Abstract
Parkinson disease (PD) is the most common age-dependent neurodegenerative movement disorder. Accumulated evidence indicates both environmental and genetic factors play important roles in PD pathogenesis, but the potential interaction between environment and genetics in PD etiology remains largely elusive. Here, we report that PD-related neurotoxins induce both expression and acetylation of multiple sites of histones in cultured human cells and mouse midbrain dopaminergic (DA) neurons. Consistently, levels of histone acetylation are markedly higher in midbrain DA neurons of PD patients compared to those of their matched control individuals. Further analysis reveals that multiple histone deacetylases (HDACs) are concurrently decreased in 1-methyl-4-phenylpyridinium (MPP+)-treated cells and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-treated mouse brains, as well as midbrain tissues of human PD patients. Finally, inhibition of histone acetyltransferase (HAT) protects, whereas inhibition of HDAC1 and HDAC2 potentiates, MPP+-induced cell death. Pharmacological and genetic inhibition of autophagy suppresses MPP+-induced HDACs degradation. The study reveals that PD environmental factors induce HDACs degradation and histone acetylation increase in DA neurons via autophagy and identifies an epigenetic mechanism in PD pathogenesis.
Keywords: epigenetics, histone acetylation, histone deacetylase (HDAC), neurodegenerative disease, neurotoxin
Introduction
Parkinson disease (PD)2 is pathologically characterized as loss of DA neurons in substantial nigra of midbrain and Lewy body formation in the remaining neurons (1). Genetic studies of familial cases have identified mutations in at least 14 genes that are associated with the disease (2). Nevertheless, the majority of PD cases are sporadic with unidentified etiology. It is well accepted that environmental factors play an important role in PD. In addition to aging, exposure to environmental toxins, such as certain pesticides and herbicides, results in parkinsonism resembling the idiopathic PD (3). Thus, both genetic and environmental factors contribute to PD pathogenesis. However, the molecular basis of parkinsonia induced by environmental factors remains unclear. Environmental factors are known to cause abnormal epigenetic modifications resulting in human diseases, including neurodegenerative diseases (4–6). Such modifications regulate gene expression by mechanisms other than DNA sequence changes (7). These types of regulation are heritable, self-perpetuating, and reversible (7–9). The most studied epigenetic regulations include DNA methylation, RNA modification, and histone modification (8).
Acetylation of histone proteins associated with chromatin plays a pivotal role in the epigenetic regulation of transcription and other functions in cells, including neurons (10). Reduced histone acetylation in animal models has been reported in neurodegeneration characterized by cognitive decline, including models of Alzheimer disease (AD) (11). Similar findings have been reported with PD models (3). Valproic acid, a histone deacetylase inhibitor, demonstrates protection against rotenone in a rat model of PD (12). Inhibitors of sirtuin-2 rescues α-synuclein-mediated neurotoxicity both in vitro in cell cultures and in vivo in a Drosophila PD model (13). These findings suggest that dysregulation of acetylation of histone or non-histone protein is a common mechanism of neurodegeneration in different neurodegenerative diseases.
In this study, we aim to investigate a role of histone acetylation in PD pathogenesis. Our results reveal that both levels of histone and histone acetylation are up-regulated in cells treated with PD-related neurotoxins and in brains of mice injected with 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP). Increased detection of histone acetylation is also observed in midbrain DA neurons of PD patients. Further analysis suggests that reduced expression of HDACs is likely responsible for changes of histone acetylation induced by PD-related neurotoxins. Moreover, inhibition of autophagy suppresses 1-methyl-4-phenylpyridinium (MPP+)-induced HDACs degradation. The results reveal that PD-related environmental toxins regulate autophagy resulting in abnormal histone acetylation to contribute to PD pathogenesis.
Experimental Procedures
Materials
Antibodies including histone 2A (Cell Signaling, 2578), histone 2B (Cell Signaling, 2934), histone 3 (Cell Signaling, 9715), histone 4 (Cell Signaling, 2935), acetylated lysine (Cell Signaling, 9441), acetyl-H2Ak5 (Cell Signaling, 2576), acetyl-H2Bk5 (Cell Signaling, 2574), acetyl-H2Bk15 (Cell Signaling, 5435), acetyl-H2Bk20 (Cell Signaling, 2571), acetyl-H3k9 (Cell singling, 9671), acetyl-H3k18 (Cell Signaling, 9675), acetyl-H3k27 (Cell Signaling, 4353), acetyl-H3k56 (Cell Signaling, 4243), acetyl-H4k5 (Cell Signaling, 9672), acetyl-H4k8 (Cell Signaling, 2594), acetyl-H4k12 (Cell Signaling, 2591), HDAC1 (Cell Signaling, 5356), HDAC4 (Cell Signaling, 5392), HDAC6 (Cell Signaling, 7558), and SirT1 (Cell Signaling, 2310) were purchased from Cell Signaling. Gcn5 (Epitomics, Q92830), cAMP response element-binding protein (CBP) (Abcam, ab83857), HDAC2 (Abcam, ab51832), tyrosine hydroxylase (Millipore, ab152; ab9702), Tip60 (Millipore, 07-038) were commercially purchased. All chemicals were from Sigma. Human tissues were obtained from the NICHD, National Institutes of Health, Brain and Tissue Bank for Developmental Disorders at the University of Maryland, Baltimore, MD (Tables 1 and 2).
TABLE 1.
Information of brain tissues from PD patients and their controls
| Sample No. | Tissue Bank ID | Sex | Age | Race | Postmortem interval | Disease condition |
|---|---|---|---|---|---|---|
| h | ||||||
| C1 | 5028 | M | 67.8 | Caucasian | 18 | Control |
| C2 | 4735 | M | 73.5 | Caucasian | 21 | Control |
| C3 | 4789 | F | 72.1 | Caucasian | 19 | Control |
| C4 | 5171 | M | 79.2 | Caucasian | 5 | Control |
| C5 | 1818 | M | 76.8 | Caucasian | 3 | Control |
| PD1 | 1947 | M | 70.7 | Caucasian | 17 | PD |
| PD2 | 1741 | M | 70.9 | Caucasian | 20 | PD |
| PD3 | 4977 | F | 76.2 | Caucasian | 14 | PD |
| PD4 | 4879 | M | 75.9 | Caucasian | 15 | PD |
| PD5 | 4526 | M | 78.5 | Caucasian | 1 | PD |
TABLE 2.
Information of brain tissues from AD patients and their controls
| Sample No. | Tissue Bank ID | Sex | Age | Race | Postmortem interval | Disease condition |
|---|---|---|---|---|---|---|
| h | ||||||
| C1 | 4921 | F | 74 | Caucasian | 13 | Control |
| C2 | 5082 | M | 68 | Caucasian | 19 | Control |
| C3 | 5171 | M | 79 | Caucasian | 5 | Control |
| C4 | 5246 | F | 78 | Caucasian | 19 | Control |
| C5 | 5352 | M | 81 | Caucasian | 17 | Control |
| AD1 | 1212 | F | 73 | Caucasian | 18 | AD |
| AD2 | 4693 | M | 70 | Caucasian | 12 | AD |
| AD3 | 4697 | M | 78 | Caucasian | 23 | AD |
| AD4 | 5007 | F | 77 | Caucasian | 15 | AD |
| AD5 | 5222 | F | 80 | Caucasian | 7 | AD |
Differentiation of SH-SY5Y Cells
SH-SY5Y cells were from ATCC and maintained according to the manufacturer's instructions. To differentiate, cells were treated with 10 μm retinoic acid (Sigma, R2625) for 10 days.
