Abstract
The morphology of the vallate papillae from postmortem human samples was investigated with immunohistochemistry. Microscopically, taste buds were present along the inner wall of the papilla, and in some cases in the outer wall as well. The typical taste cell markers PLCβ2, GNAT3 (gustducin) and the T1R3 receptor stain elongated cells in human taste buds consistent with the Type II cells in rodents. In the human tissue, taste bud cells that stain with Type II cell markers, PLCβ2 and GNAT3, also stain with villin antibody. Two typical immunochemical markers for Type III taste cells in rodents, PGP9.5 and SNAP25, fail to stain any taste bud cells in the human postmortem tissue, although these antibodies do stain numerous nerve fibers throughout the specimen. Car4, another Type III cell marker, reacted with only a few taste cells in our samples. Finally, human vallate papillae have a general network of innervation similar to rodents and antibodies directed against SNAP25, PGP9.5, acetylated tubulin and P2X3 all stain free perigemmal nerve endings as well as intragemmal taste fibers. We conclude that with the exception of certain molecular features of Type III cells, human vallate papillae share the structural, morphological, and molecular features observed in rodents.
Key words: glossopharyngeal, gustducin, P2X3, PLCb2, SNAP25, taste bud
Introduction
Taste buds in the circumvallate papillae of rodents have been described in detail both in terms of morphology and molecular features, whereas taste buds in humans are less well characterized. In rodents, each taste bud, comprises 50–80 elongate, mature taste cells divisible into 3 morphological types: Type I, Type II, and Type III (Finger 2005; Chaudhari and Roper 2010). Each of the types displays characteristic molecular and functional features. Type I cells are glial-like, expressing mechanisms for reuptake (GLAST) or destruction of neurotransmitters (Bartel et al. 2006) and extending lamellate processes to surround other types of cells and nerve fibers. Type II cells, also called “receptor cells,” express the G-protein coupled taste receptors (T1R and T2R family members) and their associated downstream partners including gustducin (GNAT3), PLCβ2, and TrpM5 (Kataoka et al. 2008; Chaudhari and Roper 2010). Type III cells, which are responsive to acids (sour) (Huang et al. 2008), synthesize and release several neurotransmitters, including serotonin and GABA (Huang et al. 2011). In addition, proliferative basal cells lie along the basal margin of the taste bud and provide a reservoir for replacement of taste cells over time.
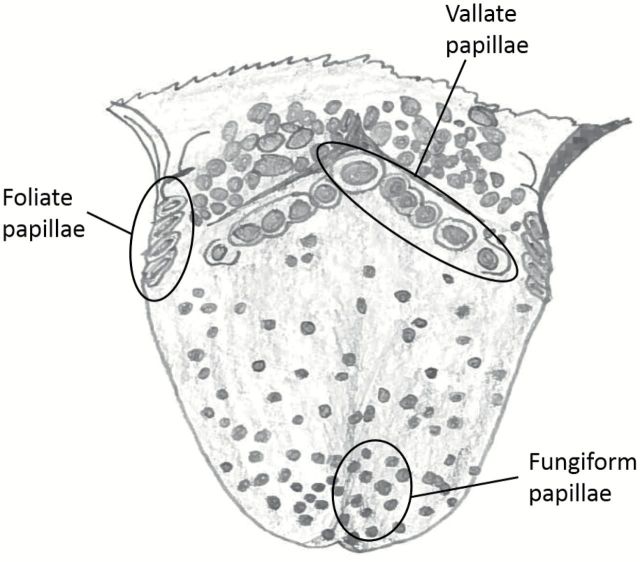
Human taste buds are histologically similar to and share many molecular features with taste buds in rodents (Paran et al. 1975; Takami et al. 1994; Azzali, 1997; Huque et al. 2009). Unlike rodents, humans have multiple circumvallate papillae arrayed as a posteriorly directed chevron of 6 or more papillae across the rear one-third of the tongue (Figure 1). Humans are, however, similar to rodents, in having taste buds in foliate papillae situated laterally along the side of the tongue and in fungiform papillae distributed broadly across the anterior two-thirds of the tongue. Multiple morphological types of taste cells are described in human taste buds but the vast majority of reports deal with taste buds in fungiform papillae, perhaps because of ease of sampling these papillae. Many reports and reviews describe the taste buds of human circumvallate papillae as lying only on the interior wall of the papilla (Gray et al. 1918; Smith and Margolskee 2001; Bear et al. 2007; Felten et al. 2010; Mescher 2013), in contrast to the situation in rodents where taste buds occupy both walls of the circumvallate papilla. In this article, we sought to re-examine taste buds of human circumvallate papillae utilizing contemporary immunohistochemical reagents to characterize suitable tools for studying human postmortem tissue.
