Abstract
Adequate membrane fluidity is required for a variety of key cellular processes and in particular for proper function of membrane proteins. In most eukaryotic cells, membrane fluidity is known to be regulated by fatty acid desaturation and cholesterol, although some cells, such as insect cells, are almost devoid of sterol synthesis. We show here that insect and mammalian cells present similar microviscosity at their respective physiological temperature. To investigate how both sterols and phospholipids control fluidity homeostasis, we quantified the lipidic composition of insect SF9 and mammalian HEK 293T cells under normal or sterol-modified condition. As expected, insect cells show minimal sterols compared with mammalian cells. A major difference is also observed in phospholipid content as the ratio of phosphatidylethanolamine (PE) to phosphatidylcholine (PC) is inverted (4 times higher in SF9 cells). In vitro studies in liposomes confirm that both cholesterol and PE can increase rigidity of the bilayer, suggesting that both can be used by cells to maintain membrane fluidity. We then show that exogenously increasing the cholesterol amount in SF9 membranes leads to a significant decrease in PE:PC ratio whereas decreasing cholesterol in HEK 293T cells using statin treatment leads to an increase in the PE:PC ratio. In all cases, the membrane fluidity is maintained, indicating that both cell types combine regulation by sterols and phospholipids to control proper membrane fluidity.
Keywords: cholesterol, homeostasis, membrane bilayer, membrane lipid, phosphatidylethanolamine
Introduction
The maintenance of proper cell membrane fluidity is of critical importance for adequate diffusion of membrane components from lipids to proteins and influences dynamics and function of integral membrane proteins. It is also responsible for their remarkable flexibility and thus required for a multitude of morphological transformations necessary for cellular functions including division, differentiation, and general adaptation to the environment (1, 2).
In bacteria, membrane fluidity is mostly regulated by adapting the length and saturation of acyl chains (3). For instance, upon lowering of the growth temperature, bacteria will typically increase the content of unsaturated acyl chains, shorten chain length, and modify branching or cyclization to lower the temperature of phase transition from gel to liquid-crystalline state, a process called homeoviscous adaptation (4). In mammalian cells, membrane fluidity is known to be also regulated by cholesterol, which has the ability to render membrane fluid by interfering with acyl chain packing, inhibiting the transition to the solid gel state but at the same time rigidifying fluid membranes by reducing the flexibility of neighboring unsaturated acyl chains (5, 6).
Membranes of cell organelles need to adapt their properties and thus their composition according to the function they need to fulfill (2, 5). It is indeed known that total percentage of cholesterol varies extensively among the organelles implicated in cell trafficking; it is low in endoplasmic reticulum (5% of lipids), and its amount increases in the trans-Golgi and plasma membrane, allowing a tighter packing compatible with the plasma membrane function as a barrier (5, 7). Thus, animal cells are submitted to a fine-tuned regulation of cholesterol and fatty acid synthesis through the sterol regulator element-binding protein (SREBP) pathway (8). In this context, an imbalance in membrane lipid composition could affect membrane properties and lead to pathological consequences as described for erythrocyte membranes in hypercholesterolemia patients and in type 2 diabetes (9, 10). Indeed, an increased cholesterol:phospholipid (PL)4 ratio leads to a decreased membrane fluidity and is associated with a reduced binding of insulin (9).
Conversely, some higher eukaryotes, such as insects, do not synthesize sterols. Although mass spectrometry studies have shown that insect cell membranes contain the main classes of PL found in mammalian cells, the presence of sterols is strictly diet-dependent (11).
How cells sense and regulate the complex mixture and distribution of lipids to preserve membranes characteristics is, therefore, an important and challenging question. In this work, we compared the membrane lipid distribution regulation and its consequence on membrane fluidity of two extensively used eukaryotic cell models, insect SF9 and human HEK 293T cells. Using a liquid chromatography system coupled to a high resolution mass spectrometer, we determined the lipid composition of both membranes. We show that despite the differences in their cholesterol and phosphatidylethanolamine (PE) contents the fluidity of both membranes was similar. We show that fluidity in SF9 and HEK 293T membranes is similarly regulated. Indeed, a change in cholesterol amounts did not affect membrane fluidity of both cells but resulted in an inverse modulation of PE content. Our findings point to a conserved compensation balance between cholesterol and PE metabolism that might be needed for membrane fluidity control.
Experimental Procedures
Cell Culture
SF9 cells were grown in ESF 921 protein-free medium from Expression Systems at 27 °C with 130-rpm agitation. For cholesterol amount enhancement, cells were cultured for five passages (7–10 days) in ESF 921 supplemented with 10% fetal calf serum (FBS) (Lonza). HEK 293T cells were grown in DMEM (Lonza) supplemented with 10% FBS, 4.5 mg/ml glucose, 1 mm sodium pyruvate, 2 mm l-glutamine at 37 °C with 5% CO2. For statin treatment, simvastatin stock solution was prepared and activated by the following method. First 54 mg of simvastatin was dissolved in 1.04 ml of 95% ethanol, and then 813 μl of 1 n NaOH was added. The resulting solution was neutralized with 1 n HCl to a pH of 7.2 and brought up to 13 ml with distilled water. The stock of 10 mm was then aliquoted and stored at −20 °C until used. Prior to simvastatin treatment, cells to be treated and controls were cultured for 48 h in serum-free DMEM supplemented with insulin, transferrin, and selenium (all from Gibco), 0.1 mg/ml BSA, 4.5 mg/ml glucose, 1 mm sodium pyruvate, 2 mm l-glutamine at 37 °C with 5% CO2. Next 75 μm simvastatin was added only for statin-treated cells; control cells remained statin-free. Cells were then harvested after 48–50 h and washed with phosphate-buffered saline, and pellets were stored at −80 °C for membrane preparation.
Lipid Standards
The following standards were used: for phosphatidylcholine (PC), PE, phosphatidylglycerol (PG), phosphatidylserine (PS), and sphingomyelin (SM), C12:0 C12:0 and C17:0 C17:0; for phosphatidylinositol (PI), C18:0 C20:4 and [2,3,4-13C3]cholesterol. Standard curves were made by MS analysis using dilution series of standards (from 0.02 to 5 × 10−5 mg/ml for phospholipids and from 0.2 to 0.002 mg/ml for cholesterol). All phospholipid standards were purchased from Avanti® Polar lipids, Inc. [2,3,4-13C3]Cholesterol was purchased from Sigma.
Membrane Preparation and Membrane Lipid Extraction
Cell pellets (∼20 × 106 cells for HEK 293T and 60 × 106 cells for SF9) were transferred into hypotonic lysis buffer (10 mm Tris, pH 7.4, 1 mm EDTA) and stirred for 15 min to break cells. Lysed cells were centrifuged at 25,000 × g for 10 min, and the pellets containing the membranes were resuspended in phosphate-buffered saline. This procedure was carried out at 4 °C. Standards for each phospholipid class and [13C3]cholesterol were added to the membrane, and lipids were then extracted from membranes using a methanol:chloroform solution (50:50, v/v) as described by Bligh and Dyer (31) with the following modifications. 10 μl of 6 n HCl was added to the mixture, and extraction was repeated three times on the same sample to have maximum recovery of all types of lipids. Corresponding organic phases were pooled together, the solvent was evaporated under a stream of nitrogen, and lipids were then dissolved in dichloromethane:isopropanol (1:4, v/v) prior to MS analysis.
