Abstract
The intervertebral disc is an important mechanical structure that allows range of motion of the spinal column. Degeneration of the intervertebral disc, incited by aging, traumatic insult, genetic predisposition, or other factors, is often defined by functional and structural changes in the tissue, including excessive breakdown of the extracellular matrix, increased disc cell senescence and death, and compromised biomechanical function of the tissue. Intervertebral disc degeneration is strongly correlated with low back pain, which is a highly prevalent and costly condition, significantly contributing to loss in productivity and health care costs. Disc degeneration is a chronic, progressive condition, and current therapies are limited and often focused on symptomatic pain relief rather than curtailing the progression of the disease. Inflammatory processes, exacerbated by cytokines TNF-α and IL-1β are believed to be key mediators of disc degeneration and low back pain. In this review, we describe the contributions of TNF-α and IL-1β to changes seen during disc degeneration at the cellular and tissue level, new evidence suggesting a link between infection of the spine and low back pain, and the emerging therapeutic modalities aimed at combating these processes.
Keywords: intervertebral disc, nucleus pulposus, cytokines, extracellular matrix, tumor necrosis factor, interleukin-1, toll-like receptor
Introduction
The intervertebral disc (IVD) comprises an outer circumferential annulus fibrosus (AF) and an inner cell-sparse, matrix-rich nucleus pulposus (NP), bordered superiorly and inferiorly by two cartilaginous endplates. The AF is a lamellar fibrocartilagenous structure rich in collagen I, derived from embryonic sclerotome, and is responsible for withstanding large hoop stress from the pressurized NP, and tensile and torsional stresses from motion of adjacent vertebrae (Guterl et al., 2013). The NP encompasses a physiologically hypoxic and hyperosmotic niche; cells residing within the NP are derived from the embryonic notochord (Risbud et al., 2010; Risbud and Shapiro, 2011; Johnson et al., 2014; Choi et al., 2015). NP cells are phenotypically defined in part by the distinct ratio of synthesized aggrecan and collagen II (Mwale et al., 2004; Risbud et al., 2015). The hydrated, aggrecan-rich ECM resists large compressive loads from the trunk, allowing us to remain upright (Vergroesen et al., 2015). Degeneration of the NP, AF, and IVD as a structure is often defined by functional and structural changes in the tissue, including excessive breakdown of ECM by proteases and increased cell senescence and death (Roughley, 2004; Adams and Roughley, 2006). IVD degeneration is strongly correlated with low back pain (LBP) (Chou et al., 2011; Livshits et al., 2011), which is a highly prevalent, costly, and crippling condition across the globe (Katz, 2006; Hoy et al., 2012). Inflammatory processes, exacerbated by cytokines tumor necrosis factor-α (TNF-α and interleukin -1β (IL-1β are believed to be key events during disc degeneration and associated LBP (Risbud and Shapiro, 2014). In this review, we describe the specific contributions of TNF-α and IL-1β to cellular and tissue level changes seen during disc degeneration, discuss new evidence suggesting a link between infection of the spine and LBP, and the emerging therapeutic modalities aimed at combating these processes. Specifically, the effects of cytokines on breakdown of disc ECM and potential activation of toll like receptors, the immune response, disc cell homeostasis, and resolution of disc herniation are discussed as well as potential triggering mechanisms for this cytokine activity.
Initiating events that may contribute to elevated cytokine production by disc cells and spinal tissues
It has been well established that inflammatory changes in the disc contribute to degeneration, and that trauma and genetic predisposition may contribute to some aspects of this cascade. However, the question remains of the initiating event(s) that promotes production of these cytokines by NP and AF cells, especially in the absence of acute trauma or herniation. One possible initiator is chronic overload of the disc, which has been shown to cause degenerative changes in several models (Adams et al., 2000). Discs from rats subjected to in vivo dynamic compression overloading over 8 weeks showed increased MMP-associated aggrecan degradation products in both the AF and NP compared to sham discs (Iatridis et al., 2011). Some evidence exists correlating bacterial infection with degenerative disc disease and LBP. In one study, 53% of surgical microdiscectomy samples from patients with sciatica were positive for bacterial cultures (Stirling et al., 2001). In another recent study, herniated nuclear tissue removed during surgery was positive for microbial cultures in 46% of patients, and significantly correlated with Modic changes in adjacent vertebral endplates, which are highly associated with LBP (Jensen et al., 2008; Albert et al., 2013a). Additional studies have also shown evidence of bacterial infection in some painful degenerate discs, even in the absence of overt clinical infection (Stirling et al., 2002; Fritzell et al., 2004; Agarwal et al., 2011; Arndt et al., 2012).
In 2008, Albert et al. proposed the hypothesis that some cases of degenerative disc disease and low back pain have an infectious cause (Albert et al., 2008a), supported by a pilot study investigating the efficacy of antibiotics for the treatment of patients with persistent LBP and Modic changes that did not respond to initial conservative therapy (Albert et al., 2008b). In this pilot study, there was a statistically and clinically significant improvement in patients receiving a course of amoxicillin-clavulanate antibiotics. A larger double-blind randomized clinical trial followed and similarly reported significant improvement in disability and pain in patients treated with antibiotics compared to placebo (Albert et al., 2013b). Importantly, some evidence suggests that clavulanate has anti-inflammatory and analgesic properties, which may confound interpretation of clinical findings by Albert et al. (Casellas et al., 1998; Hajhashemi and Dehdashti, 2014). In addition, some investigators have suggested that the presence of bacteria in disc samples arises from contamination of skin commensals (e.g. Proprionibacterium acnes) during non-sterile surgical and collection procedures (McLorinan et al., 2005; Ben-Galim et al., 2006; Carricajo et al., 2007; Wedderkopp et al., 2009); it may also arise from infected endplate and bony avulsions (Rajasekaran et al., 2013). In spite of the controversial nature, subclinical bacterial infection of endplate/vertebrae and/or discitis as a cause for disc degeneration and LBP warrants further consideration and careful investigation.
Bacterial infection of the disc may initiate inflammatory cascades through activation of components of the innate immune response. Toll-like receptors (TLRs) are plasma- and endolysosomal-bound pattern recognition receptors, best characterized as expressed on the surface of cells of the immune system (De Nardo, 2015). TLRs recognize pathogen-associated molecular patterns (PAMPs), and activate inflammatory signaling cascades (Kumar et al., 2009). Most relevant to bacterial infection are TLR2 and TLR4. TLR2 recognizes a range of PAMPs including peptidoglycans expressed in the cell wall of Gram-positive bacteria (Zähringer et al., 2008), while TLR4 is activated by lipopolysaccharide (LPS) in the cell wall of Gram-negative bacteria (Poltorak et al., 1998). Activation both TLR2 and TLR4 signaling results in transcription of many pro-inflammatory cytokines through the MyD88 pathway (Kawai et al., 1999).
Interestingly, NP cells also express TLRs with a recent study identifying expression of TLR1/2/3/4/5/6/9/10 in isolated human IVD cells. Importantly, expression levels of TLR2 and TLR4 were raised with increasing grade of degeneration (Klawitter et al., 2014). Another study showed that treatment of IVD cells with LPS resulted in increased expression of TNF-α, IL-1β, and IL-6, and decreased expression of aggrecan and collagen II. Furthermore, injection of LPS into discs in vivo induced degenerative changes (Rajan et al., 2012). Ellman et al. demonstrated that inhibition of MyD88 attenuated the changes in catabolic gene expression incurred by LPS in vitro as well as in an ex vivo organ culture model (Ellman et al., 2012). These studies suggest that not only can innate immunity initiate degenerative changes in the disc, but it can also perpetuate inflammatory cascades and promote progressive disease.