MTS Assay and ATP Measurement
CellTiter 96® AQueous Non-radioactive Cell Proliferation Assay (Promega, G5421), and ATPlite 1step Luminescence Assay System (PerkinElmer, 6016731) were used to measure cell viability. The assays were performed according to the manufacturer's protocol. Absorbance for the MTS assay was measured with a microplate reader (Thermo Electron Co.). ATP luminescence was detected with an Analyst HT (Molecular Devices, CA).
FACS Analysis
For FACS analysis, adherent cells treated with MPP+ and garcinol (Enzo Life Sciences, BML-GR343-0050) were harvested by trypsinization followed by neutralization with FBS containing culture medium. The cells were washed briefly with PBS followed by staining with propidium iodide for 15 min at room temperature in a Ca2+-enriched binding buffer. The stained cells were immediately analyzed using a BD FACSCanto flow cytometer (BD Biosciences, San Jose, CA) at the excitation wavelength of 585 nm. For each sample, a minimum of 30,000 events was collected on logarithmic scales. FACS data were analyzed by FlowJo software (TreeStar).
Transfection of Plasmid DNA and siRNA
SH-SY5Y cells were split with 70% confluence at 1 day before transfection. Plasmids HDAC1 and HDAC2, EGFP, and mock were transfected using Lipofectamine® 2000 DNA Transfection Reagent (Invitrogen, 11668) according to the manufacturer's protocol. Nonspecific control siRNA, HDAC1 siRNA, HDAC2 siRNA, and ATG5 siRNA were purchased from Shanghai GenePharma Co., Ltd. siRNAs were transfected using Lipofectamine® RNAiMax according to the manufacturer's protocol. Cells were incubated for 24 h followed by treatment with MPP+ for 2 days.
RNA Extraction and Real-time PCR
RNA extraction was performed with RNA extraction kit, RNeasy Mini Kit (Qiagen, 74104). Real-time PCR was performed with Dual-labeled probe (TaqMan Assay), using a real-time PCR detection system (Bio-Rad). The relative quantity of immunoprecipitated DNA fragment was adjusted by using the comparative CT method. Results were compared by a standard curve generated by serial dilutions of input DNA. Data were derived from three independent amplifications. Primers were as follows: HDAC1, F5′-ACTACTACGACGGGGATGTTGGA-3′ and R5′-GATGGAGCGCAAGAATTTAATGT-3′; HDAC2, F5′-GTCTGCTACTACTACGACGGTGA-3′ and R5′-AGTGGCTTTATGGGGCCTATATA-3′; HDAC4, F5′-CCAAAGCCATCCAGATGGACTTT-3′ and R5′-AGGCGCAGGTCCATGGGCACTGC-3′; HDAC6, F5′-CCCCAGTCGCCCCCTCAGGACTC-3′ and R5′-CACGATTAGGTCTTCTTCCATTG-3′; SIRT1, F5-′GATGAAATTATCACTAATGGTTT-3′ and R5′-TCGAGGATCTGTGCCAATCATAA-3′.
MPTP Injection
MPTP (Sigma, M0896) was dissolved in saline and intraperitoneally injected in a volume of 10 mg/kg body weight, 3 times per a day, every 3 h for 1 week (acute model, aMPTP) and 5 mg/kg body weight, 1 time per a day for 1 month (chronic model, cMPTP). Saline was used as control injection.
Immunoassays
Immunoblotting and immunofluorescent staining was performed essentially as described in the protocol of Abcam and previously described (14). For mouse brains, Rodent Block M blocking reagent (Biocare Medical) was used for blocking endogenous mouse IgG, according to the manufacturer's protocol. The slides were scanned using a automated high-throughput scanning system (ScanScope® XT system) and analyzed with a confocal microscope (Zeiss LSM-710).
Statistical Analysis
Immunoblotting, cytotoxicity assay, PI staining, and immunostaining results were quantified using ImageJ. Data are analyzed with Student's t test and one-way analysis of variance using GraphPad. All error bars indicate mean ± S.E. A probability less than 0.05 was considered statistically significant.
Results
PD Environmental Toxins Induce Histone Acetylation
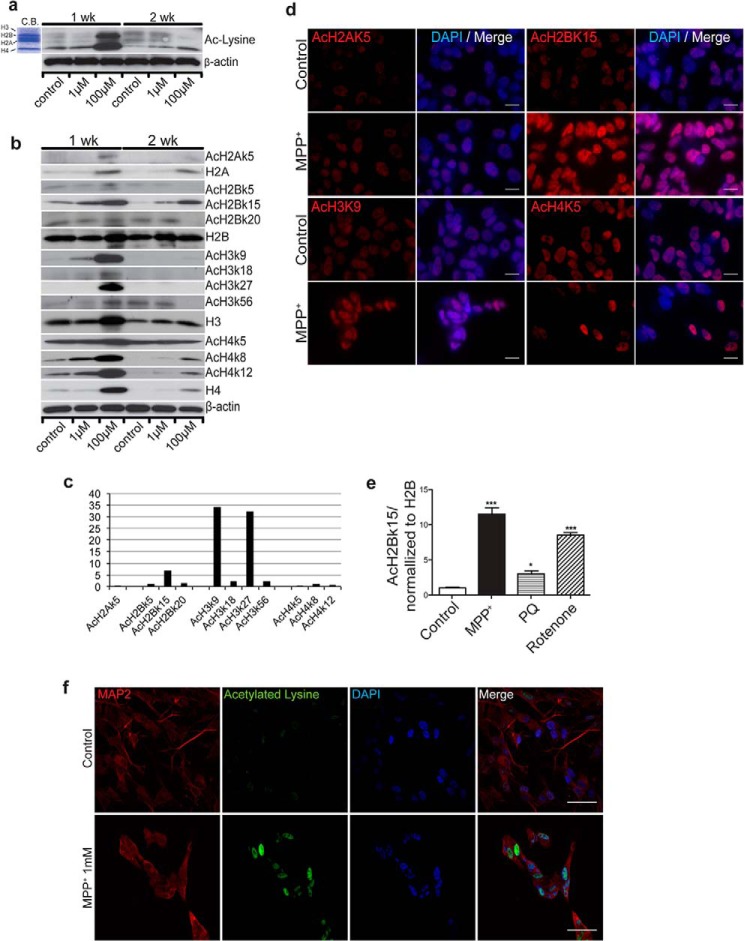
To investigate environmental effects on PD pathogenesis, we first defined a window of time and toxin concentration that resulted in minimal cell morphological changes and cell death using human SH-SY5Y neuroblastoma cells, thus settling on MPP+ at 100 μm for 7 days. Cells treated with MPP+ (100 μm) for 7 days showed a notable up-regulation of protein amount and acetylation status of several histones compared with the untreated controls (Fig. 1, a and b). Further characterization revealed markedly increased detection of H2A, H3, and H4 as well as a transient dose-dependent induction of histone acetylation on multiple sites, including H2Ak5, H2Bk5, H2Bk15, H2Bk20, H3k9, H3k18, H3k27, H3k56, H4k5, H4k8, and H4k12 with MPP+ treatment (Fig. 1, b and c). The apparent increase in histone acetylation is due in part to increased histone expression. Nevertheless, quantitative analysis of pixel density from 4 independent experiments indicated a 6–30-fold increase of acetylation of H2Bk15, H3k9, and H3k27 after normalizing to the level of corresponding histones (Fig. 1c). Immunofluorescent staining verified increased acetylation of H2Ak5, H2Bk15, H3k9, and H4k5 in MPP+-treated cells compared to their untreated controls (Fig. 1d). At higher concentrations (2 mm) that induces substantial cell death, MPP+ treatment also induced histone acetylation before cell death (data not shown). Two additional neurotoxins known to induce parkinsonism, paraquat and rotenone, showed similar effects by inducing histone acetylation in cultured SH-SY5Y cells (Fig. 1e). Immunofluorescent staining reveals a markedly increased detection of histone acetylation with MPP+ treatment in SH-SY5Y cells differentiated to a neuron-like morphology with retinoic acid (Fig. 1f). The results suggest that PD-associated environmental toxins aberrantly up-regulate histone expression and induce histone acetylation.