Mass Spectrometry Analysis
Two microliters of each sample was injected in a rapid resolution liquid chromatography (LC) system (1200 series from Agilent Technologies) fitted with a Zorbax XDB Eclipse Plus column (C18, 4.6 × 50 mm, 1.8-μm particle size). The run was 30 min long with the following characteristics: flow rate, 0.3 ml/min; column temperature, 40 °C. Mobile phase A was 0.1% formic acid (positive MS analysis) or 5 mm ammonium acetate, pH 5 (negative MS analysis), and mobile phase B was an isopropanol gradient, which started with 90% solvent A, directly increased to 20% in 10 min, stayed at 20% solvent A for 15 min, and was re-equilibrated to starting conditions in 5 min. A 6520 series electrospray ion source (ESI)-quadrupole time-of-flight (QTOF) high resolution mass spectrometer from Agilent Technologies was used. For the MS/MS analyses, auto-MS/MS mode was used, and parameters were as follows: positive or negative mode; high resolution acquisition mode (4 GHz); gas temperature, 330 °C; drying gas, 7 liters/min; nebulizer pressure, 50 p.s.i.g.; capillary voltage, −4500 V; fragmentor, 210 V; fixed collision energy, 25 V; MS scan range and rate, 100–1700 at four spectra per second; MS/MS scan range and rate, 50–1700 at three spectra per second; auto-MS/MS, three maximum precursors; precursor absolute threshold, 200 counts; active exclusion on two repeats and released after 0.5 min; fixed exclusion range, 100–499 and 1500–1700; preferred charge state, 1 and 2. Data were acquired by Mass Hunter Acquisition® for TOF and QTOF version B.04 SP3 (Agilent Technologies). For quantification, single MS analyses were performed with the following parameters: positive or negative mode; extended dynamic range mode (2 GHz); gas temperature, 330 °C; drying gas, 7 liters/min; nebulizer pressure, 50 p.s.i.g.; capillary voltage, 4500 V; fragmentor, 210 V; MS scan range and rate, 100–1700 at twp spectra per second. Data were acquired by Mass Hunter Acquisition for TOF and QTOF version B.04 SP3. Phosphatidylcholines and cholesterol were analyzed in positive mode, whereas the other PLs were analyzed in negative mode.
Data Analysis and Quantification
The data were first analyzed by Mass Hunter Qualitative Analysis version B.06 SP1 based on a fragment-based searching mode. Briefly, the MS/MS spectra obtained during the run time were selected according to the specific fragmentation of each PL. In negative mode, the search mode was focused on the fatty acid fragments (C16:0; C16:1; C14:0; C14:1, C18:0 … ). In positive mode, the search mode was focused on the fragments of the polar head of the PL. Maximal accepted error on detected m/z was 5 ppm. Regarding quantification, samples were run in simple MS mode. Ion chromatograms were extracted based on the exact masses of the lipids observed during auto-MS/MS analyses. Phospholipids were quantified by the standard curves (with mass concentrations) run during the set of experiments.
Liposome Formation and Size Determination by Dynamic Light Scattering
Lipids used for liposome formation (POPE, POPC, and cholesterol) were all purchased from Avanti Polar Lipids, Inc. Lipids mixtures were prepared with the following procedure. POPC was mixed with 0, 10, 20, or 30% (w/w) cholesterol; with 40, 50, or 60% (w/w) POPE; or with 10% cholesterol in addition to 30 or 60% (w/w) POPE to a final lipid concentration of 3 mg/ml in 100 mm HEPES, pH 7.4. Liposomes were then sonicated in an ice/water bath for 5 min and immediately used for size determination or 1,6-diphenyl-1,3,5-hexatriene (DPH) fluorescence measurements. Mean diameter of liposomes was assessed by dynamic light scattering using a Zetasizer (Malvern Instruments Ltd.).
Membrane Fluidity Monitoring by DPH Fluorescence Polarization Measurements
Lipids extracted from membranes were dried in preweighed vials, then weighed, and resuspended in the correct volume of 100 mm HEPES, pH 7.4 to have 0.3 mg/ml. For liposomes, we took the volume of lipids from chloroform stock corresponding to 0.3 mg in total according to the concentration provided by the manufacturer and then dried them before resuspension in 1 ml of 100 mm HEPES, pH 7.4. Membranes and liposomes were sonicated prior to DPH fluorescence polarization measurements. Next 1.5 ml of bilayer at 0.3 mg/ml final concentration in 100 mm HEPES, pH 7.4 was mixed with 0.2 μg/ml final concentration DPH (from a stock of 0.1 mg/ml in N,N-dimethylformamide). This mixture was then vortexed and heated for 15 min at 45 °C to allow homogeneous integration of DPH in the bilayer. Fluorescence polarization of DPH was measured on a Photon Technology International fluorometer with the following protocol: time-based polarization, digital mode, excitation wavelength of 358 nm, emission wavelength of 429 nm, and 4-nm bandpass filter. Polarization was assessed using a temperature gradient from 16 to 50 °C with a 2 °C increase with stabilization of temperature for 1 min and three measurements taken per temperature (one measurement every 6 s). Fluorescence polarization of non-DPH-labeled control bilayers was used for correction.
Results
Mammalian and Insect Cell Membranes Display Similar Fluidity at Physiologically Relevant Temperatures
We initially analyzed the viscosity of membranes using polarization measurements of the DPH dye. The probe is incorporated into membranes in parallel to the acyl chains of membrane lipids, and its fluorescence polarization depends on the bilayer viscosity. We measured DPH polarization of membranes extracted from SF9 and HEK 293T cells at a range of temperatures between 15 and 50 °C (Fig. 1). Remarkably, we observed almost identical values (0.219 ± 0.002 for SF9 cells and 0.211 ± 0.008 for HEK 293T) when comparing the fluidity of both membranes at relevant temperatures (i.e. temperature used for cell culture), namely 27 °C for SF9 and 37 °C for HEK 293T (Fig. 1, gray and black arrows, respectively). Interestingly, the temperature dependence of the fluidity is much less pronounced in insect cell membranes compared with mammalian cell membranes (see below).
FIGURE 1.
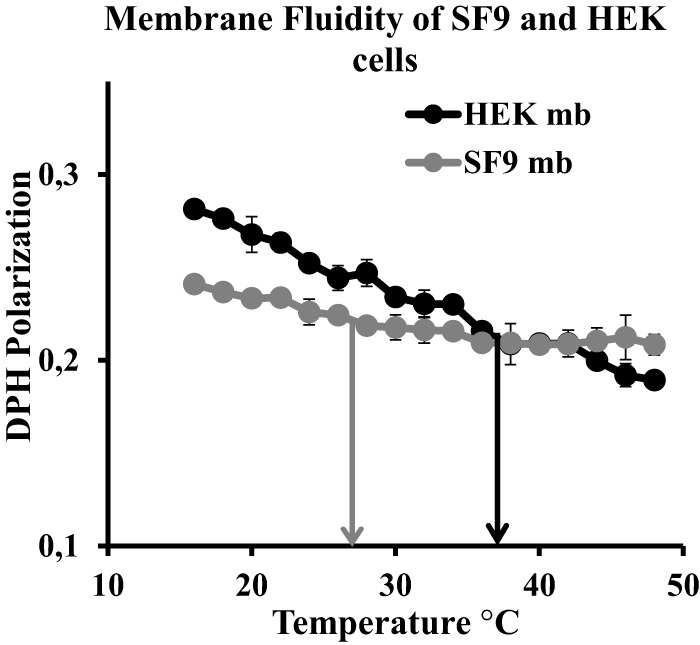
SF9 and HEK 293T membrane fluidity. DPH fluorescence polarization in a temperature range from 15 to 50 °C on membranes (mb) prepared from SF9 (gray) and HEK 293T (black) cells is shown. Error bars indicates S.D. (n = 3). Arrows indicate growth temperature for SF9 (gray; 27 °C) and HEK 293T (black; 37 °C).
