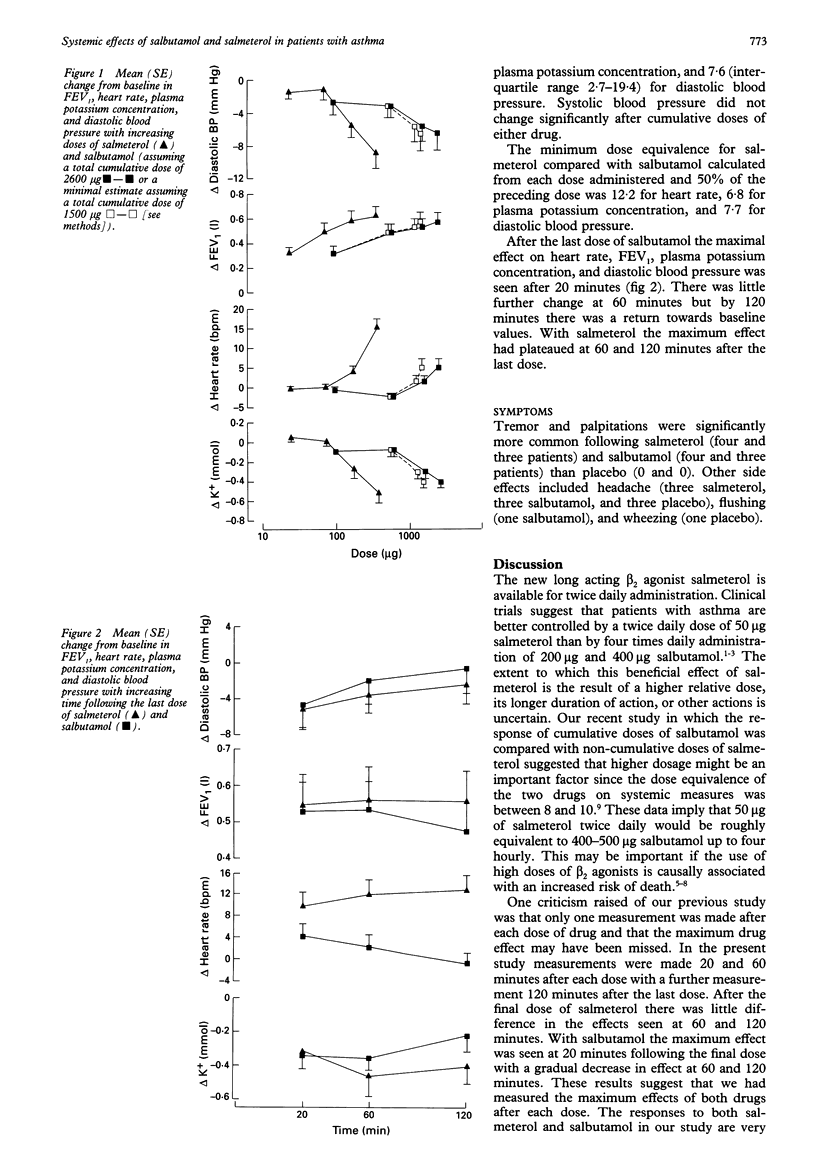
Abstract
BACKGROUND--Knowing the extent of the systemic effects of a new beta 2 agonist relative to an established drug is important for the prediction and interpretation of side effects. A recent study in which the effect of cumulative doses of salbutamol was compared with single doses of salmetreol suggested that, weight for weight, salmeterol may be up to 10 times more potent than salbutamol. This current study was designed to investigate further the dose equivalence of salmeterol and salbutamol. METHODS--Twelve patients with mild asthma inhaled cumulative doses of placebo, salmeterol 25, 50, 100, and 200 micrograms, and salbutamol 100, 500, 1000, and 1000 micrograms on separate days at hourly intervals in a randomised double blind crossover study. Changes in forced expiratory volume in one second (FEV1), heart rate, plasma potassium concentration, systolic and diastolic blood pressure were measured. Dose equivalence was determined as the dose ratio of salmeterol to salbutamol for the 50% maximum response to salbutamol. RESULTS--No important changes occurred in any measurements following placebo. Salmeterol and salbutamol caused a near maximum increase in FEV1 following the first dose so the dose equivalence for the airway effects could not be estimated. Heart rate increased and plasma potassium concentration and diastolic blood pressure decreased in a dose dependent manner following salmeterol and salbutamol, with median dose equivalences for salmeterol compared with salbutamol of 17.7, 7.8, and 7.6, respectively. CONCLUSIONS--These results confirm that the systemic activity of salmeterol compared with salbutamol is higher than would be expected from in vitro data, particularly for heart rate. Whether this is because of the relatively high dose of salmeterol used or pharmacokinetic differences between the two drugs is uncertain.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ball D. I., Brittain R. T., Coleman R. A., Denyer L. H., Jack D., Johnson M., Lunts L. H., Nials A. T., Sheldrick K. E., Skidmore I. F. Salmeterol, a novel, long-acting beta 2-adrenoceptor agonist: characterization of pharmacological activity in vitro and in vivo. Br J Pharmacol. 1991 Nov;104(3):665–671. doi: 10.1111/j.1476-5381.1991.tb12486.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castle W., Fuller R., Hall J., Palmer J. Serevent nationwide surveillance study: comparison of salmeterol with salbutamol in asthmatic patients who require regular bronchodilator treatment. BMJ. 1993 Apr 17;306(6884):1034–1037. doi: 10.1136/bmj.306.6884.1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung D., Timmers M. C., Zwinderman A. H., Bel E. H., Dijkman J. H., Sterk P. J. Long-term effects of a long-acting beta 2-adrenoceptor agonist, salmeterol, on airway hyperresponsiveness in patients with mild asthma. N Engl J Med. 1992 Oct 22;327(17):1198–1203. doi: 10.1056/NEJM199210223271703. [DOI] [PubMed] [Google Scholar]
- Collier J. G., Dobbs R. J., Williams I. Salbutamol aerosol causes a tachycardia due to the inhaled rather than the swallowed fraction. Br J Clin Pharmacol. 1980 Mar;9(3):273–274. doi: 10.1111/j.1365-2125.1980.tb04837.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane J., Pearce N., Flatt A., Burgess C., Jackson R., Kwong T., Ball M., Beasley R. Prescribed fenoterol and death from asthma in New Zealand, 1981-83: case-control study. Lancet. 1989 Apr 29;1(8644):917–922. doi: 10.1016/s0140-6736(89)92505-1. [DOI] [PubMed] [Google Scholar]
