Abstract
Aims
While mineralocorticoid receptor antagonists (MRAs) have been shown to benefit patients with reduced left ventricular ejection fraction (LVEF), spironolactone did not reduce the primary endpoint of cardiovascular death, heart failure hospitalization, or aborted cardiac arrest in patients with heart failure with preserved ejection fraction (HFpEF) in the TOPCAT trial, which enrolled patients with LVEF of 45% or greater. We utilized data from TOPCAT to assess the relationship between LVEF as well as outcomes and efficacy of spironolactone.
Methods and results
We assessed differences in baseline characteristics and outcomes across LVEF categories in 3444 patients with HFpEF, and determined whether LVEF modified the treatment effect of spironolactone. Ejection fraction ranged from 44 to 85%. Patients with higher ejection fraction were older, more likely to be female, less likely to have a history of myocardial infarction, and more likely to have a history of hypertension and diabetes. The incidence of the primary endpoint and cardiovascular death was highest in patients at the lower end of the ejection fraction spectrum. Ejection fraction modified the spironolactone treatment effect, particularly in the patients enrolled in the Americas, for the primary outcome (P = 0.046) and for heart failure hospitalization (P = 0.039), with stronger estimated benefits of spironolactone at the lower end of the ejection fraction spectrum with respect to the primary endpoint (LVEF <50%: HR 0.72, 95% CI 0.50, 1.05; LVEF ≥60%: HR 0.97, 95% CI 0.76, 1.23) and heart failure hospitalization (LVEF <50%: HR 0.76, 95% CI 0.46, 1.27; LVEF ≥60%: HR 0.98, 95% CI 0.74, 1.30).
Conclusion
In patients with HFpEF enrolled in TOPCAT, patient characteristics and outcomes varied substantially by LVEF. The potential efficacy of spironolactone was greatest at the lower end of the LVEF spectrum.
ClinicalTrials.gov number
Keywords: Heart failure with preserved ejection fraction, Spironolactone
See page 463 for the editorial comment on this article (doi:10.1093/eurheartj/ehv561)
Introduction
Heart failure with preserved ejection fraction (HFpEF) is associated with substantial morbidity and mortality, and to date no clinical outcome trials have definitively shown benefit with any therapy.1 The definition of ‘preserved’ ejection fraction has been subject to debate and has varied among trials of HFpEF, ranging from ≥40 to ≥55%.2,3 As a consequence, these studies have included patients with left ventricular ejection fraction (LVEF) well below the normal range. Although ejection fraction is an important predictor of outcomes in patients with heart failure with reduced ejection fraction (HFrEF), LVEF becomes less prognostic when higher than 40–45%.4,5 The extent to which the effectiveness of therapies for HFpEF is modified by LVEF is unclear.
The recently completed TOPCAT trial enrolled HFpEF patients with LVEF ≥45%.6 Spironolactone did not reduce the primary outcome of heart failure hospitalization, cardiovascular death, or aborted cardiac arrest, but was associated with reduced hospitalization for heart failure.7 Left ventricular ejection fraction above or below 50%, or above or below the median, were two of several prespecified subgroups, and we did not observe any heterogeneity based on these cut-off, although the number of patients with LVEF <50% in TOPCAT was low. We therefore sought to determine whether efficacy of spironolactone varied by left ventricular ejection fraction assessed continuously.
Methods
Patients
TOPCAT enrolled patients with HFpEF (LVEF ≥45%), who had either had a hospitalization within the past year for which heart failure was a major component, or had an elevated natriuretic peptide level (BNP >150 or NT-proBNP >360). Patients were eligible if they were 50 years of age or older, with at least one sign and one symptom of heart failure, controlled blood pressure, and a serum potassium level of <5.0 mmol/L. Patients were randomized to spironolactone (titrated to 45 mg/day if tolerated) or placebo and followed for a median of 3.4 years. The detailed study design as well as inclusion and exclusion criteria have been previously described,5 as have the primary results.6
Exposure and outcomes
Left ventricular ejection fraction was available in 3444 patients at baseline. Ejection fraction for enrolment was determined at the sites by either echocardiography or radionuclide ventriculography, and measurement methods were not specified by the protocol. The primary outcome was a composite of cardiovascular death, aborted cardiac arrest, or hospitalization for heart failure. All endpoints were adjudicated centrally by a clinical endpoint committee blinded to treatment assignment using prespecified criteria.
Statistical methods
Patients were categorized into four groups based on ejection fraction: <50, 50–54.9, 55–59.9, and >60%, although interaction analyses are based on LVEF as a continuous variable. Baseline characteristics were summarized for each group, and trend tests were conducted using linear regression and χ2 tests to examine trends in continuous and binary data, respectively. All analyses were performed using the primary endpoint, the components of heart failure hospitalization and cardiovascular death, and all-cause death. Incidence rates were calculated for each endpoint across LVEF categories, and hazard ratios using the Cox proportional hazards model with the LVEF>60% group serving as the referent group. Multivariable models were adjusted for the following covariates: enrolment stratum and region (Americas vs. Russia/Georgia), age, race, NYHA class (1–2 vs. 3–4), smoking status, diabetes status, creatinine, heart rate, QRS duration, history of atrial fibrillation, history of peripheral artery disease, and treatment assignment. Owing to previously noted substantial regional differences in patient demographics, outcomes, and response to spironolactone, these analyses were also conducted separately for patients enrolled in the Americas (n = 1766) and for those enrolled in Russia/Georgia (n = 1678).8 Estimates and 95% confidence intervals obtained from Poisson regression models using restricted cubic splines with four knots were used to plot the flexible relationship between ejection fraction as a continuous variable and the incidence of each endpoint. We assessed treatment effect modification for LVEF as a linear continuous variable using Cox proportional hazards models and Poisson regression models. Treatment effects were assessed without further adjustment for covariates.