Lipidic Composition of Insect and Mammalian Membranes
Quantitative analysis of the lipid composition of membranes prepared from insect SF9 and mammalian HEK 293T cells was performed by MS. For quantification purposes, internal standards for each of the PL species were added prior to the lipid extraction. All main eukaryotic lipids were considered: PC (and plasmalogen PCO-), PE (and plasmalogen PEO-), PG, PI, and PS as well as SM and cholesterol (see “Experimental Procedures”). The method allows evaluation and correction of the experimental loss for each lipid class in a specific manner. Extracted lipids were then subjected to rapid resolution LC followed by high resolution mass spectrometry (ESI-QTOF 6520 series). We first analyzed the samples by auto-MS/MS to identify the components (headgroups and acyl chains) and then by single MS to quantify each of the lipids identified by auto-MS/MS using the standard curves and internal standards.
Both cell membranes contain comparable amounts of all classes of phospholipids (Fig. 2A). Total membrane lipid contents from both SF9 and HEK 293T cells were composed of 18.4–23.4% PS, 7–13% PI, 3% PG, and around 11% SM (Fig. 2A). As expected, a larger amount of cholesterol was found in mammalian membranes, expressed as the cholesterol:PL ratio (9 times higher in HEK 293T membranes when compared with SF9) (Fig. 2B). The small amount of cholesterol identified in insect cells most likely comes from the cell culture medium, which indeed was found to contain low amounts of cholesterol (about 0.2% of total medium mass; Table 1). However, a significant difference was observed when comparing PC and PE amounts. PE represented 38% and PC represented 16% of total membrane lipids in SF9 cells (no plasmalogens were found), whereas HEK 293T membranes contained 16% PE, 14% PEO-, 36% PC, and 5%PCO-. Thus, the PE:PC ((PE + PEO-):(PC + PCO-)) ratio is 4 times larger in HEK 293T than in SF9 cells (Fig. 2C).
FIGURE 2.
Phospholipid and cholesterol distribution in SF9 and HEK 293T membranes. A–E, lipids extracted from SF9 (gray) and HEK 293T (black) membranes were analyzed on a rapid resolution LC system coupled to an ESI-QTOF mass spectrometer. Species were identified by Mass Hunter Qualitative Analysis software of auto-MS/MS data with an accepted maximal error of 5 ppm for detected m/z and quantification made by automatic integration of peaks from extracted ion chromatograms. Bars represent values normalized to 100% (w/w). A, phospholipid distribution; B, cholesterol to phospholipid ratio; C, PE (including PEO-) to PC (including PCO-) ratio; D, fatty acid chain length distribution; E, unsaturation(s) per acyl chain in main fatty acid chains. Error bars indicate S.D. (n = 3). p values were estimated by Student's t test (n = 3): ***, p < 0.001; **, p < 0.005.
TABLE 1.
Quantification of sterols in SF9 medium
Sterols present in SF9 cell culture medium (ESF 921) were normalized to total medium mass and are represented by mean ± S.D. (n = 3). N.F., not found.
| ESF 921 | |
|---|---|
| Cholesterol (%) | 0.18 ± 0.01 |
| Stigmasterol (%) | N.F. |
| β-Sitosterol (%) | N.F. |
| Ergosterol (%) | N.F. |
| Campesterol (%) | 0.01 ± 0.0 |
Analysis of fatty acid chains shows for both cell types a large dominance of acyl chains composed of 16 and 18 carbons (Fig. 2D and Table 2) with an equal distribution of 44% between C16 and C18 in SF9 membranes and a majority of 58% C18 acyl chains versus 30% C16 in HEK 293T. Interestingly, C16 acyl chains contained more unsaturation in SF9 than in HEK 293T cells (77 versus 43%) (Fig. 2E).
TABLE 2.
Fatty acid chain length distribution in single PL species of SF9 and HEK 293T membranes
Fatty acid chain length of lipids extracted from SF9 and HEK 293T membranes in single PL species PC, PE, PG, PS, PI, and SM was normalized to 100% (w/w) and represented by mean ± S.D. (n = 3). N.F., not found.
| SF9 | HEK | |
|---|---|---|
| PC | ||
| C14 | 5.3 ± 1.7 | 6.2 ± 1.6 |
| C16 | 44.1 ± 7.8 | 48.6 ± 4.3 |
| C18 | 48.9 ± 7.1 | 43.1 ± 0.1 |
| C20 | 0.6 ± 0.3 | 1.5 ± 2.1 |
| C22 | N.F. | 0.1 ± 0.1 |
| Others | 1.0 ± 1.3 | 0.5 ± 0.6 |
| PE | ||
| C14 | N.F. | N.F. |
| C16 | 41.0 ± 0.2 | 32.2 ± 2.8 |
| C18 | 45.9 ± 0.1 | 59.9 ± 4.7 |
| C20 | 11.0 ± 0.4 | 7.5 ± 7 |
| C22 | 2.0 ± 0.1 | 0.4 ± 0.3 |
| Others | N.F. | N.F. |
| PG | ||
| C14 | N.F. | N.F. |
| C16 | 80.2 ± 3.3 | 12.2 ± 4.4 |
| C18 | 19.8 ± 3.3 | 87.8 ± 4.4 |
| C20 | N.F. | N.F. |
| C22 | N.F. | N.F. |
| Others | N.F. | N.F. |
| PS | ||
| C14 | N.F. | N.F. |
| C16 | 29.4 ± 3.7 | 24.4 ± 4.8 |
| C18 | 33.4 ± 0.9 | 32.0 ± 3 |
| C20 | 10.7 ± 2.3 | 18.6 ± 2.7 |
| C22 | 20.0 ± 1.4 | 23.8 ± 0.8 |
| Others | 6.5 ± 0.8 | 1.2 ± 1.9 |
| PI | ||
| C14 | N.F. | N.F. |
| C16 | 44.8 ± 1.3 | 14.5 ± 4.3 |
| C18 | 53.5 ± 0.7 | 76.7 ± 3.4 |
| C20 | 1.6 ± 0.7 | 8.5 ± 0.9 |
| C22 | 0.1 ± 0.0 | 0.3 ± 0 |
| Others | N.F. | N.F. |
| SM | ||
| C14 | 2.3 ± 3.3 | 2.1 ± 2.4 |
| C16 | 27.5 ± 0.4 | 44.7 ± 3.4 |
| C18 | 68.0 ± 3.5 | 50.2 ± 2.7 |
| C20 | N.F. | N.F. |
| C22 | N.F. | N.F. |
| Others | 2.2 ± 0.0 | 3.0 ± 3.3 |
Our data largely agree with published studies analyzing the lipid composition of insect cell membranes (11), in particular SF9 cells (12), especially regarding limited amounts of cholesterol as well as for their high PE content. In contrast, we found that SF9 similarly to HEK 293T cells contain all major PL species, notably PG, PS, and SM, which was described for Drosophila but not found in previously published studies on SF9 cells. Epithelial mammalian cultured cells (Madin-Darby canine kidney cells) have already been studied for their lipid composition by MS. The obtained results are in agreement with our data concerning overall PL distribution (13). Nevertheless, the saturation state of acyl chains differed between both studies. Reasonable amounts of polyunsaturated fatty acids were found in Madin-Darby canine kidney cells, whereas our data show only traces of polyunsaturated fatty acids in HEK 293T cells. This difference in polyunsaturated fatty acids could be due to methodological differences (sample preparation and quantification method) or to intrinsic differences in the cell lines used.