The TNF and IL-1 signaling pathways
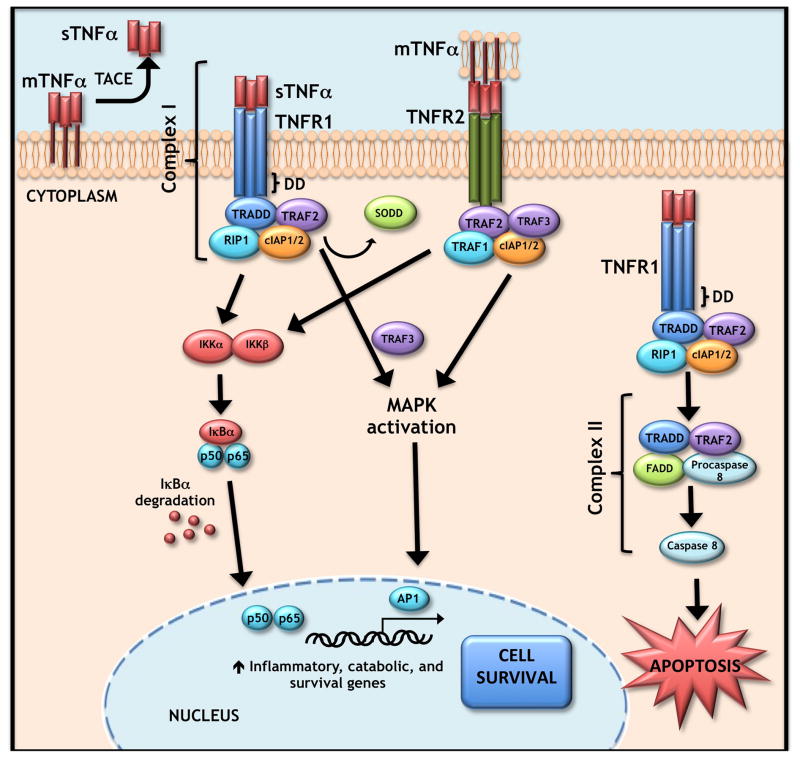
TNF-α is synthesized by cells as a 26 kDa type II transmembrane protein termed mTNF. This membrane-bound form is processed to a 17 kDa soluble form, sTNF, by the metalloproteinase tumor necrosis-α-converting enzyme (TACE) also known as ADAM metallopeptidase domain 17 (ADAM17). The trimeric form of both mTNF and sTNF are biologically active, although monomeric and dimeric forms also exist (Black et al., 1997). TNF-α interacts with either of two receptors of the tumor necrosis factor receptor (TNFR) superfamily, TNFR1 and TNFR2. mTNF can bind either receptor, while sTNF can bind TNFR1 only. TNFR1 activation by TNF-α leads to formation of two distinct TNF signaling complexes: Complex 1 has anti-apoptotic functions, while Complex II/death inducing signaling complex (DISC) induces apoptosis after receptor internalization. TNFR2, on the other hand, lacks an intracellular death domain. Therefore, signaling through this complex is considered anti-apoptotic. Recent evidence, however, suggests that TNFR2 may induce degradation of TNF receptor-associated factor 2 (TRAF2), resulting in crosstalk between the TNFR1 and TNFR2 pathways. Signaling through Complex I activates the nuclear factor κB (NF-κB) and mitogen activated protein kinase (MAPK) pathways (Cabal-Hierro and Lazo, 2012) (See Figure 1). Relevant to this review, high levels of TNF-α have been associated with disc degeneration (discussed in the following sections).
Figure 1. TNF-α signaling pathway.
Membrane-bound TNF (mTNF) is processed by the metalloproteinase TACE/ADAM-17 to the soluble form (sTNF). sTNF-α or mTNF-α may bind the transmembrane TNFR1 receptor, causing a conformational change that releases the inhibitory SODD protein. Binding results in the recruitment of several facors including TRADD, RIP1, TRAF2, and cIAP 1 and 2 resulting in formation of Complex I that signals through either the NF-κB or MAPK pathways to activate p65 or AP1, respectively. Complex I signaling results in transcription of inflammatory (chemokines, cytokines) and matrix catabolic genes (MMPs, ADAMTSs) as well as pro-survival genes (cIAP1 and 2, cFLIP, TRAF1, TRAF2). Alternatively, mTNF-α may activate the TNFR2 receptor to form a similar complex and downstream signaling cascade. In some instance, TNFR1 bound to sTNF-α may be internalized initiating Complex II or DISC formation that leads to cleavage of procaspase 8 and finally cell apoptosis.
Abbreviations: TNF-α, tumor necrosis factor α ; TACE, TNF-α converting enzyme; ADAM-17, a disintegrin and metalloproteinase domain containing protein 17; TNFR1, TNF receptor 1; SODD, silencer of death domains; TNFR2, TNF receptor 2; TRADD, TNFR1-assoicated death domain protein; RIP1, receptor-interacting protein 1; TRAF2, TNF-receptor-associated factor 2; cIAP, baculoviral IAP repeat containing; DISC, death-inducing signaling complex; NF-κB, nuclear factor κB; MAPK, mitogen-activated protein kinase; DD, death domain.
IL-1α and IL-1β, members of the IL-1 family of 11 cytokines, are first synthesized as precursor proteins and then activated through intracellular proteolytic cleavage by calpain and caspase-1, respectively. Secreted pro-IL-1β can also be activated extracellularly by neutrophil proteases. While pro-IL-1β requires this activation, membrane-bound pro-IL-1α can signal through Interleukin 1 Receptor, Type I (IL-1R1) on adjacent cells without proteolytic cleavage. In addition, the 16 kDa N-terminal propeptide cleavage product of pro-IL-1α (ppIL-1α) can translocate into the nucleus and is thought to function as a transcriptional modulator. Both IL-1α and IL-1β through binding IL-1R1, can stimulate NF-κB, JNK, and p38 MAPK signaling pathways, resulting in phosphorylation of various proteins involved in transcription of inflammatory and catabolic genes such as IL-6, IL-8, MCP-1, COX-2, IκBα, IL-1α, IL-1β, and MKP-1 (Gabay et al., 2010; Weber et al., 2010).
TNF and IL-1 are closely associated with intervertebral disc degeneration
Associations between herniation, disc degeneration, and the inflammatory response have well been established. Herniated disc tissue exhibits a large inflammatory cell response with macrophages being predominant players and increased TNF-α, IL-1β, and many other cytokines detected at the site of herniation (Grönblad et al., 1994; Takahashi et al., 1996). A 2012 study of NP samples from adolescent patients found an increased number of TNF-α-immunoreactive cells in painful herniated samples compared to non-painful scoliotic controls (Ohtori et al., 2012). Importantly, during disc degeneration TNF-α and IL-1β are not only produced by leukocytes, but also by IVD cells themselves (Le Maitre et al., 2005; Le Maitre et al., 2007b). In addition, TNFR1, TNFR2, TACE, interleukin-1 receptor antagonist (IL-1ra), and IL-1R1 are expressed in human nucleus pulposus tissue, and expression of TNF-α and IL-1β increases with age and severity of degeneration in both humans and animal models (Oda et al., 2004; Le Maitre et al., 2005; Bachmeier et al., 2007; Le Maitre et al., 2007b; Wang et al., 2013b). Futhermore, another study confirmed expression of both TNFR1 and TNFR2 in NP tissue from patients with disc herniation, with levels of TNFR1 being positively correlated with degree of pain (Andrade et al., 2011). In addition, IL-1 gene family polymorphisms, IL-1RN G1812A, IL-1α C889T, and IL-1β C3954T, are associated with increased risk of LBP (Solovieva et al., 2004). Importantly, expression of these cytokines is not merely correlated with degeneration, but is believed to be causative of disease.
TNF and IL-1 contribute to disc disease through degradation of ECM
One meaningful characteristic of disc degeneration is disruption of the ECM. TNF-α and IL-1β have been shown in many contexts to induce degenerative changes to the ECM. Induction of catabolic enzymes A Disintegrin And Metalloproteinase with Thrombospondin Motifs (ADAMTS) -4, and -5, and matrix metalloproteinases (MMPs) -1, -2, -3, -4, -13, and -14, and concurrent decrease in expression of anabolic ECM proteins aggrecan and collagen II is mediated by both IL-1β and TNF-α (Doita et al., 2001; Shen et al., 2003; Jimbo et al., 2005; Le Maitre et al., 2005; Séguin et al., 2005; Murata et al., 2006; Le Maitre et al., 2007a; Bachmeier et al., 2009; Pockert et al., 2009; Wang et al., 2011; Wang et al., 2014a). Séguin et al. showed that TNF-α-induced increase in MMP2 activity was specifically associated with upregulation of membrane type MT1-MMP (Séguin et al., 2008). A recent study by Ye et al. showed that unlike chondrocytes, NP cell expression of xylosyltransferase-1 (XT-1), a key enzyme in glycosaminoglycan synthesis, is unaffected by TNF-α and IL-1β treatment, suggesting that the effects of these cytokines on the ECM homeostasis are fairly cell-type-specific (Ye et al., 2015). Further studies have shown that TNF-mediated activation of ADAMTS4 occurs through ERK1, p38, and NF-κB pathways in NP cells (Tian et al., 2013). In organ culture models, treatment with TNF-α resulted in suppression of multiple collagen types, aggrecan, fibromodulin, increased expression of MMPs and pain-associated molecule nerve growth factor (NGF) (Ponnappan et al., 2011), and compromised disc biomechanics (Walter et al., 2015a). These results contrast with that of Hoyland et al. which showed that TNF-α treatment had little effect on matrix degradation as assessed by in situ zymography on gelatin, collagen II, and casein, although changes were seen after treatment with IL-1β (Hoyland et al., 2008). This differential response may be explained by differences in concentration, duration of TNF-α treatment, culture system, and/or species between the studies. Not surprisingly the relative importance of each of these inflammatory mediators is still debated. Nonetheless both of these cytokines have been shown under numerous conditions to control catabolic gene expression in disc tissues.
TNF-α and IL-1β also suppress expression of matricellular protein connective tissue growth factor (CCN2/CTGF), an important regulator of cellular adhesion, proliferation, migration, and ECM synthesis (Tran et al., 2010; Tran et al., 2014). Tran et al. demonstrated that the activity of these cytokines on CCN2 expression was NF-κB-dependent. In addition, CCN2 successfully suppressed IL-1β mediated induction of catabolic molecules MMP-3, ADAMTS5, syndecan-4, and prolyl hydroxylase 3 (PHD3), and this inhibition was through αvβ3 and α5β1 integrins, indicating a protective role of CCN2 in pathogenesis of disc disease (Tran et al., 2014).