Figure 1.
Semi-schematic drawing of a human tongue showing location of the lingual taste papillae. All samples for the current study were taken from the row of circumvallate papillae including both the central papilla and more lateral ones.
Using immunohistochemistry, we studied the expression of common rodent taste cell markers in human taste buds to determine the utility of common immunochemical probes in human postmortem samples. Markers for both taste cells and neural elements were employed (Table 1). In particular, we investigated immunoreactivity for Type II and III taste cell markers and for either general or taste specific innervation markers. We also stained tissue with the villin antibody, a specific marker for microvilliar cells (e.g., taste cells, solitary chemosensory cells, and brush cells).
Table 1.
Primary antisera and antibodies
| Antisera against | Marker for | Company, Catalog No., RRID No. | Host; dilution |
|---|---|---|---|
| Carbonic anhydrase 4 (Car4) | Type III cells | R&D Systems, Cat # AF2414, AB_2070332 | Goat; 1:1000 |
| GNAT3 (α-gustducin) | G-protein subunit in Type II taste cells | Aviva System Biology Corp., Cat # OAEB00418, AB_10882823 | Goat; 1:500 |
| PLCβ2 | Transduction component in Type II taste cells | Santa Cruz Biotechnology, Cat # sc-206, AB_632197 | Rabbit; 1:500 |
| Tas1R3 | Sweet/umami Type II taste cells | Sigma, Cat # Sab4503300, AB_10746492 | Rabbit; 1:500 |
| Villin | Microvillous cells | Chemicon, Cat # MAB1671, AB_11214198 | Mouse; 1:500 |
| Acetylated tubulin | Nerve fibers, ciliated cells | Sigma, Cat # T7451, AB_609894 | Mouse; 1:5000 |
| P2X3 | ATP receptor on taste nerves and pain nerves | Neuromics, Cat # GP10108, AB_2283325 | Guinea Pig; 1:500 |
| PGP9.5 | Neurons and nerve fibers; and subpopulation of Type III taste cells | AbD Serotec, Cat # 7863-0504, AB_2210505 | Rabbit; 1:500 |
| SNAP25 | Neurons and nerve fibers; and subpopulation of Type III taste cells | Sigma, Cat # S9684, AB_261576 | Rabbit; 1:500 |
| Substance P | Peptidergic polymodal nociceptors | Accurate Chemicals, Cat # YMC1021, AB_2333091 | Rat; 1:1000 |
Materials and methods
Tissue origin
Human cadaveric lingual tissue specimens were obtained from Lonetree Medical Donation Services (Littleton, CO). A total of 3 samples were utilized: 91-year-old female, fixation 6 h postmortem (cause of death: traumatic head injury); 65-year-old male, fixation 4 h postmortem (cause of death: brain cancer); 89-year-old male, fixation 7 h postmortem (dementia, cause of death: unspecified natural causes). Because these were de-identified tissue samples, our work complies with the Declaration of Helsinki for Medical Research involving Human Subjects.