Increased Unsaturation of PE Reduces Temperature Dependence of Membrane Fluidity
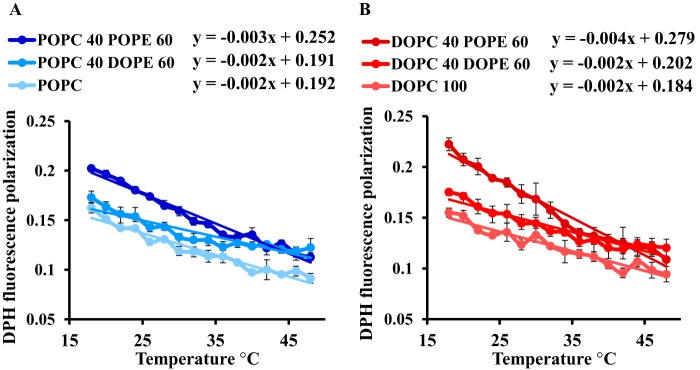
As shown in Fig. 1, the fluidity of SF9 cells shows little or no dependence on temperature in contrast to mammalian cells. Insects are generally ectotherm and poikilotherm organisms that are potentially exposed to rapid temperature changes in their environment. From an evolutionary standpoint, it would thus be sensible that membrane properties of insect cells, fluidity in particular, do not fluctuate much with temperature changes. In contrast, studies on a wide range of organism have shown that, upon temperature changes, metabolic control of membrane homeostasis (e.g. fluidity) typically involves modulation of the unsaturation state (4, 14–17). Therefore, we investigated whether differences in fatty acid unsaturation between lipids from mammalian and insect cells observed here (Fig. 2E) could be correlated to the difference in temperature sensitivity. As shown in Fig. 3, the temperature dependence is lower in the presence of DOPE compared with POPE as illustrated by the statistically significant differences in slopes of the DPH fluorescence polarization as a function of temperature. Indeed, in a linear approximation, we see that for the PC:PE ratio in the mixtures (40:60) the slope for DOPC:DOPE is half of that of DOPC:POPE (p < 0.001) and the slope of the POPC:DOPE is respectively 1.5 smaller than that of POPC:POPE (p < 0.005). This demonstrates reduced temperature dependence in the presence of diunsaturated PE when compared with monounsaturated PE. The modulation of temperature dependence by unsaturations was specific to PE because no difference in the slope of linear approximation curves was noticed between DOPC and POPC.
FIGURE 3.
Effect of unsaturation on temperature dependence of membrane fluidity. Membrane fluidity curves were measured by DPH fluorescence polarization in a temperature range from 16 to 48 °C on synthetic liposomes with POPC and POPC with 60% (w/w) DOPE or POPE (A) and synthetic liposomes with DOPC and DOPC with 60% (w/w) DOPE or POPE (B). Error bars indicate S.D. (n = 3).
Both PE and Cholesterol Affect Microviscosity in Vitro
As we have observed that the membrane microviscosity is almost identical in both SF9 and HEK 293T cells at growth temperatures, our data argue that cholesterol and acyl chain unsaturation are not the sole regulators of membrane fluidity of higher eukaryotes and that the PE:PC ratio may be involved. We thus performed microviscosity measurements on liposomes of controlled composition, varying the relative amounts of PE and PC of identical acyl chains and the amounts of cholesterol (Fig. 4). As expected, DPH measurements on liposomes of POPC containing 0, 10, 20, or 30% cholesterol showed a clear increase in DPH polarization curves, reflecting an increase in the bilayer rigidity (Fig. 4A). Similar results were obtained when we replaced cholesterol by PE: i.e. addition of increasing amounts of POPE (40, 50, and 60%) to POPC liposomes leads to a steady increase in DPH fluorescence polarization, reflecting the enhancement of the bilayer viscosity (Fig. 4B), in agreement with previous studies (18, 19). We also compared the effect of the inversion in PE:PC ratio as observed between SF9 and HEK 293T in the presence of cholesterol and noticed a clear decrease in the fluidity when the PE:PC ratio is higher (Fig. 4C). By dynamic light scattering, we determined that the size of all the liposomes was in the same range (180–300 nm), excluding a curvature-related effect in the observed changes (Table 3). In summary, DPH polarization measurements indicate that the presence of either PE or cholesterol enhances membrane viscosity, indicating that a balance and regulation between them could be involved in maintaining cell membrane properties.
FIGURE 4.
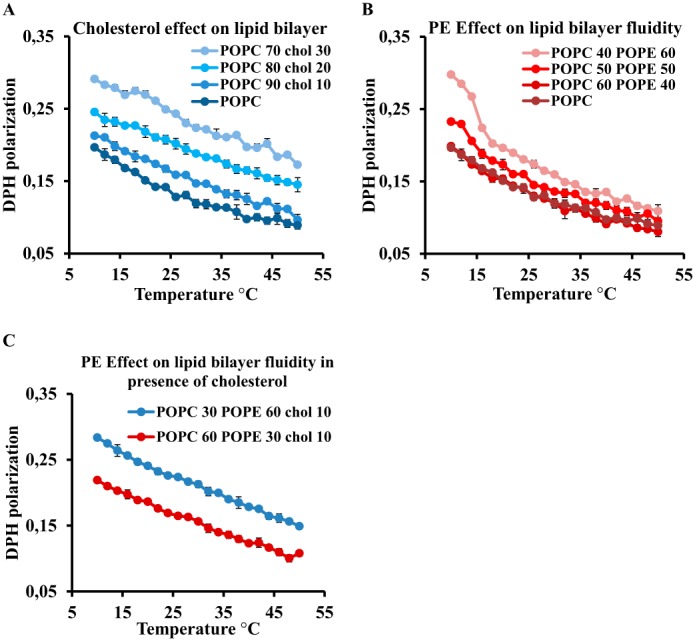
Effect of cholesterol and PE on lipid bilayer viscosity. Membrane fluidity curves were measured by DPH fluorescence polarization in a temperature range from 10 to 50 °C on synthetic liposomes with 0, 10, 20, and 30% (w/w) cholesterol (A) and synthetic liposomes with POPC and POPC with 40, 50, and 60% (w/w) POPE (B) and POPC with 30% (w/w) POPE and 10% (w/w) cholesterol (blue) and POPC with 60% (w/w) POPE and 10% (w/w) cholesterol (red) (C). Error bars indicate S.D. (n = 3).