Results
Ejection fraction in TOPCAT ranged from 44 to 85% (mean 57.1%, median 56%, IQR [51, 61%]) (Figure 1). Baseline characteristics varied considerably by ejection fraction (Table 1). Patients in higher ejection fraction categories were older, more likely to be female, less likely to have a history of myocardial infarction, and more likely to have a history of hypertension and diabetes. Both heart rate and BMI were slightly higher in those with higher ejection fraction. NYHA functional class did not differ by EF. Patients with higher EF were more likely to have reduced diastolic blood pressure, although systolic blood pressure did not differ. Patients with higher ejection fraction were less likely to be on angiotensin converting enzyme inhibitor/angiotensin receptor blocker, and more likely to be treated with diuretics. A substantially higher proportion of patients with a higher LVEF were enrolled in the USA, Canada, Argentina, and Brazil, and a substantially higher proportion of patients with lower LVEF were enrolled in Russia and the Republic of Georgia.
Figure 1.
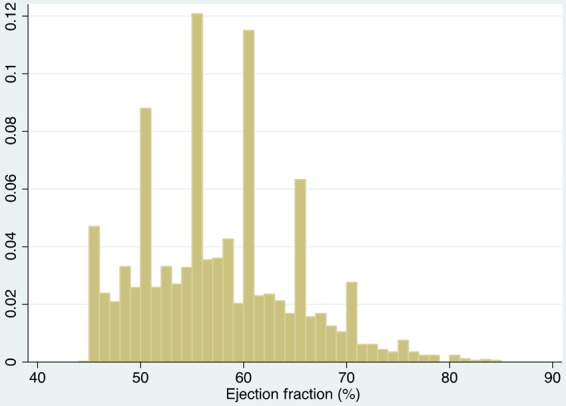
Distribution of ejection fractions.
Table 1.
Baseline characteristics by the EF group
| EF <50% (N = 520) | EF 50–54.99% (N = 712) | EF 55–59.99% (N = 879) | EF ≥60% (N = 1333) | P-value | |
|---|---|---|---|---|---|
| Age (years) | 66 ± 9 | 68 ± 10 | 68 ± 9 | 70 ± 10 | <0.001 |
| Female gender | 190 (36.5%) | 345 (48.5%) | 449 (51.1%) | 791 (59.3%) | <0.001 |
| Black race | 38 (7.3%) | 52 (7.3%) | 74 (8.4%) | 138 (10.4%) | 0.05 |
| Region | |||||
| Americas | 197 (38%) | 289 (41%) | 422 (48%) | 858 (64%) | <0.001 |
| Russia/Georgia | 323 (62%) | 423 (59%) | 457 (52%) | 475 (36%) | |
| Systolic BP (mmHg) | 128 ± 14 | 129 ± 13 | 129 ± 14 | 130 ± 14 | 0.12 |
| Diastolic BP (mmHg) | 78 ± 10 | 77 ± 10 | 76 ± 10 | 74 ± 11 | <0.001 |
| History of HF hospitalization | 374 (71.9%) | 537 (75.4%) | 662 (75.5%) | 916 (68.7%) | <0.001 |
| History of myocardial infarction | 230 (44.2%) | 200 (28.1%) | 192 (21.9%) | 271 (20.3%) | <0.001 |
| History of hypertension | 450 (86.5%) | 643 (90.3%) | 803 (91.6%) | 1251 (93.8%) | <0.001 |
| History of diabetes | 149 (28.7%) | 195 (27.4%) | 285 (32.5%) | 489 (36.7%) | <0.001 |
| NYHA class | |||||
| 1 | 18 (3.5%) | 21 (2.9%) | 23 (2.6%) | 47 (3.5%) | 0.18 |
| 2 | 318 (61.2%) | 474 (66.6%) | 580 (66.2%) | 822 (61.8%) | |
| 3 | 181 (34.8%) | 217 (30.5%) | 268 (30.6%) | 455 (34.2%) | |
| 4 | 3 (0.6%) | 0 (0.0%) | 5 (0.6%) | 7 (0.5%) | |
| Heart rate (b.p.m.) | 69.98 ± 10.17 | 69.49 ± 9.89 | 69.58 ± 10.12 | 68.16 ± 10.86 | <0.001 |
| Body mass index (mg/m2) | 31.5 ± 7.2 | 31.2 ± 6.6 | 32.1 ± 7.3 | 32.9 ± 7.5 | <0.001 |
| ACE/ARB | 458 (88.1%) | 620 (87.1%) | 749 (85.3%) | 1073 (80.6%) | <0.001 |
| Beta-blockers | 407 (78.3%) | 577 (81.0%) | 682 (77.7%) | 1010 (75.8%) | 0.06 |
| Diuretics | 396 (76.2%) | 603 (84.7%) | 717 (81.7%) | 1101 (82.7%) | 0.001 |
| eGFR (mL/min/1.78 m2) | 69.6 ± 19.9 | 68.0 ± 21.4 | 68.0 ± 19.9 | 66.5 ± 19.6 | 0.021 |
EF, ejection fraction; BP, blood pressure; HF, heart failure; ACE/ARB, angiotensin converting enzyme inhibitor/angiotensin receptor blocker; eGFR, estimated glomerular filtration rate.