Many of the effects of TNF-α and IL-1β on the ECM are mediated through the NF-κB signaling pathway and the heparan-sulfate proteoglycan syndecan-4. Fujita et al. showed that prolyl hydroxylase 3 (PHD3) acts as a coactivator of p65 to propagate matrix catabolism induced by TNF-α. TNF-α treatment of NP cells induces expression of PHD3, which then interacts with p65 to promote transcription of syndecan-4, ADAMTS5, MMP-13, and COX2 (Fujita et al., 2012). More recent work has confirmed that PHD2 plays a similar role in the NP in promoting p65-mediated transcription (Li et al., 2015). Syndecan-4 is required for TNF-α and IL-1β-dependent increase in expression and activity of ADAMTS5 and MMP-3 in rat NP cells (Wang et al., 2011; Wang et al., 2014a). Adding another dimension to cytokine action on disc cells, Ye et al. demonstrated a positive relationship between TNF-α and Wnt/β-catenin signaling in NP cells and showed that TNF-α activates the Wnt/β-catenin pathway, thereby increasing expression of MMP-13 (Ye et al., 2011). Additionally, Wnt signaling induces TNF-α expression in NP cells, possibly leading to a pro-degenerative feed-forward loop between the two signaling pathways (Hiyama et al., 2013).
In vivo studies support the role of these endogenous cytokines in the degenerative process of the IVD. Mice lacking functional IL-1ra, an endogenous antagonist for IL-1R1, demonstrated loss of proteoglycans and increased expression of MMP-3, -7, and ADAMTS4 in their IVD. Compared to wild type mice, discs of IL-1ra−/− mice had higher histological grade of degeneration, and disc cells exhibited diminished proliferative capacity (Phillips et al., 2013). Similarly, Kang et al. showed that injection of TNF-α in a porcine model was sufficient to induce early-stage disc degeneration, characterized by matrix loss, annular fissure formation, and vascularization (Kang et al., 2015).
Activation of TLRs by endogenous matrix ligands may exacerbate the disease progression through cytokine production
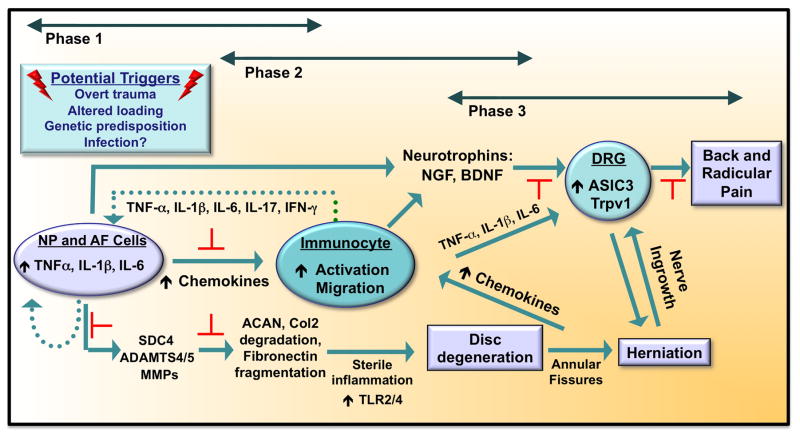
Additionally, matrix catabolism products may contribute to progression of disc disease by serving as endogenous ligands of TLRs and, thus, exacerbating the inflammatory state. Biglycan is a small, leucine-rich proteoglycan expressed in the IVD. Proteolytically-cleaved biglycan has been shown to activate proinflammatory cascades through binding to TLR2 and TLR4 in macrophages (Schaefer et al., 2005). Relevant to this discussion, biglycan has been shown to be extensively fragmented in pathological human IVDs (Brown et al., 2012). Similarly, fibronectin fragments can act as endogenous ligands for TLR4 (Okamura et al., 2001), fibronectin fragmentation increases in degenerated discs possibly due to increased ADAM8-mediated cleavage (Ruel et al., 2014), and injection of 30 kDa N-terminal Fn-f in rabbit discs promotes degeneration (Anderson et al., 2005). Similarly, versican, a large aggregating proteoglycan that is expressed in the disc (Sivan et al., 2014), is shown to activate TLR2 and TLR6 (Kim et al., 2009). While the in vivo evidence is limited, there are indeed studies showing activation of TLR by ECM components in NP, giving credence to the hypothesis. Fragments of hyaluronic acid (HA) increase expression of IL-1β, IL-6, IL-8, MMP-1, and MMP-13 by NP cells by binding to TLR2 (Quero et al., 2013). Additionally, excessive mechanical loading of IVD cells, a contributor to disc disease, has also been shown to upregulate TLR2 and TLR4 expression (Gawri et al., 2014). Thus, ECM proteins, particularly fragments generated during the degenerative process, binding to TLRs on NP cells can potentiate the pathogenesis of disc degeneration (See schematic in Figure 2 for various phases of disc degeneration and possible areas for intervention, as indicated by red blocking symbols).
Figure 2. Schematic showing the major phases of intervertebral disc degeneration and discogenic back pain.
Potential triggering events include overt trauma to the disc, altered loading, genetic predisposition and spinal infection. These triggers result in NP and AF cell-mediated synthesis of cytokines such as TNF-α, IL-1β, and IL-6. These cytokines have at least three major effects: increased matrix breakdown through production of SDC4, ADAMTS4/5, and MMPs, activation and chemotaxis of immune cells to the disc, induction of neurotrophins (NGF, BDNF) by disc cells and immunocytes. NGF and BDNF induce production of pain-associated channels ASIC3 and Trpv1 in the DRG. Presence of these factors in the inflammatory microenvironment of the degenerated disc is thought to promote DRG sensitization and pain. Blocking of cytokine production in Phases 1 and 2 may have most the profound positive effect on disease progression and back pain. Red blocking symbols indicate possible areas for clinical intervention.
Abbreviations: ADAMTS4/5, a disintegrin and metalloproteinase with thrombospondin motifs 4/5; ASIC3, acid-sensing ion channel 3; BDNF, brain-derived neurotrophic factor; β-NGF, β-nerve growth factor; DRG, dorsal root ganglion; IFN-γ, interferon-γ; MMPs, matrix metalloproteinases; SDC4, Syndecan-4; TrpV1, transient receptor potential cation channel subfamily V member 1. Adapted from Risbud and Shapiro, 2014.
Immune cells are activated by disc cells in response to TNF and IL-1
In addition to affecting matrix homeostasis, cytokines also induce NP cell to synthesize many cytokines and chemokines that can further enhance the inflammatory state by recruiting and activating immune cells. Gruber et al. showed that production of IL-17 is correlated with severity of degeneration and was induced by TNF-α and IL-1β (Gruber et al., 2013). Human IVD cells, in response to IL-1β treatment, increase production of COX-2 and IL-6 as well as IL-1β itself, creating a positive feedback loop (Jimbo et al., 2005). When exposed to TNF-α, human IVD cells increased production of the tachykinin peptide Substance P, which subsequently induced expression of IL-1β, IL-6, and IL-8, a chemoattractant for neutrophils (Kepler et al., 2012). Similarly, IVD cells following treatment with TNF-α and IL-1β produce CCL5/RANTES, a chemoattractant for macrophages and eosinophils, and the expression is correlated to both discogenic pain and severity of degeneration (Kepler et al., 2013; Gruber et al., 2014a). When treated with IL-1β or TNF-α, both NP and AF cells produced monocyte chemoattractant protein-1 (MCP-1/CCL2) in a dose- and time-dependent manner. This was also demonstrated in surgically-induced rabbit IVD herniation model (Yoshida et al., 2002; Yoshida et al., 2005). Human AF cells also induced expression of MCP-1/CCL2 in response to IL-1β treatment (Gruber et al., 2015). Other macrophage chemoattractants, such as CCL3/MIP-1α and CCL4/MIP-1β are expressed by NP cells, and their expression is regulated by TNF-α or IL-1β through MAPK and NF-κB pathways. Interestingly, while p65 induces the expression of CCL3, p50 showed inhibitory effects on CCL3 expression. Importantly, expression of CCL3 has been correlated to severity of degeneration in human NP tissue (Wang et al., 2013b).