TABLE 3.
Liposome diameter sizes of DPH-tested liposomes
Average bilayer diameter in synthetic liposomes was measured by dynamic light scattering and is represented by mean ± S.D. (n = 3). Chol, cholesterol.
| Liposomes | Average diameter |
|---|---|
| nm | |
| POPC | 185.9 ± 5.2 |
| POPC, 10% Chol | 210.8 ± 7.4 |
| POPC, 20% Chol | 270.6 ± 4.3 |
| POPC, 30% Chol | 296.4 ± 11.5 |
| 60% POPC, 40% POPE | 193.3 ± 6.2 |
| 40% POPC, 60% POPE | 205.4 ± 9.4 |
| 60% POPC, 40% POPE, 10% Chol | 227.3 ± 6.6 |
| 40% POPC, 60% POPE, 10% Chol | 219 ± 5.5 |
Insect Cells Modulate PE Amounts to Balance Exogenous Sterols
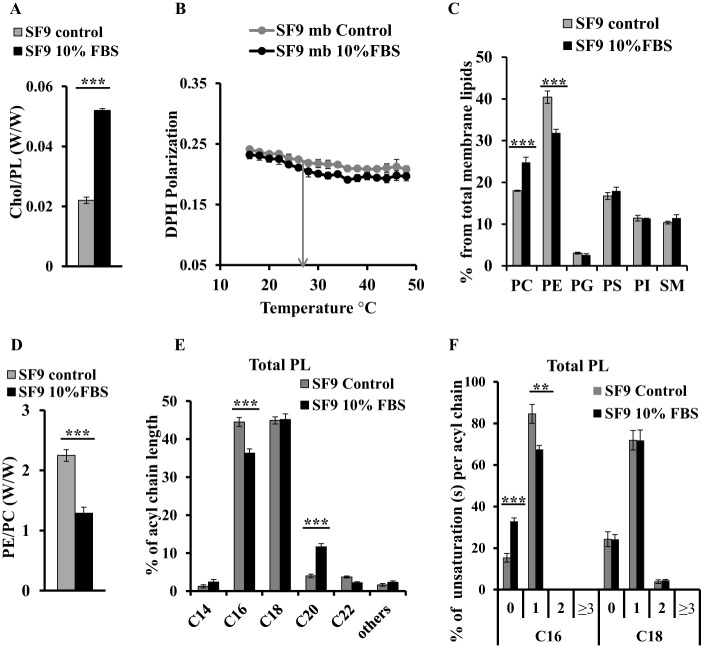
To establish the biological relevance of the above observations, we analyzed how insect cells adapt their membrane upon increase of the sterol content through diet supplementation. We cultured SF9 cells with or without 10% FBS containing cholesterol for 7 days, leading to a >2-fold increase in membrane cholesterol (Fig. 5A). Surprisingly, despite the increase in cholesterol levels, membrane fluidity of FBS-cultured cells is almost unchanged compared with the control with DPH polarization values at 27 °C of 0.210 ± 0.006 and 0.219 ± 0.002, respectively (Fig. 5B).
FIGURE 5.
Membrane fluidity maintained by cholesterol and PE in SF9 membranes upon FBS addition. Lipid distribution and membrane fluidity of membranes (mb) of SF9 control (gray) and SF9 cultured with 10% FBS (black) are shown. A, cholesterol to phospholipid ratio; B, DPH fluorescence polarization; C, phospholipid distribution; D, PE to PC ratio; E, fatty acid chain length distribution; F, unsaturation(s) per acyl chain in main fatty acid chains. Bars represent values normalized to 100% (w/w). Error bars indicate S.D. (n = 3). p values were estimated by Student's t test (n = 3): ***, p < 0.001; **, p < 0.005.
We then measured the PL distribution of both samples by MS and observed that the PE:PC ratio is significantly decreased by 1.8× (Fig. 5D), reflecting a decrease in PE content of the membranes from 40 to 32% together with an enrichment in PC from 18 to 25% in FBS-cultured cells compared with controls (Fig. 5C). The amounts of the other lipid components of membranes remained unchanged. Significant differences were observed in acyl chain length distribution of total lipids, i.e. a decrease in C16 fatty acids together with an increase of C20 chains (Fig. 5E). Analysis of the acyl chain saturation state (total lipids and in each of the PL classes) shows a clear shift in unsaturation distributions (Figs. 5F and 6). Indeed, although controls contained a majority of C16 with one unsaturation (85%) and only a minority of saturated C16 (15%), SF9 cells supplemented with FBS showed increased saturated C16 acyl chains (33%) along with a decrease in monounsaturated C16 (67%). No difference in saturation state of acyl chains of C18 was observed. These shifts toward increased saturation were also distributed among the different individual phospholipid species, i.e. PE, PG, PS, and PI (Fig. 6). These results suggest that the increase in cholesterol in SF9 membranes could be compensated by a decrease in PE content together with a decrease in unsaturated C16 fatty acids to maintain similar fluidity of cell membranes.
FIGURE 6.
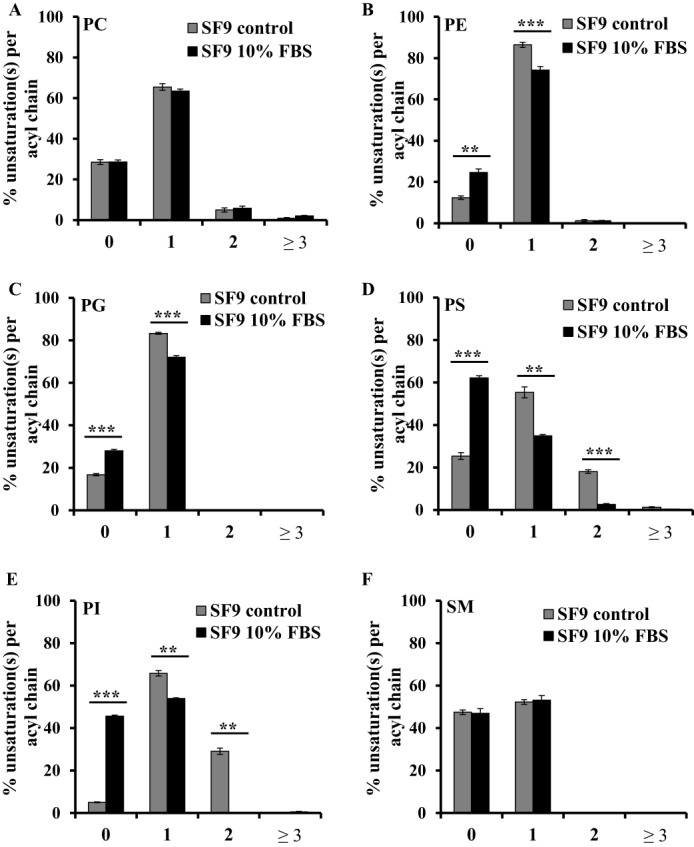
Unsaturation distribution per acyl chain of different PL species in SF9 control and cultured in 10% FBS. Unsaturation(s) per acyl chain distribution in different PL species from SF9 control (gray) and SF9 cultured with 10% FBS (black) is shown. A, PC; B, PE; C, PG; D, PS; E, PI; F, SM. Bars represent values normalized to 100% (w/w). Error bars indicate S.D. (n = 3). p values were estimated by Student's t test (n = 3): ***, p < 0.001; **, p < 0.005.