Immunohistochemistry
Lingual tissues were immersion-fixed using 4% paraformaldehyde (PFA) in 0.1M phosphate buffer (PB), pH 7.2–7.4 for 2–3 days. Following fixation, both the central and some of the lateral circumvallate papillae were removed from each specimen, then the tissues were cryoprotected in 20% sucrose 0.1M PB for 3–4 days prior to sectioning on a cryostat at 16 μm. Cryostat sections were collected and dried onto Superfrost Plus slides (Fisher Scientific). Slides were rinsed in 0.1M phosphate-buffered saline (PBS: 150mM sodium chloride, 25mM sodium phosphate dibasic anhydrous, 75mM sodium phosphate monobasic monohydrate; pH 7.2) and nonspecific binding was blocked for 1h in blocking solution (2% normal goat serum, 1% bovine serum albumin, 0.3% Triton in PBS). Next, the slides were incubated overnight with primary antibodies in blocking solution (Table 1).
Following incubation with the primary antibodies, the tissue samples were rinsed with PBS and incubated for 4h with fluorescent secondary antibodies at 1:400 dilution (Table 2). The slides then were washed 2×10min in 0.1M PBS and one time for 10min in 0.1M PB before coverslipping slides with Fluormount G (Southern Biotechnology Associates). All images were collected with an Olympus epifluorescence microscope equipped with Q-imaging monochrome camera or with an Olympus Fluoview confocal laser scanning microscope (LSCM) FV300 (Olympus Corporation). For each image, the channels were collected sequentially with single wavelength excitation and then merged to produce the composite image using the native acquisition software for each device. This avoids the problem resulting from side-band excitation of the fluorochromes. Brightness, contrast and gamma were adjusted in Adobe Photoshop to approximate the appearance of the original histological samples.
Table 2.
Secondary antisera
| Primary antibody | Secondary antibody | Antisera against |
|---|---|---|
| Car4 | Alexa 488 | Donkey anti-goat |
| GNAT3 (α-gustducin) | Alexa 488 | Donkey anti-goat |
| GNAT3 (α-gustducin) | Alexa 568 | Donkey anti-goat |
| GNAT3 (α-gustducin) | Alexa 649 | Donkey anti-goat |
| PLCβ2 | Alexa 488 | Donkey anti-rabbit |
| PLCβ2 | Alexa 568 | Donkey anti-rabbit |
| T1R3 | Alexa 568 | Donkey anti-rabbit |
| Villin | Alexa 568 | Donkey anti-mouse |
| Acetylated tubulin | Alexa 568 | Donkey anti-mouse |
| P2X3 | Alexa 488 | Donkey anti-guinea pig |
| PGP9.5 | Alexa 568 | Donkey anti-rabbit |
| PGP9.5 | Alexa 488 | Donkey anti-rabbit |
| SNAP25 | Alexa 568 | Donkey anti-rabbit |
| Substance P | Alexa 488 | Donkey anti-rat |
Results
Taste buds and taste cells
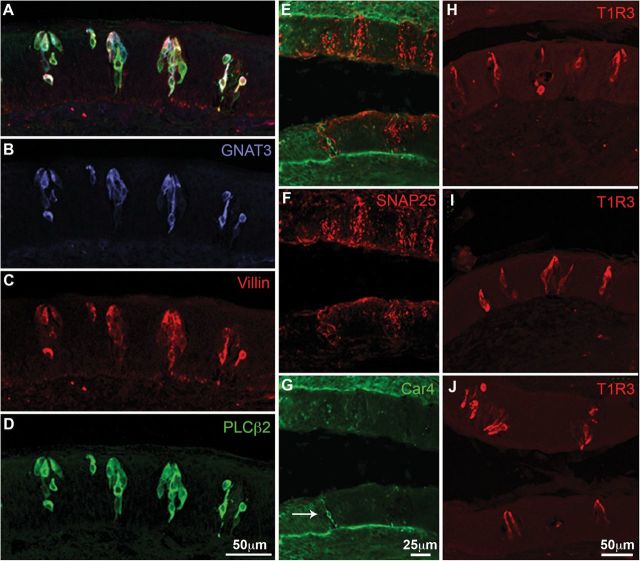
Our results show that postmortem samples of human vallate papillae are largely similar to rodent circumvallate papillae prepared with conventional perfusion or immersion fixation. Immunocytochemistry with appropriate probes reveals both taste cells immunoreactive for the rodent taste markers and innervation of the taste epithelium. In our samples, taste bud profiles in human circumvallate papilla averaged 66.7 µm × 31.1 µm (n = 15; range: 46 µm × 26 µm–89 µm × 38 µm) (Figure 2) although we did not undertake rigorous quantification of the variety of sizes and shapes observed.
