Abstract
The National Cancer Institute-Molecular Analysis for Therapy Choice trial is a clinical trial that will analyze various genetic statuses of patients' tumors to determine whether they contain abnormalities which can be a target for an available drug. National Cancer Institute-Molecular Analysis for Therapy Choice seeks to determine whether improved outcomes can be achieved when cancer treatments are personalized based on molecular abnormalities found in individual patients. As a master protocol, or basket trial, National Cancer Institute-Molecular Analysis for Therapy Choice can add or remove treatments as indicated over the duration of the study. Each treatment will be used in a unique arm, or sub-study, of the trial. The trial initially has 10 arms, each of which will enroll patients to a specific molecularly targeted treatment. It is ultimately anticipated that 20–25 drugs or combination treatments will be tested. To be eligible for the study, participants must have an advanced solid tumor or lymphoma that is no longer responding or never responded to the standard therapy. National Cancer Institute-Molecular Analysis for Therapy Choice investigators plan to obtain tumor biopsy specimens from as many as 3000 patients initially. To identify multiple genetic abnormalities that may respond to the targeted drugs selected for the trial, next-generation deoxyribonucleic acid and ribonucleic acid sequencing will be done in the genetic testing laboratories, analyzing for >4000 different variants across 143 genes. The drugs included in the trial have all either been approved by the US Food and Drug Administration for another cancer indication or are still being tested in other clinical trials, but have shown some clinical levels of evidence against tumors with a particular genetic alteration.
Keywords: precision medicine, National Cancer Institute (NCI), NCI-MATCH, National Clinical Trials Network (NCTN)
Precision medicine
Precision medicine is a medical model that proposes the customization of healthcare—with medical decisions, practices and/or products being tailored to the individual patient. The prospect of applying this concept broadly has been dramatically improved by the recent development of large-scale biologic databases, powerful methods for characterizing patients and computational tools for analyzing large sets of data. Oncology is a clear choice for enhancing the impact of precision medicine. Research in oncology so far has already revealed many of the molecular lesions that drive cancers, showing that each cancer has its own genomic signature, with some features tumor-specific and others common to multiple types (1).
To push the development of precision medicine in the USA, the Federal Government has begun the precision medicine initiative (PMI) which will be funded through a $215 million request in the President's 2016 Budget directed at the NIH, the FDA and the Office of the National Coordinator for Health Information Technology (1). The PMI contains a budget request of $70 million for NCI to scale-up efforts to identify genomic drivers in cancer and apply that knowledge in the development of more effective approaches to cancer treatment.
NCI-Molecular Analysis for Therapy Choice Program (NCI-MATCH)
NCI-Molecular Analysis for Therapy Choice (NCI-MATCH) is a Phase II clinical trial that will analyze patients' tumors to determine whether they contain genetic abnormalities for which a targeted drug exists and assign treatment based on the abnormality (NCT02465060). NCI-MATCH seeks to determine whether treating cancers according to their molecular abnormalities will show evidence of effectiveness.
NCI-MATCH is considered the largest and most scientifically rigorous precision medicine cancer trial to date based on the number of patients, treatment options and types of cancer being studied in a single clinical trial. NCI-MATCH investigators plan to obtain tumor biopsy specimens from ∼3000 patients initially. The specimens will undergo deoxyribonucleic acid (DNA) and ribonucleic acid (RNA) sequencing to identify 143 genetic abnormalities that may respond to the targeted drugs selected for the trial. The 143 genetic abnormalities were composed of 73 hotspot genes, 49 copy number alterations, 26 tumor suppressor genes and 22 fusion drivers including 183 assays. Those were selected as pan-cancer, recurrently altered abnormalities using Oncomine® database supplemented with data from COSMIC (2). The drugs included in the trial have all either been approved by the US Food and Drug Administration (FDA) for another cancer indication or are still being tested in other clinical trials but have shown some effectiveness against tumors with a particular genetic alterations (3) (Fig. 1).
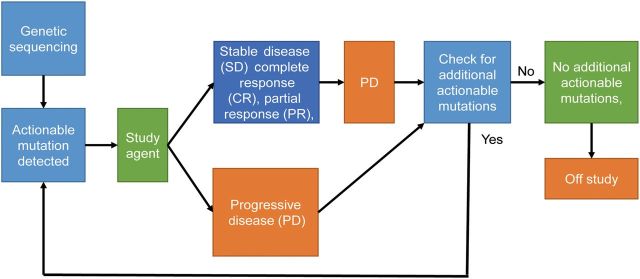
Figure 1.