It is worth noting that IVD cells themselves express chemokine receptors, although their physiologic function and precise role in degeneration is not yet fully elucidated. IVD cells express CCL5 receptors CCR1, CCR3, and CCR5 (Gruber et al., 2014a). In a recent report, Phillips et al. showed expression of CCR1, CXCR1, and CXCR2 by human NP cells in situ; expression of CXCR2 significantly correlated with the grade of degeneration. Surprisingly, NP cells were also positive for CD4, a receptor exclusively expressed by immune cells, more prominently by the CD4+ T helper cells, an observation that requires further careful investigation. Even though human NP cells expressed these chemokine receptors, treatment of primary human NP cells with IL-16, CCL2, CCL3, CCL7, or CXCL8 did not induce inflammatory responses or ECM remodeling. Rather, IL-1β treatment was able to induce cytokines and chemokines as well as ECM remodeling, suggesting IL-1β as a key player in modulating inflammatory process in intervertebral disc (Phillips et al., 2015)
TNF and IL-1 affect homeostatic activities of IVD cells
In addition to the effects on the matrix, inflammatory cytokines can also directly effect disc cell survival and functionality. A study from Purmessur et al. showed in a bovine organ culture model that TNF-α induced cell senescence along with anti-anabolic and pro-catabolic activities of the resident cells (Purmessur et al., 2013). In a rat organ culture model of disc degeneration, tissues were cultured with TNF-α, IL-1β, and serum limiting conditions, and showed differential expression of many genes involved in cell cycle, cell death, cellular growth, and proliferation (Ponnappan et al., 2011). In addition, human AF cells exposed to both IL-1β and TNF-α significantly downregulated growth differentiation factor 5 (GDF5) (Gruber et al., 2014b), which has previously been demonstrated to be an important anabolic factor in disc (Li et al., 2004). Microarray analysis of human outer and inner AF tissue revealed higher GDF5 expression in herniated lumbar discs, compared to non-herniated lumbar discs (Gruber et al., 2014b). Another central mediator critical to disc cell proliferation is the Notch signaling pathway (Hiyama et al., 2011). TNF-α and IL-1β treatment of NP cells increased expression of NOTCH1 and NOTCH2 receptors along with their ligand Jagged-2, and downstream transcription factors HES1, HEY1, and HEY2. In addition, levels of NOTCH2 were increased in degenerative human NP tissues compared to non-degenerative samples, suggesting cytokine-mediated aberration in Notch signaling may affect cellular proliferation and differentiation in disc degeneration (Wang et al., 2013a).
There is emerging evidence that even transient exposure to cytokines may have lasting effects on the IVD. In a 2014 study, Maidhof et al. demonstrated that inflammatory stimuli have a permanent effect on IVD cell biophysical properties. After 24 hours of treatment with TNF-α, hydraulic permeability and cell radius of NP cells were altered for 1 week (Maidhof et al., 2014). These authors also noted a decrease in the expression of water channel Aquaporin 1, which has been recently shown to be associated with severity of degenerative disc disease (Maidhof et al., 2014; Johnson et al., 2015). Similarly, in a bovine disc organ culture model, short-term TNF-α treatment has been shown to result in long-term catabolic effects without negatively affecting cell viability (Purmessur et al., 2013). Likewise, human AF cells treated with IL-1β displayed greater and sustained increase in intracellular calcium concentration in response to laminar fluid flow, indicating IL-1β “sensitizes” the AF to mechanical loading and sheer stress (Elfervig et al., 2001).
TNF and IL-1 may play a critical role in resolution of disc herniation
While the high cytokines levels associated with chronic inflammation negatively affect the ECM and cell viability during disc degeneration, it is also important to note that TNF-α and IL-1β (Genevay et al., 2009) plays an important role in natural resolution of disc herniation. It is well known that majority of patients with disc herniation do not develop symptoms of chronic LBP and radiculopathy due to neutrophil and macrophage-mediated timely resorption of the extruded NP tissue (Iwabuchi et al., 2008). One study has shown that TNF-α was required to induce MMP-3 in the herniated tissue, which acted as a chemoattractant for macrophages necessary for resorption (Haro et al., 2000). Immunoreactivity to TNF-α in the dorsal root ganglion (DRG) was increased following exposure to NP material in a rat model of disc herniation (Murata et al., 2004). Application of TNF-α inhibitor during surgery abrogated macrophage infiltration and VCAM-1 expression in the DRG. These results suggest that TNF-α plays a critical role in the coordination of macrophage infiltration following herniation of NP tissue (You et al., 2013). Likewise, inhibition of IL-1 by IL-1ra resulted in decreased active MMP-3 in human disc herniation samples, suggesting a contribution of IL-1 to this process (Genevay et al., 2009).
While these results demonstrate the importance of TNF-α and IL-1 in resolution of disc herniation, as discussed above, an unchecked inflammatory response is likely deleterious to the disc. It is therefore desirable to devise a strategy to carefully control timing and magnitude of the inflammatory response by using agents such as sTNFRII (Sinclair et al., 2011), IL-1ra (Le Maitre et al., 2006; Shamji et al., 2007; Gorth et al., 2012), and anti-chemotactic agents (Wang et al., 2013b).
New therapies and future directions for the treatment of disc disease
Several approaches have been studied to counteract the effects of TNF-α on disc degeneration. BMP-7 is able to counteract TNF-α-induced activation of NF-κB and subsequent induction of ADAMTS4 and ADAMTS5, thereby preventing TNF-induced matrix loss in human disc cells (Wang et al., 2014b). Platelet rich plasma, or PRP, has also been shown to reduce the TNF- and IL-1-mediated decreases in collagen II and aggrecan expression levels in human NP cells (Kim et al., 2014). Finally, LIM mineralization protein-1 (LMP-1) has recently been shown to prevent TNF-α-mediated induction of MMP-3 and -13 in rat NP cells (Liu et al., 2015).
A recent study by Walter et al. demonstrated that production of IL-1β, IL-6, and IL-8 by diseased human NP cells in response to TNF-α was at vastly different rates and magnitudes, suggesting different roles these cytokines may play in disc disease. Unlike reported by previous studies, blocking IL-6 or IL-1β did not affect the expression of other pro-inflammatory cytokines in response to TNF-α treatment. Anti-TNF-α therapy, given at the same time as TNF-α stimulation, was most effective at inhibiting expression of these cytokines (Walter et al., 2015b).
Other studies have suggested a possible role for anti-IL-1β therapy as a treatment modality, however. Co-treatment of agarose-encapsulated bovine NP cells with both IL-1β and IL-1Ra abolished the catabolic effect caused by IL-1β (Smith et al., 2011). When IL-1Ra was introduced to degenerate human IVD explants matrix degradation as well as expression of MMPs and ADAMTSs were successfully blocked (Le Maitre et al., 2007a). Similarly, medium conditioned with IL-1Ra encapsulated in PLGA microspheres was effective in attenuating the effect of IL-1β on bovine NP cells (Gorth et al., 2012). Shamji et al. used recombinant human elastin-like polypeptide (ELP)-IL-1Ra fusion protein to promote prolonged release of IL-1Ra, and were successful in decreasing TNF-α expression and ADAMTS4 and MMP-3 transcription in human IVD cells (Shamji et al., 2007). In addition, Krupkova et al. demonstrated that degenerate human IVD cells treated in vitro with epigallocatechin 3-gallate (EPCG) significantly inhibited the expression of pro-inflammatory cytokines and MMPs in response to IL-1β treatment, and also showed in vivo reduction of radiculopathic pain in rats treated with EPCG (Krupkova et al., 2014).
A study by Gu et al. showed miR-146a has a protective effect on IL-1-induced IVD degeneration. When bovine NP cells transfected with a miR-146a mimic were treated with IL-1, induction of inflammatory cytokines and matrix protease gene expression was decreased. Additionally, IVDs from miR-146a−/− mice ex vivo showed greater degradation of proteoglycan and increased level of MMP-13 and ADAMTS-5 after treatment with IL-1, suggesting a new potential therapeutic approach in modulating IL-1 activity in disc disease (Gu et al., 2015). Importantly, in addition to these laboratory-based investigations, few clinical studies have tested efficacy of anti-TNF-α and anti-IL-1β therapies for treatment of back and radicular pain (see Table I for details). These clinical studies suggest that anti-cytokine therapies as well as, in a subset of patients, antibiotic regimens hold promise in treating low back and radicular pain.
Table 1.