- Grainger J., Woodman K., Pearce N., Crane J., Burgess C., Keane A., Beasley R. Prescribed fenoterol and death from asthma in New Zealand, 1981-7: a further case-control study. Thorax. 1991 Feb;46(2):105–111. doi: 10.1136/thx.46.2.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipworth B. J., Brown R. A., McDevitt D. G. Assessment of airways, tremor and chronotropic responses to inhaled salbutamol in the quantification of beta 2-adrenoceptor blockade. Br J Clin Pharmacol. 1989 Jul;28(1):95–102. doi: 10.1111/j.1365-2125.1989.tb03510.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundback B., Rawlinson D. W., Palmer J. B. Twelve month comparison of salmeterol and salbutamol as dry powder formulations in asthmatic patients. European Study Group. Thorax. 1993 Feb;48(2):148–153. doi: 10.1136/thx.48.2.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maconochie J. G., Forster J. K. Dose-response study with high-dose inhaled salmeterol in healthy subjects. Br J Clin Pharmacol. 1992 Mar;33(3):342–345. doi: 10.1111/j.1365-2125.1992.tb04049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan D. J., Paull J. D., Richmond B. H., Wilson-Evered E., Ziccone S. P. Pharmacokinetics of intravenous and oral salbutamol and its sulphate conjugate. Br J Clin Pharmacol. 1986 Nov;22(5):587–593. doi: 10.1111/j.1365-2125.1986.tb02939.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce N., Grainger J., Atkinson M., Crane J., Burgess C., Culling C., Windom H., Beasley R. Case-control study of prescribed fenoterol and death from asthma in New Zealand, 1977-81. Thorax. 1990 Mar;45(3):170–175. doi: 10.1136/thx.45.3.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearlman D. S., Chervinsky P., LaForce C., Seltzer J. M., Southern D. L., Kemp J. P., Dockhorn R. J., Grossman J., Liddle R. F., Yancey S. W. A comparison of salmeterol with albuterol in the treatment of mild-to-moderate asthma. N Engl J Med. 1992 Nov 12;327(20):1420–1425. doi: 10.1056/NEJM199211123272004. [DOI] [PubMed] [Google Scholar]
- Smyth E. T., Pavord I. D., Wong C. S., Wisniewski A. F., Williams J., Tattersfield A. E. Interaction and dose equivalence of salbutamol and salmeterol in patients with asthma. BMJ. 1993 Feb 27;306(6877):543–545. doi: 10.1136/bmj.306.6877.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer W. O., Suissa S., Ernst P., Horwitz R. I., Habbick B., Cockcroft D., Boivin J. F., McNutt M., Buist A. S., Rebuck A. S. The use of beta-agonists and the risk of death and near death from asthma. N Engl J Med. 1992 Feb 20;326(8):501–506. doi: 10.1056/NEJM199202203260801. [DOI] [PubMed] [Google Scholar]
- Ullman A., Hedner J., Svedmyr N. Inhaled salmeterol and salbutamol in asthmatic patients. An evaluation of asthma symptoms and the possible development of tachyphylaxis. Am Rev Respir Dis. 1990 Sep;142(3):571–575. doi: 10.1164/ajrccm/142.3.571. [DOI] [PubMed] [Google Scholar]
- Ullman A., Svedmyr N. Salmeterol, a new long acting inhaled beta 2 adrenoceptor agonist: comparison with salbutamol in adult asthmatic patients. Thorax. 1988 Sep;43(9):674–678. doi: 10.1136/thx.43.9.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White P. C., New M. I., Dupont B. Cloning and expression of cDNA encoding a bovine adrenal cytochrome P-450 specific for steroid 21-hydroxylation. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1986–1990. doi: 10.1073/pnas.81.7.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong C. S., Pavord I. D., Williams J., Britton J. R., Tattersfield A. E. Bronchodilator, cardiovascular, and hypokalaemic effects of fenoterol, salbutamol, and terbutaline in asthma. Lancet. 1990 Dec 8;336(8728):1396–1399. doi: 10.1016/0140-6736(90)93099-b. [DOI] [PubMed] [Google Scholar]