NCI-Molecular Analysis for Therapy Choice (NCI-MATCH) schema. NCI-MATCH investigators obtain tumor biopsy specimens from ∼3000 patients. The specimens will undergo deoxyribonucleic acid and ribonucleic sequencing to identify 143 genetic abnormalities that may respond to the targeted drugs selected for the trial. If genetic abnormalities are detected, study drugs will be allocated to the patients. The patients will be treated with the targeted drug for as long as their tumor shrinks or remains stable. If genetic testing showed that the patient has a second abnormality that is targeted by a drug being studied in the trial, and if there is an open slot in the other study that is testing that drug, the patient may be eligible for the study.
Study design
NCI-MATCH began enrollment in August 2015 with 10 arms, each enrolling up to 35 patients; the number of experimental arms could increase to 25 or 30 within a year (4) (Table 1). Each arm will enroll adult patients 18 years of age and older with advanced solid tumors and lymphomas that are no longer responding to the standard therapy and have begun to grow.
Table 1.
Initial substudies planned in NCI-MATCH
| Agent(s) | Molecular target(s) | Estimated prevalence |
|---|---|---|
| Afatiniba | EGFR activating mutations | 1–4% |
| Afatiniba | HER2 activating mutations | 2–5% |
| Crizotinib | ALK rearrangement | 4% |
| AZD9291a | EGFR T790M mutations and rare EGFR activating mutations | 1–2% |
| Crizotinib | ROS1 translocations | 5% |
| Dabrafenib and Trametinib | BRAF V600E and V600K mutations | 7% |
| Trametinib | BRAF fusions or non-V600E, non-V600K BRAF mutations | 2.8% |
| TDM1a | HER2 amplification | 5% |
| VS-6063a | NF2 loss | 2% |
| Sunitiniba | cKIT mutations | 4% |
EGFR, epidermal growth factor receptor; HER2, human epidermal growth factor receptor type2; ALK, anaplastic lymphoma kinase; BRAF, v-Raf murine sarcoma viral oncogene homolog B.
aAgents and associated targets are pending final regulatory review.
The study uses a master protocol, which allows investigators to run concurrent studies under one overarching protocol and to add or terminate arms based on patients' responses. Patients with more than one actionable mutation may switch arms if they do not respond to their initial therapy.
The primary and secondary endpoints for the trial will be overall response rate (ORR) and 6-month progression-free survival (PFS), respectively. Investigators will look for an ORR of at least 16–25% and 6-month PFS of at least 35% as indications that a particular drug or drug combination may merit further study (4). This threshold was chosen to minimize the chance of a treatment outcome being a false positive, while maximizing the chance that a treatment that is actually effective will appear as a true positive. In addition to assessing ORR and PFS, researchers will also determine time to progression, PFS, overall survival (OS) and evaluate the side effects of the treatments.
The FDA will consider approving drugs for specific cancers based on Phase II evidence, especially if the drug has already been approved for another cancer with the same mutation. In cases where an unapproved drug shows effectiveness—judged by a response rate of 50% or higher in a targeted population—investigators might launch a larger, confirmatory trial to validate the findings before seeking approval (5).
Trial collaborators
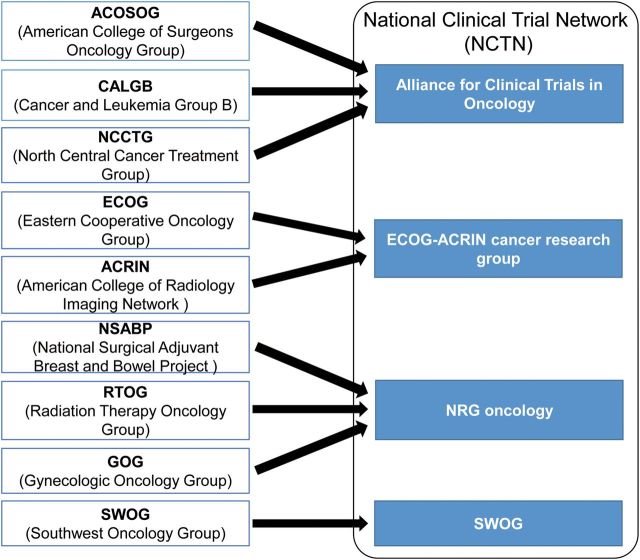
In this new era of Precision Medicine, NCI dramatically restructured its trial network systems. The nine former adult Cooperative Groups (ACOSOG, SWOG, RTOG, CALGB, ECOG, ACRIN, GOG, NSABP, and NCCTG) have been consolidated into four adult groups (Alliance, SWOG, ECOG-ACRIN and NRG), and the Canadian Network Group in the new National Clinical Trials Network (NCTN) to improve efficiency in completing trials and to rapidly take advantage of the technological changes and increased understanding of cancer biology (6) (Fig. 2).
Figure 2.
