gonçalves Basso F, Hebling J, de Souza Costa CA. Effect of hydrogen-peroxide-mediated oxidative stress on human dental pulp cells. J Dent. 2015;43:750–756. doi: 10.1016/j.jdent.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 4.Soares DG, Marcomini N, Basso FG, Pansani TN, Hebling J, de Souza Costa CA. Indirect cytocompatibility of a low-concentration hydrogen peroxide bleaching gel to odontoblast-like cells. Int Endod J. 2016;49:26–36. doi: 10.1111/iej.12426. [DOI] [PubMed] [Google Scholar]
- 5.de Almeida LC, Costa CA, Riehl H, dos Santos PH, Sundfeld RH, Briso AL. Occurrence of sensitivity during at-home and in-office tooth bleaching therapies with or without use of light sources. Acta Odontol Latinoam. 2012;25:3–8. [PubMed] [Google Scholar]
- 6.Bonafe E, Bacovis CL, Iensen S, Loguercio AD, Reis A, Kossatz S. Tooth sensitivity and efficacy of in-office bleaching in restored teeth. J Dent. 2013;41:363–369. doi: 10.1016/j.jdent.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 7.Costa CA, Riehl H, Kina JF, Sacono NT, Hebling J. Human pulp responses to in-office tooth bleaching. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;109:e59–e64. doi: 10.1016/j.tripleo.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 8.Paula E, Kossatz S, Fernandes D, Loguercio A, Reis A. The effect of perioperative ibuprofen use on tooth sensitivity caused by in-office bleaching. Oper Dent. 2013;38:601–608. doi: 10.2341/12-107-C. [DOI] [PubMed] [Google Scholar]
- 9.Soares DG, Ribeiro AP, Lima AF, Sacono NT, Hebling J, de Souza Costa CA. Effect of fluoride-treated enamel on indirect cytotoxicity of a 16% carbamide peroxide bleaching gel to pulp cells. Braz Dent J. 2013;24:121–127. doi: 10.1590/0103-6440201302161. [DOI] [PubMed] [Google Scholar]
- 10.Reis A, Dalanhol AP, Cunha TS, Kossatz S, Loguercio AD. Assessment of tooth sensitivity using a desensitizer before light-activated bleaching. Oper Dent. 2011;36:12–17. doi: 10.2341/10-148-CR. [DOI] [PubMed] [Google Scholar]
- 11.Lima AF, Lessa FC, Hebling J, de Souza Costa CA, Marchi GM. Protective effect of sodium ascorbate on MDPC-23 odontoblast-like cells exposed to a bleaching agent. Eur J Dent. 2010;4:238–244. [PMC free article] [PubMed] [Google Scholar]
- 12.Lima AF, Lessa FC, Mancini MN, Hebling J, Costa CA, Marchi GM. Transdentinal protective role of sodium ascorbate against the cytopathic effects of H2O2 released from bleaching agents. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;109:e70–e76. doi: 10.1016/j.tripleo.2009.12.020. [DOI] [PubMed] [Google Scholar]
- 13.Vargas Fda S, Soares DG, Basso FG, Hebling J, Costa CA. Dose-response and time-course of α-tocoferol mediating the cytoprotection of dental pulp cells against hydrogen peroxide. Braz Dent J. 2014;25:367–371. doi: 10.1590/0103-6440201302434. [DOI] [PubMed] [Google Scholar]
- 14.de Paula EA, Kossatz S, Fernandes D, Loguercio AD, Reis A. Administration of ascorbic acid to prevent bleaching-induced tooth sensitivity: a randomized triple-blind clinical trial. Oper Dent. 2014;39:128–135. doi: 10.2341/12-483-C. [DOI] [PubMed] [Google Scholar]
- 15.de Silveira Vargas F, Soares DG, Ribeiro AP, Hebling J, de Souza Costa CA. Protective effect of alphatocopherol isomer from vitamin E against the H2O2 induced toxicity on dental pulp cells. Biomed Res Int. 2014;2014:895049. doi: 10.1155/2014/895049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lima AF, Fonseca FM, Freitas MS, Palialol AR, Aguiar FH, Marchi GM. Effect of bleaching treatment and reduced application time of an antioxidant on bond strength to bleached enamel and subjacent dentin. J Adhes Dent. 2011;13:537–542. doi: 10.3290/j.jad.a19813. [DOI] [PubMed] [Google Scholar]
- 17.Whang HJ, Shin DH. Effects of applying antioxidants on bond strength of bleached bovine dentin. Restor Dent Endod. 2015;40:37–43. doi: 10.5395/rde.2015.40.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park JY, Kwon TY, Kim YK. Effective application duration of sodium ascorbate antioxidant in reducing microleakage of bonded composite restoration in intracoronally-bleached teeth. Restor Dent Endod. 2013;38:43–47. doi: 10.5395/rde.2013.38.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ekuni D, Tomofuji T, Sanbe T, Irie K, Azuma T, Maruyama T, Tamaki N, Murakami J, Kokeguchi S, Yamamoto T. Vitamin C intake attenuates the degree of experimental atherosclerosis induced by periodontitis in the rat by decreasing oxidative stress. Arch Oral Biol. 2009;54:495–502. doi: 10.1016/j.archoralbio.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 20.Tomofuji T, Ekuni D, Sanbe T, Irie K, Azuma T, Maruyama T, Tamaki N, Murakami J, Kokeguchi S, Yamamoto T. Effects of vitamin C intake on gingival oxidative stress in rat periodontitis. Free Radic Biol Med. 2009;46:163–168. doi: 10.1016/j.freeradbiomed.2008.09.040. [DOI] [PubMed] [Google Scholar]
