Abstract
Aims
In the dual antiplatelet therapy (DAPT) study, continued thienopyridine beyond 12 months after drug-eluting stent placement was associated with increased mortality compared with placebo. We sought to evaluate factors related to mortality in randomized patients receiving either drug-eluting or bare metal stents in the DAPT study.
Methods and results
Patients were enrolled after coronary stenting, given thienopyridine and aspirin for 12 months, randomly assigned to continued thienopyridine or placebo for an additional 18 months (while taking aspirin), and subsequently treated with aspirin alone for another 3 months. A blinded independent adjudication committee evaluated deaths. Among 11 648 randomized patients, rates of all-cause mortality rates were 1.9 vs. 1.5% (continued thienopyridine vs. placebo, P = 0.07), cardiovascular mortality, 1.0 vs. 1.0% (P = 0.97), and non-cardiovascular mortality, 0.9 vs. 0.5% (P = 0.01) over the randomized period (Months 12–30). Rates of fatal bleeding were 0.2 vs. 0.1% (P = 0.81), and deaths related to any prior bleeding were 0.3 vs. 0.2% (P = 0.36), Months 12–33). Cancer incidence did not differ (2.0 vs. 1.6%, P = 0.12). Cancer-related deaths occurred in 0.6 vs. 0.3% (P = 0.02) and were rarely related to bleeding (0.1 vs. 0, P = 0.25). After excluding those occurring in patients with cancer diagnosed before enrolment, rates were 0.4 vs. 0.3% (P = 0.16).
Conclusion
Bleeding accounted for a minority of deaths among patients treated with continued thienopyridine. Cancer-related death in association with thienopyridine therapy was mainly not related to bleeding and may be a chance finding. Caution is warranted when considering extended thienopyridine in patients with advanced cancer.
Trial Registration
clinicaltrials.gov Identifier: NCT00977938.
Keywords: Dual antiplatelet therapy, Mortality, Cancer, Thienopyridine
See page 386 for the editorial comment on this article (doi:10.1093/eurheartj/ehv610)
Introduction
With prevention and treatment, age-adjusted mortality from cardiovascular disease in the USA has declined dramatically.1,2 In populations with coronary artery disease, deaths due to non-cardiovascular causes now rival those due to cardiovascular aetiologies.3,4 Contemporary cardiovascular trials are therefore often designed to detect events that impact quality of life or morbidity, rather than mortality alone. Nonetheless, mortality, from any cause, has great significance to patients and physicians.
Anticoagulants and anti-thrombotics may be associated with cardiovascular benefits, yet they also increase bleeding risk. Among individuals treated for coronary artery disease, bleeding and subsequent mortality are associated.5,6 The degree to which medical therapies increase the risk of bleeding and subsequent mortality beyond what can be attributed to intrinsic patient risk factors may be difficult to determine from observational analysis.7,8
The dual antiplatelet therapy (DAPT) study was an international, blinded, placebo-controlled, and randomized trial comparing two durations of thienopyridine therapy to prevent stent thrombosis and/or the composite endpoint of mortality, myocardial infarction or stroke (MACCE: major adverse cardiovascular and cerebrovascular events) among patients treated with coronary stents. While continued thienopyridine therapy was associated with reductions in the co-primary endpoints of stent thrombosis and MACCE, it was also associated with an increase in moderate or severe bleeding.9,10 Among patients treated with drug-eluting stents (DES), all-cause mortality, a secondary endpoint, was increased in those treated with continued thienopyridine therapy plus aspirin over the combined randomized and aspirin-only treatment periods (12–33 months).9 In populations with cardiovascular disease, prior large trials evaluating extended thienopyridine therapy have not observed higher mortality in combined meta-analysis compared with aspirin alone,11–13 yet meta-analyses of the subset of DES-treated subjects, predominantly driven by the DAPT DES-treated cohort, have reached varying conclusions using different methods.14,15 Based on the premise that a mortality risk with thienopyridine therapy would not be unique to a particular stent type, we performed an exploratory analysis of all randomized patients [DES and bare metal stent (BMS) treated] in the DAPT study to closely analyse causes of death and to identify a putative mechanism of mortality associated with continued thienopyridine plus aspirin therapy.
Methods
Study background
The DAPT study compared the effects of continued thienopyridine therapy vs. placebo beyond 12 months in patients who had coronary stent placement and who were taking aspirin.16 Primary outcomes have been described and included concomitant reduction in definite or probable stent thrombosis and MACCE, and increase in moderate/severe bleeding.9 Among randomized DES-treated patients (n = 9961), continued thienopyridine reduced MACCE (hazard ratio 0.71, P < 0.001), with myocardial infarction being the component most favourably affected (hazard ratio 0.47, P < 0.001). However, continued thienopyridine (vs. placebo) was associated with increased moderate or severe bleeding (2.5 vs. 1.6%, respectively, P = 0.001) and all-cause mortality (2.0 vs. 1.5%, respectively, hazard ratio 1.36, 95% confidence interval [CI] 1.00–1.85; P = 0.05) from 12 to 30 months, and all-cause mortality from 12 to 33 months (2.3 vs. 1.8%, respectively, hazard ratio 1.36, 95% CI 1.02–1.82, P = 0.04). Among BMS-treated patients who were randomized (n = 1687), all-cause mortality was 1.0 and 1.2%, respectively, from 12 to 30 months (hazard ratio 0.90, 95% CI 0.35–2.33, P = 0.83)10 and 1.5 vs. 1.7% from 12 to 33 months.