FIGURE 1.
PD-related neurotoxin MPP+ induces histone acetylations. a, SH-SY5Y cells treated with MPP+ or solvent (Control) for 1 (1 week) to 2 weeks (2 weeks) followed by immunoblotting with an anti-pan histone acetylation antibody. Migration of protein-stained histones is shown in the left panel (C.B., Coomassie Blue staining). β-Actin was used as a loading control. Note that MPP+ (100 μm) markedly increases histone acetylation with the 1-week treatment. b, expression and acetylation of histones following MPP+ treatment. SH-SY5Y cells were treated with MPP+ or solvent (Control) for 1 (1 week) to 2 weeks (2 weeks) followed by immunoblotting with histone and histone acetylation antibodies. Expression of histone H2A, H2B, H3, and H4 are shown. Acetylation of histone H2A lysine 5 (AcH2Ak5), H2B lysine 5, 15, and 20 (AcH2Bk5, AcH2Bk15, AcH2bk20), H3 lysine 9, 18, 27, and 56 (AcH3k9, AcH3k18, AcH3k27, AcH3k56), and H4 lysine 5, 8, and 12 (AcH4k5, AcH4k8, AcH4k12) are shown. c, quantitation of histone acetylation. Signal intensity of immunoblot from 4 independent experiments was measured by ImageJ software and normalized to each expression of histone. d, immunofluorescent detection of acetylated histones. SH-SY5Y cells were treated with MPP+ or solvent (Control) for 1 week (1 week) followed by immunofluorescent staining with histone acetylation antibodies (red). Cell nuclei were counterstained with DAPI (blue). Acetylation of histone H2A lysine 5 (AcH2Ak5), H2B lysine 15 (AcH2Bk15), H3 lysine 9 (AcH3k9), and H4 lysine 5 (AcH4k5) are shown. Scale bar = 8 μm. e, PD-related environmental toxins induce histone acetylation. SH-SY5Y cells were treated with MPP+ (100 μm), Paraquat (PQ, 10 μm), rotenone (100 nm), or solvent (Control) for 1 week followed by detection using a histone 2B acetyl-lysine 15 (AcH2Bk15) antibody. Quantitation of AcH2Bk15 with normalization to histone 2B from three independent experiments is shown. *, p < 0.05; ***, p < 0.001. f, MPP+ induces histone acetylation in neurons differentiated from SH-SY5Y cells. SH-SY5Y cells were differentiated with retinoic acid for 10 days followed by MPP+ treatment for 5 days. Cells were immunofluorescent detected with acetylated lysine antibody (green), neuronal marker MAP2 (red), and nuclei (DAPI, blue). Bar = 30 μm.
MPP+ Promotes Histone Acetylation in Dopaminergic Mouse Brain Neurons
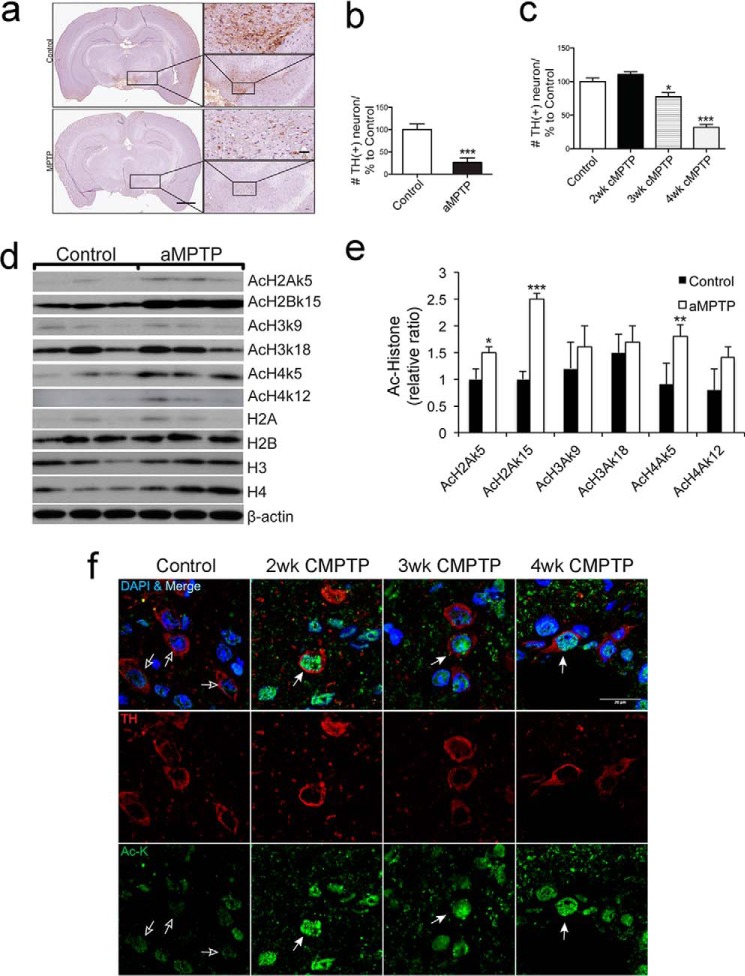
To investigate whether PD neurotoxins up-regulate histone acetylation in vivo, we analyzed histone acetylation in brain tissues of mice treated with MPTP. Two established protocols were employed to treat mice, including a 1-week acute treatment (aMPTP: 10 mg/kg, 3 injections/day for 1 week) and a 4-week chronic treatment (cMPTP: 5 mg/kg, 1 injection/day for 4 weeks) (15). The effectiveness of MPTP treatment was demonstrated by a 74 and 68.4% reduction of tyrosine hydroxylase (TH)-positive neurons in midbrains with aMPTP and cMPTP treatments, respectively (Fig. 2, a–c). Histone acetylation is significantly up-regulated in midbrain tissues compared with controls injected with saline (Fig. 2, d and e). In the cMPTP model, a notable increase of histone acetylation was detected in DA neurons of mouse midbrains as early as 2 weeks of treatment and persisted through 4 weeks treatment (Fig. 2f). Consistent with findings of cells treated with MPP+, expression levels of H2A, H3, and H4 were up-regulated by acute treatment (Fig. 2d). Together, these results indicate that environmental toxins induce expression and acetylation of histones in dopaminergic neurons of mouse brains in vivo.