Clinical trials of different therapeutic modalities for low back and radicular pain
| Drug | Study design | Outcome |
|---|---|---|
| Etanercept (TNF-α decoy receptor) | Multicenter, double-blind, RCT for treating symptomatic lumbar disc herniation | Two transforaminal injections of etanercept resulted in clinically and statistically significant reduction in worst back pain and mean daily worst leg pain at 4 weeks post-treatment (Freeman et al., 2013) |
| Prospective, randomized trial for treating radicular pain in patients with lumbar spinal stenosis | Epidural injection of etanercept was more effective than dexamethasone for improving low back pain, leg pain, and leg numbness (Ohtori et al., 2012) | |
| Multicenter, 3-group, RCT for treating pain due to lumbosacral radiculopathy | No improvement when compared to placebo, and had worse efficacy than epidural steroid injection in improving pain and functionality (Cohen et al., 2012) | |
| Triple-blind RCT for treating acute sciatica secondary to lumbar disc herniation | The ODI and VAS improved at 6 weeks and at a 3- month follow-up (Okoro et al., 2010) | |
| Double-blind, controlled study for treating patients with subacute lumbosacral radiculopathy | Significant improvements in leg and back pain at 6 months (Cohen et al., 2009) | |
| Open cohort, historical group controlled study for treating patients with severe sciatica | Improvement of leg pain, low back pain at 6 weeks, as well as Roland Morris disability questionnaire and the ODI (Genevay et al., 2004) | |
| Adalimumab (Anti-TNF-α antibody) | Multicenter, double-blind, RCT for treating acute and severe sciatica and imaging-confirmed lumbar disc herniation | Small but significant improvement in sciatica, and fewer surgical procedures in short term, and markedly reduced back surgery in a 3-year follow-up (Genevay et al., 2010; Genevay et al., 2012) |
| Infliximab (Anti-TNF-α antibody) | RCT for treating patients with acute/subacute sciatica due to herniated disc | No difference compared to placebo group in improving pain at 1 year, but shortened symptom duration and less straight leg raising restriction in cases of L4- L5/L3-L4 herniation with Modic change (Korhonen et al., 2006) |
| IL-1Ra-enriched Authologous Conditioned Serum (ACS; Orthokine) | Prospective, double-blind, reference-controlled, single center, investigator-initiated study for treatment of lumbar back pain | Significant clinical improvements and VAS score, and reduction in pain and disability, ACS showed statistically significant superiority over triamcinolone at week 22 (Becker et al., 2007) |
| Bioclavid (Amoxicillin-clavulanate) | Double-blind, RCT for treatment of chronic low back pain and Modic type 1 changes Pilot prospective uncontrolled study for treating patients with low back pain and Modic type 1 changes | Statistically significant improvement on lumbar pain, leg pain, and disease-specific disability in antibiotic- treated group at 1 year (Albert et al., 2013) Clinically and statistically significant improvement in low back pain intensity, number of days with pain, disease-specific and patient-specific function, and global perceived effect at long-term follow up (mean 10.8 months) (Albert et al., 2008) |
Abbreviations: RCT, randomized controlled trial; VAS, visual analog scale; ODI, Oswestry disability index
Current treatment modalities for disc degeneration and LBP are limited and lack efficacy (Martin et al., 2008; Williams et al., 2014). Therefore, these new, targeted therapeutic strategies hold much promise. Careful consideration must be taken, however, when using anti-cytokine treatment. As mentioned above, TNF-α and IL-1β play necessary positive roles in natural resolution of NP herniation. Additionally, also mentioned above, even transient exposure to LPS or TNF-α results in lasting biophysical changes in NP cells (Maidhof et al., 2014). In light of this evidence, the timing of targeted anti-inflammatory treatment modalities is likely to have a critical impact on overall efficacy and success of therapy. Although a definitive “cure” for disc degeneration is far in the future, current research is providing hope for researchers and clinicians for improving patient outcomes for degenerative disc disease and LBP.
Acknowledgments
Research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Numbers R01AR055655, R01AR064733, R01AR050087, T32AR052273 (ZIJ) and F30AR066506 (ZRS). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- Adams MA, Freeman BJ, Morrison HP, Nelson IW, Dolan P. Mechanical initiation of intervertebral disc degeneration. Spine (Phila Pa 1976) 2000;25:1625–36. doi: 10.1097/00007632-200007010-00005. [DOI] [PubMed] [Google Scholar]
- Adams MA, Roughley PJ. What is intervertebral disc degeneration, and what causes it? Spine (Phila Pa 1976) 2006;31:2151–2161. doi: 10.1097/01.brs.0000231761.73859.2c. [DOI] [PubMed] [Google Scholar]
- Agarwal V, Golish SR, Alamin TF. Bacteriologic culture of excised intervertebral disc from immunocompetent patients undergoing single level primary lumbar microdiscectomy. J Spinal Disord Tech. 2011;24:397–400. doi: 10.1097/BSD.0b013e3182019f3a. [DOI] [PubMed] [Google Scholar]
- Albert HB, Kjaer P, Jensen TS, Sorensen JS, Bendix T, Manniche C. Modic changes, possible causes and relation to low back pain. Med Hypotheses. 2008a;70:361–368. doi: 10.1016/j.mehy.2007.05.014. [DOI] [PubMed] [Google Scholar]
- Albert HB, Lambert P, Rollason J, Sorensen JS, Worthington T, Pedersen MB, Norgaard HS, Vernallis A, Busch F, Manniche C, Elliott T. Does nuclear tissue infected with bacteria following disc herniations lead to Modic changes in the adjacent vertebrae? Eur Spine J. 2013a;22:690–696. doi: 10.1007/s00586-013-2674-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert HB, Manniche C, Sorensen JS, Deleuran BW. Antibiotic treatment in patients with low-back pain associated with Modic changes Type 1 (bone oedema): a pilot study. Br J Sports Med. 2008b;42:969–973. doi: 10.1136/bjsm.2008.050369. [DOI] [PubMed] [Google Scholar]
- Albert HB, Sorensen JS, Christensen BS, Manniche C. Antibiotic treatment in patients with chronic low back pain and vertebral bone edema (Modic type 1 changes): A double-blind randomized clinical controlled trial of efficacy. Eur Spine J. 2013b;22:697–707. doi: 10.1007/s00586-013-2675-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson DG, Li X, Balian G. A fibronectin fragment alters the metabolism by rabbit intervertebral disc cells in vitro. Spine (Phila Pa 1976) 2005;30:1242–6. doi: 10.1097/01.brs.0000164097.47091.4c. [DOI] [PubMed] [Google Scholar]
- Andrade P, Visser-Vandewalle V, Philippens M, Daemen MA, Steinbusch HWM, Buurman WA, Hoogland G. Tumor necrosis factor-α levels correlate with postoperative pain severity in lumbar disc hernia patients: Opposite clinical effects between tumor necrosis factor receptor 1 and 2. Pain. 2011;152:2645–2652. doi: 10.1016/j.pain.2011.08.012. [DOI] [PubMed] [Google Scholar]
- Arndt J, Charles YP, Koebel C, Bogorin I, Steib J-P. Bacteriology of Degenerated Lumbar Intervertebral Disks. J Spinal Disord Tech. 2012;25:E211–E216. doi: 10.1097/BSD.0b013e318269851a. [DOI] [PubMed] [Google Scholar]
- Bachmeier BE, Nerlich A, Mittermaier N, Weiler C, Lumenta C, Wuertz K, Boos N. Matrix metalloproteinase expression levels suggest distinct enzyme roles during lumbar disc herniation and degeneration. Eur Spine J. 2009;18:1573–1586. doi: 10.1007/s00586-009-1031-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmeier BE, Nerlich AG, Weiler C, Paesold G, Jochum M, Boos N. Analysis of tissue distribution of TNF-α, TNF-α-receptors, and the activating TNF-α-converting enzyme suggests activation of the TNF-α system in the aging intervertebral disc. Ann N Y Acad Sci. 2007;1096:44–54. doi: 10.1196/annals.1397.069. [DOI] [PubMed] [Google Scholar]
- Becker C, Heidersdorf S, Drewlo S, de Rodriguez SZ, Krämer J, Willburger RE. Efficacy of epidural perineural injections with autologous conditioned serum for lumbar radicular compression: an investigator-initiated, prospective, double-blind, reference-controlled study. Spine (Phila Pa 1976) 2007;32:1803–1808. doi: 10.1097/BRS.0b013e3181076514. [DOI] [PubMed] [Google Scholar]
