Abstract
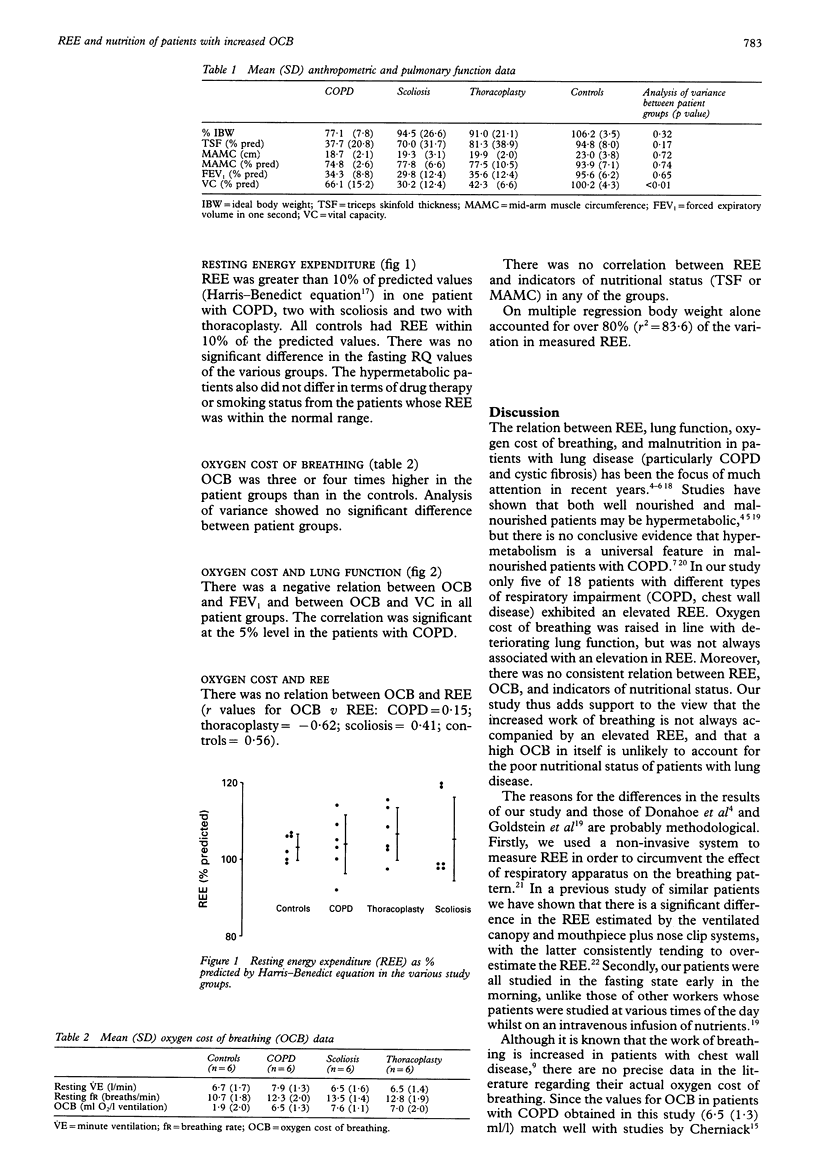
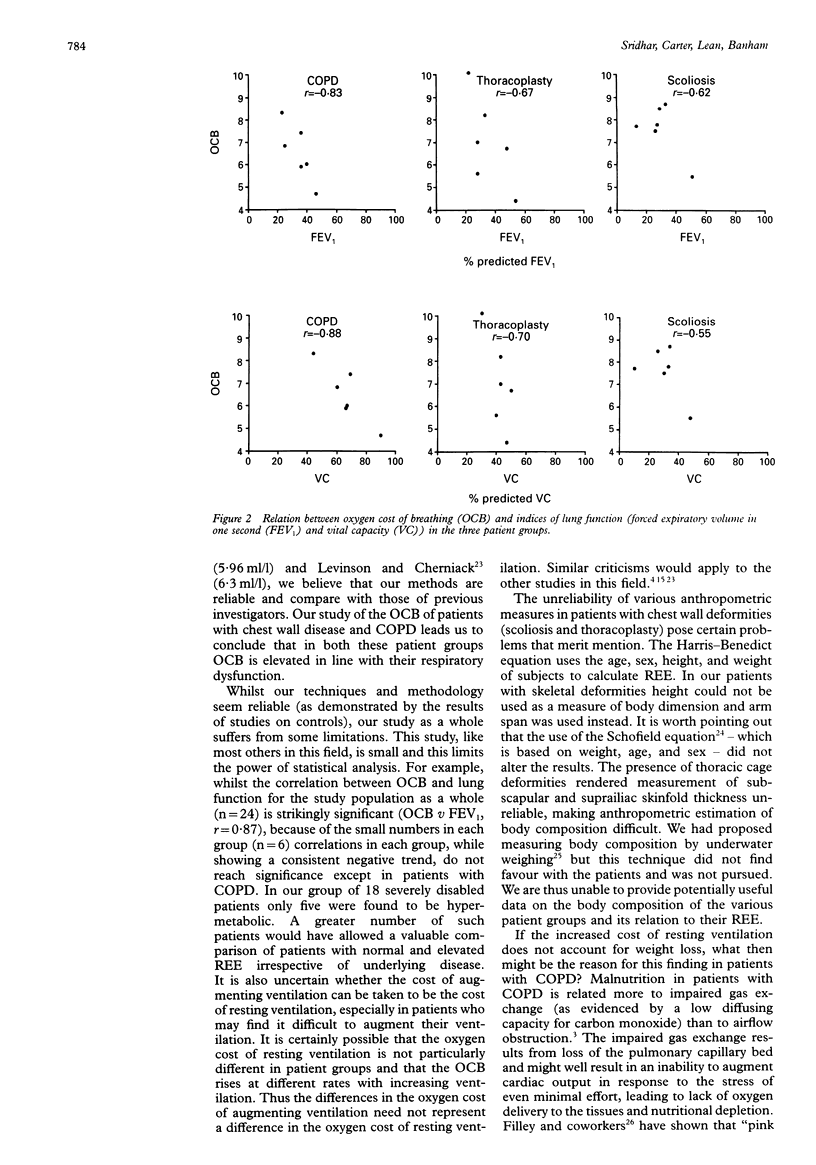
BACKGROUND--Weight loss is a well recognised feature of patients with emphysematous chronic obstructive pulmonary disease (COPD). It has been suggested that this weight loss could be due to a hypermetabolic state resulting from the increased oxygen cost of breathing (OCB). To clarify the relation between resting energy expenditure (REE), nutritional state, and OCB these indices were measured in patients with respiratory impairment and an increased OCB due to COPD, scoliosis, and thoracoplasty. METHODS--Eighteen patients (six COPD, six scoliosis, six thoracoplasty) of mean (SD) age 59.9 (8.6) years (8M, 10F) and six controls (45.5 (9.9) years; 2M, 4F) were studied. OCB was estimated by the addition of dead space to the breathing circuit and REE was measured by indirect calorimetry using a ventilated canopy system. Height, arm span, weight, triceps skin fold thickness (TSF), mid-arm muscle circumference (MAMC), forced expiratory volume in one second (FEV1), and vital capacity (VC) were measured in all study subjects. RESULTS--OCB was elevated in all patient groups (mean 7.0 ml/l) compared with controls (1.9 ml/l). All patients with COPD, four with scoliosis, three with thoracoplasty, and none of the controls were < 90% ideal body weight. Mean (SD) measured REE as % predicted (Harris-Benedict equation) was 103.8 (7.6) in patients with COPD, 105.5 (10.9) in those with scoliosis, 106.3 (6.9) in the thoracoplasty patients, and 103.3 (3.4) in controls. One patient with COPD, two with scoliosis, two with thoracoplasty, but no controls were hypermetabolic (REE > 110% predicted). In all groups there was a negative relation between OCB and lung function (OCB v FEV1 r = -0.83 in COPD, -0.62 in scoliosis, -0.67 in thoracoplasty, and -0.76 in controls). There was no correlation between REE and OCB or MAMC. CONCLUSIONS--In patients with respiratory disease OCB (augmented ventilation) is related to lung function but not to REE. This is evidence against the hypothesis that hypermetabolism due to increased oxygen cost of breathing at rest is the sole or major cause of malnutrition in patients with lung disease.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Askanazi J., Silverberg P. A., Foster R. J., Hyman A. I., Milic-Emili J., Kinney J. M. Effects of respiratory apparatus on breathing pattern. J Appl Physiol Respir Environ Exerc Physiol. 1980 Apr;48(4):577–580. doi: 10.1152/jappl.1980.48.4.577. [DOI] [PubMed] [Google Scholar]
