In a nationwide Danish register-based cohort study, the live oral polio vaccine was associated with fewer admissions for lower respiratory infections compared with the inactivated DTaP-IPV-Hib vaccine. There was no difference between oral polio vaccine and measles-mumps-rubella vaccine.
Keywords: heterologous immunity, immunization, nonspecific effects, nontargeted effects, oral polio vaccination
Abstract
Background. Live vaccines may have nonspecific beneficial effects on morbidity and mortality. This study examines whether children who had the live-attenuated oral polio vaccine (OPV) as the most recent vaccine had a different rate of admissions for infectious diseases than children with inactivated diphtheria-tetanus-pertussis-polio-Haemophilus influenzae type b vaccine (DTaP-IPV-Hib) or live measles-mumps-rubella vaccine (MMR) as their most recent vaccine.
Methods. A nationwide, register-based, retrospective cohort study of 137 403 Danish children born 1997–1999, who had received 3 doses of DTaP-IPV-Hib, were observed from 24 months (first OPV dose) to 36 months of age.
Results. Oral polio vaccine was associated with a lower rate of admissions with any type of non-polio infection compared with DTaP-IPV-Hib as most recent vaccine (adjusted incidence rate ratio [IRR], 0.85; 95% confidence interval [CI], .77–.95). The association was separately significant for admissions with lower respiratory infections (adjusted IRR, 0.73; 95% CI, .61–.87). The admission rates did not differ for OPV versus MMR.
Conclusions. Like MMR, OPV was associated with fewer admissions for lower respiratory infections than having DTaP-IPV-Hib as the most recent vaccination. Because OPV is now being phased-out globally, further studies of the potential beneficial nonspecific effects of OPV are warranted.
There is increasing evidence that vaccines may have not only specific effects on the target disease, but also nonspecific effects on the incidence of unrelated diseases. The World Health Organization's Strategic Advisory Group of Experts on immunizations (SAGE) recently concluded that further research on nonspecific effects of vaccines is needed [1]. This conclusion was based on a review of Bacille Calmette-Guérin vaccine (BCG), measles vaccine (MV), and diphtheria, tetanus, and pertussis vaccine (DTP), which indicated that the 2 live vaccines, BCG and MV, were associated with almost a halving of mortality that was not explained merely by the prevention of the target diseases [2].
The SAGE review did not include the live oral polio vaccine (OPV). However, OPV may also have beneficial nonspecific effects; previous studies have found that it was associated with lower child mortality [3–6], lower risk of acute otitis media [7], and acute respiratory virus infections [8]. Further assessments are important now because the global health community is planning to replace OPV with inactivated polio vaccine (IPV) as part of the endgame to eradicate polio virus [9].
Inactivated polio vaccine was introduced in Denmark in 1955 after a polio epidemic, and in 1968 OPV was added to the Danish vaccination program [10]. For many years, Denmark gave both IPV in 3 infant doses and OPV at 2, 3, and 4 years of age. The last case of endogenous polio occurred in 1976, and the last imported case was in 1983 [10]. In 2001 a phase-out of OPV began, whereas IPV was maintained [11]. Using data on vaccination and admissions from national Danish registers, the potential nonspecific effects of OPV can be examined for the birth cohorts 1997–1999, who were born after vaccinations became registered on their personal identification number and who were still entitled to OPV. In addition to OPV, the recommended vaccination program for these birth cohorts included 3 doses of the inactivated vaccine against diphtheria, tetanus, acellular pertussis, polio, and Haemophilus influenzae type b (DTaP-IPV-Hib) at ages 3, 5, and 12 months, and the live vaccine against measles, mumps, and rubella (MMR) at age 15 months.
We tested the hypothesis that OPV was associated with a lower rate of admissions with any type of non-polio infection compared with DTaP-IPV-Hib as most recent vaccine. In addition, we compared OPV with MMR and with simultaneous administration of MMR and OPV. Finally, we examined whether the associations differed by type of infection and sex as in previous studies of nonspecific effects of vaccines [12–15].
METHODS
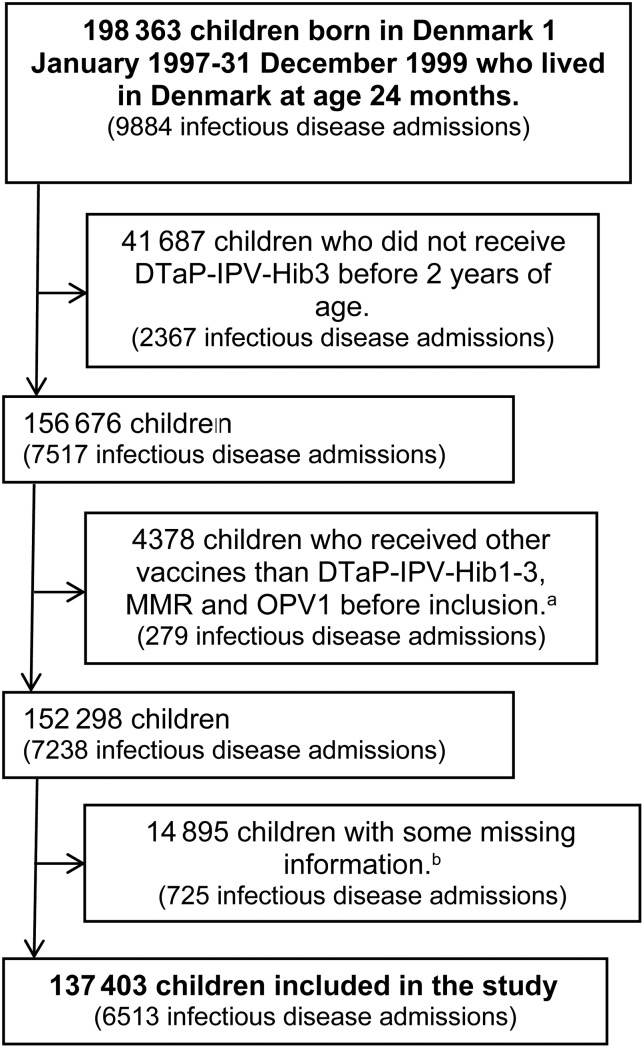
We included children who were born in Denmark from January 1, 1997 to December 31, 1999 and who were alive and living in Denmark at 24 months of age. We only included children who had received DTaP-IPV-Hib3 before 2 years of age to limit the potential bias related to low vaccination coverage; see Figure 1 for further inclusion criteria. All Danish residents have a unique personal identification number and are registered with date of birth and whereabouts in the Danish Civil Registration System [16] enabling identification of the cohort. All national Danish registries record the unique personal identification number [17], which makes it possible to retrieve additional information about the cohort as described below.