FIGURE 2.
MPTP induces histone acetylation in vivo in mouse midbrain. a, representative immunohistology images of dopaminergic neuron in acute MPTP mouse model: DA neurons (dark brown), nucleus (violet). Low magnification (left panel: scale bar = 500 μm); medium magnification (right lower panel: scale bar = 100 μm); high magnification (right upper panel = 60 μm). b, quantitative analysis of DA neurons of acute MPTP mouse model (aMPTP) and the control solvent-treated mouse (Control). ***, p < 0.001, n = 3. c, quantitative analysis of DA neurons of chronic MPTP mouse model (cMPTP) and the control solvent-treated mouse midbrain (Control). *, p < 0.05; ***, p < 0.001, n = 3. d and e, MPTP induces histone acetylation in mouse substantial nigra. Mice were either acutely treated with MPTP (aMPTP) or solvent (Control). Midbrain tissues mainly containing substantia nigra were dissected and followed by immunoblot analysis of histone acetylation. Acetylation of H2Ak5, H2Bk15, H3k9, H3k18, H4K5, and H4k12 is shown for 3 MPTP-treated mice and 3 solvent-treated control mice. β-Actin was detected as loading control. Quantitative analysis of acetyl-histone with normalization to corresponding histones is shown. *, p < 0.05; **, p < 0.05; ***, p < 0.001, n = 3. f, MPTP induces histone acetylation in mouse midbrain DA neurons. Midbrains of mice treated with chronic concentration of MPTP (cMPTP) for different time frames were immunostained with an anti-tyrosine hydroxylase antibody (TH, red) and an anti-acetyl lysine antibody (Ac-K, green). Cell nuclei were counterstained with DAPI (blue). Control: mouse injected with saline for 2 weeks; 2, 3, and 4 weeks stand for mouse treated with MPTP for 2, 3, and 4 weeks, respectively. Empty arrows: DA neuron, solid arrows: DA neurons with histone acetylation. Bar = 20 μm.
Specific Up-regulation of Histone Acetylation in DA Neurons of PD Patient Brain
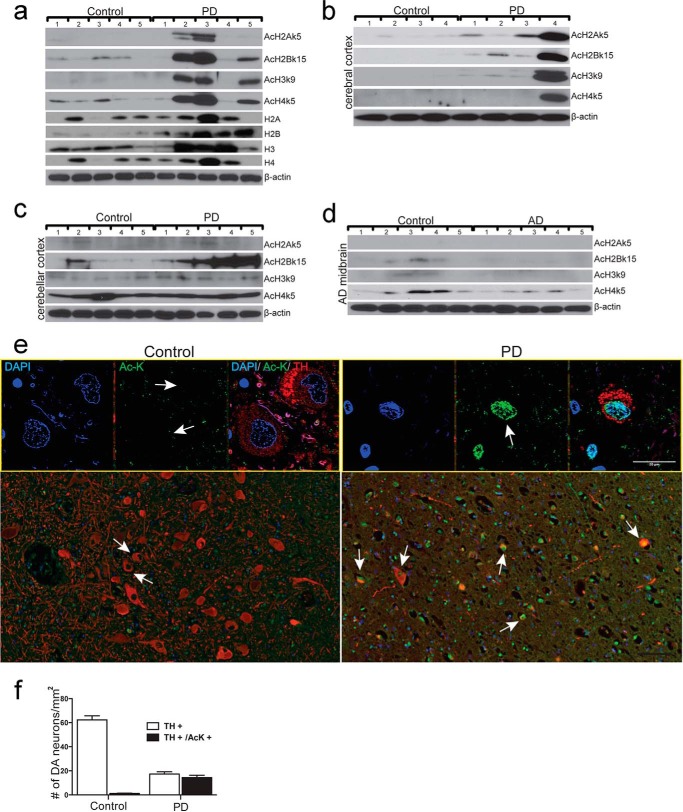
We next examined histone acetylation in postmodern brain tissues of PD patients. Midbrain tissues from 5 PD patients and 5 age, sex, and postmodern interval-matched control individuals were analyzed (Table 1). The results show a marked increase of acetylation of H2Ak5, H2Bk15, H3k9, and H4k5 in midbrain tissues of 3 of 5 PD patients compared with that of 5 control individuals (Fig. 3a). Importantly, increased histone acetylation was only observed in cerebral tissue of one PD compared with controls (Fig. 3b). Acetylation of H2Bk15, but not H2Ak5, H3k9, and H4k5, was increased in the cerebellar cortex of PD patients over their controls (Fig. 3c). In contrast, acetylation of H2Bk15 and H4k5 are notably decreased in the midbrain of AD patients compared with that of matched controls (Fig. 3d, Table 2). Immunostaining analysis specifically detected high levels of histone acetylation (Ac-K) in midbrain DA neurons of PD patients. Conversely, histone acetylation is barely detectable in non-DA cells of PD patients or in the midbrain DA neurons of the control individuals (Fig. 3e). Quantitative analysis by pixel density revealed detection of Ac-K in 82.7% TH-positive neurons of PD patients (n = 5). However, only 2% TH-positive neurons are AcK positive in the midbrain of normal control individuals (n = 5, Fig. 3f). The results suggest that specific histone acetylation is involved in dopaminergic neurodegeration in the midbrain of PD patients. Moreover, histone acetylation is differentially regulated in PD and AD. Together, histone acetylation is regulated in the midbrain of PD patients with cell type, brain region, and disease specificity.
FIGURE 3.
Aberrant up-regulation of histone acetylation in midbrain DA neuron of PD patients. a-c, immunodetection of histone expression and histone acetylation in midbrain (a), histone acetylation in cerebral cortex (b), and cerebellar cortex (c) of PD patients and their matched controls. Numbers on the top of each panel represent different individuals. Expression of H2A, H2B, H3, and H4 and acetylation of H2Ak5 (AcH2Ak5), H2Bk15 (AcH2Bk15), H3k9 (AcH3k9), and H4K5 (AcH4K5) was shown. β-Actin was detected as a loading control. d, immunodetection of histone acetylation in midbrains of AD patients and their matched controls. Numbers on the top of each panel represent different individuals. Acetylation of H2Ak5 (AcH2Ak5), H2Bk15 (AcH2Bk15), H3k9 (AcH3k9), and H4K5 (AcH4K5) was shown. β-Actin was detected as a loading control. e, representative immunofluorescent images of histone acetylation in midbrain of PD patients. TH+, DA neurons (red); Ac-K+, acetylated lysine (green). Cell nuclei are stained with DAPI (blue). Low magnification (scale bar = 100 μm) in the lower panel and high magnification (scale bar = 20 μm) in the upper panel are shown. White arrows, DA neurons with acetylated lysine signal. f, quantitative analysis of the number of DA neurons (TH+) and DA neurons with histone acetylation positive signal (TH+/Ac-K+) in midbrains of PD patients and their matched control individuals (Control). The data for each condition represents an average of 10 independent microscopic fields.