Figure 2.
Immunocytochemistry for cell-type specific markers. Horizontal sections through the vallate papilla. (A–D) Triple label for Villin (red; A and C) and the Type II markers GNAT3 (gustducin; blue; A and B), and PLCβ2 (green; A and D). (E–J) Longitudinal sections of vallate papillae where dorsal is to the left. (E–G) Double label for SNAP25 (red; E and F) and Car4 (green; E and G). SNAP25 labels nerve fibers well but does not obviously label any taste cells. Car4 labels only rare taste cells (arrow G). (H–J) T1R3 (Tas1R3) labels several elongate taste cells in nearly every taste bud. Note the presence of taste buds on both sides of the crypt in J.
Notably, taste buds were evident on both walls of the trench of several of the circumvallate specimens examined. The appearance of taste buds on both walls of the trench was not correlated with the position of the papilla examined, that is, this was not a property of just the central or lateral papillae.
Antibodies against GNAT3, PLCβ2, and the T1R3 receptor (Figure 2B,D,H–J, respectively) applied to tissue sections of human papillae stain the cytoplasm of elongate cells, approximately 10 µm wide (range = 8.2–12.1 µm; n = 7) with large round nuclei consistent with the Type II taste cells of rodents. Occasional PLCβ2 positive cells show no GNAT3 immunoreactivity (arrow Figure 2A). Villin, a protein present in microvilli of diverse types of cells including taste cells, is largely coexpressed in the PLCβ2 immunoreactive taste cell population (Figure 2A,C,D). T1R3 antiserum stains a population of taste cells with characteristics similar to the villin-positive cells, that is, an elongate cell of similar size and with a large round nucleus characteristic of Type II cells in rodents. Figure 2J shows T1R3 immunoreactive cells are present in nearly all taste buds on both sides of the trench wall (Figure 2H,I).
Neither of the usual Type III markers for rodent taste cells, PGP9.5 and SNAP25, yielded cytoplasmic staining of any taste bud cells in the postmortem human vallate papillae samples (Figure 3B,C), although Car4 did react with a few cells—likely too few to be representative of the entire Type III population (Figure 2G, white arrow) estimated by other means (Azzali 1997).
Figure 3.
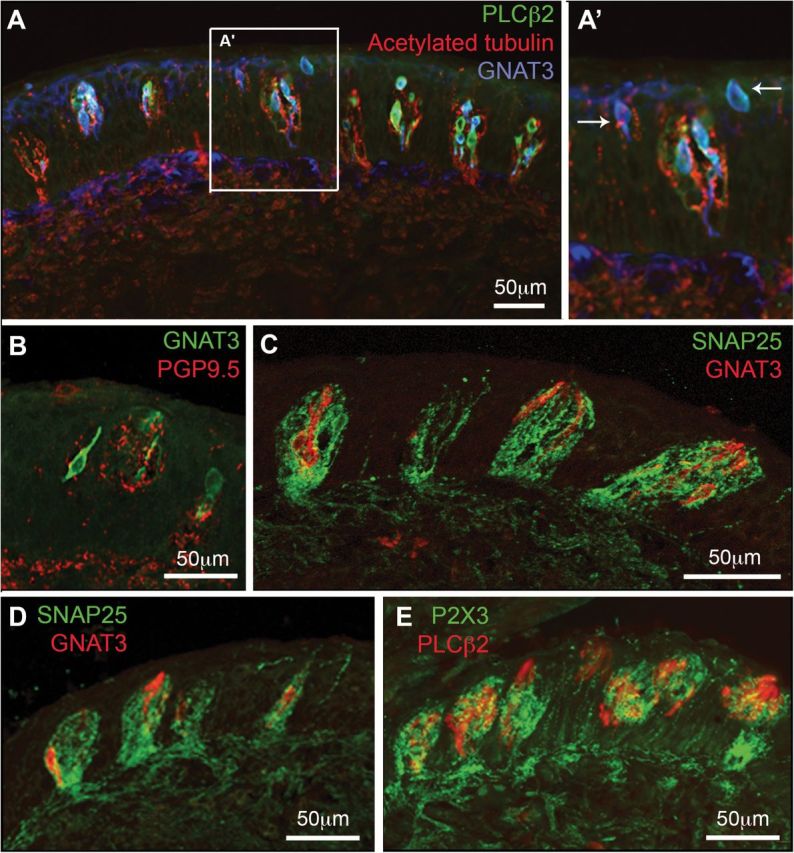
Double and triple labeling for cell and nerve fiber markers. (A, A′) Horizontal section of the papilla; (B–E) Longitudinal sections where dorsal is to the right. (A, A′) Nerve fibers stained with acetylated tubulin (red) densely innervate taste buds as well as nontaste epithelium between the taste buds. Type II taste cells, marked by staining for PLCβ2 (green) and GNAT3 (Gustducin, blue) populate virtually all taste buds. In addition scattered GNAT3-positive superficial epithelial cells (arrows A′) appear between some taste buds. (B–E) Longitudinal sections of vallate papillae where dorsal is to the right. Note that in this plane of sections, taste buds are oriented at an angle to the surface of the epithelium, so that their taste pore is somewhat more dorsal than the base of the bud. (B) PGP95 stained (red) fibers innervate taste buds as well as surrounding epithelium. Some GNAT3 positive (green) cells appear to contact the PGP9.5-positive nerve fibers. (C–E) The dense network of intragemmal nerve fibers is stained by both SNAP25 (green; C and D) and P2X3 (green; E). These nerve fibers surround and apparently contact the Type II cells marked by GNAT3 (red; C and D) or PLCβ2 (red; E).
Innervation of taste buds and papillary epithelium
Antibodies directed against SNAP25, PGP9.5, acetylated tubulin, and P2X3 all stain free perigemmal nerve endings as well as intragemmal taste fibers (Figures 2F and 3). Fibers immunoreactive for each of these antibodies densely innervate the taste buds and come to lie apposed to PLCβ2 and GNAT3 positive taste cells (Figure 3). In summary, human vallate taste buds have a general network of innervation not dissimilar from rodents and these taste buds are highly innervated by intragemmal fibers (Figure 3).
Antibodies for the purinergic receptor P2X3 that preferentially stain taste fibers in rodent taste buds also react robustly with intragemmal fibers in human vallate papillae (Figure 4). These P2X3-immunoreactive fibers appear to be closely associated with PLCβ2 positive taste cells (Figure 3D) as they are in rodents. PGP9.5 and SNAP25 stain fibers innervating human vallate papilla taste buds, but unlike the situation in rodents these antibodies do not stain Type III taste cells (Figure 3B,C).
Figure 4.
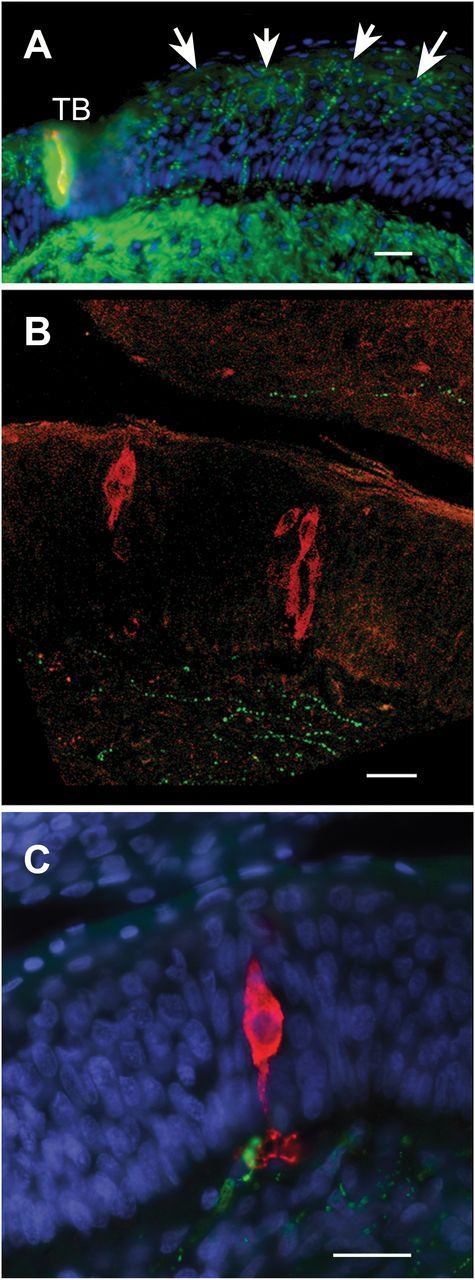
Innervation of the papillary epithelium. Longitudinal sections; dorsal to the left. (A) PGP immunoreactive (green) fibers (arrows) extensively innervate the papillary trench wall below the taste bud (TB) shown at the left side of the image. PLCβ2 red. (B) Substance P immunoreactive fibers (green) sparsely innervate the epithelium and are mostly confined to basal regions or the subepithelial mucosa. PLCβ2 red. (C) In rare cases, Substance P immunoreactive nerve fibers (green) appear to contact the basal process of PLCβ2 immunoreactive (red) taste cells. Scale bar = 25 µm all panels.
In some cases isolated immunoreactive PLCβ2 and GNAT3 taste cells are not associated with organized taste bud structures but are nonetheless innervated by nerve endings (Figure 3A, white arrows).