Figure 1.
Flowchart of inclusion in the study. aFourth dose of inactivated vaccine against diphtheria, tetanus, pertussis (acellular), polio, and Haemophilus influenzae type b (DTaP-IPV-Hib) (N = 3077; 70.3%), second dose of vaccination against measles, mumps, and rubella (MMR) (N = 605; 13.8%), not recommended combination of vaccines (N = 358; 8.2%), DTaP-IPV or Hib alone (N = 286; 6.5%), booster dose against different combinations of diphtheria, tetanus, pertussis (acellular), and polio (N = 32; 0.7%), or oral polio vaccine (OPV)2 (N = 20; 0.5%). bSome children had missing information on more than 1 variable. The number of children with missing information on each variable and in parentheses the percentage among the total number of children with missing information was as follows: 9732 (65.3%) with missing information on maternal smoking during pregnancy, 4315 (29.0%) with missing information on educational level for the female adult in the household, 1775 (11.9%) with missing information on birth weight, 955 (6.4%) with missing information on gestational age, 795 (5.3%) with missing information on household income, 276 (1.9%) with missing information on adult composition of the household, 107 (0.7%) with uncertain vaccine allocation for twins or triplets, 102 (0.7%) with missing information on parental place of birth, 80 (0.5%) with missing information on caesarean section, 12 (0.1%) with missing information on population density, and 6 (0.0%) with missing information on maternal age at birth of the child. (Note: Infectious disease admissions are counted from 24 months of age and until date of censoring for the children included in the study or until 36 months of age for the children excluded from the study, because these children did not have a censoring date.)
Vaccinations
In Denmark, all recommended childhood vaccinations are noncompulsory and free of charge. The general practitioners administer the vaccines and report vaccination information to the Danish National Health Service Register to receive reimbursement [18]. Before 1997, all vaccines were reported using the personal identification number of the parents, but thereafter they should be reported by the child's own personal identification number. Some childhood vaccines were still registered by the parents' personal identification number (5.6%); we assigned these vaccines to the child in the family who was closest to the recommended age of that vaccine.
Hospital Admissions With Infections
Hospital admissions are free of charge in Denmark. We obtained information on hospital admissions with infections from the Danish National Patient Register, which includes information about discharge diagnoses coded according to the Tenth Revision of the International Classification of Diseases (ICD-10) [19]. We identified dates of admission and discharge for all inpatient contacts with primary or secondary discharge diagnoses of any infection as described previously [14].
Other Register-Based Information
We obtained information on previous admissions, chronic diseases (coded according to Kristensen et al [20]), and emergency department visits registered as unintentional injuries from the Danish National Patient Register. Inclusion and follow-up was defined according to information on births, deaths, and emigration from the Danish Civil Registration System, which also contains information about a child's parents, siblings, and household [16]. Information on maternal smoking during pregnancy, birth weight, gestational age, and mode of delivery was obtained from the Danish Medical Birth Register [21]. Information on household equivalence income [22] and maternal education [23] was obtained from Statistics Denmark.
Follow-up
We started follow-up at 2 years of age, when the first dose of OPV was recommended (OPV1). The main focus was the examination of potential nonspecific effects of OPV1 compared with DTaP-IPV-Hib3 and MMR. Therefore, we did not follow the children beyond 3 years of age when OPV2 was recommended and a limited number of children had other vaccines than OPV as the most recent vaccine. Hence, the children were observed until the earliest of the following events: administration of other vaccines than DTaP-IPV-Hib, MMR, or OPV1, 3 years of age, death, emigration, or unknown whereabouts for the Danish authorities (last date of follow-up was December 31, 2002). In supplemental analyses, we examined whether additional doses of OPV compared with OPV1 affected the rate of admissions for infection between 36 months and 60 months of age (Supplementary Methods).
Statistical Methods
We used Cox regression to estimate the incidence rate ratios (IRRs) and 95% confidence intervals (CIs) for hospital admissions according to the most recent vaccine (vaccination status was a time-varying variable). The assumption of proportional hazards between vaccination types was evaluated by Schoenfeld residuals, and no violations were detected. All models had age as the underlying timescale and were stratified by date of birth to adjust completely for any effect of age, season, and calendar year. All admissions with infections were included, so children may contribute with several admissions. However, because several admissions within a short time span may be related to the same infection, we defined admissions within 14 days after a previous discharge as the same episode. We used Stata 13 for both unadjusted analyses and analyses adjusted for the following background factors: mother smoking during pregnancy, sex, birth weight, gestational age, caesarean section, chronic diseases, number of infectious disease admissions before 24 months of age, admitted to hospital for any cause within the last 30 days, maternal age at birth of the child, highest educational level for the female adult in the household, parental place of birth, adults in the household, income quintiles for the household, other children in the household, and population density. All statistical tests were 2-sided, and the threshold for statistical significance was P < .05 and 95% CI for IRRs not overlapping 1.0.