The NCI National Clinical Trials Network (NCTN) structure. In the era of Precision Medicine, the nine former adult Cooperative Groups (ACOSOG, SWOG, RTOG, CALGB, ECOG, ACRIN, GOG, NSABP and NCCTG) have been consolidated into four adult groups (Alliance, SWOG, ECOG-ACRIN and NRG), and the Canadian Network Group in the new National Clinical Trials Network (NCTN) to improve efficiency in completing trials and to rapidly take advantage of the technological changes and understanding of cancer biology.
NCI-MATCH is supported by NCI and is coordinated by the ECOG-ACRIN Cancer Research Group. Personnel from NCI, ECOG-ACRIN, and the other adult trial groups in the NCI-supported NCTN—the Alliance for Clinical Trials in Oncology, SWOG and the NRG Oncology Group—have collaborated in the development of NCI-MATCH (http://www.cancer.gov/about-cancer/treatment/clinical-trials/nci-supported/nci-match). The NCTN includes researchers, physicians and health-care professionals at public and private institutions across the USA. They conduct clinical trials on all types of adult cancers (4).
In addition to the institutions belonging to the NCTN, NCI-MATCH will be open to all institutions and sites that participate in the NCI Community Oncology Research Program (NCORP), bringing the total of possible institutions to nearly 2400 nationwide. Therefore, patients may not need to travel far from home to enroll in the trial (http://www.cancer.gov/news-events/press-releases/2015/nci-match).
Patients
The NCI-MATCH investigators plan to screen ∼3000 patients during the full course of the trial to enroll ∼1000 patients in the various treatment arms. A goal for the trial is for at least 25% of the ∼1000 patients enrolled in the trial to have rare cancers (3). Rare cancers can include cancers at sites in the body where cancer infrequently occurs, such as the eye, ureter and pituitary gland, along with cancers that are classified as rare because the primary location of the tumor could not be determined at the time of diagnosis. Common cancers are non-small-cell lung, breast, colorectal and prostate cancers.
The NCI-MATCH trial has two enrollment steps. First, each patient will initially enroll for screening. In this step, a biopsy procedure will be used to collect tumor samples that will undergo DNA and RNA sequencing to detect genetic abnormalities that may be driving tumor growth. Second, if a molecular abnormality is detected that is targeted by one of the drugs being studied in the trial, patients will be further evaluated to determine whether they meet the specific eligibility requirements of that arm and can be accepted into NCI-MATCH. Once enrolled, patients will be treated with the targeted drug for as long as their tumor shrinks or remains stable.
Patients can be considered for entering a second arm of NCI-MATCH if the first treatment they received was not successful. If genetic testing showed that the patient has a second abnormality that is targeted by a drug being studied in the trial, and if there is an open slot in the other study that is testing that drug, the patient may be eligible for the study. There may be additional tests that the patient would need before being registered into the new arm to receive treatment.
Next-generation sequencing
To ensure consistent testing for genomic variants across all sites, researchers will first send tumor formalin-fixed paraffin-embedded samples to a laboratory at the University of Texas, MD Anderson Cancer Center in Houston to ensure the sample includes an adequate amount of DNA and RNA samples will then go to next-generation sequencing (NGS) laboratories at one of four Clinical Laboratory Improvement Amendments (CLIA) certified laboratories: MD Anderson, Yale Comprehensive Cancer Center, Massachusetts General Hospital and the NCI testing facility in Frederick, MD. (4).
To identify multiple genetic abnormalities, NCI collaborated with Thermo Fisher Scientific and developed the Oncomine® Cancer Research Panel), which consists of amplicon-based panels and an informatics pipeline for Ion Torrent® NGS. In the four genetic testing laboratories, they will be analyzed for >4000 different variants across 143 genes. The trial will also use standard procedures for the collection of specimens and for preparing specimens for analysis. The estimated turn-around time from tumor collections to receive of qualified reports will be 11–14 days. In case immunohistochemistry or fluorescence in situ hybridization testing is needed, they will be done at the same CLIA-certified laboratories.
Collaborations with pharmaceutical companies
NCI-MATCH will incorporate 25 or 30 different study drugs or drug combinations, each targeting a specific gene mutation, in order to match each patient in the trial with a therapy that targets a molecular abnormality in their tumor. Many pharmaceutical companies are collaborating in NCI-MATCH and have also contributed their expertise.
Since NCI-MATCH is designed to explore whether drugs are effective against specific molecular abnormalities, patients who have tumors that can be treated with a drug already approved by the FDA for their molecular abnormality will not be eligible to use the same drug in NCI-MATCH. They could be considered for other drugs within NCI-MATCH if they have already received an approved therapy and have a different genetic abnormality that could be targeted with a new drug.
There are three levels of evidence for consideration in selecting drugs that will be used in the trial. Level 1 is the drug approved by FDA as a cancer treatment, with a companion diagnostic test. Level 2 is the drug met a clinical endpoint (ORR or PFS) in previous studies with evidence of target inhibition along with plausible evidence of a predictive molecular test. Level 3 is the drug demonstrated evidence of clinical activity with evidence of target inhibition plus some evidence of a predictive molecular test.