Study population
Inclusion criteria required that eligible subjects were adults undergoing coronary stent placement who had ≥3 years life expectancy, were candidates for dual antiplatelet therapy, and were not on oral anticoagulants. Patients treated with either BMS or DES were eligible for randomization, and were pre-specified for analysis of the primary endpoints and their components.
Study procedures
Patients enrolled in the DAPT study received thienopyridine (clopidogrel or prasugrel) and aspirin therapy (open-label period). At 12 months, patients free of myocardial infarction, stroke, moderate or severe bleeding, and who were treatment compliant, continued aspirin and were randomized to continued thienopyridine or placebo for an additional 18 months (randomized treatment period; Months 12–30). After 18 months of randomized treatment, the study drug was discontinued and patients were followed for an additional 3 months during which they received aspirin alone (aspirin only period; Months 30–33).
This study was conducted in accordance with the Declaration of Helsinki; the locally appointed ethics committee approved the research protocol at each institution and informed consent was obtained from the subjects (or their guardians).
Clinical event adjudication
All possible primary endpoint events, including death, underwent independent, blinded adjudication by a Clinical Events Committee (CEC) as previously described.16 Deaths were classified as cardiac, vascular, or non-cardiovascular, according to Academic Research Consortium definition.17 Bleeding was defined by both Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Arteries18 and Bleeding Academic Research Consortium (BARC) criteria, including BARC Type 3C to define intracranial haemorrhage, and Type 5 to define fatal bleeding.19 Cardiovascular procedure-related bleeding or bleeding related to primarily cardiac or vascular conditions was considered cardiovascular death, other fatal bleeds were considered non-cardiovascular.
Upon recognition of a mortality signal within the primary analysis (DES-treated) cohort,9 a second blinded CEC composed of two cardiologists and four oncologists was established to evaluate the potential contribution of specific pathologic mechanisms to mortality (see Appendix). The classification of cardiac, vascular, and non-cardiovascular death was not readjudicated.
All deaths were reviewed regardless of initial adjudicated cause. Bleeding-related death was adjudicated as any death that was possibly, probably, or definitely related to a prior bleeding event. Cancer-related deaths were similarly adjudicated as any death that was possibly, probably, or definitely related to a malignancy or complications from treatments specifically administered for the malignancy. In addition, the second CEC adjudicated characteristics of the malignancy, including date of diagnosis, stage, location, and treatment type.
Statistical analysis
Analyses planned and conducted after the primary analysis of the DES-treated cohort had been completed were intended to investigate mortality, and should be considered exploratory. Analyses were performed on the intention-to-treat population (all randomized patients). Analysis periods included the randomized period, to evaluate mortality while patients were taking study drug, and the combined randomized plus aspirin-only periods (Months 12–33), to include the possibility of delayed effects on mortality. Outcomes included all-cause, cardiovascular, and non-cardiovascular mortality. Kaplan–Meier estimates of the cumulative incidence are presented according to randomized treatment group along with hazard ratios (continued thienopyridine vs. placebo) derived from Cox proportional hazards model, and their two-sided 95% CIs, all of which are stratified by randomization strata. Patients without a given event were censored at 33 months or last known follow-up, whichever was earlier. Treatments were compared using the log-rank test. Other subtypes of mortality are presented as absolute frequencies and compared between randomized treatment groups using Fisher's exact tests.
We repeated the analyses using multiple-imputation logistic-regression modelling (50 imputations) to impute missing mortality status using baseline covariates from Table 1 in the imputation model. We assessed the consistency of the randomized treatment effect on all-cause mortality using Cox proportional hazards regression with tests of subgroup-by-treatment interactions across 15 factors of interest, all previously defined for tests of interaction on the primary study endpoints. In addition, we evaluated the results regarding mortality types using competing risk models. All statistical analyses were conducted with the use of SAS version 9.2 (SAS Institute Inc., Cary, NC, USA). A two-sided P-value of ≤0.05 was considered statistically significant.
Table 1.
Patient characteristicsa
| Characteristic | Continued thienopyridine (n = 5862) | Placebo (n = 5786) |
|---|---|---|
| Patients | ||
| Age (years) | 61.4 ± 10.3 | 61.2 ± 10.3 |
| Female sex | 1457 (24.9) | 1468 (25.4) |
| Non-White Raceb | 500 (8.7) | 480 (8.5) |
| Hispanic or Latino ethnic groupb | 199 (3.5) | 207 (3.6) |
| Weight (kg) | 91.0 ± 19.6 | 91.1 ± 19.4 |
| Body mass indexc | 30.4 ± 5.7 | 30.4 ± 5.8 |
| Diabetes mellitus | 1737 (29.8) | 1654 (28.7) |
| Hypertension | 4330 (74.1) | 4192 (72.6) |
| Cigarette smoker | 1582 (27.4) | 1560 (27.4) |
| Stroke or TIA | 198 (3.4) | 203 (3.5) |
| Congestive heart failure | 273 (4.7) | 251 (4.4) |
| Peripheral arterial disease | 319 (5.5) | 330 (5.8) |
| Renal insufficiency/failure | 251 (4.3) | 217 (3.8) |
| Prior myocardial infarction | 1252 (21.7) | 1204 (21.1) |
| Prior cancer | 547 (9.4) | 523 (9.1) |
| Indication for Index PCI | ||
| STEMI | 845 (14.4) | 835 (14.4) |
| NSTEMI | 960 (16.4) | 936 (16.2) |
| Unstable anginad | 915 (15.6) | 906 (15.7) |
| Stable angina | 2081 (35.5) | 2068 (35.7) |
| Other | 1061 (18.1) | 1041 (18.0) |
| Thienopyridine at randomization | ||
| Clopidogrel | 4008 (68.4) | 3954 (68.3) |
| Prasugrel | 1854 (31.6) | 1832 (31.7) |
| Type of stent at index procedure | ||
| Drug eluting | 5020 (85.6) | 4941 (85.4) |
| Bare metal | 842 (14.4) | 845 (14.6) |
CABG, coronary-artery bypass grafting; NSTEMI, non-ST-segment elevation myocardial infarction; PCI, percutaneous coronary intervention; STEMI, ST-segment elevation myocardial infarction; TIA, transient ischaemic attack.