We examined whether the sequence of vaccines was associated with the rate of admissions with any type of infection. We performed the analyses separately according to type of infection and duration of admission. Because a Finish study has reported that OPV compared with IPV was associated with a 24% (95% CI, 6%–41%) lower risk of otitis media [7], we tested specifically whether there was any association between most recent vaccine and admissions with otitis media (ICD-10 codes DH65.0–DH66.9).
Control Outcome
We would not expect any association between vaccination status and emergency department visits after unintentional injury. Therefore, we performed an analysis with emergency department visits after unintentional injury as outcome to examine whether differential health-seeking behavior could explain associations between vaccination status and admissions with infections.
Interaction
We examined whether the association between the most recent vaccine and admissions differed according to sex using Wald test statistics.
Sensitivity Analyses
First, we excluded admissions related to diseases targeted by any of the included vaccines. Second, vaccines are only registered by the week of vaccination in the Danish National Health Service Register, and so we coded the date of vaccination as Wednesday of the specified week. Therefore, we excluded admissions and person-time occurring during the week of vaccination to uncover potential misclassification of vaccination information. Third, we repeated the analyses including only children who had all vaccines reported with their own personal identification number.
RESULTS
Inclusion and Censoring
The study included 137 403 children (Figure 1). Follow-up ended at age 3 years (N = 124 451; 90.6%), administration of other vaccines than MMR or OPV1 (mainly OPV2) (N = 12 876; 9.4%), migration (N = 64; 0.0%), death (N = 6; 0.0%), and unknown whereabouts for the Danish authorities (N = 6; 0.0%).
Vaccination
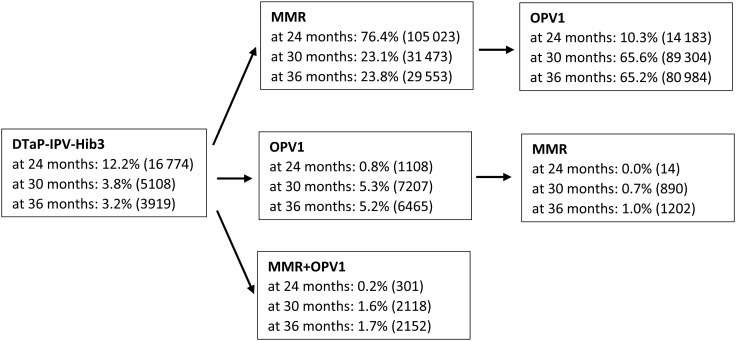
At 24 months of age, 76.4% of the children had received MMR after DTaP-IPV-Hib3 as the most recent vaccine, and at 36 months of age 65.2% of the children had received OPV1 after MMR as the most recent vaccine, as expected according to the recommended vaccination schedule (Figure 2). However, some children did not follow the recommended vaccination sequence and received OPV1 after DTaP-IPV-Hib3 or MMR and OPV1 together (Figure 2). The distribution of the most recent vaccine was statistically significantly uneven for most background factors in a data set of this size, and we adjusted for all background factors in Supplementary Table 1 in the following analyses.
Figure 2.
Distribution of the children on the sequence of vaccine groups at 24, 30, and 36 months of age (in parentheses the number of children). (Note: At 24 months of age 137 403 children were included in the study, but at 30 months of age 1303 children had been censored leaving 136 100 children in the study. At 36 months of age another 11 825 children had been censored leaving 124 275 children in the study.) Abbreviations: DTaP-IPV-Hib, inactivated vaccine against diphtheria, tetanus, pertussis (acellular), polio, and Haemophilus influenzae; MMR, vaccination against measles, mumps, and rubella; OPV, oral polio vaccine.
Admissions With Infections
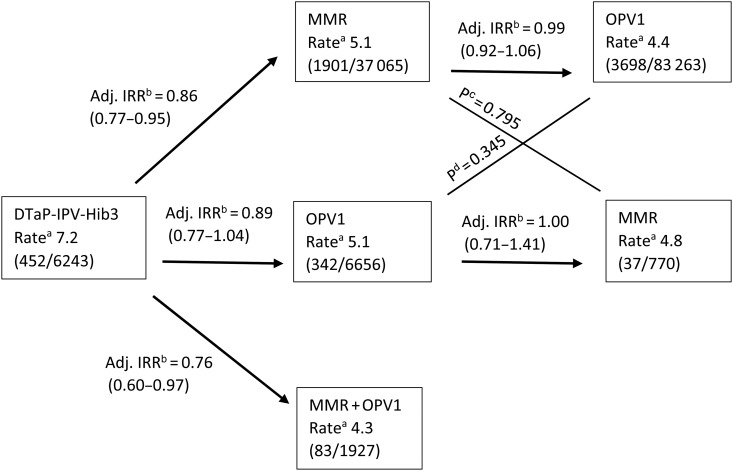
The study included 6513 admissions with any type of infection during 135 924 person years of risk, the incidence rate being 4.8 per 100 person years. Figure 3 shows the incidence rates for different sequences of vaccinations. There was no statistically significant difference in the rate of admissions with any type of infection after OPV1 or MMR as the most recent vaccine, no matter if OPV1 was administered before or after MMR (Figure 3). Furthermore, the rate of admissions did not differ for the 2 OPV1 groups, ie, OPV1 after DTaP-IPV-Hib3 or OPV1 after MMR (P equality, .345; Figure 3), or for the 2 MMR groups, ie, MMR after DTaP-IPV-Hib3 or MMR after OPV1 (P equality, .795; Figure 3). The same applied when admissions were analyzed by type of infections (Supplementary Figure 1). Hence, in all further analyses, OPV1 as most recent vaccine is grouped together no matter which vaccine the child had before receiving OPV1, and the same applies to MMR.