The nongustatory epithelium in the trench walls of the papillae are heavily innervated by free nerve endings as revealed by immunocytochemistry for acetylated tubulin, PGP9.5, and SNAP25 (Figure 4A). Although the density of innervation in these regions is not as high as within the taste buds proper, it is considerably denser than the degree of innervation of the dorsum of the papilla or of other regions of nearby lingual epithelium. These free nerve endings extend outward to reach the uppermost layers of the papillary epithelium. Substance P immunoreactivity, which marks populations of peptidergic polymodal nociceptors, does not show this same pattern of staining. Sparse substance P fibers are evident (Figure 4B) but these are mostly confined to the mucosal layers or the bottom most layers of the epithelium. Rarely, a substance P positive fiber can be observed to make apparent contact with the basal processes of PLC-positive taste cells (Figure 4C).
Discussion
Using the technique of immunocytochemistry, we have explored the pattern of expression of taste cell and neuronal markers in human vallate papillae. The circumvallate taste buds from humans are similar in many respects to those in rodents both in terms of size and cellular constituents. Like taste buds of the circumvallate papilla in rodents, circumvallate taste buds in humans line both sides of the trench of the papillae in most specimens that we examined. Although many reviews and chapters describe circumvallate taste buds in humans as populating only the inner trench wall (Gray et al. 1918; Smith and Margolskee 2001; Bear et al. 2007; Felten et al. 2010), the presence of taste buds on both walls of the trench is well documented in the literature. For example, Arey et al. (1935) report that the probability of finding taste buds on both sides of the trench wall increases with increasing age of the subjects. This is in keeping with our findings from the specimens we examined—all from relatively old subjects. The notion that circumvallate taste buds in humans populate only the inner trench wall may have been popularized by the commonly used illustration in Gray’s classic anatomical work (Gray et al. 1918).
Taste buds in humans, like those in rodents, react with antisera directed against components of the canonical G-protein coupled receptor cascade involving gustducin (GNAT3) and PLCβ2, which are implicated in detecting bitter, umami, and sweet tastants. We show that particular antibodies for taste bud and neuronal cells characterized in rodents also react with taste cells obtained from human postmortem samples. This observation is consistent with the few previous reports utilizing similar antisera on human tissue (Takami et al. 1994; Huque et al. 2009; Behrens et al. 2012). In addition, we find that the Sigma antiserum directed against T1R3 reacts well with human postmortem tissue, similar to another Tas1R3 antiserum employed by another team of investigators (Raliou et al. 2009).