aPlus–minus values are means ± SD; all other numbers are n (%). There were no significant differences between the two groups. For most variables, <2% of patients had missing values.
bRace and ethnic groups were self-reported.
cBody-mass index is the weight in kilograms divided by the square of the height in meters.
dIncludes unstable angina without reported elevation of cardiac enzymes.
Results
Study population
Between 13 August 2009 and 1 July 2011 a total of 25 682 patients were enrolled in the DAPT study; after 12 months of open-label treatment, 11 648 were randomized to continued thienopyridine (n = 5862) or placebo (n = 5786). At least 30 months follow-up was complete or vital status was available in 5588 (95.3%) vs. 5510 (95.2%) of patients receiving continued thienopyridine or placebo, respectively, and in 5526 (94.3%) vs. 5451 (94.2%), respectively, at 33 months (see Supplementary material online, Figure S1). Patient and lesion characteristics did not differ between treatment arms (Table 1), nor did rates of study drug discontinuation (21.0% with thienopyridine vs. 19.7% with placebo, P = 0.12) through 30 months.
Mortality
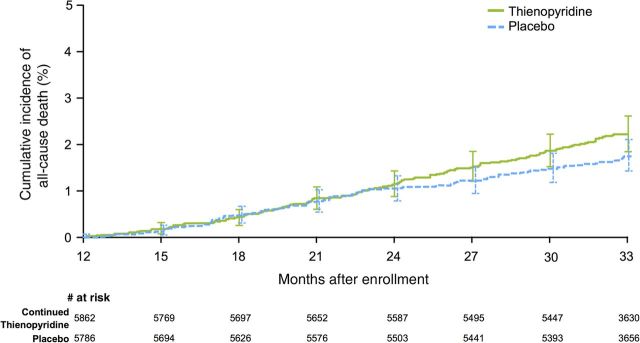
During the randomized period, patients assigned to continued thienopyridine had a rate of all-cause mortality of 1.9 vs. 1.5% in those receiving placebo (hazard ratio 1.31, 95% CI 0.97–1.75; P = 0.07; Figure 1, Table 2). There was no difference in cardiovascular death (1.0 vs. 1.0%, respectively; hazard ratio 1.01, 95% CI 0.69–1.47; P = 0.97), but there was a difference in non-cardiovascular death (0.9 vs. 0.5%, respectively; hazard ratio 1.94, 95% CI 1.20–3.15; P = 0.01, Figure 2, Table 2). In the aspirin alone period (30–33 months), there were no significant differences in mortality (0.4 vs. 0.3%, hazard ratio 1.38, 95% CI 0.67–2.81, P = 0.38), but the rate of cardiovascular death was numerically higher in the continued thienopyridine arm when active treatment was stopped (0.3 vs. 0.1%, hazard ratio 2.39, 95% CI 0.84–6.77; P = 0.09). Over the combined randomized treatment plus aspirin alone periods (12–33 months), the rate of all-cause mortality was 2.2% with continued thienopyridine and 1.8% with placebo (hazard ratio 1.32, 95% CI 1.00–1.73; P = 0.05). Multiple imputation for missing data showed a similar directional trend without statistical significance for mortality comparing continued thienopyridine vs. placebo at 30 and 33 months (hazard ratio 1.21, 95% CI 0.91–1.62, P = 0.19 at 30 months; 1.16, 95% CI 0.92–1.48, P = 0.21 at 33 months). No subgroup appeared to have a significantly higher or lower hazard ratio for mortality with continued thienopyridine, including those with a prior history of cancer (see Supplementary material online, Table S1). Similar results were obtained using competing risk models (see Supplementary material online, Table S2).
Figure 1.
Cumulative incidence curves for all-cause mortality. Randomization occurred at 12 months after stenting. The primary analysis period was the period from Months 12 to 30 after percutaneous coronary intervention, during which subjects received the randomized study drug. Patients were followed for an observational period of an additional 3 months after discontinuation of the study drug (i.e. to 33 months after enrolment). From 12 to 30 months, 1.9% in the thienopyridine arm died compared with 1.5% in the placebo arm (hazard ratio 1.31, P = 0.07). From 12 to 33 months, 2.2 vs. 1.8% mortality was observed in the thienopyridine and placebo arms, respectively (hazard ratio 1.32, P = 0.05).
Table 2.