Figure 3.
Incidence rate and incidence rate ratio (IRR) according to the most recent vaccine and sequence. aThe incidence rate per 100 person years and in brackets the number of admissions and number of person years. bThe IRRs for the vaccine type the arrow points at relative to the previous vaccine type in the sequence and in brackets 95% confidence interval. Estimated from Cox proportional hazards model with age as underlying time, stratified by date of birth and adjusted for maternal smoking during pregnancy, sex, birth weight, gestational age, caesarean section, chronic diseases, number of infectious disease admissions before 24 months of age, admitted to hospital for any cause within the last 30 days, maternal age at birth of the child, highest educational level for the female adult in the household, parental place of birth, adults in the household, income quintiles for the household, other children in the household, and population density. cP value from a Wald test of the equality of rates for live vaccine against measles, mumps, and rubella (MMR) as most recent vaccine in the adjusted model. dP value from a Wald test of the equality of rates for live oral polio vaccine (OPV)1 as most recent vaccine in the adjusted model. Abbreviations: Adj, adjusted; DTaP-IPV-Hib, inactivated vaccine against diphtheria, tetanus, pertussis (acellular), polio, and Haemophilus influenzae type b.
Having OPV1 as the most recent vaccine was associated with a significantly lower rate of admission with any type of infection compared with having DTaP-IPV-Hib3 as most recent vaccine (adjusted IRR, 0.85; 95% CI, .77–.95; Table 1a); this association was strongest for admissions with lower respiratory infections and for admissions lasting 2 days or more (Table 1b and c). The association with admissions for lower respiratory infections was found for all durations of admissions (Supplementary Table 2). There were no statistically significant differences in rates of admissions for those having OPV1 as the most recent vaccine compared with MMR (adjusted IRR, 0.99; 95% CI, .93–1.07) and MMR + OPV1 (adjusted IRR, 1.12; 95% CI, .90–1.39); this was also the case when the data were analyzed for different types of admissions and durations of admissions (data available on request).
Table 1.
Incidence Rate and Incidence Rate Ratio for Infectious Disease Hospital Admissions for the Most Recent Vaccine for Any Type of Infection (a), Type of Infection (b), and Duration of Admission (c)
| Outcomes and Most Recent Vaccine | Admissions per 100 Person-Years (Admissions/Person-Years) | Unadjusted IRRa (95% CI) | Adjusted IRRb (95% CI) |
|---|---|---|---|
| (a) Any Type of Infection | |||
| DTaP-IPV-Hib3 | 7.2 (452/6243) | 1 (ref) | 1 (ref) |
| MMR | 5.1 (1938/37 835) | 0.72 (.65–.80) | 0.86 (.77–.95) |
| OPV1 | 4.5 (4040/89 919) | 0.65 (.59–.72) | 0.85 (.77–.95) |
| MMR + OPV1 | 4.3 (83/1927) | 0.64 (.51–.81) | 0.76 (.60–.97) |
| (b) Type of Infectionc | |||
| Upper respiratory infections | |||
| DTaP-IPV-Hib3 | 2.8 (172/6243) | 1 (ref) | 1 (ref) |
| MMR | 2.1 (785/37 835) | 0.76 (.64–.90) | 0.91 (.77–1.08) |
| OPV1 | 1.8 (1644/89 919) | 0.72 (.61–.85) | 0.93 (.79–1.10) |
| MMR + OPV1 | 1.5 (28/1927) | 0.59 (.40–.89) | 0.70 (.47–1.06) |
| Lower respiratory infections | |||
| DTaP-IPV-Hib3 | 2.7 (170/6243) | 1 (ref) | 1 (ref) |
| MMR | 1.6 (618/37 835) | 0.61 (.51–.72) | 0.77 (.64–.92) |
| OPV1 | 1.3 (1157/89 919) | 0.51 (.43–.61) | 0.73 (.61–.87) |
| MMR + OPV1 | 1.5 (29/1927) | 0.62 (.42–.92) | 0.79 (.52–1.19) |
| Gastrointestinal infections | |||
| DTaP-IPV-Hib3 | 0.9 (58/6243) | 1 (ref) | 1 (ref) |
| MMR | 0.7 (248/37 835) | 0.78 (.58–1.04) | 0.86 (.64–1.15) |
| OPV1 | 0.7 (650/89 919) | 0.77 (.58–1.01) | 0.89 (.67–1.18) |
| MMR + OPV1 | 0.7 (14/1927) | 0.78 (.43–1.41) | 0.87 (.48–1.58) |
| Other type infections | |||
| DTaP-IPV-Hib3 | 1.4 (86/6243) | 1 (ref) | 1 (ref) |
| MMR | 1.2 (436/37 835) | 0.80 (.64–1.02) | 0.97 (.76–1.23) |
| OPV1 | 0.9 (828/89 919) | 0.70 (.55–0.88) | 0.94 (.74–1.20) |
| MMR + OPV1 | 0.8 (15/1927) | 0.59 (.34–1.03) | 0.69 (.39–1.21) |
| (c) Duration of Admission With Any Type of Infection | |||
| 0 d | |||
| DTaP-IPV-Hib3 | 1.9 (117/6243) | 1 (ref) | 1 (ref) |
| MMR | 1.5 (573/37 835) | 0.81 (.66–.99) | 0.98 (.79–1.21) |
| OPV1 | 1.3 (1131/89 919) | 0.70 (.58–.86) | 0.93 (.76–1.14) |
| MMR + OPV1 | 1.0 (19/1927) | 0.58 (.36–.95) | 0.73 (.45–1.20) |
| 1 d | |||
| DTaP-IPV-Hib3 | 2.0 (127/6243) | 1 (ref) | 1 (ref) |
| MMR | 1.6 (605/37 835) | 0.80 (.66–.97) | 0.93 (.76–1.13) |
| OPV1 | 1.5 (1306/89 919) | 0.76 (.63–.92) | 0.94 (.77–1.15) |
| MMR + OPV1 | 1.3 (26/1927) | 0.71 (.46–1.09) | 0.81 (.53–1.25) |
| 2 d or more | |||
| DTaP-IPV-Hib3 | 3.3 (208/6243) | 1 (ref) | 1 (ref) |
| MMR | 2.0 (760/37 835) | 0.62 (.53–.72) | 0.74 (.63–.88) |
| OPV1 | 1.8 (1603/89 919) | 0.56 (.48–.65) | 0.75 (.64–.88) |
| MMR + OPV1 | 2.0 (38/1927) | 0.63 (.44–.90) | 0.75 (.52–1.07) |
Abbreviations: CI, confidence interval; DTaP-IPV-Hib, inactivated vaccine against diphtheria, tetanus, pertussis (acellular), polio, and Haemophilus influenzae type b; ICD-10, Tenth Revision of the International Classification of Diseases; IRR, incidence rate ratio; MMR, live vaccine against measles, mumps, and rubella; OPV, live oral polio vaccine; ref, reference group.