Down-regulation of HDACs in Brain of PD Patients
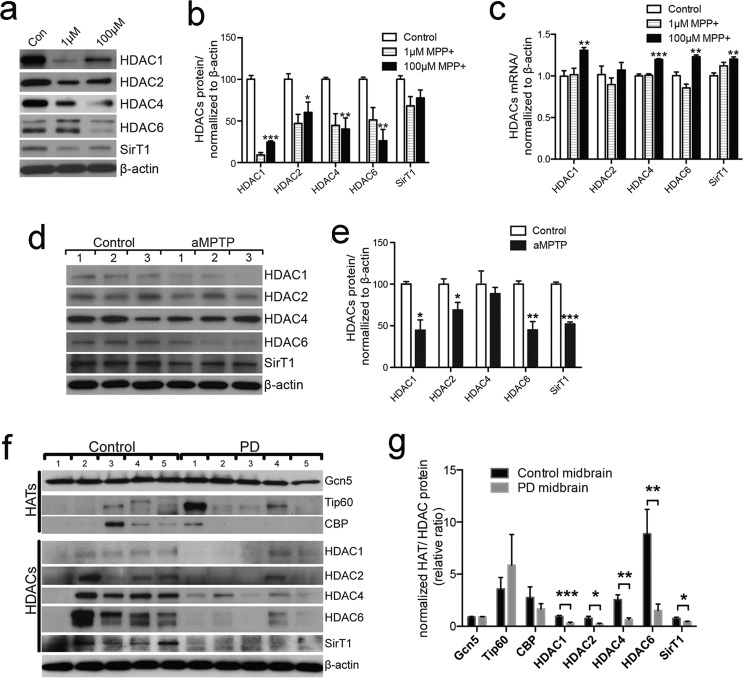
We next investigated the mechanism of abnormal histone acetylation induced by PD-related neurotoxins. SH-SY5Y cells treated with low doses of 1 μm and 100 μm MPP+ exhibited significantly reduced levels of HDAC1, HDAC2, HDAC4, and HDAC6 (Fig. 4, a and b). These changes are unlikely due to transcriptional regulation because mRNA levels of these HDACs were either not altered with 1 μm MPP+ treatment or up-regulated with 100 μm MPP+ treatment (Fig. 4c). Consistent with findings from cultured cells, HDAC1, HDAC2, HDAC6, and SirT1 were decreased in midbrains of mice treated with aMPTP compared with that of vehicle-treated controls. In contrast, the level of HDAC4 was not notably affected (Fig. 4, d and e). Furthermore, levels of HDAC1, HDAC2, HDAC4, HDAC6, and SirT1 were also markedly lower in midbrain tissues of PD patients than those in midbrain tissues of their matched controls (Fig. 4f). Consequently, decreased HDACs and increased histone acetylation seem to be correlated in the midbrains of PD patients. Histone acetyltransferases Gcn5 and Tip60 were either not changed or not consistently changed between the disease and control groups (Fig. 4f). However, CBP was detected in 1 of 5 PD patient midbrains and 3 of 5 control midbrains (Fig. 4f). These results indicate that PD-associated up-regulation of histone acetylation is likely a result of reduced HDACs in midbrain tissues.
FIGURE 4.
Down-regulation of HDACs in MPP+-treated cells, MPTP-treated mice, and the midbrain of PD patients. a and b, immunoblotting detection of HDAC1, -2, -4, -6, and SirT1 in cells treated with 1 or 100 μm MPP+ to those in cells treated with solvent (a). Quantitative analysis of 3 independent experiments is shown (b). Results are normalized to β-actin. *, p < 0.05; **, p < 0.01; ***, p < 0.001. c, quantitative RT-PCR analysis of expression of HDAC1, -2, -4, -6, and SirT1 in cells treated with 1 or 100 μm MPP+ to those in cells treated with solvent. Results are normalized to β-actin. **, p < 0.01; ***, p < 0.001. d and e, immunoblotting detection of HDAC1, -2, -4, -6, and SirT1 in midbrain of aMPTP-treated mice to those in the midbrain of solvent-treated mice (d). Quantitative analysis of 3 independent experiments is shown (e). Results are normalized to β-actin. *, p < 0.05; **, p < 0.01; ***, p < 0.001. f, immunodetection of HATs (Gcn5, Tip60, and CBP), HDACs (HDAC1, HDAC2, HDAC4, HDAC6, and SirT1), and acetylated histone H3k27 (AcH3k27) in human PD midbrain (PD) and their matched controls (Control). Numbers on the top of each panel represent different individuals.
MPP+ Promotes Degradation of HDAC1 and HDAC2 via Induction of Autophagy
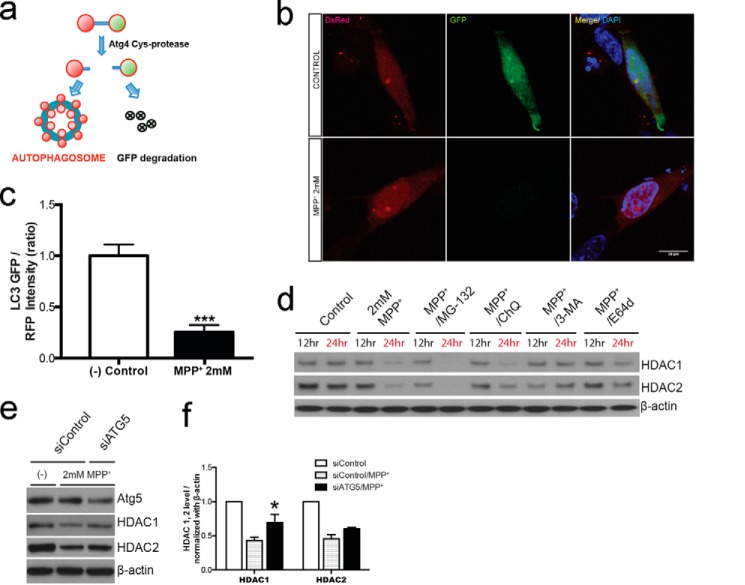
Previous studies suggested that MPP+ treatment induces autophagy (16, 17). We explored the mechanism of HDACs degradation by employing a modified fluorescent protein-tagged LC3 reporter system (18). This dual color system provides two readouts for autophagy activity, including the number of DsRed-LC3 puncta and measurable autophagy index. MPP+ treatment resulted in loss of GFP detection in SH-SY5Y cells (Fig. 5, a–c). The results support induction of autophagy by MPP+ treatment. Meanwhile, MPP+ treatment led to markedly reduced detection of HDAC1 and HDAC2 (Fig. 5, d and e). Three autophagy inhibitors, including chloroquine, 3-methyladenine, and E-64d, suppressed MPP+-induced degradation of HDAC1 and HDAC2. In contrast, the proteasomal inhibitor MG-132 had little effect on MPP+-induced degradation of HDAC1 and HDAC2 (Fig. 5d). Consistent with results of pharmacological inhibition, ATG5 siRNA showed a notable inhibitory effect on MPP+-induced degradation of HDAC1 and HDAC2 (Fig. 5, e and f). Together, MPP+ treatment induces degradation of HDAC1 and HDAC2 via autophagy.