Mortality rates by treatment arm during randomised treatment and extended follow-up
| Outcome | Continued thienopyridine (n = 5862), n (%) | Placebo (n = 5786), n (%) | HR (95% CI) | Log-rank P-value |
|---|---|---|---|---|
| 12–30 months (randomized period) | ||||
| Death | 106 (1.9) | 84 (1.5) | 1.31 (0.97, 1.75) | 0.07 |
| Cardiovascular | 54 (1.0) | 57 (1.0) | 1.01 (0.69, 1.47) | 0.97 |
| Cardiac | 49 (0.9) | 52 (0.9) | 1.01 (0.68, 1.50) | 0.97 |
| Vascular | 5 (0.1) | 5 (0.1) | 0.99 (0.29, 3.40) | 0.98 |
| Non-cardiovascular | 52 (0.9) | 27 (0.5) | 1.94 (1.20, 3.15) | 0.01 |
| 30–33 months (aspirin alone period) | ||||
| Death | 19 (0.4) | 13 (0.3) | 1.38 (0.67,2.81) | 0.38 |
| Cardiovascular | 13 (0.3) | 5 (0.1) | 2.39 (0.84,6.77) | 0.09 |
| Cardiac | 10 (0.2) | 3 (0.1) | 2.98 (0.81,10.99) | 0.09 |
| Vascular | 3 (0.1) | 2 (0.1) | 1.50 (0.25,9.00) | 0.65 |
| Non-cardiovascular | 6 (0.1) | 8 (0.2) | 0.74 (0.26,2.14) | 0.58 |
| 12–33 months (combined randomized and aspirin alone periods) | ||||
| Death | 125 (2.2) | 97 (1.8) | 1.32 (1.00,1.73) | 0.05 |
| Cardiovascular | 67 (1.2) | 62 (1.1) | 1.12 (0.79,1.60) | 0.51 |
| Cardiac | 59 (1.1) | 55 (1.0) | 1.12 (0.77,1.64) | 0.54 |
| Vascular | 8 (0.1) | 7 (0.1) | 1.13 (0.41,3.12) | 0.81 |
| Non-cardiovascular | 58 (1.0) | 35 (0.7) | 1.65 (1.07,2.54) | 0.02 |
Event rates are expressed as Kaplan–Meier estimates.
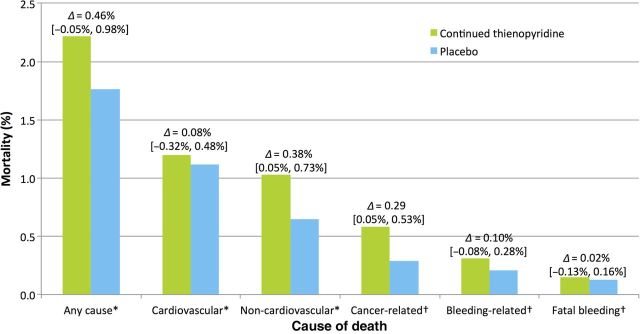
Figure 2.
Causes of mortality in all randomized patients. Adjudicated causes of mortality by randomized treatment arm from 12 to 33 months are shown. All deaths were adjudicated for each category and may be represented in more than one category. Non-cardiovascular causes were identified in more than half of deaths overall. The deltas and their 95% confidence intervals represent the difference either Kaplan–Meier estimates asterisk or absolute event rates dagger between randomized treatment arms.
Events preceding mortality
Bleeding
During the combined randomized and aspirin only treatment periods, moderate or severe bleeding events were more frequent with continued thienopyridine (2.7 vs. 1.8%, P = 0.002). However, fatal bleeding (0.2 vs. 0.1%, P = 0.81) and fatal or non-fatal intracranial haemorrhage (0.4 vs. 0.4%, P = 0.97; see Supplementary material online, Table S3) did not differ between treatment groups. Death that was definitely, probably, or possibly related to bleeding occurred in 0.3 vs. 0.2% (P = 0.36, Table 3) in the continued thienopyridine and placebo arms, respectively. Among the 30 bleeding-related deaths, 13 were related to trauma, usually major. There was no difference in rates of death preceded by any bleeding within 30 days (defined as BARC Type 2, 3 or 5) (0.2 vs. 0.2%) or death preceded by any bleeding at any point after randomization (0.4 vs. 0.4%).
Table 3.
Relatedness of death at 12–33 months to bleeding, cancer, and trauma according to blinded adjudication
| Continued thienopyridine (n = 5862), n (%) | Placebo (n = 5786), n (%) | Difference | P-value | |
|---|---|---|---|---|
| Relatedness to bleeding, cancer, and/or trauma | ||||
| All bleeding-related death | 18 (0.3) | 12 (0.2) | +6 (0.1) | 0.36 |
| Bleeding-related death without cancera or trauma | 7 (0.1) | 7 (0.1) | +0 (0.0) | |
| Bleeding-related death with cancer | 3 (0.1) | 0 (0.0) | +3 (0.1) | |
| Bleeding-related death with trauma | 8 (0.1) | 5 (0.1) | +3 (0.1) | |
| All cancer-related death | 34 (0.6) | 17 (0.3) | +17 (0.3) | 0.02 |
| Cancer-related death without bleedingb | 31 (0.5) | 17 (0.3) | +14 (0.2) | |
| All trauma-related deathc | 10 (0.2) | 5 (0.1) | +5 (0.1) | 0.30 |
| Trauma-related death without bleedingb | 2 (0.0) | 0 (0.0) | +2 (0.0) | |
Event rates are expressed as absolute percentages.
aWithout possible, probable, or definite cancer-related death.
bWithout possible, probably, or definite bleeding-related death.
cTrauma included five motor vehicle accidents, three suicides/gun shots, four major trauma/crush injuries, two falls/head trauma, and one thigh haematoma without documented history of trauma.