a Cox proportional hazards model with age as underlying time and stratified by date of birth thereby controlling for age and season.
b Cox proportional hazards model with age as underlying time, stratified by date of birth and adjusted for mother smoking during pregnancy, sex, birth weight, gestational age, caesarean section, chronic diseases, number of infectious disease admissions before 24 months of age, admitted to hospital for any cause within the last 30 days, maternal age at birth of the child, highest educational level for the female adult in the household, parental place of birth, adults in the household, income quintiles for the household, other children in the household, and population density.
c The total number of admissions for the 4 types of admissions add to more than the total number of admissions, because admissions including ICD-10 codes from more than 1 type of infection were included in both groups.
An OPV1 as most recent vaccine was associated with an insignificant lower rate of admissions with otitis media compared with having DTaP-IPV-Hib3 as the most recent vaccine (adjusted IRR, 0.81; 95% CI, .60–1.08; Supplementary Table 3).
Additional Doses of Oral Polio Vaccine
The distribution of the different OPV doses was statistically significantly uneven for most background factors (Supplementary Table 4). Compared with OPV1, having OPV2 or OPV3 as most recent vaccine was associated with significantly lower rate of admissions with any type of infection for children aged 36–60 months (adjusted IRR, 0.92; 95% CI, .86–.99; Supplementary Results 1 and Supplementary Table 5).
Control Outcome: Emergency Department Visits After Unintentional Injury
There was no association between the most recent vaccine and the rate of emergency department visits after unintentional injury (Supplementary Table 6 and Supplementary Table 5f).
Interaction and Sensitivity Analyses
There was no statistically significant interaction between most recent vaccine and sex for any type of infection (Table 2). None of the sensitivity analyses revealed any important differences from the main analysis (Supplementary Results 2).
Table 2.
Results for Interaction Between Most Recent Vaccine and Sex for Admissions due Any Type of Infection (a), Upper Respiratory Infections (b), Lower Respiratory Infections (c), Gastrointestinal Infections (d), and Other Types of Infections (e)
| Type of Infection, Sex, and Most Recent Vaccine | Admissions per 100 Person-Years (Admissions/Person-Years) | Unadjusted IRRa (95% CI) | Adjusted IRRb (95% CI) |
|---|---|---|---|
| (a) Any Type of Infection | |||
| Female | |||
| DTaP-IPV-Hib3 | 5.8 (178/3043) | 1 (ref) | 1 (ref) |
| MMR | 4.2 (777/18 511) | 0.72 (.61–.85) | 0.86 (.73–1.02) |
| OPV1 | 3.6 (1601/44 062) | 0.65 (.56–.76) | 0.83 (.71–.98) |
| MMR + OPV1 | 3.2 (29/893) | 0.60 (.40–.89) | 0.72 (.49–1.08) |
| Male | |||
| DTaP-IPV-Hib3 | 8.6 (274/3200) | 1 (ref) | 1 (ref) |
| MMR | 6.0 (1161/19 325) | 0.72 (.63–.82) | 0.85 (.74–.98) |
| OPV1 | 5.3 (2439/45 857) | 0.66 (.58–.75) | 0.86 (.76–.99) |
| MMR + OPV1 | 5.2 (54/1034) | 0.66 (.49–.88) | 0.78 (.58–1.06) |
| P equality for MMR | 0.918 | 0.917 | |
| P equality for OPV1 | 0.941 | 0.711 | |
| P equality for MMR + OPV1 | 0.709 | 0.748 | |
| (b) Upper Respiratory Infection | |||
| Female | |||
| DTaP-IPV-Hib3 | 2.1 (63/3043) | 1 (ref) | 1 (ref) |
| MMR | 1.4 (262/18 511) | 0.68 (.52–.90) | 0.82 (.62–1.09) |
| OPV1 | 1.3 (572/44 062) | 0.67 (.52–.88) | 0.86 (.65–1.12) |
| MMR + OPV1 | 0.9 (8/893) | 0.48 (.23–1.00) | 0.59 (.28–1.24) |
| Male | |||
| DTaP-IPV-Hib3 | 3.4 (109/3200) | 1 (ref) | 1 (ref) |
| MMR | 2.7 (523/19 325) | 0.81 (.66–1.00) | 0.97 (.78–1.20) |
| OPV1 | 2.3 (1072/45 857) | 0.75 (.61–.92) | 0.98 (.79–1.21) |
| MMR + OPV1 | 1.9 (20/1034) | 0.65 (.40–1.05) | 0.77 (.47–1.25) |
| P equality for MMR | 0.315 | 0.376 | |
| P equality for OPV1 | 0.513 | 0.437 | |