- Ben-Galim P, Rand N, Giladi M, Schwartz D, Ashkenazi E, Millgram M, Dekel S, Floman Y. Association between sciatica and microbial infection: true infection or culture contamination? Spine (Phila Pa 1976) 2006;31:2507–2509. doi: 10.1097/01.brs.0000238657.13263.b2. [DOI] [PubMed] [Google Scholar]
- Black RA, Rauch CT, Kozlosky CJ, Peschon JJ, Slack JL, Wolfson MF, Castner BJ, Stocking KL, Reddy P, Srinivasan S, Nelson N, Boiani N, Schooley Ka, Gerhart M, Davis R, Fitzner JN, Johnson RS, Paxton RJ, March CJ, Cerretti DP. A metalloproteinase disintegrin that releases tumour-necrosis factor-alpha from cells. Nature. 1997;385:729–733. doi: 10.1038/385729a0. [DOI] [PubMed] [Google Scholar]
- Brown S, Melrose J, Caterson B, Roughley P, Eisenstein SM, Roberts S. A comparative evaluation of the small leucine-rich proteoglycans of pathological human intervertebral discs. Eur Spine J. 2012;21:S154–159. doi: 10.1007/s00586-012-2179-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabal-Hierro L, Lazo PS. Signal transduction by tumor necrosis factor receptors. Cell Signal. 2012;24:1297–1305. doi: 10.1016/j.cellsig.2012.02.006. [DOI] [PubMed] [Google Scholar]
- Carricajo A, Nuti C, Aubert E, Hatem O, Fonsale N, Mallaval FO, Vautrin aC, Brunon J, Aubert G. Propionibacterium acnes contamination in lumbar disc surgery. J Hosp Infect. 2007;66:275–277. doi: 10.1016/j.jhin.2007.04.007. [DOI] [PubMed] [Google Scholar]
- Casellas F, Borruel N, Papo M, Guarner F, Antolín M, Videla S, Malagelada JR. Antiinflammatory effects of enterically coated amoxicillin-clavulanic acid in active ulcerative colitis. Inflamm Bowel Dis. 1998;4:1–5. doi: 10.1097/00054725-199802000-00001. [DOI] [PubMed] [Google Scholar]
- Choi H, Johnson ZI, Risbud MV. Understanding Nucleus Pulposus Cell Phenotype: A Prerequisite for Stem Cell Based Therapies to Treat Intervertebral Disc Degeneration. Curr Stem Cell Res Ther. 2015 doi: 10.2174/1574888x10666150113112149. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou D, Samartzis D, Bellabarba C, Patel A, Luk KDK, Kisser JMS, Skelly AC. Degenerative magnetic resonance imaging changes in patients with chronic low back pain: a systematic review. Spine (Phila Pa 1976) 2011;36:S43–53. doi: 10.1097/BRS.0b013e31822ef700. [DOI] [PubMed] [Google Scholar]
- Cohen SP, Bogduk N, Dragovich A, Buckenmaier CC, Griffith S, Kurihara C, Raymond J, Richter PJ, Williams N, Yaksh TL. Randomized, double-blind, placebo-controlled, dose-response, and preclinical safety study of transforaminal epidural etanercept for the treatment of sciatica. Anesthesiology. 2009;110:1116–1126. doi: 10.1097/ALN.0b013e3181a05aa0. [DOI] [PubMed] [Google Scholar]
- Cohen SP, White RL, Kurihara C, Larkin TM, Chang A, Griffith SR, Gilligan C, Larkin R, Morlando B, Pasquina PF, Yaksh TL, Nguyen C. Epidural steroids, etanercept, or saline in subacute sciatica: a multicenter, randomized trial. Ann Intern Med. 2012;156:551–559. doi: 10.7326/0003-4819-156-8-201204170-00397. [DOI] [PubMed] [Google Scholar]
- Doita M, Kanatani T, Ozaki T, Matsui N, Kurosaka M, Yoshiya S. Influence of macrophage infiltration of herniated disc tissue on the production of matrix metalloproteinases leading to disc resorption. Spine (Phila Pa 1976) 2001;26:1522–1527. doi: 10.1097/00007632-200107150-00004. [DOI] [PubMed] [Google Scholar]
- Elfervig MK, Minchew JT, Francke E, Tsuzaki M, Banes AJ. IL-1β sensitizes intervertebral disc annulus cells to fluid-induced shear stress. J Cell Biochem. 2001;82:290–298. doi: 10.1002/jcb.1153. [DOI] [PubMed] [Google Scholar]
- Ellman MB, Kim JS, An HS, Chen D, Kc R, An J, Dittakavi T, van Wijnen AJ, Cs-Szabo G, Li X, Xiao G, An S, Kim SG, Im HJ. Toll-like receptor adaptor signaling molecule MyD88 on intervertebral disk homeostasis: In vitro, ex vivo studies. Gene. 2012;505:283–290. doi: 10.1016/j.gene.2012.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman BJC, Ludbrook GL, Hall S, Cousins M, Mitchell B, Jaros M, Wyand M, Gorman JR. Randomized, double-blind, placebo-controlled, trial of transforaminal epidural etanercept for the treatment of symptomatic lumbar disc herniation. Spine (Phila Pa 1976) 2013;38:1986–1994. doi: 10.1097/01.brs.0000435140.61593.4c. [DOI] [PubMed] [Google Scholar]
- Fritzell P, Bergström T, Welinder-Olsson C. Detection of bacterial DNA in painful degenerated spinal discs in patients without signs of clinical infection. Eur Spine J. 2004;13:702–706. doi: 10.1007/s00586-004-0719-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita N, Gogate SS, Chiba K, Toyama Y, Shapiro IM, Risbud MV. Prolyl hydroxylase 3 (PHD3) modulates catabolic effects of tumor necrosis factor-α (TNF-α) on cells of the nucleus pulposus through co-activation of nuclear factor κB (NF-κB)/p65 signaling. J Biol Chem. 2012;287:39942–39953. doi: 10.1074/jbc.M112.375964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabay C, Lamacchia C, Palmer G. IL-1 pathways in inflammation and human diseases. Nat Rev Rheumatol. 2010;6:232–241. doi: 10.1038/nrrheum.2010.4. [DOI] [PubMed] [Google Scholar]
- Gawri R, Rosenzweig DH, Krock E, Ouellet JA, Stone LS, Quinn TM, Haglund L. High mechanical strain of primary intervertebral disc cells promotes secretion of inflammatory factors associated with disc degeneration and pain. Arthritis Res Ther. 2014;16:R21. doi: 10.1186/ar4449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genevay S, Finckh A, Mezin F, Tessitore E, Guerne PA. Influence of cytokine inhibitors on concentration and activity of MMP-1 and MMP-3 in disc herniation. Arthritis Res Ther. 2009;11:R169. doi: 10.1186/ar2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genevay S, Finckh A, Zufferey P, Viatte S, Balagué F, Gabay C. Adalimumab in acute sciatica reduces the long-term need for surgery: a 3-year follow-up of a randomised double-blind placebo-controlled trial. Ann Rheum Dis. 2012;71:560–562. doi: 10.1136/annrheumdis-2011-200373. [DOI] [PubMed] [Google Scholar]
- Genevay S, Stingelin S, Gabay C. Efficacy of etanercept in the treatment of acute, severe sciatica: a pilot study. Ann Rheum Dis. 2004;63:1120–1123. doi: 10.1136/ard.2003.016451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genevay S, Viatte S, Finckh A, Zufferey P, Balagué F, Gabay C. Adalimumab in severe and acute sciatica: a multicenter, randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2010;62:2339–2346. doi: 10.1002/art.27499. [DOI] [PubMed] [Google Scholar]
- Gorth DJ, Mauck RL, Chiaro JA, Mohanraj B, Hebela NM, Dodge GR, Elliott DM, Smith LJ. IL-1Ra delivered from poly(lactic-co-glycolic acid) microspheres attenuates IL-1β mediated degradation of nucleus pulposus in vitro. Arthritis Res Ther. 2012;14:R179. doi: 10.1186/ar3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grönblad M, Virri J, Tolonen J, Seitsalo S, Kääpä E, Kankare J, Myllynen P, Karaharju EO. A controlled immunohistochemical study of inflammatory cells in disc herniation tissue. Spine (Phila Pa 1976) 1994;19:2744–2751. doi: 10.1097/00007632-199412150-00002. [DOI] [PubMed] [Google Scholar]
- Gruber HE, Hoelscher GL, Ingram JA, Bethea S, Norton HJ, Hanley EN. Production and expression of RANTES (CCL5) by human disc cells and modulation by IL-1β and TNF-α in 3D culture. Exp Mol Pathol. 2014a;96:133–138. doi: 10.1016/j.yexmp.2014.01.002. [DOI] [PubMed] [Google Scholar]
- Gruber HE, Hoelscher GL, Ingram JA, Bethea S, Cox M, Hanley EN. Proinflammatory cytokines modulate the chemokine CCL2 (MCP-1) in human annulus cells in vitro: CCL2 expression and production. Exp Mol Pathol. 2015;98:102–105. doi: 10.1016/j.yexmp.2014.12.002. [DOI] [PubMed] [Google Scholar]
- Gruber HE, Hoelscher GL, Ingram JA, Bethea S, Hanley EN. Growth and differentiation factor-5 (GDF-5) in the human intervertebral annulus cells and its modulation by IL-1β and TNF-α in vitro. Exp Mol Pathol. 2014b;96:225–229. doi: 10.1016/j.yexmp.2014.02.005. [DOI] [PubMed] [Google Scholar]
- Gruber HE, Hoelscher GL, Ingram JA, Norton HJ, Hanley EN. Increased IL-17 expression in degenerated human discs and increased production in cultured annulus cells exposed to IL-1β and TNF-α. Biotech Histochem. 2013;88:302–310. doi: 10.3109/10520295.2013.783235. [DOI] [PubMed] [Google Scholar]