Cancer
Cancer incidence between enrolment and randomization (0.4% for continued thienopyridine vs. 0.5% for placebo, P = 0.57), and after randomization (12–33 months) did not differ (2.0 vs. 1.6%, respectively, P = 0.12). Cancer-related death occurred in 0.6 vs. 0.3% respectively, P = 0.02 (Figure 2). Cancer location in patients with cancer-related death was diverse. Lung cancer was most common (n = 10 vs. 9), followed by prostate (5 vs. 0), pancreas (4 vs. 1), and colorectal cancer (4 vs. 0). There were no differences in the occurrence of haematologic malignancies between treatment arms (2 vs. 1). Cancers were metastatic in >33% of the subjects at the time of diagnosis (see Supplementary material online, Table S4). Study drug had been discontinued prior to death at a median 60 and 118 days in subjects treated with continued thienopyridine or placebo, respectively. Cancer-related deaths were more commonly not associated with bleeding (Table 3). Of three cancer-related deaths attributed to bleeding, two were in the setting of surgical procedures. Cancer was diagnosed prior to enrolment in nine patients who subsequently died, eight of whom were assigned to continued thienopyridine. Exclusion of these 9 patients in a sensitivity analysis resulted in 26 (0.4%) vs. 16 (0.3%) cancer-related deaths with continued thienopyridine and placebo, respectively (P = 0.16).
Discussion
This analysis was prompted by the unexpected observation of an increase in non-cardiovascular event-related death, which entirely accounted for the increase in total mortality (+0.4%) among patients assigned to continued thienopyridine (vs. placebo) in the DAPT study. No difference in cardiovascular-related death rate was observed during the randomized treatment period despite a 71% relative reduction in stent thrombosis and a 53% relative reduction in myocardial infarction with continued thienopyridine therapy (vs. placebo), although over the 3-month period when subjects discontinued thienopyridine, there was a trend toward increased cardiovascular mortality.9
In this analysis of the mechanisms of deaths occurring within the DAPT study, we found that fatal bleeding was rare and accounted for only 6.8% (15 of 222) of all deaths while relatedness of death to any (possible, probable, and definite) bleeding accounted for 13.5% (30 of 222) of all deaths. Continued thienopyridine therapy was associated with increased bleeding risk, particularly in the setting of unexpected major trauma, but bleeding accounted for a small fraction of the observed difference in mortality between randomly assigned treatment arms in the DAPT study. The major portion of mortality difference was observed in clopidogrel-treated patients, although thienopyridine type was not randomized (the clopidogrel group was larger in sample size and of older average age than the prasugrel group). A recent large randomized trial of ticagrelor vs. placebo showed no hazard for non-cardiovascular mortality despite a higher risk of bleeding,20 and a recent meta-analysis suggested a reduction in cardiovascular mortality with no increase in non-cardiovascular mortality with P2Y12 inhibition following myocardial infarction.21 Importantly, only patients expected to tolerate thienopyridine plus aspirin therapy were enrolled into these studies, and patients requiring oral anticoagulation were excluded.
In the DAPT study, subjects with major bleeding events during the first year following coronary stenting while receiving thienopyridine plus aspirin therapy were considered ineligible for randomization. Furthermore, if medical reasons for discontinuing thienopyridine plus aspirin therapy arose after randomization, patients were permitted to stop study drug and receive open-label therapy according to their physician's discretion. Under these conditions, despite the known bleeding risk of thienopyridine plus aspirin therapy, bleeding was the attributed cause of death in a minority of patients and fatal bleeding was rare. While it is possible that undetected bleeding may have contributed mortality risk, adjudication by the blinded committee included any possible precedent bleeding before death. Trauma-related death was unusual, but most often associated with severe rather than minor trauma.
The difference in mortality between randomized treatment arms was primarily attributable to cancer-related deaths that occurred without clinically evident bleeding. In this regard, several points deserve mention. First, a history of cancer was not an exclusion from DAPT study enrolment and was present in ∼∼10% of all randomized patients. Second, a larger number of patients with pre-existing cancer, in some cases metastatic at the time of initial diagnosis, were randomly assigned to continued thienopyridine therapy, and there was no significant difference in the incidence of new cancer diagnosis was observed after randomization. Third, cancer-related deaths were most commonly due to solid tumours without predilection for specific cell type or location. The grouping of these various cancers together as one common clinical condition may have limited pathophysiologic rationale, despite being a simple construct for analysis. Finally, cancer-related death was rarely related to bleeding (n = 3), and in most cases antiplatelet therapy had been discontinued well in advance of death. Given these observations, and the intended but incomplete exclusion of life expectancy limiting cancers from enrolment, it seems prudent to conclude that continued thienopyridine should be considered very carefully among patients with limited life expectancy due to cancer, as the impact of cancer may be more important than cardiovascular event reduction over the immediate course of treatment. Furthermore, the observation of increased cancer-related mortality may be a chance finding either related to enrolment imbalance or related to false-positive results.