| P equality for MMR + OPV1 | 0.496 | 0.562 | |
| (c) Lower Respiratory Infection | |||
| Female | |||
| DTaP-IPV-Hib3 | 2.0 (60/3043) | 1 (ref) | 1 (ref) |
| MMR | 1.4 (257/18 511) | 0.71 (.53–.94) | 0.91 (.68–1.22) |
| OPV1 | 1.1 (465/44 062) | 0.58 (.44–.77) | 0.81 (.61–1.08) |
| MMR + OPV1 | 1.1 (10/893) | 0.65 (.33–1.27) | 0.83 (.42–1.64) |
| Male | |||
| DTaP-IPV-Hib3 | 3.4 (110/3200) | 1 (ref) | 1 (ref) |
| MMR | 1.9 (361/19 325) | 0.55 (.44–.68) | 0.68 (.54–.86) |
| OPV1 | 1.5 (692/45 857) | 0.48 (.39–.59) | 0.68 (.55–.85) |
| MMR + OPV1 | 1.8 (19/1034) | 0.59 (.36–.97) | 0.76 (.46–1.27) |
| P equality for MMR | 0.156 | 0.129 | |
| P equality for OPV1 | 0.257 | 0.333 | |
| P equality for MMR + OPV1 | 0.841 | 0.851 | |
| (d) Gastrointestinal Infection | |||
| Female | |||
| DTaP-IPV-Hib3 | 0.8 (25/3043) | 1 (ref) | 1 (ref) |
| MMR | 0.6 (112/18 511) | 0.81 (.52–1.25) | 0.92 (.59–1.44) |
| OPV1 | 0.6 (280/44 062) | 0.76 (.50–1.15) | 0.90 (.59–1.37) |
| MMR + OPV1 | 0.7 (6/893) | 0.81 (.33–1.99) | 0.92 (.37–2.27) |
| Male | |||
| DTaP-IPV-Hib3 | 1.0 (33/3200) | 1 (ref) | 1 (ref) |
| MMR | 0.7 (136/19 325) | 0.76 (.52–1.12) | 0.81 (.55–1.20) |
| OPV1 | 0.8 (370/45 857) | 0.77 (.54–1.11) | 0.88 (.61–1.28) |
| MMR + OPV1 | 0.8 (8/1034) | 0.76 (.35–1.65) | 0.84 (.38–1.83) |
| P equality for MMR | 0.840 | 0.672 | |
| P equality for OPV1 | 0.956 | 0.953 | |
| P equality for MMR + OPV1 | 0.910 | 0.870 | |
| (e) Other Types of Infections | |||
| Female | |||
| DTaP-IPV-Hib3 | 1.3 (41/3043) | 1 (ref) | 1 (ref) |
| MMR | 1.1 (207/18 511) | 0.80 (.57–1.13) | 0.92 (.65–1.31) |
| OPV1 | 0.9 (375/44 062) | 0.66 (.48–.92) | 0.84 (.60–1.18) |
| MMR + OPV1 | 0.7 (6/893) | 0.53 (.22–1.25) | 0.62 (.26–1.48) |
| Male | |||
| DTaP-IPV-Hib3 | 1.4 (45/3200) | 1 (ref) | 1 (ref) |
| MMR | 1.2 (229/19 325) | 0.81 (.58–1.11) | 1.00 (.72–1.41) |
| OPV1 | 1.0 (453/45 857) | 0.73 (.54–1.01) | 1.03 (.74–1.43) |
| MMR + OPV1 | 0.9 (9/1034) | 0.64 (.31–1.31) | 0.76 (.36–1.58) |
| P equality for MMR | 0.990 | 0.733 | |
| P equality for OPV1 | 0.649 | 0.390 | |
| P equality for MMR + OPV1 | 0.746 | 0.729 | |
Abbreviations: CI, confidence interval; DTaP-IPV-Hib, inactivated vaccine against diphtheria, tetanus, pertussis (acellular), polio, and Haemophilus influenzae type b; IRR, incidence rate ratio; MMR, live vaccine against measles, mumps, and rubella; OPV, live oral polio vaccine; ref, reference group.
a Cox proportional hazards model with age as underlying time scale and stratified by date of birth thereby controlling for age and season.
b Cox proportional hazards model with age as underlying time, stratified by date of birth and adjusted for mother smoking during pregnancy, sex, birth weight, gestational age, caesarean section, chronic diseases, number of infectious disease admissions before 24 months of age, admitted to hospital for any cause within the last 30 days, maternal age at birth of the child, highest educational level for the female adult in the household, parental place of birth, adults in the household, income quintiles for the household, other children in the household, and population density.
DISCUSSION
The OPV1 was associated with a lower rate of admissions with infection compared with DTaP-IPV-Hib as most recent vaccine, mainly due to fewer admissions with lower respiratory infections and admissions lasting 2 days or more. There were no significant differences between OPV1 and MMR as most recent vaccine.
We believe the reliability of the vaccination information is high because it is reported by general practitioners for reimbursement purposes and control measures for overreporting are in place [18], but we cannot exclude some underreporting as found in a validation study of the Danish register-based vaccination information on DTaP-IPV booster vaccine [24]. Any nondifferential misclassification of vaccinations would bias the estimates toward no association.