Here, we uniquely show for the first time the presence of human taste cells immunoreactive for villin. The presence of villin-immunoreactivity as a marker for taste-like cells in gastrointestinal and respiratory brush cells is well characterized (Höfer and Drenckhahn 1992; Höfer et al. 1996; Krasteva et al. 2011). Thus, villin may prove a useful marker for taste-like cells elsewhere in the human respiratory and gastrointestinal tracts.
Typical Type III cell markers used in mice and rats, PGP9.5 and SNAP25 fail to stain taste cells in the human postmortem specimens we examined although these antisera were effective at staining nerve fibers. Another Type III cell marker, Car4, stained only a few scattered elongate taste cells in our samples. Nonetheless, Type III cells are present in human taste buds (Azzali 1997) based on both ultrastructural criteria and on immunohistochemistry for serotonin at the electron microscopic level. Thus, our failure to demonstrate Type III cells may be attributable to several possibilities: 1) the relevant markers degrade in taste cells before adequate fixation is obtained in these postmortem samples, 2) the Type III cells undergo autolysis or other severe morphological alteration making them impossible to identify in this postmortem material, or, perhaps least likely, 3) that human Type III cells do not express either PGP9.5 or SNAP25.
Antisera showing general innervation, including PGP9.5 and SNAP25, as well as acetylated tubulin, reveal dense innervation of both taste buds and regions of trench wall epithelium lacking taste buds (see also Astbäck et al. 1995; Yamagishi et al. 1995; Kusakabe et al. 1998). This general mucosal innervation, derived from the glossopharyngeal nerve, is not immunoreactive for substance P and therefore is unlikely to represent peptidergic polymodal nociceptors which express Trp channels responsive to either capsaicin (TrpV1) or wasabi and many other chemical irritants (TrpA1). Similarly, other investigators (Yamagishi et al. 1995; Kusakabe et al. 1998) detected few taste bud-associated fibers immunoreactive for CGRP, which would be expected to also delineate the peptidergic polymodal nociceptive population. Taste buds in human fungiform papilla also show a paucity of peptidergic fibers (Astbäck et al. 1995). The exact functional nature of the heavy nonpeptidergic mucosal innervation is unclear. The occasional close relationship between substance P expressing fibers and the base of taste buds may indicate a means by which the peptidergic polymodal nociceptors may interact with taste buds in generating a complex perception of oral chemical stimulation.
In summary, we find that many antisera commonly used for delineating particular features of rodent taste buds are suitable for use in postmortem human tissue samples. The postmortem period for our samples ranged from 4 to 8h before the tissue was immersion fixed in buffered PFA. Other fixatives and a shorter postmortem extraction time are likely to yield higher quality immunohistochemical results with a wider variety of antisera.
Funding
This work was supported by the National Institute on Deafness and Other Communication Disorders at the National Institutes of Health (P30DC004657 to D. Restrepo, R21DC013186 and R01DC014728 to T.E.F., and T32DC012280 to Sue Kinnamon) and by the American Academy of Otolaryngology ARS Resident Research Grant (235218 to H.P. Barham].