The observed prominence of cancer as a cause of death following coronary stenting is consistent with current trends. Over the past 30 years, cancer has supplanted cardiovascular diagnoses as the dominant cause of mortality following percutaneous coronary intervention.3,4 This likely reflects improvement in procedural outcomes and stent technology as well as more comprehensive, intensive associated medical therapy. As death due to cardiovascular disease has declined, the relative contribution of cancer to all-cause mortality has increased. In fact, we did not observe a reduction in cardiovascular mortality associated with continued thienopyridine therapy despite reduction in ischaemic events (stent thrombosis, myocardial infarction), possibly due to a decline in acute mortality following such events.22,23
Recent meta-analysis of 14 randomized trials involving almost 70 000 patients and almost 140 000 patients/years of follow-up, with a median 2 years follow-up, showed no difference in mortality with thienopyridine and aspirin compared with aspirin alone [hazard ratio 1.04, (95% credible interval 0.96–1.18)], and meta-regression analysis demonstrated no relationship between duration of combination therapy and mortality.11 In addition, a recent large-scale clinical trial suggests that continuation of aspirin plus ticagrelor therapy beyond 1 year after myocardial infarction is not associated with increased mortality.20 Additionally, recent studies have not demonstrated a relationship between thienopyridine therapy and cancer-related mortality.24–26
Limitations
Cancer endpoints were not pre-specified, and histologic data were not available in most patients. A common pathophysiologic mechanism is unlikely across the types and locations of cancer observed in this trial. The analysis was performed with pooled types and locations of cancer to provide statistical power, but this, and multiple testing, may have increased the risk of a false-positive finding. Similarly, while all-cause mortality and was not significantly increased in this analysis, observations on mortality in a single trial are equally subject to either lack of power or the risk of a false-positive finding as are other endpoints. Sub-analyses for relatedness to bleeding and cancer were designed as exploratory, and not pre-specified before examining the primary study results. These event rates are small and effect estimates unstable.
Conclusion/summary
As cardiovascular mortality has decreased with iterative improvements in revascularization procedures, better risk factor management, and adoption of effective medical therapies, the relative contribution of non-cardiovascular events to total mortality in patients with coronary artery disease has increased.
In the DAPT study, there was a trend toward increased overall mortality contributed to by a higher rate of non-cardiovascular death during treatment with continued thienopyridine, and a trend to higher cardiovascular death during the period of discontinuation of therapy. Non-fatal bleeding was increased, although fatal bleeding was rare, and did not explain a difference in non-cardiovascular mortality. The relationship between cancer-related death and continued thienopyridine treatment was not evident after cancer-related deaths in patients diagnosed with cancer prior to enrolment were excluded, and may have been attributable to chance. Nevertheless, clinicians should carefully consider the use of extended thienopyridine therapy in patients with limited life expectancy due to cancer on an individual basis.
Supplementary material
Supplementary material is available at European Heart Journal online.
Funding
This work was supported by Abbott, Boston Scientific, Cordis, Medtronic, Bristol-Myers Squibb Company/Sanofi Pharmaceuticals Partnership, Eli Lilly and Company, and Daiichi Sankyo Company and the US Department of Health and Human Services (1RO1FD003870-01).
Conflict of interest: L.M.: Grants to institution-Abbott, Boeringher Ingleheim, Boston Scientific, Cordis, Medtronic Vascular, Eli Lilly, Daiichi Sankyo, Bristol Myers Squibb, Sanofi Aventis, Biotronik (all significant); Consulting-Medtronic Vascular, Biotronik, Boeringher Ingleheim, St. Jude Medical, Eli Lilly, Recor, Biotronik (all modest); Honoraria-Sanofi-Aventis, modest. S.M.: Grants to institution-Harvard Clinical Research Institute, Siemens. R.W.Y.: Harvard Clinical Research Institute, salary, significant; Merck, other, significant; Abbott vascular, consultant, modest; Boston Scientific, consultant, modest. D.E.C.: Medtronic, grant, modest; Boston Scientific, grant, modest; Celonova, grant, consultant, modest. P.G.S.: Research grant-Sanofi, Servier (both significant); Ownership-Aterovax, modest; Other-Astra Zeneca, Sanofi, Servier (all significant); Other-Amarin, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, CSL-Behring, Daiichi-Sankyo, Eli Lilly, Merck Sharpe Dohme, Janssen, Novartis, Medtronic, Pfizer, The Medicines Company, GlaxoSmithKline (all modest). S.W.: Research grants to institution-Abbott Vascular, Boston Scientific, Biosensors, Biotronik, Medtronic, Edwards; Honoraria-AstraZeneca, Eli Lilly, Boston Scientific, Biotronik, Medtronic, Edwards. S.D.W.: Research grants-Arena, AstraZeneca, Bristol-Myers Squibb, Eisai, Eli Lilly/Daiichi Sankyo, Merck, Sanofi Aventis; Consulting-Aegerion, Angelmed, Eli Lilly/Daiichi Sankyo, Xoma, ICON Clinical, Boston Clinical Research Institute, Janssen, AstraZeneca, Bristol-Myers Squibb, Eisai, Arena. D.J.C.: Research grant support-Eli Lilly, Astra Zeneca, Daiichi Sankyo, Medtronic, Abbott Vascular, Boston Scientific (all significant); Consulting fees-Eli Lilly, Astra Zeneca, Medtronic, Abbott Vascular (all modest); Speaking honoraria-Astra Zeneca (modest). J.M.M.: Harvard Clinical Research Institute, salary, significant. E.B.: Research grant to institution-AstraZeneca, Bristol-Meyers Squib, Daiichi Sankyo, Duke University, Johnson & Johnson, Merck, Sanofi Aventis; Honoraria-Bayer, Daiichi Sankyo, Medscape, Menarini International; Consultancy-Sanofi Aventis, The Medicines Company.