- Askanazi J., Silverberg P. A., Foster R. J., Hyman A. I., Milic-Emili J., Kinney J. M. Effects of respiratory apparatus on breathing pattern. J Appl Physiol Respir Environ Exerc Physiol. 1980 Apr;48(4):577–580. doi: 10.1152/jappl.1980.48.4.577. [DOI] [PubMed] [Google Scholar]
- Bergofsky E. H. Respiratory failure in disorders of the thoracic cage. Am Rev Respir Dis. 1979 Apr;119(4):643–669. doi: 10.1164/arrd.1979.119.4.643. [DOI] [PubMed] [Google Scholar]
- Bishop C. W., Bowen P. E., Ritchey S. J. Norms for nutritional assessment of American adults by upper arm anthropometry. Am J Clin Nutr. 1981 Nov;34(11):2530–2539. doi: 10.1093/ajcn/34.11.2530. [DOI] [PubMed] [Google Scholar]
- Donahoe M., Rogers R. M., Wilson D. O., Pennock B. E. Oxygen consumption of the respiratory muscles in normal and in malnourished patients with chronic obstructive pulmonary disease. Am Rev Respir Dis. 1989 Aug;140(2):385–391. doi: 10.1164/ajrccm/140.2.385. [DOI] [PubMed] [Google Scholar]
- Durnin J. V., Rahaman M. M. The assessment of the amount of fat in the human body from measurements of skinfold thickness. Br J Nutr. 1967 Aug;21(3):681–689. doi: 10.1079/bjn19670070. [DOI] [PubMed] [Google Scholar]
- Filley G. F., Beckwitt H. J., Reeves J. T., Mitchell R. S. Chronic obstructive bronchopulmonary disease. II. Oxygen transport in two clinical types. Am J Med. 1968 Jan;44(1):26–38. doi: 10.1016/0002-9343(68)90234-9. [DOI] [PubMed] [Google Scholar]
- Fitting J. W., Frascarolo P., Jéquier E., Leuenberger P. Energy expenditure and rib cage-abdominal motion in chronic obstructive pulmonary disease. Eur Respir J. 1989 Oct;2(9):840–845. [PubMed] [Google Scholar]
- Goldstein S., Askanazi J., Weissman C., Thomashow B., Kinney J. M. Energy expenditure in patients with chronic obstructive pulmonary disease. Chest. 1987 Feb;91(2):222–224. doi: 10.1378/chest.91.2.222. [DOI] [PubMed] [Google Scholar]
- Green J. H., Muers M. F. Comparisons between basal metabolic rate and diet-induced thermogenesis in different types of chronic obstructive pulmonary disease. Clin Sci (Lond) 1992 Jul;83(1):109–116. doi: 10.1042/cs0830109. [DOI] [PubMed] [Google Scholar]