FIGURE 5.
MPP+ promotes autophagy degradation of HDAC1 and HDAC2. a, schematic illustration of pQCXI Puro Ds-Red-LC3-GFP reporter plasmid responding to autophagy signal. Once induction of autophagy, activated Atg4 Cys-protease cleaves DsRed-LC3-GFP. The GFP region goes through degradation and Red-LC3 incorporates to form autophagosome. b, representative immunofluorescent images of overexpression of the Ds-Red-LC3-GFP reporter plasmid in SH-SY5Y with 2 mm MPP+. Red-LC3 (red), GFP (green), and cell nuclei with DAPI (blue). Scale bar = 10 μm. c, quantitative analysis of the signal intensity of the GFP/Red-LC3 confocal image (10 cells are analyzed for each group). ImageJ software was used to measure the intensity. ***, p < 0.001. d, immunodetection of HDAC1 and HDAC2 in 2 mm MPP+, proteasome inhibitor (MG-132) in 5 μm, and autophagy inhibitors (chloroquine (ChQ) in 10 μm, 3-methyladenine (3-MA) in 10 mm, and E-64d in 1 μm) with controls (Control) for 12 and 24 h. Numbers on the top of each panel show different time points. β-Actin was detected as a loading control. e, inhibition of autophagy (siRNA ATG5) recoveries the dysregulation of HDAC1 and HDAC2 by 2 mm MPP+. f, quantitative analysis of experiments in e is shown. The results are normalized by β-actin. *, p < 0.05, n = 4.
Inhibition of HATs or HDACs Regulates MPP+-induced Cytotoxicity
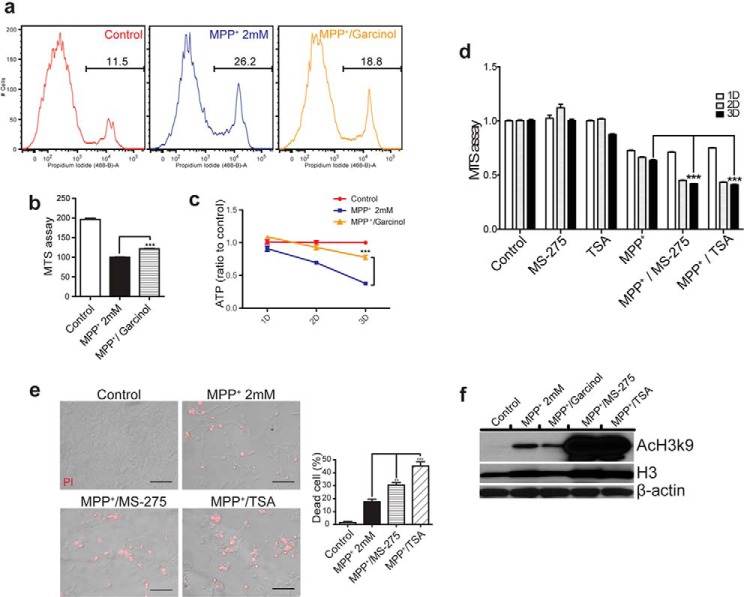
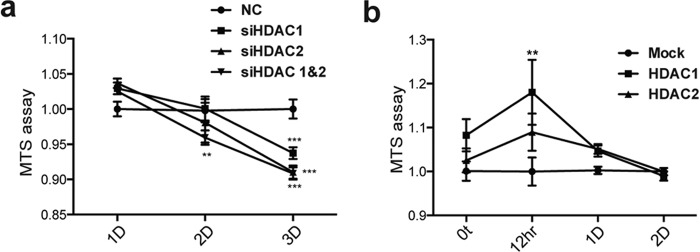
To determine whether histone acetylation regulates MPP+-induced cell death, we employed a commercial HAT inhibitor and two HDAC inhibitors. Pharmacological inhibition of HATs by garcinol significantly suppresses MPP+-induced cell death and ATP content reduction (Fig. 6, a–c). Conversely, HDAC inhibitors, MS-275 and trichostatin A (TSA), promote MPP+-induced cell death (Fig. 6, d and e). Immunoblotting analysis revealed that pan-histone and H3k9 acetylation induced by MPP+ were down-regulated by garcinol and greatly up-regulated by MS-275 and TSA, respectively (Fig. 6f). MS-275 and TSA are known to inhibit HDAC1 and HDAC2(19). Likewise, knockdown of HDAC1 and HDAC2 individually or in combination using specific siRNAs promoted MPP+-induced cytotoxicity (Fig. 7a). Consistently, overexpression of HDAC1 and HDAC2 in SH-SY5Y cells significantly suppressed MPP+-induced cytotoxicity (Fig. 7b). The results suggest that reduced HDACs promote histone acetylation likely contributing to neurodegeneration induced by PD neurotoxins.
FIGURE 6.
Pharmacological inhibition of HATs or HDACs modulates MPP+ toxicity. a–c, HAT inhibitor, garcinol, suppresses MPP+ cytotoxicity. Cells treatedwith solvent (Control), MPP+ (MPP+, 2 mm), or MPP+ and garcinol (MPP+/garcinol). Cell death was quantified with FACS (a), MTS assay (b), and ATP level (c). Note that inhibition of HATs with garcinol suppresses MPP+ cytotoxicity. ***, p < 0.001. d and e, HDAC inhibitors potentiate MPP+ cytotoxicity. Cells were treated with solvent (control), MPP+ (MPP+, 2 mm), MPP+ and 10 μm MS-275 (MPP+/MS-275), or MPP+ and 100 nm TSA (MPP+/TSA). Non-lethal concentrations of HAT and HDAC inhibitors were determined by MTS assay (data not shown). Quantitations of cell death with a MTS assay (d) and representative images of PI staining by ImageJ software (e) are shown. **, p < 0.01; ***, p < 0.001. f, MPP+-induced histone H3k9 acetylation (AcH3k9) is inhibited by garcinol treatment (MPP+/Garcinol), but greatly induced by treatments of MS-275 (MPP+/MS-275) or TSA (MPP+/TSA).
FIGURE 7.