Acknowledgements
We acknowledge Deborah Schrag, MD for her guidance regarding oncology related analyses; Robert Bonow, MD for his leadership of the study data safety monitoring board; Wen Hua Hsieh for her assistance with statistical analysis; and Joanna Suomi for her assistance in preparing tables and figures for this manuscript.
References
- 1.Nabel EG, Braunwald E. A tale of coronary artery disease and myocardial infarction. N Engl J Med 2012;366:54–63. [DOI] [PubMed] [Google Scholar]
- 2.US Department of Health and Human Services. NHLBI Fact Book, Fiscal Year 2012. Bethesda, MD: National Heart, Lung, and Blood Institute, 2012. [Google Scholar]
- 3.Spoon DB, Psaltis PJ, Singh M, Holmes DR, Jr, Gersh BJ, Rihal CS, Lennon RJ, Moussa ID, Simari RD, Gulati R. Trends in cause of death after percutaneous coronary intervention. Circulation 2014;129:1286–1294. [DOI] [PubMed] [Google Scholar]
- 4.Pedersen F, Butrymovich V, Kelbaek H, Wachtell K, Helqvist S, Kastrup J, Holmvang L, Clemmensen P, Engstrom T, Grande P, Saunamaki K, Jorgensen E. Short- and long-term cause of death in patients treated with primary PCI for STEMI. J Am Coll Cardiol 2014;64:2101–2108. [DOI] [PubMed] [Google Scholar]
- 5.Eikelboom JW, Mehta SR, Anand SS, Xie C, Fox KA, Yusuf S. Adverse impact of bleeding on prognosis in patients with acute coronary syndromes. Circulation 2006;114:774–782. [DOI] [PubMed] [Google Scholar]
- 6.Mehran R, Pocock S, Nikolsky E, Dangas GD, Clayton T, Claessen BE, Caixeta A, Feit F, Manoukian SV, White H, Bertrand M, Ohman EM, Parise H, Lansky AJ, Lincoff AM, Stone GW. Impact of bleeding on mortality after percutaneous coronary intervention results from a patient-level pooled analysis of the REPLACE-2 (randomized evaluation of PCI linking angiomax to reduced clinical events), ACUITY (acute catheterization and urgent intervention triage strategy), and HORIZONS-AMI (harmonizing outcomes with revascularization and stents in acute myocardial infarction) trials. JACC Cardiovasc Interv 2011;4:654–664. [DOI] [PubMed] [Google Scholar]
- 7.Lopes RD, Subherwal S, Holmes DN, Thomas L, Wang TY, Rao SV, Magnus Ohman E, Roe MT, Peterson ED, Alexander KP. The association of in-hospital major bleeding with short-, intermediate-, and long-term mortality among older patients with non-ST-segment elevation myocardial infarction. Eur Heart J 2012;33:2044–2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spencer FA, Moscucci M, Granger CB, Gore JM, Goldberg RJ, Steg PG, Goodman SG, Budaj A, FitzGerald G, Fox KA, Investigators G. Does comorbidity account for the excess mortality in patients with major bleeding in acute myocardial infarction? Circulation 2007;116:2793–2801. [DOI] [PubMed] [Google Scholar]
- 9.Mauri L, Kereiakes DJ, Yeh RW, Driscoll-Shempp P, Cutlip DE, Steg PG, Normand SL, Braunwald E, Wiviott SD, Cohen DJ, Holmes DR, Jr, Krucoff MW, Hermiller J, Dauerman HL, Simon DI, Kandzari DE, Garratt KN, Lee DP, Pow TK, Ver Lee P, Rinaldi MJ, Massaro JM. Twelve or 30 months of dual antiplatelet therapy after drug-eluting stents. N Engl J Med 2014;371:2155–2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kereiakes DJ, Yeh RW, Massaro JM, Driscoll-Shempp P, Cutlip DE, Steg PG, Gershlick AH, Darius H, Meredith IT, Ormiston J, Tanguay JF, Windecker S, Garratt KN, Kandzari DE, Lee DP, Simon DI, Iancu AC, Trebacz J, Mauri L. Antiplatelet therapy duration following bare metal or drug-eluting coronary stents: the dual antiplatelet therapy randomized clinical trial. JAMA 2015;313:1113–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elmariah S, Mauri L, Doros G, Galper BZ, O'Neill KE, Steg PG, Kereiakes DJ, Yeh RW. Extended duration dual antiplatelet therapy and mortality: a systematic review and meta-analysis. Lancet 2014;385:792–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Faxon DP, Lawler E, Young M, Gaziano M, Kinlay S. Prolonged clopidogrel use after bare metal and drug-eluting stent placement: the Veterans Administration drug-eluting stent study. Circ Cardiovasc Interv 2012;5:372–380. [DOI] [PubMed] [Google Scholar]
- 13.Mulukutla SR, Marroquin OC, Vlachos HA, Selzer F, Toma C, Kip KE, Abbott JD, Holper E, Lee JS, Khandhar S, Kutcher M, Kelsey S, Smith C, Faxon D, Williams DO. Benefit of long-term dual anti-platelet therapy in patients treated with drug-eluting stents: from the NHLBI dynamic registry. Am J Cardiol 2013;111:486–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giustino G, Baber U, Sartori S, Mehran R, Mastoris I, Kini AS, Sharma SK, Pocock SJ, Dangas GD. Duration of dual antiplatelet therapy after drug-eluting stent implantation: a systematic review and meta-analysis of randomized controlled trials. J Am Coll Cardiol 2015;65:1298–1310. [DOI] [PubMed] [Google Scholar]