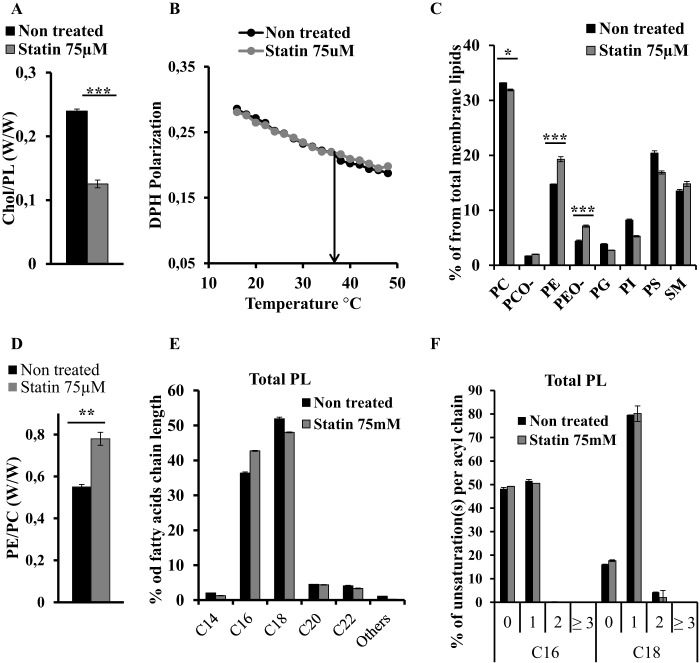
Cholesterol Decrease Leads to PE Up-regulation in Mammalian Cells
To assess whether the balance between cholesterol and PE is also operating in mammalian cells, we inhibited cholesterol synthesis in HEK 293T cells using statins. We compared PL distribution and viscosity of membranes from HEK 293T cultured in cholesterol-free medium with or without addition of 75 μm simvastatin for 48 h, a treatment that leads to a 2-fold decrease of the cholesterol:PL ratio (Fig. 7A). Despite this change, the microviscosity of the statin-treated cells is not changed compared with control cells (Fig. 7B). Remarkably, MS analysis of the PL distribution demonstrated an increase of PE and PEO- from 14 and 4% in controls to 20 and 7%, respectively, in statin-treated membranes, leading to a change in the PE:PC ratio from 0.55 in control to 0.78 in treated membranes (Fig. 7, C and D). With the exception of PC and SM that remained unchanged in both conditions, all other PLs decreased in statin-treated membranes (Fig. 7C). Surprisingly, we did not observe a significant change either in the length or in the saturation state of total lipids (Fig. 7, E and F). The saturation state of acyl chains in the different phospholipid species also remained unchanged (Fig. 8). Taken together, our data support the hypothesis that a compensatory regulation between cholesterol and PE metabolism in eukaryotic cells is involved in the control of membrane homeostasis.
FIGURE 7.
Membrane fluidity maintained by cholesterol and PE in HEK 293T membranes upon statin treatment. Lipid distribution and membrane fluidity of membranes of HEK 293T control (black) and HEK 293T treated with 75 μm simvastatin for 50 h (gray). A, cholesterol (Chol) to phospholipid ratio; B, DPH fluorescence polarization; C, phospholipid distribution; D, PE (including PEO-) to PC (including PCO-) ratio; E, fatty acid chain length distribution; F, unsaturation(s) per acyl chain in main fatty acid chains. Bars represent values normalized to 100% (w/w). Error bars indicate S.D. (n = 3). p values were estimated by Student's t test (n = 3): ***, p < 0.001; **, p < 0.005; *, p < 0.01.
FIGURE 8.
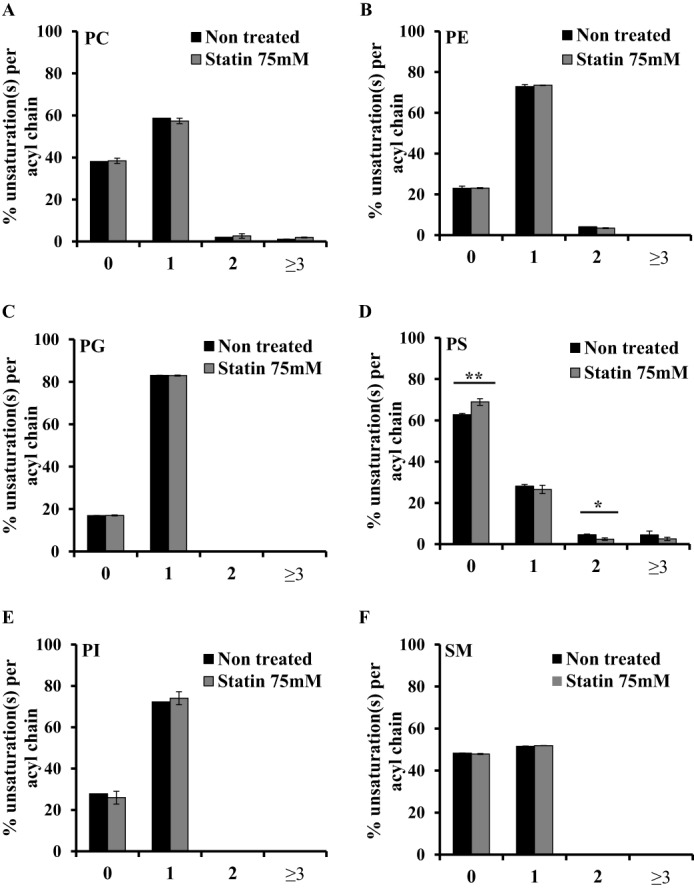
Unsaturation distribution in acyl chains of different PL species in HEK 293T control and statin-treated cells. Unsaturation(s) per acyl chain distribution in different PL species from HEK 293T control (black) and HEK 293T treated with 75 μm simvastatin for 50 h (gray) is shown. A, PC; B, PE; C, PG; D, PS; E, PI; F, SM. Bars represent values normalized to 100% (w/w). Error bars indicate S.D. (n = 3). p values were estimated by Student's t test (n = 3): ***, p < 0.001; **, p < 0.005; *, p < 0.01.
Discussion
The main finding of this study is that both mammalian and insect cells achieve membrane fluidity homeostasis not only by modulating unsaturation levels and (in mammalian cells) sterols but also by changing phosphatidylethanolamine amounts. Specifically, changes in the sterol content are compensated in both cell types by changes in PE quantities. This was particularly surprising for SF9 cells as insects do not synthesize sterols, suggesting a complex sterol-sensitive (or viscosity-sensitive) regulation mechanism (see below).
In the case of SF9 membranes, an exogenous increase of sterol led to a reduction in PE percentage along with a clear shift in acyl chain saturation state. Correspondingly, blocking cholesterol synthesis in HEK 293T cells cultured in cholesterol-free medium results in an enhancement in PE amounts although with no detectable change in saturation state, suggesting that modulating PE amounts is a main mechanism for regulating fluidity. In this experiment, the extent of the effects observed in statin-treated HEK 293T cells were somewhat moderate, but this is likely due to the fact that statin treatment was intentionally limited to 48 h. Indeed, extending such treatment over 48–60 h leads to significant increase in cell death (20, 21). Our data imply a metabolic control of HEK 293T and SF9 membrane lipid composition to maintain membrane properties.