- 21.Sanbe T, Tomofuji T, Ekuni D, Azuma T, Irie K, Tamaki N, Yamamoto T, Morita M. Vitamin C intake inhibits serum lipid peroxidation and osteoclast differentiation on alveolar bone in rats fed on a high-cholesterol diet. Arch Oral Biol. 2009;54:235–240. doi: 10.1016/j.archoralbio.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 22.Patra RC, Swarup D, Dwivedi SK. Antioxidant effects of alpha tocopherol, ascorbic acid and L-methionine on lead induced oxidative stress to the liver, kidney and brain in rats. Toxicology. 2001;162:81–88. doi: 10.1016/s0300-483x(01)00345-6. [DOI] [PubMed] [Google Scholar]
- 23.Chandra Jagetia G, Rajanikant GK, Rao SK, Shrinath Baliga M. Alteration in the glutathione, glutathione peroxidase, superoxide dismutase and lipid peroxidation by ascorbic acid in the skin of mice exposed to fractionated gamma radiation. Clin Chim Acta. 2003;332:111–121. doi: 10.1016/s0009-8981(03)00132-3. [DOI] [PubMed] [Google Scholar]
- 24.Nandi D, Patra RC, Swarup D. Effect of cysteine, methionine, ascorbic acid and thiamine on arsenic-induced oxidative stress and biochemical alterations in rats. Toxicology. 2005;211:26–35. doi: 10.1016/j.tox.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 25.Jagetia GC, Rajanikant GK, Rao SK. Evaluation of the effect of ascorbic acid treatment on wound healing in mice exposed to different doses of fractionated gamma radiation. Radiat Res. 2003;159:371–380. doi: 10.1667/0033-7587(2003)159[0371:eoteoa]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 26.Kaplan B, Gönül B, Dinçer S, Dincer Kaya FN, Babül A. Relationships between tensile strength, ascorbic acid, hydroxyproline, and zinc levels of rabbit full-thickness incision wound healing. Surg Today. 2004;34:747–751. doi: 10.1007/s00595-004-2827-0. [DOI] [PubMed] [Google Scholar]
- 27.Kadirvel R, Sundaram K, Mani S, Samuel S, Elango N, Panneerselvam C. Supplementation of ascorbic acid and alpha-tocopherol prevents arsenic-induced protein oxidation and DNA damage induced by arsenic in rats. Hum Exp Toxicol. 2007;26:939–946. doi: 10.1177/0960327107087909. [DOI] [PubMed] [Google Scholar]
- 28.Chandler NP. The radiographic assessment of pulp size: validity and clinical implications. N Z Dent J. 1989;85:23–26. [PubMed] [Google Scholar]
- 29.Kina JF, Huck C, Riehl H, Martinez TC, Sacono NT, Ribeiro AP, Costa CA. Response of human pulps after professionally applied vital tooth bleaching. Int Endod J. 2010;43:572–580. doi: 10.1111/j.1365-2591.2010.01713.x. [DOI] [PubMed] [Google Scholar]
- 30.Roderjan DA, Stanislawczuk R, Hebling J, de Souza Costa CA, Soares DG, Reis A, Loguercio AD. Histopathological features of dental pulp tissue from bleached mandibular incisors. J Mater Sci Eng B. 2014;6:178–185. [Google Scholar]
- 31.Costa CA, Oliveira MF, Giro EM, Hebling J. Biocompatibility of resin-based materials used as pulpcapping agents. Int Endod J. 2003;36:831–839. doi: 10.1111/j.1365-2591.2003.00702.x. [DOI] [PubMed] [Google Scholar]
- 32.Accorinte Mde L, Loguercio AD, Reis A, Muench A, de Araújo VC. Response of human pulp capped with a bonding agent after bleeding control with hemostatic agents. Oper Dent. 2005;30:147–155. [PubMed] [Google Scholar]
- 33.de Souza Costa CA, Hebling J, Scheffel DL, Soares DG, Basso FG, Ribeiro AP. Methods to evaluate and strategies to improve the biocompatibility of dental materials and operative techniques. Dent Mater. 2014;30:769–784. doi: 10.1016/j.dental.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 34.Kadirvel R, Sundaram K, Mani S, Samuel S, Elango N, Panneerselvam C. Supplementation of ascorbic acid and alpha-tocopherol prevents arsenic-induced protein oxidation and DNA damage induced by arsenic in rats. Hum Exp Toxicol. 2007;26:939–946. doi: 10.1177/0960327107087909. [DOI] [PubMed] [Google Scholar]
- 35.Moores J. Vitamin C: a wound healing perspective. Br J Community Nurs. 2013;(Suppl):S6, S8, S11. doi: 10.12968/bjcn.2013.18.sup12.s6. [DOI] [PubMed] [Google Scholar]
- 36.Levine M, Padayatty SJ, Espey MG. Vitamin C: a concentration-function approach yields pharmacology and therapeutic discoveries. Adv Nutr. 2011;2:78–88. doi: 10.3945/an.110.000109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Padayatty SJ, Sun H, Wang Y, Riordan HD, Hewitt SM, Katz A, Wesley RA, Levine M. Vitamin C pharmacokinetics: implications for oral and intravenous use. Ann Intern Med. 2004;140:533–537. doi: 10.7326/0003-4819-140-7-200404060-00010. [DOI] [PubMed] [Google Scholar]
- 38.Schmalz G, Smith AJ. Pulp development, repair, and regeneration: challenges of the transition from traditional dentistry to biologically based therapies. J Endod. 2014;40:S2–S5. doi: 10.1016/j.joen.2014.01.018. [DOI] [PubMed] [Google Scholar]