- 15.Palmerini T, Benedetto U, Bacchi-Reggiani L, Riva DD, Biondi-Zoccai G, Feres F, Abizaid A, Hong MK, Kim BK, Jang Y, Kim HS, Park KW, Genereux P, Bhatt DL, Orlandi C, De Servi S, Petrou M, Rapezzi C, Stone GW. Mortality in patients treated with extended duration dual antiplatelet therapy after drug-eluting stent implantation: a pairwise and Bayesian network meta-analysis of randomised trials. Lancet 2015;385:2371–2382. [DOI] [PubMed] [Google Scholar]
- 16.Mauri L, Kereiakes DJ, Normand SL, Wiviott SD, Cohen DJ, Holmes DR, Bangalore S, Cutlip DE, Pencina M, Massaro JM. Rationale and design of the dual antiplatelet therapy study, a prospective, multicenter, randomized, double-blind trial to assess the effectiveness and safety of 12 versus 30 months of dual antiplatelet therapy in subjects undergoing percutaneous coronary intervention with either drug-eluting stent or bare metal stent placement for the treatment of coronary artery lesions. Am Heart J 2010;160:1035–1041, 1041 e1. [DOI] [PubMed] [Google Scholar]
- 17.Cutlip DE, Windecker S, Mehran R, Boam A, Cohen DJ, van Es GA, Steg PG, Morel MA, Mauri L, Vranckx P, McFadden E, Lansky A, Hamon M, Krucoff MW, Serruys PW. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation 2007;115:2344–2351. [DOI] [PubMed] [Google Scholar]
- 18.The GUSTO Investigators. An international randomized trial comparing four thrombolytic strategies for acute myocardial infarction. N Engl J Med 1993;329:673–682. [DOI] [PubMed] [Google Scholar]
- 19.Mehran R, Rao SV, Bhatt DL, Gibson CM, Caixeta A, Eikelboom J, Kaul S, Wiviott SD, Menon V, Nikolsky E, Serebruany V, Valgimigli M, Vranckx P, Taggart D, Sabik JF, Cutlip DE, Krucoff MW, Ohman EM, Steg PG, White H. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the Bleeding Academic Research Consortium. Circulation 2011;123:2736–2747. [DOI] [PubMed] [Google Scholar]
- 20.Bonaca MP, Bhatt DL, Cohen M, Steg PG, Storey RF, Jensen EC, Magnani G, Bansilal S, Fish MP, Im K, Bengtsson O, Oude Ophuis T, Budaj A, Theroux P, Ruda M, Hamm C, Goto S, Spinar J, Nicolau JC, Kiss RG, Murphy SA, Wiviott SD, Held P, Braunwald E, Sabatine MS. Long-term use of ticagrelor in patients with prior myocardial infarction. N Engl J Med 2015;372:1791–1800. [DOI] [PubMed] [Google Scholar]
- 21.Udell JA, Bonaca MP, Collet JP, Lincoff AM, Kereiakes DJ, Costa F, Lee CW, Mauri L, Valgimigli M, Park SJ, Montalescot G, Sabatine MS, Braunwald E, Bhatt DL. Long-term dual antiplatelet therapy for secondary prevention of cardiovascular events in the subgroup of patients with previous myocardial infarction: a collaborative meta-analysis of randomized trials. Eur Heart J 2015. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 22.Yeh RW, Sidney S, Chandra M, Sorel M, Selby JV, Go AS. Population trends in the incidence and outcomes of acute myocardial infarction. N Engl J Med 2010;362:2155–2165. [DOI] [PubMed] [Google Scholar]
- 23.Secemsky EA, Matteau A, Yeh RW, Steg PG, Camenzind E, Wijns W, McFadden E, Mauri L. Comparison of short- and long-term cardiac mortality in early versus late stent thrombosis (from pooled PROTECT trials). Am J Cardiol 2015;115:1678–1684. [DOI] [PubMed] [Google Scholar]
- 24.Unger EF. Weighing benefits and risks – the FDA's review of prasugrel. N Engl J Med 2009;361:942–945. [DOI] [PubMed] [Google Scholar]
- 25.Roe MT, Armstrong PW, Fox KA, White HD, Prabhakaran D, Goodman SG, Cornel JH, Bhatt DL, Clemmensen P, Martinez F, Ardissino D, Nicolau JC, Boden WE, Gurbel PA, Ruzyllo W, Dalby AJ, McGuire DK, Leiva-Pons JL, Parkhomenko A, Gottlieb S, Topacio GO, Hamm C, Pavlides G, Goudev AR, Oto A, Tseng CD, Merkely B, Gasparovic V, Corbalan R, Cinteza M, McLendon RC, Winters KJ, Brown EB, Lokhnygina Y, Aylward PE, Huber K, Hochman JS, Ohman EM, Investigators TA. Prasugrel versus clopidogrel for acute coronary syndromes without revascularization. N Engl J Med 2012;367:1297–1309. [DOI] [PubMed] [Google Scholar]
- 26.Hicks BM, Murray LJ, Hughes C, Cardwell CR. Clopidogrel use and cancer-specific mortality: a population-based cohort study of colorectal, breast and prostate cancer patients. Pharmacoepidemiol Drug Saf 2015;24:830–840. [DOI] [PubMed] [Google Scholar]