It is important to note that the analyses were performed in a Cox regression with age as underlying time scale to secure complete adjustment for age, because only children of the exact same age are compared in this model. Furthermore, we adjusted for a considerable number of other background factors, but residual confounding or confounding from unmeasured factors cannot be excluded. A particular concern is healthy vaccinée bias, ie, the most healthy children receive the next vaccine first, biasing the estimates toward lower rates of admissions for children who have received most vaccines. However, we did not find any indication of a lower rate of admissions with infection when OPV1 was given after MMR or vice versa (Figure 3). This suggests that healthy vaccinée bias is an unlikely explanation for the lower rate of admissions in children with OPV as the most recent vaccine. In addition, if healthy vaccinée bias was a major problem, the effect would probably be similar for all types of infections and durations of admissions, which was not the case in the present study. Furthermore, differences in health-seeking behavior was unlikely to explain the results because there was no association between the most recent vaccine and emergency department visits after unintentional injury.
The present study found that both MMR and OPV were associated with less admissions for lower respiratory infection compared with DTaP-IPV-Hib as most recent vaccine. We have previously shown for children aged 11–24 months that compared with DTaP-IPV-Hib as most recent vaccine, MMR was associated with a 14% (95% CI, 12%–16%) lower rate of admissions with any type of infection, the association being strongest for lower respiratory infection with a reduction of 20% (95% CI, 16%–24%) [14]. In the present study, most children with OPV as the most recent vaccine had previously received MMR. This could suggest that the apparent beneficial nonspecific effect of OPV was in fact caused by the previous MMR vaccination. However, OPV was also associated with fewer admissions for lower respiratory infections compared with DTaP-IPV-Hib as most recent vaccine among children who had not yet received MMR (Supplementary Figure 1B).
One Finish open trial found that OPV compared with IPV was associated with a lower risk of acute otitis media at 6–18 months of age [7]. We found a similar but not statistically significant association.
In low-income countries, there have been very few studies of the association between OPV and child mortality because OPV is nearly always given with other vaccines (BCG or DTP). However, 1 study from Guinea-Bissau showed that the case fatality at the pediatric ward was significantly reduced for children who, due to shortage of DTP, had received OPV alone compared with those children who had received the recommended combination of DTP and OPV [5]. Another study from Guinea-Bissau showed that children below 6 months of age vaccinated with OPV during a national immunization campaign had significantly lower mortality and lower rate of hospital admission within the first months after the campaign compared with those who did not receive OPV [6]. Today, OPV is only used in low-income countries, and the first dose is recommended at birth together with BCG. Previous observational studies have reported conflicting results regarding the association between OPV at birth (OPV0) and infant mortality [25, 26]. However, in the only randomized trial, OPV0 administered with BCG at birth was associated with lower infant mortality than receiving BCG only (hazard ratio, 0.68; 95% CI, .45–1.00) when the effect of subsequent OPV campaigns was censored [27].
In previous studies of possible nonspecific effects of OPV, it has been hypothesized that OPV could interfere with other enteroviruses [3, 4, 7, 8]. In this study, OPV was only statistically significantly associated to admissions with lower respiratory infections and admissions lasting 2 days or more, which are likely to be the most severe infections. These infections are probably not related to enterovirus. Hence, the association between OPV and these diseases indicate that other mechanisms might be involved. Both changes in the adaptive immune responses to unrelated pathogens after vaccination and trained innate immunity have been suggested as mechanisms underlying nonspecific effects of vaccines [12, 15], but this need to be further explored. It would be particularly interesting to investigate mechanisms that are related to lower respiratory infections because both OPV, MMR, and MV have the strongest association with reduction in lower respiratory infections [8, 14, 28, 29].
The present study showed that the rate of admissions with infections was similar for children who had OPV and MMR as the most recent vaccine. The MMR vaccination is still included in the vaccination program in Denmark. Because OPV1 was primarily used after MMR, our study indicates that the phasing out of OPV probably had only limited effects on overall morbidity levels for children aged 24–36 months in the Danish population. However, additional doses of OPV were associated with lower rates of admissions with infections, and the second dose of OPV was recommended at 3 years of age, so the morbidity levels for children above 36 months of age might have been affected by the phasing out of OPV.
CONCLUSION
Oral polio vaccine is associated with lower rate of admissions with non-polio infections compared with DTaP-IPV-Hib as most recent vaccine. The association was significant for all admissions, for admissions with lower respiratory infections, and for admissions lasting 2 days or more. Hence, the phase-out of OPV may affect morbidity levels, and it is important that the potential beneficial nonspecific effects of OPV be examined in other studies to give a better basis for assessing the likely consequences of stopping OPV globally.
Supplementary Data
Supplementary material is available online at Open Forum Infectious Diseases online (http://OpenForumInfectiousDiseases.oxfordjournals.org/)
Acknowledgments
Financial support. This work was funded by the Danish Council for Independent Research (DFF- 4183-00316; to S. S.); Fonden til Lægevidenskabens Fremme (to S. S.); the Novo Nordisk Foundation (research professorship grant to P. A.); and European Research Council Starting Grant (ERC-StG-243149; to C. S. B.). The Danish National Research Foundation supports the Research Center for Vitamins and Vaccines (DNRF108).
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1.Meeting of the Strategic Advisory Group of Experts on immunization, April 2014 – conclusions and recommendations. Wkly Epidemiol Rec 2014; 89:221–36. [PubMed] [Google Scholar]
- 2.Systematic review of the non-specific effects of BCG, DTP and measles containing vaccines. Available at: http://www.who.int/immunization/sage/meetings/2014/april/3_NSE_Epidemiology_review_Report_to_SAGE_14_Mar_FINAL.pdf?ua=1 Accessed 23 October 2014.