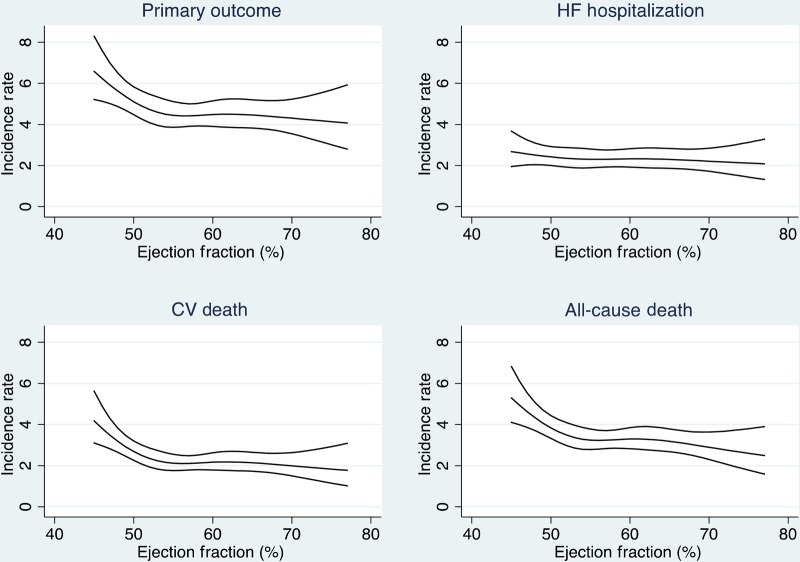
The incidence of the primary endpoint, heart failure hospitalization, cardiovascular death, and non-cardiovascular death is shown for each LVEF category in Table 2. Figure 2 shows adjusted incidence rates for the same endpoints across the continuous LVEF spectrum. The crude incidence rate of the primary endpoint and cardiovascular death was highest in patients at the lower end of the ejection fraction spectrum. In contrast, the crude incidence rate of heart failure hospitalization was highest in those with an ejection fraction of 60% or greater, though this observation is no longer apparent after adjustment for geographic region (Tables 3 and 4). With adjustment for baseline covariates (Figure 2), the incidence of the primary composite endpoint, cardiovascular death, and all-cause death varied by ejection fraction in a cubic spline model, with the adjusted incidence of cardiovascular death declining rapidly until an LVEF of ∼55%. Similarly, the incidence of cardiovascular death was highest in the group of patients with ejection fraction <50% and was similar across the spectrum above 50%. Results were similar when adjusting for baseline covariates. Heart failure hospitalization did not vary by ejection fraction after adjustment for covariates in the overall population, but LVEF was found to be independently associated with heart failure hospitalization in the Russia/Georgia cohort (Table 4).
Table 2.
Event rates and crude and adjusted hazard ratios for primary endpoint, heart failure hospitalization, cardiovascular death, and all-cause death by the ejection fraction group
| All patients (N = 3444) | Ejection fraction group |
Continuous LVEF × treatment interaction | |||
|---|---|---|---|---|---|
| EF <50% (N = 520) | EF 50–54.99% (N = 712) | EF 55–59.99% (N = 879) | EF ≥60% (N = 1333) | ||
| Primary endpoint | |||||
| Event rate (per 100 patient-years) | 7.2 (6.0, 8.7) | 6.0 (5.0, 7.0) | 5.5 (4.7, 6.4) | 6.7 (5.9, 7.5) | |
| HR (unadjusted) | 1.09 (0.88, 1.36) | 0.92 (0.75, 1.13) | 0.84 (0.69, 1.02) | Referent | |
| HR (adjusteda) | 1.37 (1.09, 1.72) | 1.19 (0.97, 1.47) | 1.02 (0.83, 1.25) | Referent | |
| Treatment effect (HR) | 0.72 (0.50, 1.05) | 0.85 (0.61, 1.18) | 0.94 (0.68, 1.29) | 0.97 (0.76, 1.23) | P = 0.046 |
| HF hospitalization | |||||
| Event rate (per 100 patient-years) | 3.8 (2.9, 5.0) | 4.1 (3.3, 5.0) | 3.7 (3.0, 4.5) | 4.9 (4.2, 5.6) | |
| HR (unadjusted) | 0.79 (0.59, 1.06) | 0.88 (0.69, 1.12) | 0.78 (0.62, 1.00) | Referent | |
| HR (adjusteda) | 1.06 (0.79, 1.44) | 1.20 (0.93, 1.53) | 0.96 (0.75, 1.23) | Referent | |
| Treatment effect (HR) | 0.76 (0.46, 1.27) | 0.70 (0.47, 1.04) | 0.73 (0.49, 1.08) | 0.98 (0.74, 1.30) | P = 0.039 |
| CV death | |||||
| Event rate (per 100 patient-years) | 4.1 (3.2, 5.2) | 2.8 (2.2, 3.6) | 2.7 (2.2, 3.3) | 2.7 (2.2, 3.2) | |
| HR (unadjusted) | 1.53 (1.13, 2.06) | 1.04 (0.77, 1.39) | 0.98 (0.74, 1.31) | Referent | |
| HR (adjusteda) | 1.86 (1.35, 2.55) | 1.24 (0.91, 1.67) | 1.19 (0.89, 1.59) | Referent | |
| Treatment effect (HR) | 0.69 (0.43, 1.12) | 0.95 (0.60, 1.51) | 1.09 (0.70, 1.68) | 0.90 (0.62, 1.29) | P = 0.61 |
| Death | |||||
| Event rate (per 100 patient-years) | 5.6 (4.5, 6.8) | 4.0 (3.3, 4.8) | 4.3 (3.6, 5.0) | 4.3 (3.7, 4.9) | |
| HR (unadjusted) | 1.30 (1.02, 1.66) | 0.92 (0.72, 1.17) | 0.99 (0.79, 1.24) | Referent | |
| HR (adjusteda) | 1.70 (1.31, 2.20) | 1.13 (0.88, 1.44) | 1.21 (0.96, 1.52) | Referent | |
| Treatment effect (HR) | 0.73 (0.49, 1.10) | 0.96 (0.66, 1.41) | 1.09 (0.78, 1.53) | 0.88 (0.66, 1.17) | P = 0.99 |
The treatment by ejection fraction interaction with LVEF modelled continuously is shown for each endpoint.