- Gu S-X, Li X, Hamilton JL, Chee A, Kc R, Chen D, An HS, Kim J-S, Oh C, Ma Y-Z, van Wijnen AJ, Im H-J. MicroRNA-146a reduces IL-1 dependent inflammatory responses in the intervertebral disc. Gene. 2015;555:80–87. doi: 10.1016/j.gene.2014.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guterl CC, See EY, Blanquer SBG, Pandit A, Ferguson SJ, Benneker LM, Grijpma DW, Sakai D, Eglin D, Alini M, Iatridis JC, Grad S. Challenges and strategies in the repair of ruptured annulus fibrosus. Eur Cell Mater. 2013;25:1–21. doi: 10.22203/ecm.v025a01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häcker H, Redecke V, Blagoev B, Kratchmarova I, Hsu L-C, Wang GG, Kamps MP, Raz E, Wagner H, Häcker G, Mann M, Karin M. Specificity in Toll-like receptor signalling through distinct effector functions of TRAF3 and TRAF6. Nature. 2006;439:204–207. doi: 10.1038/nature04369. [DOI] [PubMed] [Google Scholar]
- Hajhashemi V, Dehdashti Kh. Antinociceptive effect of clavulanic acid and its preventive activity against development of morphine tolerance and dependence in animal models. Res Pharm Sci. 2014;9:315–21. [PMC free article] [PubMed] [Google Scholar]
- Haro H, Crawford HC, Fingleton B, Shinomiya K, Spengler DM, Matrisian LM. Matrix metalloproteinase-7-dependent release of tumor necrosis factor-α in a model of herniated disc resorption. J Clin Invest. 2000;105:143–150. doi: 10.1172/JCI7091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiyama A, Skubutyte R, Markova D, Anderson DG, Yadla S, Sakai D, Mochida J, Albert TJ, Shapiro IM, Risbud MV. Hypoxia activates the notch signaling pathway in cells of the intervertebral disc: Implications in degenerative disc disease. Arthritis Rheum. 2011;63:1355–1364. doi: 10.1002/art.30246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiyama A, Yokoyama K, Nukaga T, Sakai D, Mochida J. A complex interaction between Wnt signaling and TNF-α in nucleus pulposus cells. Arthritis Res Ther. 2013;15:R189. doi: 10.1186/ar4379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoy D, Bain C, Williams G, March L, Brooks P, Blyth F, Woolf A, Vos T, Buchbinder R. A systematic review of the global prevalence of low back pain. Arthritis Rheum. 2012;64:2028–2037. doi: 10.1002/art.34347. [DOI] [PubMed] [Google Scholar]
- Hoyland JA, Le Maitre CL, Freemont AJ. Investigation of the role of IL-1 and TNF in matrix degradation in the intervertebral disc. Rheumatology. 2008;47:809–814. doi: 10.1093/rheumatology/ken056. [DOI] [PubMed] [Google Scholar]
- latridis JC, Godburn K, Wuertz K, Alini M, Roughley PJ. Region-dependent aggrecan degradation patterns in the rat intervertebral disc are affected by mechanical loading in vivo. Spine (Phila Pa 1976) 2011;36:203–9. doi: 10.1097/BRS.0b013e3181cec247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwabuchi S, Ito M, Chikanishi T, Azuma Y, Haro H. Role of the tumor necrosis factor-alpha, cyclooxygenase-2, prostaglandin E2, and effect of low-intensity pulsed ultrasound in an in vitro herniated disc resorption model. J Orthop Res. 2008;26:1274–1278. doi: 10.1002/jor.20525. [DOI] [PubMed] [Google Scholar]
- Jensen TS, Karppinen J, Sorensen JS, Niinimäki J, Leboeuf-Yde C. Vertebral endplate signal changes (Modic change): A systematic literature review of prevalence and association with non-specific low back pain. Eur Spine J. 2008;17:1407–1422. doi: 10.1007/s00586-008-0770-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimbo K, Park JS, Yokosuka K, Sato K, Nagata K. Positive feedback loop of interleukin-1β upregulating production of inflammatory mediators in human intervertebral disc cells in vitro. J Neurosurg Spine. 2005;2:589–595. doi: 10.3171/spi.2005.2.5.0589. [DOI] [PubMed] [Google Scholar]
- Johnson ZI, Gogate SS, Day R, Binch A, Markova DZ, Chiverton N, Cole A, Conner M, Shapiro IM, Le Maitre CL, Risbud MV. Aquaporin 1 and 5 expression decreases during human intervertebral disc degeneration: novel HIF-1-mediated regulation of aquaporins in NP cells. Oncotarget. 2015 doi: 10.18632/oncotarget.3631. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson ZI, Shapiro IM, Risbud MV. Extracellular osmolarity regulates matrix homeostasis in the intervertebral disc and articular cartilage: Evolving role of TonEBP. Matrix Biol. 2014;40:10–16. doi: 10.1016/j.matbio.2014.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang R, Li H, Rickers K, Ringgaard S, Xie L, Bünger C. Intervertebral disc degenerative changes after intradiscal injection of TNF-α in a porcine model. Eur Spine J. 2015 doi: 10.1007/s00586-015-3926-x. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Katz JN. Lumbar disc disorders and low-back pain: socioeconomic factors and consequences. J Bone Joint Surg Am. 2006;88(Suppl 2):21–24. doi: 10.2106/JBJS.E.01273. [DOI] [PubMed] [Google Scholar]
- Kawai T, Adachi O, Ogawa T, Takeda K, Akira S. Unresponsiveness of MyD88-deficient mice to endotoxin. Immunity. 1999;11:115–122. doi: 10.1016/s1074-7613(00)80086-2. [DOI] [PubMed] [Google Scholar]
- Kepler CK, Markova D, Hilibrand AS, Vaccaro AR, Risbud MV, Albert TJ, Anderson DG. Substance P Stimulates Production of Inflammatory Cytokines in Human Disc Cells. Spine J. 2012;12:S109. doi: 10.1097/BRS.0b013e3182a42bc2. [DOI] [PubMed] [Google Scholar]
- Kepler CK, Markova DZ, Dibra F, Yadla S, Vaccaro AR, Risbud MV, Albert TJ, Anderson DG. Expression and relationship of proinflammatory chemokine RANTES/CCL5 and cytokine IL-1β in painful human intervertebral discs. Spine (Phila Pa 1976) 2013;38:873–80. doi: 10.1097/BRS.0b013e318285ae08. [DOI] [PubMed] [Google Scholar]
- Kim H-J, Yeom JS, Koh Y-G, Yeo J-E, Kang K-T, Kang Y-M, Chang B-S, Lee C-K. Anti-inflammatory effect of platelet-rich plasma on nucleus pulposus cells with response of TNF-α and IL-1. J Orthop Res. 2014;32:551–6. doi: 10.1002/jor.22532. [DOI] [PubMed] [Google Scholar]
- Kim S, Takahashi H, Lin W-W, Descargues P, Grivennikov S, Kim Y, Luo J-L, Karin M. Carcinoma-produced factors activate myeloid cells through TLR2 to stimulate metastasis. Nature. 2009;457:102–106. doi: 10.1038/nature07623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klawitter M, Hakozaki M, Kobayashi H, Krupkova O, Quero L, Ospelt C, Gay S, Hausmann O, Liebscher T, Meier U, Sekiguchi M, Konno S-I, Boos N, Ferguson SJ, Wuertz K. Expression and regulation of toll-like receptors (TLRs) in human intervertebral disc cells. Eur Spine J. 2014;30:1878–1891. doi: 10.1007/s00586-014-3442-4. [DOI] [PubMed] [Google Scholar]
- Korhonen T, Karppinen J, Paimela L, Malmivaara A, Lindgren K-A, Bowman C, Hammond A, Kirkham B, Järvinen S, Niinimäki J, Veeger N, Haapea M, Torkki M, Tervonen O, Seitsalo S, Hurri H. The treatment of disc-herniation-induced sciatica with infliximab: one-year follow-up results of FIRST II, a randomized controlled trial. Spine (Phila Pa 1976) 2006;31:2759–2766. doi: 10.1097/01.brs.0000245873.23876.1e. [DOI] [PubMed] [Google Scholar]
- Krupkova O, Sekiguchi M, Klasen J, Hausmann O, Konno S, Ferguson SJ, Wuertz-Kozak K. Epigallocatechin 3-gallate suppresses interleukin-1β-induced inflammatory responses in intervertebral disc cells in vitro and reduces radiculopathic pain in rats. Eur Cell Mater. 2014;28:372–386. doi: 10.22203/ecm.v028a26. [DOI] [PubMed] [Google Scholar]
- Kumar H, Kawai T, Akira S. Toll-like receptors and innate immunity. Biochem Biophys Res Commun. 2009;388:621–625. doi: 10.1016/j.bbrc.2009.08.062. [DOI] [PubMed] [Google Scholar]
- Li J, Yuan W, Jiang S, Ye W, Yang H, Shapiro IM, Risbud MV. Prolyl-4-hydroxylase Domain Protein 2 Controls NF-κB/p65 Transactivation and Enhances the Catabolic Effects of Inflammatory Cytokines on cells of the Nucleus Pulposus. J Biol Chem. 2015;290:7195–7207. doi: 10.1074/jbc.M114.611483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Leo BM, Beck G, Balian G, Anderson GD. Collagen and proteoglycan abnormalities in the GDF-5-deficient mice and molecular changes when treating disk cells with recombinant growth factor. Spine (Phila Pa 1976) 2004;29:2229–2234. doi: 10.1097/01.brs.0000142427.82605.fb. [DOI] [PubMed] [Google Scholar]