- 3.Contreras G. Effect of the administration of oral poliovirus vaccine on infantile diarrhoea mortality. Vaccine 1989; 7:211–2. [DOI] [PubMed] [Google Scholar]
- 4.Contreras G. Sabin's vaccine used for nonspecific prevention of infant diarrhea of viral etiology. Bull Pan Am Health Organ 1974; 8:123–32. [PubMed] [Google Scholar]
- 5.Aaby P, Rodrigues A, Biai S et al. Oral polio vaccination and low case fatality at the paediatric ward in Bissau, Guinea-Bissau. Vaccine 2004; 22:3014–7. [DOI] [PubMed] [Google Scholar]
- 6.Aaby P, Hedegaard K, Sodemann M et al. Childhood mortality after oral polio immunisation campaign in Guinea-Bissau. Vaccine 2005; 23:1746–51. [DOI] [PubMed] [Google Scholar]
- 7.Seppala E, Viskari H, Hoppu S et al. Viral interference induced by live attenuated virus vaccine (OPV) can prevent otitis media. Vaccine 2011; 29:8615–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Voroshilova MK. Potential use of nonpathogenic enteroviruses for control of human disease. Prog Med Virol 1989; 36:191–202. [PubMed] [Google Scholar]
- 9.World Health Organization. Polio Eradication & Endgame Strategic Plan 2013–2018. Available at: http://www.polioeradication.org/Portals/0/Document/Resources/StrategyWork/PEESP_EN_A4.pdf Accessed 27 August 2014.
- 10.Plesner AM, Ronne T. [The childhood vaccination program. Background, status and future]. Ugeskr Laeger 1994; 156:7497–503. [PubMed] [Google Scholar]
- 11.Andersen P, Rønne T, Bro-Jørgensen K. Childhood vaccination program -change by July 1, 2001. EPI-NYT 2001; 23.
- 12.Benn CS, Netea MG, Selin LK, Aaby P. A small jab - a big effect: nonspecific immunomodulation by vaccines. Trends Immunol 2013; 34:431–9. [DOI] [PubMed] [Google Scholar]
- 13.Aaby P, Whittle H, Benn CS. Vaccine programmes must consider their effect on general resistance. BMJ 2012; 344:e3769. [DOI] [PubMed] [Google Scholar]
- 14.Sorup S, Benn CS, Poulsen A et al. Live vaccine against measles, mumps, and rubella and the risk of hospital admissions for nontargeted infections. JAMA 2014; 311:826–35. [DOI] [PubMed] [Google Scholar]
- 15.Aaby P, Kollmann TR, Benn CS. Nonspecific effects of neonatal and infant vaccination: public-health, immunological and conceptual challenges. Nat Immunol 2014; 15:895–9. [DOI] [PubMed] [Google Scholar]
- 16.Pedersen CB. The Danish Civil Registration System. Scand J Public Health 2011; 39:22–5. [DOI] [PubMed] [Google Scholar]
- 17.Thygesen LC, Ersboll AK. Danish population-based registers for public health and health-related welfare research: introduction to the supplement. Scand J Public Health 2011; 39:8–10. [DOI] [PubMed] [Google Scholar]
- 18.Andersen JS, Olivarius ND, Krasnik A. The Danish National Health Service Register. Scand J Public Health 2011; 39:34–7. [DOI] [PubMed] [Google Scholar]
- 19.Lynge E, Sandegaard JL, Rebolj M. The Danish National Patient Register. Scand J Public Health 2011; 39:30–3. [DOI] [PubMed] [Google Scholar]
- 20.Kristensen K, Hjuler T, Ravn H et al. Chronic diseases, chromosomal abnormalities, and congenital malformations as risk factors for respiratory syncytial virus hospitalization: a population-based cohort study. Clin Infect Dis 2012; 54:810–7. [DOI] [PubMed] [Google Scholar]
- 21.Knudsen LB, Olsen J. The Danish Medical Birth Registry. Dan Med Bull 1998; 45:320–3. [PubMed] [Google Scholar]
- 22.Baadsgaard M, Quitzau J. Danish registers on personal income and transfer payments. Scand J Public Health 2011; 39:103–5. [DOI] [PubMed] [Google Scholar]
- 23.Jensen VM, Rasmussen AW. Danish Education Registers. Scand J Public Health 2011; 39:91–4. [DOI] [PubMed] [Google Scholar]
- 24.Wojcik OP, Simonsen J, Molbak K, Valentiner-Branth P. Validation of the 5-year tetanus, diphtheria, pertussis and polio booster vaccination in the Danish childhood vaccination database. Vaccine 2013; 31:955–9. [DOI] [PubMed] [Google Scholar]
- 25.Benn CS, Fisker AB, Rodrigues A et al. Sex-differential effect on infant mortality of oral polio vaccine administered with BCG at birth in Guinea-Bissau. A natural experiment. PLoS One 2008; 3:e4056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lund N, Andersen A, Monteiro I et al. No effect of oral polio vaccine administered at birth on mortality and immune response to BCG. A natural experiment. Vaccine 2012; 30:6694–9. [DOI] [PubMed] [Google Scholar]
- 27.Lund N, Andersen A, Hansen AS et al. The effect of oral polio vaccine at birth on infant mortality: a randomized trial. Clin Infect Dis 2015; 61:1504–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martins CL, Benn CS, Andersen A et al. A randomized trial of a standard dose of Edmonston-Zagreb measles vaccine given at 4.5 months of age: effect on total hospital admissions. J Infect Dis 2014; 209:1731–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sorup S, Benn CS, Stensballe LG et al. Measles-mumps-rubella vaccination and respiratory syncytial virus-associated hospital contact. Vaccine 2015; 33:237–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.