HR, hazard ratio; HF, heart failure; CV, cardiovascular; EF, ejection fraction; LVEF, left ventricular ejection fraction.
aAdjusted for region of enrolment, NYHA class, diabetes status, creatinine, heart rate, age, race, smoking status, QRS duration, enrolment stratum, atrial fibrillation, peripheral arterial disease, and assignment to spironolactone vs. placebo.
Figure 2.
Multivariable adjusted incidence rate (per 100 patient-years) by left ventricular ejection fraction for primary endpoint (upper left, P = 0.02), heart failure hospitalization (upper right, P = 0.79), cardiovascular death (lower left, P = 0.002), and all-cause death (lower right, P = 0.004).
Table 3.
Event rates and crude and adjusted hazard ratios for primary endpoint, heart failure hospitalization, cardiovascular death, and all-cause death by the ejection fraction group for patients enrolled in the Americas
| Americas (N = 1766) | Ejection fraction group |
Continuous LVEF × treatment interaction | |||
|---|---|---|---|---|---|
| EF <50% (N = 197) | EF 50–54.99% (N = 289) | EF 55–59.99% (N = 422) | EF ≥60% (N = 858) | ||
| Primary endpoint | |||||
| Event rate (per 100 patient-years) | 13.8 (10.8, 17.6) | 13.3 (10.9, 16.3) | 11.6 (9.7, 13.9) | 10.4 (9.1, 11.7) | |
| HR (unadjusted) | 1.31 (0.99, 1.72) | 1.28 (1.01, 1.62) | 1.12 (0.90, 1.39) | Referent | |
| HR (adjusteda) | 1.08 (0.81, 1.44) | 1.13 (0.89, 1.44) | 1.05 (0.84, 1.31) | Referent | |
| Treatment effect (HR) | 0.55 (0.33, 0.91) | 0.83 (0.56, 1.25) | 0.85 (0.60, 1.21) | 0.89 (0.69, 1.15) | P = 0.069 |
| HF hospitalization | |||||
| Event rate (per 100 patient-years) | 9.1 (6.7, 12.3) | 10.7 (8.6, 13.4) | 8.8 (7.2, 10.8) | 8.1 (7.0, 9.3) | |
| HR (unadjusted) | 1.10 (0.79, 1.54) | 1.32 (1.02, 1.72) | 1.09 (0.85, 1.40) | Referent | |
| HR (adjusteda) | 0.91 (0.65, 1.28) | 1.14 (0.87, 1.50) | 1.00 (0.77, 1.28) | Referent | |
| Treatment effect (HR) | 0.60 (0.32, 1.10) | 0.80 (0.51, 1.25) | 0.70 (0.47, 1.06) | 0.95 (0.71, 1.26) | P = 0.037 |
| CV death | |||||
| Event rate (per 100 patient-years) | 6.5 (4.6, 9.0) | 4.4 (3.2, 6.1) | 4.6 (3.5, 5.9) | 3.6 (3.0, 4.4) | |
| HR (unadjusted) | 1.83 (1.24, 2.71) | 1.22 (0.84, 1.78) | 1.26 (0.91, 1.76) | Referent | |
| HR (adjusteda) | 1.65 (1.10, 2.49) | 1.08 (0.74, 1.59) | 1.27 (0.91, 1.78) | Referent | |
| Treatment effect (HR) | 0.46 (0.23, 0.94) | 0.76 (0.40, 1.45) | 0.97 (0.57, 1.64) | 0.73 (0.49, 1.10) | P = 0.93 |
| Death | |||||
| Event rate (per 100 patient-years) | 10.4 (8.1, 13.5) | 7.2 (5.6, 9.2) | 7.7 (6.3, 9.4) | 6.1 (5.2, 7.1) | |
| HR (unadjusted) | 1.77 (1.31, 2.39) | 1.18 (0.88, 1.57) | 1.28 (0.99, 1.64) | Referent | |
| HR (adjusteda) | 1.62 (1.19, 2.23) | 1.03 (0.77, 1.38) | 1.26 (0.98, 1.63) | Referent | |
| Treatment effect (HR) | 0.58 (0.34, 0.99) | 0.92 (0.56, 1.50) | 1.12 (0.75, 1.66) | 0.75 (0.55, 1.03) | P = 0.54 |
The treatment by ejection fraction interaction with LVEF modelled continuously is shown for each endpoint.
HR, hazard ratio; HF, heart failure; CV, cardiovascular; EF, ejection fraction; LVEF, left ventricular ejection fraction.
aAdjusted for NYHA class, diabetes status, creatinine, heart rate, age, race, smoking status, QRS duration, enrolment stratum, atrial fibrillation, peripheral arterial disease, and assignment to spironolactone vs. placebo.