NCI-MATCH for children
The NCI-MATCH trial will also have a pediatric version that will enroll children with advanced cancers that have progressed on the standard therapy (http://www.cancer.gov/about-cancer/treatment/clinical-trials/nci-supported/nci-match). As in the adult NCI-MATCH trial, DNA and RNA sequencing will be used to identify children whose tumors have a genetic abnormality for which either an FDA-approved or investigational targeted therapy exists. Known as Pediatric MATCH, this trial is a key element of NCI's PMI. NCI is working with numerous pharmaceutical companies to make promising drugs available for Pediatric MATCH that will be offered in the adult NCI-MATCH trial. Pediatric MATCH, which will be led by the NCI-supported NCTN Children's Oncology Group (COG), is still under development but is expected to launch in 2016.
Discussions
The efforts to catalog driver mutations across over 30 different types of cancer in The Cancer Genome Atlas (TCGA) project have shown that only a small proportion of patients with any particular cancer type have a specific driver mutation (7). It is estimated that up to 10 000 patients with any given histologic type of cancer would need to be screened to capture the true prevalence of the rarer mutations. Most of the known driver mutations that may have active treatments are found in only 2–8% of patients across all types of cancer (http://www.cancer.gov/about-cancer/treatment/clinical-trials/nci-supported/nci-match) (Table 1). Thus, to efficiently investigate the effectiveness of molecularly targeted therapies for patients with the corresponding driver mutations, a broad-based genomic pre-screening effort is necessary to find and assign patients whose tumors harbor these mutations, regardless of tumor origin (http://www.cancer.gov/news-events/press-releases/2015/nci-match).
NCI-MATCH is a national project which involves 2400 clinical sites across the USA, whereas most other trials are conducted at only a few sites. Therefore, patients may not need to travel far from home to enroll in the trial. The trial also employs the expertise of the NCI and of specialized investigators and scientists within National Clinical Trials Network (NCTN) and the NCI Community Oncology Research Program (NCORP) who are at the cutting edge of precision oncology, as well as clinical oncology practices that are experienced in clinical trials.
Basket trials are expected to evolve as investigators gain more data and experience with their challenges (4,8). One direction may be toward use of combination therapies. Patients develop resistance to targeted therapies just as they do to the standard treatment, so combining a targeted therapy with one aimed at a different target or with a chemotherapy agent could make it possible to overcome the gained resistances. A secondary added drug also might target the molecular mechanisms of resistance as they become known. Another challenge for basket trial is emerging evidence that treatments directed at driver mutations can work across multiple tumor types that share common driver mutations, as well as evidence that some targeted treatments work better in some tumor types than others, even if the same mutation is present. For instance, V600E BRAF-mutated melanoma and hairy-cell leukemia respond to drugs that inhibit BRAF, but those drugs have low response rates in colon cancers with the same mutation if they are given as a single agent. Because it is difficult to perform molecularly targeted clinical trials except in the most prevalent types of cancers, NCI-MATCH is designed to be able to detect responses to the inhibition of driver mutations in more than one tumor type. Such findings can then be followed up with additional clinical trials to ascertain patient benefit.
Conflict of interest statement
None declared.
References
- 1.Collins FS, Varmus H. A new initiative on precision medicine. N Engl J Med 2015;372:793–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hovelson DH, McDaniel AS, Cani AK, et al. Development and validation of a scalable next-generation sequencing system for assessing relevant somatic variants in solid tumors. Neoplasia 2015;17:385–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.NCI Prepares to Launch MATCH Trial. Cancer Discov 2015;5:685. [Google Scholar]
- 4.McNeil C. NCI-MATCH launch highlights new trial design in precision-medicine era. J Natl Cancer Inst 2015;107:4–5. [DOI] [PubMed] [Google Scholar]
- 5.Darrow JJ, Avorn J, Kesselheim AS. New FDA breakthrough-drug category—implications for patients. N Engl J Med 2014;371:89–90. [DOI] [PubMed] [Google Scholar]
- 6.Abrams J, Conley B, Mooney M, et al. National Cancer Institute's Precision Medicine Initiatives for the new National Clinical Trials Network. Am Soc Clin Oncol Educ Book/ASCO American Society of Clinical Oncology Meeting 2014;34:71–6. [DOI] [PubMed] [Google Scholar]
- 7.Chin L, Andersen JN, Futreal PA. Cancer genomics: from discovery science to personalized medicine. Nature Med 2011;17:297–303. [DOI] [PubMed] [Google Scholar]
- 8.Redig AJ, Janne PA. Basket trials and the evolution of clinical trial design in an era of genomic medicine. J Clin Oncol 2015;33:975–7. [DOI] [PubMed] [Google Scholar]