DPH fluorescence polarization measurement has been traditionally used as a reliable tool to assess membrane fluidity, but when applied to lipid mixtures, it is reasonable to raise the issues of portioning and sidedness. As DPH is a symmetrical hydrophobic molecule that has been shown to be deeply buried in the acyl chain region of the bilayer (22), it is likely be evenly distributed in both layers of the membranes. In this same study, the authors show that the changes in DPH fluorescence polarization in the presence of cholesterol are likely due to a change in motion and in localization of the probe.
Conversely, in vitro studies investigating the behavior of PE:PC have shown that phase separation may occur at a high molar ratio of PE (i.e. POPE >30%), suggesting that domains may form in SF9 cells where we detected about 38% total PE (mostly DOPE). Although we do see a transition effect at 60% POPE (40% POPC) below 15 °C, such behavior was not observed in a physiologically relevant range of temperatures (25–40 °C). Considering these various points, we deem DPH fluorescence polarization reasonable to use as a reliable assessment of membrane fluidity for complex lipid mixtures.
Membrane fluidity and/or lipid composition alteration can be responsible for pathological behavior of the cells. The effect of lipid composition modulation on red blood cells has been extensively studied. Indeed, the rheology of erythrocytes is altered in patients with hypercholesterolemia due to an increased cholesterol:PL ratio and impaired membrane fluidity (23, 24). Statin treatment lowers the cholesterol amount in the membranes and normalizes erythrocyte membrane fluidity (10, 25). Similarly, insulin-resistant type 2 diabetes may result from modified lipid composition of the erythrocyte membranes, notably fatty acid saturation and the PE:PC ratio (9). Hence, the lipid homeostasis of cell membranes is crucial for the accomplishment of their functions. Because erythrocytes lack nuclei, they might lack regulation elements allowing maintenance of their membrane properties, although cholesterol exchange with low density proteins has been described (26).
Our data beg the questions of how membrane fluidity is controlled in these cells and whether there is a direct fluidity sensor in the membrane. Considering that insect cells share similar PL composition as mammalian cells, how do they regulate their membrane fluidity in the absence of endogenous sterol? Thermal and fluidity sensors regulated by the lipid composition have been long described in bacterial membranes. For example, the two-component pathway (DesK/DesR) of Bacillus subtilis is able to sense the change in membrane properties upon temperature change and trigger the expression of acyl-lipid desaturase to increase the amounts of unsaturated fatty acids in the membrane and thus maintain the fluidity (3). Membrane fluidity control pathways were also described in yeast cells. The Rho/PKC1/MAPK signaling pathway appears to be implicated in membrane fluidity homeostasis as shown by genetic studies of yeast mutants and is regulated by PL acyl chains (27).
IRE1 is a conserved endoplasmic reticulum stress sensor in eukaryotes that detects, via its transmembrane domain, perturbations such as increased lipid saturation and alerts the lipid biosynthesis machinery to restore the endoplasmic reticulum membrane homeostasis by synthesizing unsaturated lipids. Any uncontrolled modification of the saturated/unsaturated lipid balance (such as an increase in saturated acid content in the endoplasmic reticulum) can lead to liver failure in chronic diseases like obesity and diabetes (5, 28).
Previous studies from the Brown and Goldstein laboratories (8, 29, 30) have described that the SREBP pathway implicated in cholesterol and fatty acid homeostasis in mammalian cells is present in insect cells where it is involved in control of fatty acid synthesis and can be regulated by a phospholipid with a palmitate (most probably POPE). Indeed, in HEK 293T cells, the down-regulation of the SREBP cascade can be triggered by accumulation of cholesterol and/or unsaturated fatty acids in the cells to maintain the metabolic homeostasis of both. In contrast, studies on Drosophila cells show that PE and palmitated PE in particular control the release of SREBP and exert feedback on synthesis of other phospholipids and fatty acids (29). This regulation could be required to maintain a certain lipid composition of cell membranes and thus for the adjustment of lipid synthesis as the authors suggested. Nevertheless, a direct relationship or balance between palmitated PE and cholesterol amounts in the same cells has never been established. Moreover, the link between SREBP regulation by cholesterol/palmitated PE to adjust lipid composition and preservation of membrane properties, such as fluidity, was suggested but never investigated.
The similarity in behavior observed between insect and mammalian cells could indicate that they rely on comparable molecular mechanisms to maintain membrane properties and homeostasis not only by regulating the saturation state of the newly synthesized phospholipids but also by a fine-tuned balance between PL (especially PE) and cholesterol contents of the membranes. The implication of a membrane fluidity sensor in this regulation is the key question that needs to be investigated in the future. Evidently, establishing the molecular basis of this regulation could bring new perspectives in the comprehension of the physiopathological processes related to hypercholesterolemia.
Author Contributions
R. D. performed most sample preparation and analysis with the help of C. T. C. D., C. N., and P. V. A. performed mass spectrometry. J.-M. R., P. V. A., and C. G. designed and supervised the work and wrote the paper with R. D. All authors analyzed the results and approved the final version of the manuscript.
Acknowledgment
We thank Prof Brian Kobilka for providing the HEK 293T and SF9 cell lines.
This work was supported in part by Fonds de la Recherche Scientifique-Fonds National de la Recherche Scientifique (FRS-FNRS) Grants F.4523.12, 34553.08, and T.0136.13 and a grant from “Fond Extraordinaire de Recherche” 2007 of the Université Libre de Bruxelles. The authors declare that they have no conflicts of interest with the contents of this article.
- PL
- phospholipid
- SF9
- Spodoptera frugiperda cells
- DPH
- 1,6-diphenyl-1,3,5-hexatriene
- PC
- phosphatidylcholine
- PCO-
- phosphatidylcholine plasmalogen
- PE
- phosphatidylethanolamine
- PEO-
- phosphatidylethanolamine plasmalogen
- PG
- phosphatidylglycerol
- PI
- phosphatidylinositol
- PS
- phosphatidylserine
- SM
- sphingomyelin
- ESI
- electrospray ion source
- QTOF
- quadrupole time-of-flight
- POPE
- palmitoyloleoylphosphatidylethanolamine
- POPC
- palmitoyloleoylphosphatidylcholine
- DOPE
- dioleoylphosphatidylethanolamine
- DOPC
- dioleoylphosphatidylcholine
- SREBP
- sterol regulator element-binding protein.