Table 4.
Event rates and crude and adjusted hazard ratios for primary endpoint, heart failure hospitalization, cardiovascular death, and all-cause death by the ejection fraction group for patients enrolled in Russia/Georgia
| Russia/Georgia (N = 1678) | Ejection fraction group |
Treatment × EF interaction | |||
|---|---|---|---|---|---|
| EF <50% (N = 323) | EF 50–54.99% (N = 423) | EF 55–59.99% (N = 457) | EF ≥60% (N = 475) | ||
| Primary endpoint | |||||
| Event rate (per 100 patient-years) | 4.5 (3.4, 5.9) | 2.7 (2.0, 3.6) | 1.7 (1.2, 2.5) | 1.6 (1.1, 2.3) | |
| HR (unadjusted) | 2.88 (1.79, 4.64) | 1.75 (1.08, 2.84) | 1.12 (0.66, 1.89) | Referent | |
| HR (adjusteda) | 2.38 (1.45, 3.91) | 1.76 (1.08, 2.87) | 1.23 (0.72, 2.09) | Referent | |
| Treatment effect (HR) | 0.84 (0.48, 1.48) | 1.26 (0.69, 2.32) | 1.12 (0.55, 2.29) | 1.26 (0.58, 2.73) | P = 0.37 |
| HF hospitalization | |||||
| Event rate (per 100 patient-years) | 1.6 (1.0, 2.5) | 1.1 (0.7, 1.7) | 0.5 (0.3, 1.0) | 0.4 (0.2, 0.9) | |
| HR (unadjusted) | 3.67 (1.52, 8.84) | 2.73 (1.14, 6.53) | 1.28 (0.47, 3.42) | Referent | |
| HR (adjusteda) | 2.50 (0.99, 6.32) | 2.67 (1.10, 6.49) | 1.38 (0.51, 3.74) | Referent | |
| Treatment effect (HR) | 0.92 (0.35, 2.38) | 0.81 (0.32, 2.04) | 0.56 (0.14, 2.25) | 0.44 (0.08, 2.25) | P = 0.20 |
| CV death | |||||
| Event rate (per 100 patient-years) | 3.0 (2.2, 4.2) | 2.0 (1.4, 2.8) | 1.4 (0.9, 2.0) | 1.2 (0.8, 1.9) | |
| HR (unadjusted) | 2.44 (1.42, 4.20) | 1.61 (0.94, 2.78) | 1.09 (0.61, 1.96) | Referent | |
| HR (adjusteda) | 2.09 (1.19, 3.70) | 1.58 (0.91, 2.73) | 1.19 (0.66, 2.14) | Referent | |
| Treatment effect (HR) | 0.93 (0.47, 1.82) | 1.46 (0.72, 2.96) | 1.33 (0.60, 2.97) | 1.78 (0.74, 4.30) | P = 0.16 |
| Death | |||||
| Event rate (per 100 patient-years) | 3.3 (2.4, 4.5) | 2.3 (1.7, 3.1) | 1.8 (1.3, 2.6) | 1.6 (1.1, 2.3) | |
| HR (unadjusted) | 2.10 (1.30, 3.42) | 1.46 (0.90, 2.36) | 1.16 (0.71, 1.92) | Referent | |
| HR (adjusteda) | 1.78 (1.07, 2.97) | 1.46 (0.89, 2.38) | 1.27 (0.77, 2.11) | Referent | |
| Treatment effect (HR) | 0.88 (0.47, 1.65) | 1.35 (0.71, 2.56) | 0.89 (0.45, 1.76) | 1.49 (0.71, 3.16) | P = 0.39 |
The treatment by ejection fraction interaction with LVEF modelled continuously is shown for each endpoint.
HR, hazard ratio; HF, heart failure; CV, cardiovascular; EF, ejection fraction.
aAdjusted for NYHA class, diabetes status, creatinine, heart rate, age, race, smoking status, QRS duration, enrolment stratum, atrial fibrillation, peripheral arterial disease, and assignment to spironolactone vs. placebo.
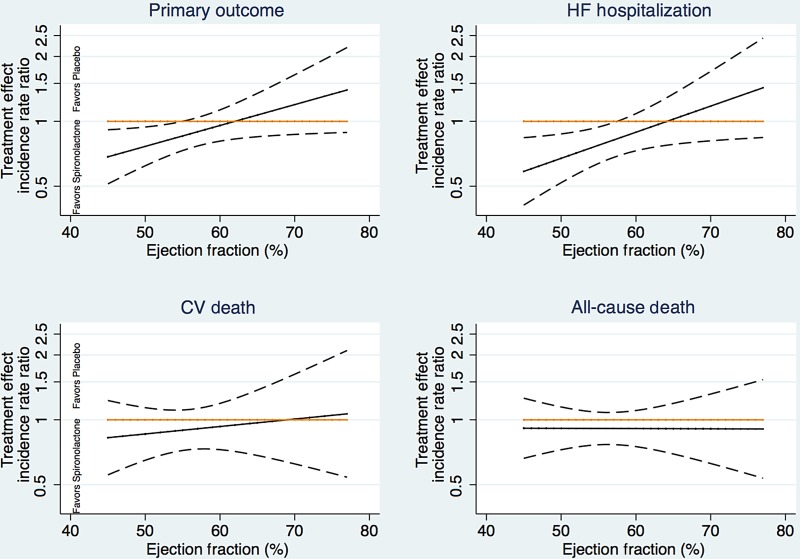
Ejection fraction modified the spironolactone treatment effect for the primary outcome and for heart failure hospitalization in the overall cohort (Table 2 and Figure 3). Patients with LVEF at the lower end of the spectrum were more likely to benefit from spironolactone with respect to the primary endpoint (interaction P = 0.046) and heart failure hospitalization (interaction P = 0.039). These treatment interactions were most prominent in the patients enrolled in the Americas (P = 0.069 for primary endpoint and P = 0.037 for HF hospitalization), and were also apparent in models adjusting for region-by-treatment and region-by-LVEF interactions (interaction P = 0.043 for primary endpoint and P = 0.093 for HF hospitalization). Treatment–LVEF interactions for both the primary outcome and for heart failure hospitalization were somewhat more pronounced in males (P = 0.01 for each outcome) than in females (interaction P > 0.80 for each outcome; Figure 4). Tests of whether the modification of treatment effect by LVEF further differed by gender were marginally significant (three-way interaction P = 0.077 and 0.089 for primary and heart failure hospitalization, respectively). We observed no treatment interaction by LVEF for the component endpoint of cardiovascular death (P = 0.61).