- Lanigan C., Moxham J., Ponte J. Effect of chronic airflow limitation on resting oxygen consumption. Thorax. 1990 May;45(5):388–390. doi: 10.1136/thx.45.5.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levison H., Cherniack R. M. Ventilatory cost of exercise in chronic obstructive pulmonary disease. J Appl Physiol. 1968 Jul;25(1):21–27. doi: 10.1152/jappl.1968.25.1.21. [DOI] [PubMed] [Google Scholar]
- Lukaski H. C. Methods for the assessment of human body composition: traditional and new. Am J Clin Nutr. 1987 Oct;46(4):537–556. doi: 10.1093/ajcn/46.4.537. [DOI] [PubMed] [Google Scholar]
- Naon H., Hack S., Shelton M. T., Gotthoffer R. C., Gozal D. Resting energy expenditure. Evolution during antibiotic treatment for pulmonary exacerbation in cystic fibrosis. Chest. 1993 Jun;103(6):1819–1825. doi: 10.1378/chest.103.6.1819. [DOI] [PubMed] [Google Scholar]
- Openbrier D. R., Irwin M. M., Rogers R. M., Gottlieb G. P., Dauber J. H., Van Thiel D. H., Pennock B. E. Nutritional status and lung function in patients with emphysema and chronic bronchitis. Chest. 1983 Jan;83(1):17–22. doi: 10.1378/chest.83.1.17. [DOI] [PubMed] [Google Scholar]
- Ryan C. F., Road J. D., Buckley P. A., Ross C., Whittaker J. S. Energy balance in stable malnourished patients with chronic obstructive pulmonary disease. Chest. 1993 Apr;103(4):1038–1044. doi: 10.1378/chest.103.4.1038. [DOI] [PubMed] [Google Scholar]
- Schofield W. N. Predicting basal metabolic rate, new standards and review of previous work. Hum Nutr Clin Nutr. 1985;39 (Suppl 1):5–41. [PubMed] [Google Scholar]
- Schols A. M., Fredrix E. W., Soeters P. B., Westerterp K. R., Wouters E. F. Resting energy expenditure in patients with chronic obstructive pulmonary disease. Am J Clin Nutr. 1991 Dec;54(6):983–987. doi: 10.1093/ajcn/54.6.983. [DOI] [PubMed] [Google Scholar]
- Spiro S. G. Exercise testing in clinical medicine. Br J Dis Chest. 1977 Jul;71(3):145–172. doi: 10.1016/0007-0971(77)90106-1. [DOI] [PubMed] [Google Scholar]
- Spiro S. G. Exercise testing in clinical medicine. Br J Dis Chest. 1977 Jul;71(3):145–172. doi: 10.1016/0007-0971(77)90106-1. [DOI] [PubMed] [Google Scholar]
- Wilson D. O., Rogers R. M., Hoffman R. M. Nutrition and chronic lung disease. Am Rev Respir Dis. 1985 Dec;132(6):1347–1365. doi: 10.1164/arrd.1985.132.6.1347. [DOI] [PubMed] [Google Scholar]
- Wilson D. O., Rogers R. M., Wright E. C., Anthonisen N. R. Body weight in chronic obstructive pulmonary disease. The National Institutes of Health Intermittent Positive-Pressure Breathing Trial. Am Rev Respir Dis. 1989 Jun;139(6):1435–1438. doi: 10.1164/ajrccm/139.6.1435. [DOI] [PubMed] [Google Scholar]