References
- 1. Nicolson G. L. (2014) The fluid-mosaic model of membrane structure: still relevant to understanding the structure, function and dynamics of biological membranes after more than 40 years. Biochim. Biophys. Acta 1838, 1451–1466 [DOI] [PubMed] [Google Scholar]
- 2. Lipowsky R. (2014) Remodeling of membrane compartments: some consequences of membrane fluidity. Biol. Chem. 395, 253–274 [DOI] [PubMed] [Google Scholar]
- 3. Cybulski L. E., del Solar G., Craig P. O., Espinosa M., and de Mendoza D. (2004) Bacillus subtilis DesR functions as a phosphorylation-activated switch to control membrane lipid fluidity. J. Biol. Chem. 279, 39340–39347 [DOI] [PubMed] [Google Scholar]
- 4. Mansilla M. C., Cybulski L. E., Albanesi D., and de Mendoza D. (2004) Control of membrane lipid fluidity by molecular thermosensors. J. Bacteriol. 186, 6681–6688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Holthuis J. C., and Menon A. K. (2014) Lipid landscapes and pipelines in membrane homeostasis. Nature 510, 48–57 [DOI] [PubMed] [Google Scholar]
- 6. Yeagle P. L. (1985) Cholesterol and the cell membrane. Biochim. Biophys. Acta 822, 267–287 [DOI] [PubMed] [Google Scholar]
- 7. van Meer G., Voelker D. R., and Feigenson G. W. (2008) Membrane lipids: where they are and how they behave. Nat. Rev. Mol. Cell Biol. 9, 112–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brown M. S., and Goldstein J. L. (1997) The SREBP pathway: regulation of cholesterol metabolism by proteolysis of a membrane-bound transcription factor. Cell 89, 331–340 [DOI] [PubMed] [Google Scholar]
- 9. Allen H. G., Allen J. C., Boyd L. C., Alston-Mills B. P., and Fenner G. P. (2006) Determination of membrane lipid differences in insulin resistant diabetes mellitus type 2 in whites and blacks. Nutrition 22, 1096–1102 [DOI] [PubMed] [Google Scholar]
- 10. Levy Y., Leibowitz R., Aviram M., Brook J. G., and Cogan U. (1992) Reduction of plasma cholesterol by lovastatin normalizes erythrocyte membrane fluidity in patients with severe hypercholesterolaemia. Br. J. Clin. Pharmacol. 34, 427–430 [PMC free article] [PubMed] [Google Scholar]
- 11. Carvalho M., Sampaio J. L., Palm W., Brankatschk M., Eaton S., and Shevchenko A. (2012) Effects of diet and development on the Drosophila lipidome. Mol. Syst. Biol. 8, 600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Marheineke K., Grünewald S., Christie W., and Reiländer H. (1998) Lipid composition of Spodoptera frugiperda (Sf9) and Trichoplusia ni (Tn) insect cells used for baculovirus infection. FEBS Lett. 441, 49–52 [DOI] [PubMed] [Google Scholar]
- 13. Sampaio J. L., Gerl M. J., Klose C., Ejsing C. S., Beug H., Simons K., and Shevchenko A. (2011) Membrane lipidome of an epithelial cell line. Proc. Natl. Acad. Sci. U.S.A. 108, 1903–1907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cooper B. S., Hammad L. A., Fisher N. P., Karty J. A., and Montooth K. L. (2012) In a variable thermal environment selection favors greater plasticity of cell membranes in Drosophila melanogaster. Evolution 66, 1976–1984 [DOI] [PubMed] [Google Scholar]
- 15. Cooper B. S., Hammad L. A., and Montooth K. L. (2014) Thermal adaptation of cellular membranes in natural populations of. Funct. Ecol. 28, 886–894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pan Q., Li M., Shi Y. L., Liu H., Speakman J. R., and Wang D. H. (2014) Lipidomics reveals mitochondrial membrane remodeling associated with acute thermoregulation in a rodent with a wide thermoneutral zone. Lipids 49, 715–730 [DOI] [PubMed] [Google Scholar]
- 17. Roy R., Das A. B., and Ghosh D. (1997) Regulation of membrane lipid bilayer structure during seasonal variation: a study on the brain membranes of Clarias batrachus. Biochim. Biophys. Acta 1323, 65–74 [DOI] [PubMed] [Google Scholar]
- 18. Ahn T., and Yun C. H. (1999) Phase properties of liquid-crystalline phosphatidylcholine/phosphatidylethanolamine bilayers revealed by fluorescent probes. Arch. Biochem. Biophys. 369, 288–294 [DOI] [PubMed] [Google Scholar]
- 19. Silvius J. R. (1986) Solid- and liquid-phase equilibria in phosphatidylcholine/phosphatidylethanolamine mixtures. A calorimetric study. Biochim. Biophys. Acta 857, 217–228 [DOI] [PubMed] [Google Scholar]
- 20. Jánosi J., Sebestyén A., Bocsi J., Barna G., Nagy K., Vályi-Nagy I., and Kopper L. (2004) Mevastatin-induced apoptosis and growth suppression in U266 myeloma cells. Anticancer Res. 24, 1817–1822 [PubMed] [Google Scholar]
- 21. van Vliet A. K., Nègre-Aminou P., van Thiel G. C., Bolhuis P. A., and Cohen L. H. (1996) Action of lovastatin, simvastatin, and pravastatin on sterol synthesis and their antiproliferative effect in cultured myoblasts from human striated muscle. Biochem. Pharmacol. 52, 1387–1392 [DOI] [PubMed] [Google Scholar]
- 22. Kaiser R. D., and London E. (1999) Location of diphenylhexatriene (DPH) and its derivatives within membranes: comparison of different fluorescence quenching analyses of membrane depth. Biochemistry 38, 2610. [DOI] [PubMed] [Google Scholar]
- 23. Chabanel A., Flamm M., Sung K. L., Lee M. M., Schachter D., and Chien S. (1983) Influence of cholesterol content on red cell membrane viscoelasticity and fluidity. Biophys. J. 44, 171–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Koter M., Franiak I., Strychalska K., Broncel M., and Chojnowska-Jezierska J. (2004) Damage to the structure of erythrocyte plasma membranes in patients with type-2 hypercholesterolemia. Int. J. Biochem. Cell Biol. 36, 205–215 [DOI] [PubMed] [Google Scholar]
- 25. Koter M., Broncel M., Chojnowska-Jezierska J., Klikczynska K., and Franiak I. (2002) The effect of atorvastatin on erythrocyte membranes and serum lipids in patients with type-2 hypercholesterolemia. Eur. J. Clin. Pharmacol. 58, 501–506 [DOI] [PubMed] [Google Scholar]
- 26. Quarfordt S. H., and Hilderman H. L. (1970) Quantitation of the in vitro free cholesterol exchange of human red cells and lipoproteins. J. Lipid Res. 11, 528–535 [PubMed] [Google Scholar]
- 27. Lockshon D., Olsen C. P., Brett C. L., Chertov A., Merz A. J., Lorenz D. A., Van Gilst M. R., and Kennedy B. K. (2012) Rho signaling participates in membrane fluidity homeostasis. PLoS One 7, e45049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wenk M. R. (2005) The emerging field of lipidomics. Nat. Rev. Drug Discov. 4, 594–610 [DOI] [PubMed] [Google Scholar]
- 29. Dobrosotskaya I. Y., Seegmiller A. C., Brown M. S., Goldstein J. L., and Rawson R. B. (2002) Regulation of SREBP processing and membrane lipid production by phospholipids in Drosophila. Science 296, 879–883 [DOI] [PubMed] [Google Scholar]
- 30. Seegmiller A. C., Dobrosotskaya I., Goldstein J. L., Ho Y. K., Brown M. S., and Rawson R. B. (2002) The SREBP pathway in Drosophila: regulation by palmitate, not sterols. Dev. Cell 2, 229–238 [DOI] [PubMed] [Google Scholar]
- 31. Bligh E. G., and Dyer W. J. (1959) A rapid method for total lipid extraction and purification. Can. J. Biochem. Physiol. 37, 911–917 [DOI] [PubMed] [Google Scholar]