Figure 3.
Treatment effect as a function of ejection fraction for primary outcome (upper left panel), heart failure hospitalization (upper right panel), and cardiovascular death (lower panel). Dashed lines represent 95% confidence intervals.
Figure 4.
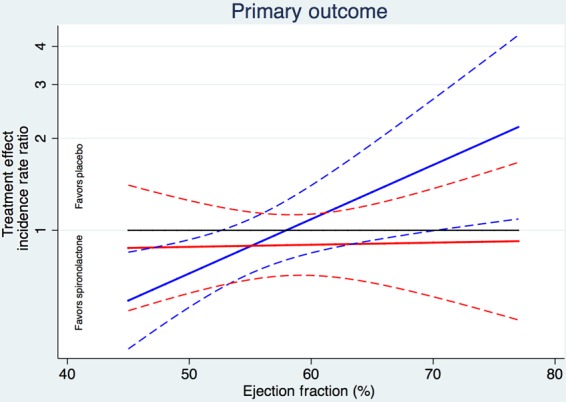
Treatment effect for the primary outcome in men (blue) and women (red). Pinteraction = 0.077.
Discussion
In patients with HFpEF enrolled in TOPCAT, we observed marked differences in baseline characteristics based on LV ejection fraction within the preserved range. Patients at the lower end of the LVEF spectrum were more likely to experience a primary endpoint [cardiovascular (CV) death, HF hospitalization, or resuscitated sudden death], which was mostly due to an increased risk of CV death in those patients. The potential benefit of spironolactone with respect to the primary outcome and HF hospitalization was greatest in patients at the lower end of the LVEF spectrum, although this relationship appeared to be most apparent in men. These findings were most prominent in those enrolled in the Americas.
Heart failure with preserved ejection fraction has traditionally been considered a heterogeneous population and the definition of ‘preserved’ has been debated. Evidence-based therapies for HF have been confined to HFrEF, defined by LVEF <40%. The term ‘preserved ejection fraction’ was originally coined to define the group above 40%,2 although more recent guidelines have used 45% and greater to define HFpEF. Nevertheless, the American Society of Echocardiography defines normal LV ejection fraction as 55% or greater.9 The extent to which HF patients with ejection fractions below 55% behave more like patients with reduced ejection fraction or preserved ejection fraction remains unclear. Still we noted substantial baseline differences in these patients based on ejection fraction and greater heterogeneity in baseline characteristics based on ejection fraction than has been previously reported in HFpEF. The most notable differences based on LVEF were a higher proportion of female patients, with a mean LVEF that was nearly three points higher in women than in men. A history of myocardial infarction was substantially lower in patients at the upper end of the EF range, suggesting that ischaemic heart disease may have contributed substantially to the aetiology of heart failure in those patients with LVEF under 50%.
Patients with LVEF under 50% demonstrated higher rates of cardiovascular death than those in the upper LVEF range, although rates of heart failure hospitalization were similar. Indeed, HF hospitalization rates were greater in the highest LVEF groups. When adjusted for baseline covariates most predictive of the primary outcome, we observed an increased risk of CV death but not HF hospitalization in the lower LVEF groups compared with the highest LVEF group. One possible explanation for this difference might be competing risk, given that patients who died were unavailable to have an HF hospitalization.
We found that the effect of spironolactone on the primary outcome and heart failure hospitalization varied by baseline LV ejection fraction such that the greatest potential benefit was observed in patients at the lower end of the LVEF spectrum. We had previously reported no heterogeneity by LVEF based on the prespecified subgroup of patients above and below an LVEF of 50%,6 an analysis that was limited by the small number of patients with LVEF <50%, and for the subgroup of patients above and below the median LVEF. While the interaction P-values were not significant for either of these analyses, the point estimates were both in favour of spironolactone in the lower EF groups and close to 1 in the placebo groups. Assessment of the relationship between LVEF and treatment effect in a more continuous fashion provides greater power to assess modification of treatment effect by ejection fraction. Mineralocorticoid receptor antagonists (MRAs) have been shown to benefit patients with LVEF <40% in the RALES,10 EMPHASIS-HF,11 and EPHESUS12 trials. While TOPCAT (with only 520 patients with LVEF <50%) was underpowered to demonstrate a treatment effect in this lower group, the hazard ratios for the primary outcome, cardiovascular death, and HF hospitalization are similar to those observed in reduced LVEF trials with MRAs, suggesting that patients in this range may behave more similarly to those with HFrEF than with higher ejection fractions.13 These data are consistent with analyses from CHARM-preserved,14 in which patients in the LVEF 40–50% range showed greater benefit from candesartan than those with LVEF ≥50%.