References
- Arey LB, Tremaine MJ, Monzingo FL. 1935. The numerical and topographical relations of taste buds to human circumv allate papillae throughout the life span. Anat Rec. 64:9–25. [Google Scholar]
- Astbäck J, Arvidson K, Johansson O. 1995. Neurochemical markers of human fungiform papillae and taste buds. Regul Pept. 59(3):389–398. [DOI] [PubMed] [Google Scholar]
- Azzali G. 1997. Ultrastructure and immunocytochemistry of gustatory cells in man. Ann Anat. 179:37–44. [DOI] [PubMed] [Google Scholar]
- Bartel DL, Sullivan SL, Lavoie EG, Sévigny J, Finger TE. 2006. Nucleoside triphosphate diphosphohydrolase-2 is the ecto-ATPase of type I cells in taste buds. J Comp Neurol. 497(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bear MF, Connors BW, Paradiso MA. 2007. Neuroscience: exploring the brain. Philadelphia (PA): Lippincott Williams & Wilkins. xxxviii, p. 857. [Google Scholar]
- Behrens M, Born S, Redel U, Voigt N, Schuh V, Raguse JD, Meyerhof W. 2012. Immunohistochemical detection of TAS2R38 protein in human taste cells. PLoS One. 7(7):e40304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhari N, Roper SD. 2010. The cell biology of taste. J Cell Biol. 190(3):285–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felten DL, Shetty AN, Felten DL. 2010. Netter’s atlas of neuroscience. Philadelphia (PA): Saunders/Elsevier. xviii, p. 438. [Google Scholar]
- Finger TE. 2005. Cell types and lineages in taste buds. Chem Senses. 30(Suppl 1):i54–i55. [DOI] [PubMed] [Google Scholar]
- Gray H, Lewis WH, Gray H. 1918. Anatomy of the human body. Philadelphia (PA): Lea & Febiger. p. 1396. [Google Scholar]
- Höfer D, Drenckhahn D. 1992. Identification of brush cells in the alimentary and respiratory system by antibodies to villin and fimbrin. Histochemistry. 98(4):237–242. [DOI] [PubMed] [Google Scholar]
- Höfer D, Püschel B, Drenckhahn D. 1996. Taste receptor-like cells in the rat gut identified by expression of alpha-gustducin. Proc Natl Acad Sci U S A. 93(13):6631–6634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YA, Maruyama Y, Stimac R, Roper SD. 2008. Presynaptic (Type III) cells in mouse taste buds sense sour (acid) taste. J Physiol. 586(Pt 12):2903–2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YA, Pereira E, Roper SD. 2011. Acid stimulation (sour taste) elicits GABA and serotonin release from mouse taste cells. PLoS One. 6(10):e25471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huque T, Cowart BJ, Dankulich-Nagrudny L, Pribitkin EA, Bayley DL, Spielman AI, Feldman RS, Mackler SA, Brand JG. 2009. Sour ageusia in two individuals implicates ion channels of the ASIC and PKD families in human sour taste perception at the anterior tongue. PLoS One. 4(10):e7347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataoka S, Yang R, Ishimaru Y, Matsunami H, Sévigny J, Kinnamon JC, Finger TE. 2008. The candidate sour taste receptor, PKD2L1, is expressed by type III taste cells in the mouse. Chem Senses. 33(3):243–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasteva G, Canning BJ, Hartmann P, Veres TZ, Papadakis T, Muhlfeld C, Schliecker K, Tallini YN, Braun A, Hackstein H, et al. 2011. Cholinergic chemosensory cells in the trachea regulate breathing. Proc Natl Acad Sci U S A. 108:9478–9483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusakabe T, Matsuda H, Gono Y, Furukawa M, Hiruma H, Kawakami T, Tsukuda M, Takenaka T. 1998. Immunohistochemical localisation of regulatory neuropeptides in human circumvallate papillae. J Anat. 192(Pt 4):557–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mescher A. 2013. Junqueira’s basic histology: text and atlas. New york (NY): McGraw-Hill. [Google Scholar]
- Paran N, Mattern CF, Henkin RI. 1975. Ultrastructure of the taste bud of the human fungiform papilla. Cell Tissue Res. 161(1):1–10. [DOI] [PubMed] [Google Scholar]
- Raliou M, Boucher Y, Wiencis A, Bézirard V, Pernollet JC, Trotier D, Faurion A, Montmayeur JP. 2009. Tas1R1-Tas1R3 taste receptor variants in human fungiform papillae. Neurosci Lett. 451(3):217–221. [DOI] [PubMed] [Google Scholar]
- Smith DV, Margolskee RF. 2001. Making sense of taste. Sci Am. 284(3):32–39. [DOI] [PubMed] [Google Scholar]
- Takami S, Getchell TV, McLaughlin SK, Margolskee RF, Getchell ML. 1994. Human taste cells express the G protein alpha-gustducin and neuron-specific enolase. Brain Res Mol Brain Res. 22:193–203. [DOI] [PubMed] [Google Scholar]
- Yamagishi M, Takami S, Getchell TV. 1995. Innervation in human taste buds and its decrease in Alzheimer’s disease patients. Acta Otolaryngol. 115(5):678–684. [DOI] [PubMed] [Google Scholar]