Genetic manipulation of HDAC1 and HDAC2 modulates MPP+-induced cytotoxicity. a, cells transfected with scramble siRNA (NC), HDAC1 siRNA (siHDAC1), HDAC2 siRNA (siHDAC2), and a combination of the two (siHDAC1 and -2) potentiate MPP+ (2 mm) induced cytotoxicity. **, p < 0.01; ***, p < 0.001. b, cells overexpressing HDAC1 and HDAC2 show reduced MPP+ (2 mm) induced cytotoxicity compared to that of cells transfected with empty vector (Mock). **, p < 0.01.
Discussion
We demonstrate in this study that PD neurotoxins specifically induce expression and acetylation of histones, in both cultured human cells and DA neurons of mouse midbrains. Substantial increases in histone acetylation at multiple sites with notably lower levels of multiple HDACs are observed to correlate in midbrain DA neurons in 3 of 5 PD patients compared with matched controls. Pharmacological and genetic inhibition of HDAC1 and HDAC2 potentiate MPP+-induced cytotoxicity. In contrast, overexpression of HDAC1 and HDAC2 suppresses MPP+-induced cell death. Further analysis reveals that PD neurotoxin-induced HDACs degradation is via an autophagy mechanism. Thus, autophagy-mediated HDACs reduction results in aberrant up-regulation of histone acetylation in DA neurons leading to eventual neurodegeneration. The study identifies an epigenetic mechanism in PD pathogenesis.
Environmental neurotoxins induce parkinsonism in both human and animal models (20–22), but the molecular basis remains largely unknown. Our results suggest that PD-related environmental neurotoxins induce broad and specific changes of both protein levels and acetylation status of histones via a mechanism of reduced expression of HDACs. These epigenetic changes likely result in abnormal gene expression, contributing to PD pathogenesis. Remarkably similar changes are identified in midbrain tissues of sporadic PD patients, suggesting that the epigenetic abnormality detected in this study is potentially a common mechanism in PD pathogenesis. It is possible that aging and different PD environmental factors induce oxidative stress via mitochondrial dysfunction. Consistent with this notion, reactive oxygen is known to induce histone acetylation in different cell types, including neurons (23). Nevertheless, it is yet to understand the functional consequence of increased histone expression and reduced HDAC6 with MPTP treatment.
Three different assays in this study indicate selected autophagic degradation of HDACs after exposure to PD neurotoxins. Previous studies have shown that PD neurotoxin MPP+ potentiates autophagy that is further confirmed by the present study (16, 17). Autophagy is proposed to play either a protective or a pathogenic role under different conditions. This is also likely the case in PD pathogenesis. PINK1 and PARKIN, two recessive PD causative genes, function to degrade damaged mitochondria via mitophagy, therefore, neuroprotective (24). A recent study shows that MPP+-induced autophagy is pro-death of DA neurons in rat brain (16). It is possible that in the early stage of disease, selective autophagy is activated to promote the clearance of damaged mitochondria and un/misfolded proteins, resulting in a protective role. With the progression of disease, health mitochondria are significantly reduced and un/misfolded proteins get accumulated, dysregulated autophagy thus accelerates degeneration of “sick” neurons. Further investigation is needed to address the potential “two-phase hypothesis” of neurondegeneration.
Previous studies have shown diverse roles of histone acetylation in neurodegeneration (3, 13, 25, 26). In cellular models, both dieldrin and paraquat induce histone acetylation (3, 27). Inhibition of HDAC1 activity by p25/Cdk5 results in DNA damage-mediated neurotoxicity, whereas overexpression of HDAC1 protects against DNA damage and neurotoxicity in cultured neurons and an ischemia rat model (25). Other studies suggest deregulation of HDAC1 and −2 as a means to induce cell death for potential cancer therapy (28, 29). Consistent with these reports, results from this study systematically demonstrate that PD-related neurotoxins promote cell death likely via inducing expression and acetylation of histones using in vitro cultured cells and in vivo both mouse model and human brain tissues. However, several HDAC inhibitors are also shown to suppress neurotoxicity induced by MPP+ and α-synuclein (30). The basis of the discrepancies among these studies remains unclear. It is possible that HDAC inhibitors have multiple targets to regulate neuronal death and survival, indeed non-histone targets of HDACs are also potentially involved in neuroprotection. HDAC inhibition-induced acetylation of α-tubulin stabilizes microtubules and increases axonal transportation leading to BDNF release and neuroprotection (31, 32). Although HDAC inhibitors are being developed to treat neurodegenerative disease, results from this study suggest that such strategies need to be cautiously examined.
Author Contributions
G. P. mainly performed the molecular, genetic and animal studies, participated in the experimental design, and drafted the manuscript. J. T. performed some molecular and genetic experiments. G. G. supported the immunohistochemistry. Y. K. assisted the FACS analysis. G. S. discussed and advised the regulation of HDAC1 and -2. Z. Z. conceived of the study, and participated in its design and helped to write the manuscript. All authors read and approved the final manuscript.
Acknowledgments
We thank Dr. S. K. Han and Dr. J. S. Han for many discussions, and Tina Nhan and Christopher Wu for technical support.
This work was supported, in whole or in part, by National Institutes of Health Grant NS057289 (to Z. Z.), Natural Science Foundation of China Grants 313300257, 81429002, and 81161120498 (to Z. Z.), “973 Program” of Ministry of Science and Technology Grant 2011CB51000 (to Z. Z.), “111 Program” of Foreign Expert Bureau of China Grant B10036 (to Z. Z.), and the Mogam Science Scholarship Foundation (to G. P.). The authors declare that they have no competing interests.
- PD
- Parkinson disease
- HDAC
- histone deacetylase
- DA
- dopaminergic
- AD
- Alzheimer disease
- MPTP
- 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- MPP
- 1-methyl-4-phenylpyridinium
- MTS
- 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium
- TH
- tyrosine hydroxylase
- aMPTP
- acute MPTP
- cMPTP
- chronic MPTP
- TSA
- trichostatin A.