The finding that patients with ejection fraction over ∼60% derived minimal benefit from spironolactone suggests that the potential mechanism of benefit of spironolactone in HFpEF may be different from a presumed improvement in cardiac structure and reversal of fibrosis, but may be a neurohormonal benefit in patients with activation of the RAAS due to a mild increase in plasma volume, as is the case in those with reduced LVEF. This notion is consistent with data from the smaller TOPCAT echocardiographic substudy, showing that left ventricular end-diastolic volume increased with declining LVEF.15 Whether similar findings would be observed with other therapies in the HFpEF population is unclear, and we cannot determine if these results are unique to spironolactone or would be more generalizable. Nevertheless, future HFpEF studies should anticipate potential differences in therapeutic efficacy at different ends of the LVEF spectrum.
The finding that the observed EF–treatment interaction was most pronounced in males was unexpected. While the overall hazard ratios were similar between men and women in TOPCAT, the treatment effect was essentially flat across the EF spectrum in women, and varied dramatically across the EF spectrum in men, such that men in the lower EF range of TOPCAT behaved more similarly to patients with HFrEF. Whether these findings reflect differences in the pathophysiology of HFpEF in men and women or whether they simply reflect inclusion biases remains to be determined.
Some limitations of this analysis should be noted. Ejection fractions were measured at the sites in TOPCAT and verified on a percentage of enrolled subjects by the core laboratory. Nevertheless, these site-derived ejection fractions are typically used to make treatment decisions in HF patients. Because TOPCAT failed to achieve its primary endpoint, these results need to be considered hypothesis-generating as with any post hoc subgroup analysis. Indeed, because the overall finding in TOPCAT was negative, we do not believe that these results should guide treatment, but do have pathophysiological implications and could potentially be informative for the design and analysis of future HFpEF trials. Nevertheless, similar post hoc analyses in CHARM show that there was a limited benefit in the upper end of the ejection fraction scale. Taken together, these data suggest that RAAS blockade alone may have limited benefit in heart failure patients with supra-normal ejection fractions. We have reported substantial geographic variation in baseline characteristics and event rates in TOPCAT, with patients in Russia and the Republic of Georgia demonstrating far lower overall event rates than those in the Americas.7 This analysis has thus stratified by region in order to ensure that these findings are apparent in the group of patients most likely to have clinical heart failure, although within each region, the lower sample size further limits our power.
In summary, we found that baseline characteristics in HFpEF vary by LV ejection fraction, and that notably, a much higher percentage of women were present in the upper LVEF categories. Moreover, the treatment effect of spironolactone, while null overall in TOPCAT, appeared to vary by ejection fraction, with virtually no benefit observed in patients with LVEF over 65%, an effect that was most apparent in men. These hypothesis-generating findings suggest that the potential benefit of spironolactone in HFpEF, if any, may be greatest in patients on the lower end of the HFpEF LVEF spectrum and with pathophysiology that most resembles HFrEF.
Funding
This work was funded by the National Heart, Lung, and Blood Institute and National Institutes of Health (contract HHSN268200425207C). The content of this article does not necessarily represent the views of the National Heart, Lung, and Blood Institute or of the Department of Health and Human Services.
Conflict of interest: S.D.S. has received consulting fees from Novartis and Bayer, and research grants from the National Heart, Lung, and Blood Institute. M.A.P. has received consulting fees from Aastrom, Abbott Vascular, Amgen, Cerenis, Concert, Daiichi Sankyo, Fibrogen, Genzyme, GlaxoSmithKline, Hamilton Health Sciences, Medtronic, Merck, Novo Nordisk, Roche, Salix, Sanderling, sanofi-aventis, Serono, Servier, and Teva, as well as research grants from New England Research Institute via subcontract from the National Institutes of Health, Amgen, Celladon, Novartis, and sanofi-aventis. The Brigham and Women's Hospital has patents for the use of inhibitors of the renin–angiotensin system in selected survivors of myocardial infarction with Novartis Pharmaceuticals on which M.A.P. is a co-inventor. His share of the licensing agreement is irrevocably transferred to charity. A.D. has received consulting fees from Novartis, Boston Scientific, Reata, Cardiomems, 5 am Ventures, Intel Corp, Coverys, and Relypsa, as well as research grants from AtCor Medical to support the Vascular Stiffness Ancillary Study to the TOPCAT trial, for which he is listed as a principal investigator. E.F.L. has received research grants from the National Heart, Lung, and Blood Institute, Novartis, and sanofi-aventis. E.O. has received consulting fees from Pfizer, Novartis, and Servier; grants from Servier; and a research grant from New England Research Institute via subcontract from the National Institutes of Health. S.J.S. has received consulting fees from Novartis, Bayer, DC Devices, Gilead, and Actelion. N.K.S. has received research grants from the National Institutes of Health. B.P. reports receiving consulting fees from Amorcyte, AstraZeneca, Aurasense, Bayer, BG Medicine, Gambro, Johnson & Johnson, Mesoblast, Novartis, Pfizer, Relypsa, and Takeda; receiving research grant support from Forest Laboratories; and holding stock in Aurasense, Relypsa, BG Medicine, and Aurasense. B.P. also reports a pending patent related to site-specific delivery of eplerenone to the myocardium.
References
- 1. Butler J, Fonarow GC, Zile MR, Lam CS, Roessig L, Schelbert EB, Shah SJ, Ahmed A, Bonow RO, Cleland JG, Cody RJ, Chioncel O, Collins SP, Dunnmon P, Filippatos G, Lefkowitz MP, Marti CN, McMurray JJ, Misselwitz F, Nodari S, O'Connor C, Pfeffer MA, Pieske B, Pitt B, Rosano G, Sabbah HN, Senni M, Solomon SD, Stockbridge N, Teerlink JR, Georgiopoulou VV, Gheorghiade M. Developing therapies for heart failure with preserved ejection fraction: current state and future directions. JACC Heart Fail 2014;2:97–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yusuf S, Pfeffer MA, Swedberg K, Granger C, Held P, McMurray JJV, Michelson EL, Olofsson B, Ostergren J; Charm Investigators. Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARMPreserved Trial . Lancet 2003;362:777–781. [DOI] [PubMed] [Google Scholar]
- 3. Massie BM, Carson PE, McMurray JJ, Komajda M, McKelvie R, Zile MR, Anderson S, Donovan M, Iverson E, Staiger C, Ptaszynska A; I-PRESERVE Investigators. Irbesartan in patients with heart failure and preserved ejection fraction. N Engl J Med 2008;359:2456–2467. [DOI] [PubMed] [Google Scholar]
- 4. Solomon SD, Anavekar N, Skali H, McMurray JJ, Swedberg K, Yusuf S, Granger CB, Michelson EL, Wang D, Pocock S, Pfeffer MA; Candesartan in Heart Failure Reduction in Mortality (CHARM) Investigators. Influence of ejection fraction on cardiovascular outcomes in a broad spectrum of heart failure patients. Circulation 2005;112:3738–3744. [DOI] [PubMed] [Google Scholar]
- 5. Meta-analysis Global Group in Chronic Heart Failure (MAGGIC). The survival of patients with heart failure with preserved or reduced left ventricular ejection fraction: an individual patient data meta-analysis. Eur Heart J 2012;33:1750–1757. [DOI] [PubMed] [Google Scholar]
- 6. Desai AS, Lewis EF, Li R, Solomon SD, Assmann SF, Boineau R, Clausell N, Diaz R, Fleg JL, Gordeev I, McKinlay S, O'Meara E, Shaburishvili T, Pitt B, Pfeffer MA. Rationale and design of the treatment of preserved cardiac function heart failure with an aldosterone antagonist trial: a randomized, controlled study of spironolactone in patients with symptomatic heart failure and preserved ejection fraction. Am Heart J 2011;162:966–972. [DOI] [PubMed] [Google Scholar]
- 7. Pitt B, Pfeffer MA, Assmann SF, Boineau R, Anand IS, Claggett B, Clausell N, Desai AS, Diaz R, Fleg JL, Gordeev I, Harty B, Heitner JF, Kenwood CT, Lewis EF, O'Meara E, Probstfield JL, Shaburishvili T, Shah SJ, Solomon SD, Sweitzer NK, Yang S, McKinlay SM; TOPCAT Investigators. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med 2014;370:1383–1392. [DOI] [PubMed] [Google Scholar]
- 8. Pfeffer MA, Claggett B, Assmann SF, Boineau R, Anand IS, Clausell N, Desai AS, Diaz R, Fleg JL, Gordeev I, Heitner JF, Lewis EF, O'Meara E, Rouleau JL, Probstfield JL, Shaburishvili T, Shah SJ, Solomon SD, Sweitzer NK, McKinlay SM, Pitt B. Regional variation in patients and outcomes in the Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist (TOPCAT) trial. Circulation 2015;131:34–42. [DOI] [PubMed] [Google Scholar]
- 9. Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ; Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. Chamber Quantification Writing Group; American Society of Echocardiography's Guidelines and Standards Committee; European Association of Echocardiography. J Am Soc Echocardiogr 2005;18:1440–1463. [DOI] [PubMed] [Google Scholar]
- 10. Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, Palensky J, Wittes J. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med 1999;341:709–717. [DOI] [PubMed] [Google Scholar]
- 11. Zannad F, McMurray JJ, Krum H, van Veldhuisen DJ, Swedberg K, Shi H, Vincent J, Pocock SJ, Pitt B; EMPHASIS-HF Study Group. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med 2011;364:11–21.21073363 [Google Scholar]
- 12. Pitt B, Remme W, Zannad F, Neaton J, Martinez F, Roniker B, Bittman R, Hurley S, Kleiman J, Gatlin M; Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study Investigators. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med 2003;348:1309–1321. [DOI] [PubMed] [Google Scholar]
- 13. Lam CS, Solomon SD. The middle child in heart failure: heart failure with mid-range ejection fraction (40–50%). Eur J Heart Fail 2014;16:1049–1055. [DOI] [PubMed] [Google Scholar]
- 14. Hogg K, McMurray J. The treatment of heart failure with preserved ejection fraction (“diastolic heart failure”). Heart Fail Rev 2006;11:141–146. [DOI] [PubMed] [Google Scholar]
- 15. Shah AM, Shah SJ, Anand IS, Sweitzer NK, O'Meara E, Heitner JF, Sopko G, Li G, Assmann SF, McKinlay SM, Pitt B, Pfeffer MA, Solomon SD; TOPCAT Investigators. Cardiac structure and function in heart failure with preserved ejection fraction: baseline findings from the echocardiographic study of the Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist trial. Circ Heart Fail 2014;7:104–115. [DOI] [PMC free article] [PubMed] [Google Scholar]