References
- 1. Lees A. J., Hardy J., and Revesz T. (2009) Parkinson's disease. Lancet 373, 2055–2066 [DOI] [PubMed] [Google Scholar]
- 2. Klein C., and Westenberger A. (2012) Genetics of Parkinson's disease. Cold Spring Harb. Perspect. Med. 2, a008888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Song C., Kanthasamy A., Anantharam V., Sun F., and Kanthasamy A. G. (2010) Environmental neurotoxic pesticide increases histone acetylation to promote apoptosis in dopaminergic neuronal cells: relevance to epigenetic mechanisms of neurodegeneration. Mol. Pharmacol 77, 621–632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Manikkam M., Guerrero-Bosagna C., Tracey R., Haque M. M., and Skinner M. K. (2012) Transgenerational actions of environmental compounds on reproductive disease and identification of epigenetic biomarkers of ancestral exposures. PLoS ONE 7, e31901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Skinner M. K., Manikkam M., and Guerrero-Bosagna C. (2010) Epigenetic transgenerational actions of environmental factors in disease etiology. Trends Endocrinol Metab. 21, 214–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Abel T., and Zukin R. S. (2008) Epigenetic targets of HDAC inhibition in neurodegenerative and psychiatric disorders. Curr. Opin. Pharmacol. 8, 57–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jaenisch R., and Bird A. (2003) Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nat. Genet. 33, 245–254 [DOI] [PubMed] [Google Scholar]
- 8. Goldberg A. D., Allis C. D., and Bernstein E. (2007) Epigenetics: a landscape takes shape. Cell 128, 635–638 [DOI] [PubMed] [Google Scholar]
- 9. Ayyanathan K., Lechner M. S., Bell P., Maul G. G., Schultz D. C., Yamada Y., Tanaka K., Torigoe K., and Rauscher F. J. 3rd. (2003) Regulated recruitment of HP1 to a euchromatic gene induces mitotically heritable, epigenetic gene silencing: a mammalian cell culture model of gene variegation. Genes Dev. 17, 1855–1869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gräff J., Kim D., Dobbin M. M., and Tsai L. H. (2011) Epigenetic regulation of gene expression in physiological and pathological brain processes. Physiol. Rev. 91, 603–649 [DOI] [PubMed] [Google Scholar]
- 11. Francis Y. I., Fà M., Ashraf H., Zhang H., Staniszewski A., Latchman D. S., and Arancio O. (2009) Dysregulation of histone acetylation in the APP/PS1 mouse model of Alzheimer's disease. J. Alzheimers Dis. 18, 131–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Monti B., Gatta V., Piretti F., Raffaelli S. S., Virgili M., and Contestabile A. (2010) Valproic acid is neuroprotective in the rotenone rat model of Parkinson's disease: involvement of α-synuclein. Neurotox. Res. 17, 130–141 [DOI] [PubMed] [Google Scholar]
- 13. Outeiro T. F., Kontopoulos E., Altmann S. M., Kufareva I., Strathearn K. E., Amore A. M., Volk C. B., Maxwell M. M., Rochet J. C., McLean P. J., Young A. B., Abagyan R., Feany M. B., Hyman B. T., and Kazantsev A. G. (2007) Sirtuin 2 inhibitors rescue α-synuclein-mediated toxicity in models of Parkinson's disease. Science 317, 516–519 [DOI] [PubMed] [Google Scholar]
- 14. Kim S. J., Park G. H., Kim D., Lee J., Min H., Wall E., Lee C. J., Simon M. I., Lee S. J., and Han S. K. (2011) Analysis of cellular and behavioral responses to imiquimod reveals a unique itch pathway in transient receptor potential vanilloid 1 (TRPV1)-expressing neurons. Proc. Natl. Acad. Sci. U.S.A. 108, 3371–3376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jackson-Lewis V., and Przedborski S. (2007) Protocol for the MPTP mouse model of Parkinson's disease. Nature Protocols 2, 141–151 [DOI] [PubMed] [Google Scholar]
- 16. Hung K. C., Huang H. J., Lin M. W., Lei Y. P., and Lin A. M. (2014) Roles of autophagy in MPP+-induced neurotoxicity in vivo: the involvement of mitochondria and α-synuclein aggregation. PLoS ONE 9, e91074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhu J. H., Horbinski C., Guo F., Watkins S., Uchiyama Y., and Chu C. T. (2007) Regulation of autophagy by extracellular signal-regulated protein kinases during 1-methyl-4-phenylpyridinium-induced cell death. Am. J. Pathol. 170, 75–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sheen J. H., Zoncu R., Kim D., and Sabatini D. M. (2011) Defective regulation of autophagy upon leucine deprivation reveals a targetable liability of human melanoma cells in vitro and in vivo. Cancer Cell 19, 613–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dokmanovic M., Clarke C., and Marks P. A. (2007) Histone deacetylase inhibitors: overview and perspectives. Mol. Cancer Res. 5, 981–989 [DOI] [PubMed] [Google Scholar]
- 20. Cicchetti F., Drouin-Ouellet J., and Gross R. E. (2009) Environmental toxins and Parkinson's disease: what have we learned from pesticide-induced animal models? Trends Pharmacol. Sci. 30, 475–483 [DOI] [PubMed] [Google Scholar]
- 21. Di Monte D. A. (2003) The environment and Parkinson's disease: is the nigrostriatal system preferentially targeted by neurotoxins? Lancet Neurol. 2, 531–538 [DOI] [PubMed] [Google Scholar]
- 22. Langston J. W., and Ballard P. A. Jr. (1983) Parkinson's disease in a chemist working with 1-methyl-4-phenyl-1,2,5,6-tetrahydropyridine. New Engl. J. Med. 309, 310. [DOI] [PubMed] [Google Scholar]
- 23. Gu X., Sun J., Li S., Wu X., and Li L. (2013) Oxidative stress induces DNA demethylation and histone acetylation in SH-SY5Y cells: potential epigenetic mechanisms in gene transcription in Abeta production. Neurobiol. Aging 34, 1069–1079 [DOI] [PubMed] [Google Scholar]
- 24. Vincow E. S., Merrihew G., Thomas R. E., Shulman N. J., Beyer R. P., MacCoss M. J., and Pallanck L. J. (2013) The PINK1-Parkin pathway promotes both mitophagy and selective respiratory chain turnover in vivo. Proc. Natl. Acad. Sci. U.S.A. 110, 6400–6405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kim D., Frank C. L., Dobbin M. M., Tsunemoto R. K., Tu W., Peng P. L., Guan J. S., Lee B. H., Moy L. Y., Giusti P., Broodie N., Mazitschek R., Delalle I., Haggarty S. J., Neve R. L., Lu Y., and Tsai L. H. (2008) Deregulation of HDAC1 by p25/Cdk5 in neurotoxicity. Neuron 60, 803–817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kontopoulos E., Parvin J. D., and Feany M. B. (2006) α-Synuclein acts in the nucleus to inhibit histone acetylation and promote neurotoxicity. Hum. Mol. Genet. 15, 3012–3023 [DOI] [PubMed] [Google Scholar]
- 27. Song C., Kanthasamy A., Jin H., Anantharam V., and Kanthasamy A. G. (2011) Paraquat induces epigenetic changes by promoting histone acetylation in cell culture models of dopaminergic degeneration. Neurotoxicology 32, 586–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Haberland M., Johnson A., Mokalled M. H., Montgomery R. L., and Olson E. N. (2009) Genetic dissection of histone deacetylase requirement in tumor cells. Proc. Natl. Acad. Sci. U.S.A. 106, 7751–7755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ropero S., and Esteller M. (2007) The role of histone deacetylases (HDACs) in human cancer. Mol Oncol 1, 19–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Harrison I. F., and Dexter D. T. (2013) Epigenetic targeting of histone deacetylase: therapeutic potential in Parkinson's disease? Pharmacol. Ther. 140, 34–52 [DOI] [PubMed] [Google Scholar]
- 31. Glozak M. A., Sengupta N., Zhang X., and Seto E. (2005) Acetylation and deacetylation of non-histone proteins. Gene 363, 15–23 [DOI] [PubMed] [Google Scholar]
- 32. Zhang Y., Li N., Caron C., Matthias G., Hess D., Khochbin S., and Matthias P. (2003) HDAC-6 interacts with and deacetylates tubulin and microtubules in vivo. EMBO J. 22, 1168–1179 [DOI] [PMC free article] [PubMed] [Google Scholar]