- Liu H, Pan H, Yang H, Wang J, Zhang K, Li X, Wang H, Ding W, Li B, Zheng Z. LIM mineralization protein-1 suppresses TNF-α induced intervertebral disc degeneration by maintaining nucleus pulposus extracellular matrix production and inhibiting matrix metalloproteinases expression. J Orthop Res. 2015;33:294–303. doi: 10.1002/jor.22732. [DOI] [PubMed] [Google Scholar]
- Livshits G, Popham M, Malkin I, Sambrook PN, Macgregor AJ, Spector T, Williams FMK. Lumbar disc degeneration and genetic factors are the main risk factors for low back pain in women: the UK Twin Spine Study. Ann Rheum Dis. 2011;70:1740–1745. doi: 10.1136/ard.2010.137836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Maitre CL, Freemont AJ, Hoyland JA. The role of interleukin-1 in the pathogenesis of human intervertebral disc degeneration. Arthritis Res Ther. 2005;7:R732–R745. doi: 10.1186/ar1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Maitre CL, Freemont AJ, Hoyland JA. A preliminary in vitro study into the use of IL-1Ra gene therapy for the inhibition of intervertebral disc degeneration. Int J Exp Pathol. 2006;87:17–28. doi: 10.1111/j.0959-9673.2006.00449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Maitre CL, Hoyland JA, Freemont AJ. Interleukin-1 receptor antagonist delivered directly and by gene therapy inhibits matrix degradation in the intact degenerate human intervertebral disc: an in situ zymographic and gene therapy study. Arthritis Res Ther. 2007a;9:R83. doi: 10.1186/ar2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Maitre CL, Hoyland JA, Freemont AJ. Catabolic cytokine expression in degenerate and herniated human intervertebral discs: IL-1beta and TNFalpha expression profile. Arthritis Res Ther. 2007b;9:R77. doi: 10.1186/ar2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maidhof R, Jacobsen T, Papatheodorou A, Chahine NO. Inflammation induces irreversible biophysical changes in isolated nucleus pulposus cells. PLoS One. 2014;9:e99621. doi: 10.1371/journal.pone.0099621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin BI, Deyo RA, Mirza SK, Turner JA, Comstock BA, Hollingworth W, Sullivan SD. Expenditures and health status among adults with back and neck problems. JAMA. 2008;299:656–664. doi: 10.1001/jama.299.6.656. [DOI] [PubMed] [Google Scholar]
- McLorinan GC, Glenn JV, McMullan MG, Patrick S. Propionibacterium acnes wound contamination at the time of spinal surgery. Clin Orthop Relat Res. 2005;437:67–73. doi: 10.1097/00003086-200508000-00012. [DOI] [PubMed] [Google Scholar]
- Murata Y, Onda A, Rydevik B, Takahashi I, Takahashi K, Olmarker K. Changes in pain behavior and histologic changes caused by application of tumor necrosis factor-alpha to the dorsal root ganglion in rats. Spine (Phila Pa 1976) 2006;31:530–535. doi: 10.1097/01.brs.0000201260.10082.23. [DOI] [PubMed] [Google Scholar]
- Murata Y, Onda A, Rydevik B, Takahashi K, Olmarker K. Distribution and appearance of tumor necrosis factor-alpha in the dorsal root ganglion exposed to experimental disc herniation in rats. Spine (Phila Pa 1976) 2004;29:2235–2241. doi: 10.1097/01.brs.0000142223.30453.e5. [DOI] [PubMed] [Google Scholar]
- Mwale F, Roughley P, Antoniou J. Distinction between the extracellular matrix of the nucleus pulposus and hyaline cartilage: a requisite for tissue engineering of intervertebral disc. Eur Cell Mater. 2004;8:58–63. doi: 10.22203/ecm.v008a06. discussion 63-4. [DOI] [PubMed] [Google Scholar]
- De Nardo D. Toll-like receptors: Activation, signalling and transcriptional modulation. Cytokine. 2015 doi: 10.1016/j.cyto.2015.02.025. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Oda H, Matsuzaki H, Tokuhashi Y, Wakabayashi K, Uematsu Y, Iwahashi M. Degeneration of intervertebral discs due to smoking: Experimental assessment in a rat-smoking model. J Orthop Sci. 2004;9:135–141. doi: 10.1007/s00776-003-0759-y. [DOI] [PubMed] [Google Scholar]
- Ohtori S, Inoue G, Eguchi Y, Orita S, Takaso M, Ochiai N, Kishida S, Kuniyoshi K, Aoki Y, Nakamura J, Ishikawa T, Arai G, Miyagi M, Kamoda H, Suzuki M, Sakuma Y, Oikawa Y, Kubota G, Inage K, Sainoh T, Toyone T, Yamauchi K, Kotani T, Akazawa T, Minami S, Takahashi K. Tumor Necrosis Factor-α-Immunoreactive Cells in Nucleus Pulposus in Adolescent Patients With Lumbar Disc Herniation. Spine (Phila Pa 1976) 2012;38:459–462. doi: 10.1097/BRS.0b013e3182739cb4. [DOI] [PubMed] [Google Scholar]
- Ohtori S, Miyagi M, Eguchi Y, Inoue G, Orita S, Ochiai N, Kishida S, Kuniyoshi K, Nakamura J, Aoki Y, Ishikawa T, Arai G, Kamoda H, Suzuki M, Takaso M, Furuya T, Toyone T, Takahashi K. Epidural administration of spinal nerves with the tumor necrosis factor-alpha inhibitor, etanercept, compared with dexamethasone for treatment of sciatica in patients with lumbar spinal stenosis: a prospective randomized study. Spine (Phila Pa 1976) 2012;37:439–444. doi: 10.1097/BRS.0b013e318238af83. [DOI] [PubMed] [Google Scholar]
- Okamura Y, Watari M, Jerud ES, Young DW, Ishizaka ST, Rose J, Chow JC, Strauss JF. The Extra Domain A of Fibronectin Activates Toll-like Receptor 4. J Biol Chem. 2001;276:10229–10233. doi: 10.1074/jbc.M100099200. [DOI] [PubMed] [Google Scholar]
- Okoro T, Tafazal SI, Longworth S, Sell PJ. Tumor necrosis alpha-blocking agent (etanercept): a triple blind randomized controlled trial of its use in treatment of sciatica. J Spinal Disord Tech. 2010;23:74–77. doi: 10.1097/BSD.0b013e31819afdc4. [DOI] [PubMed] [Google Scholar]
- Phillips KLE, Cullen K, Chiverton N, Michael ALR, Cole AA, Breakwell LM, Haddock G, Bunning RAD, Cross AK, Le Maitre CL. Potential roles of cytokines and chemokines in human intervertebral disc degeneration: interleukin-1 is a master regulator of catabolic processes. Osteoarthr Cartil. 2015 doi: 10.1016/j.joca.2015.02.017. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Phillips KLE, Jordan-Mahy N, Nicklin MJH, Le Maitre CL. Interleukin-1 receptor antagonist deficient mice provide insights into pathogenesis of human intervertebral disc degeneration. Ann Rheum Dis. 2013;72:1860–1867. doi: 10.1136/annrheumdis-2012-202266. [DOI] [PubMed] [Google Scholar]
- Pockert AJ, Richardson SM, Le Maitre CL, Lyon M, Deakin JA, Buttle DJ, Freemont AJ, Hoyland JA. Modified expression of the ADAMTS enzymes and tissue inhibitor of metalloproteinases 3 during human intervertebral disc degeneration. Arthritis Rheum. 2009;60:482–491. doi: 10.1002/art.24291. [DOI] [PubMed] [Google Scholar]
- Poltorak A, He X, Smirnova I, Liu MY, Van Huffel C, Du X, Birdwell D, Alejos E, Silva M, Galanos C, Freudenberg M, Ricciardi-Castagnoli P, Layton B, Beutler B. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- Ponnappan RK, Markova DZ, Antonio PJ, Murray HB, Vaccaro AR, Shapiro IM, Anderson DG, Albert TJ, Risbud MV. An organ culture system to model early degenerative changes of the intervertebral disc. Arthritis Res Ther. 2011;13:R171. doi: 10.1186/ar3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purmessur D, Walter BA, Roughley PJ, Laudier DM, Hecht AC, Iatridis J. A role for TNFα in intervertebral disc degeneration: A non-recoverable catabolic shift. Biochem Biophys Res Commun. 2013;433:151–156. doi: 10.1016/j.bbrc.2013.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quero L, Klawitter M, Schmaus A, Rothley M, Sleeman J, Tiaden AN, Klasen J, Boos N, Hottiger MO, Wuertz K, Richards PJ. Hyaluronic acid fragments enhance the inflammatory and catabolic response in human intervertebral disc cells through modulation of toll-like receptor 2 signalling pathways. Arthritis Res Ther. 2013;15:R94. doi: 10.1186/ar4274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajan N, Bloom O, Maidhof R, Stetson N, Sherry B, Levine M, Chahine NO. Toll-Like Receptor 4 (TLR4) Expression and Stimulation in a Model of Intervertebral Disc Inflammation and Degeneration. Spine (Phila Pa 1976) 2012;38:1343–1351. doi: 10.1097/BRS.0b013e31826b71f4. [DOI] [PubMed] [Google Scholar]
- Rajasekaran S, Bajaj N, Tubaki V, Kanna RM, Shetty AP. ISSLS Prize winner: The anatomy of failure in lumbar disc herniation: an in vivo, multimodal, prospective study of 181 subjects. Spine (Phila Pa 1976) 2013;38:1491–500. doi: 10.1097/BRS.0b013e31829a6fa6. [DOI] [PubMed] [Google Scholar]
- Risbud MV, Schipani E, Shapiro IM. Hypoxic regulation of nucleus pulposus cell survival: from niche to notch. Am J Pathol. 2010;176:1577–1583. doi: 10.2353/ajpath.2010.090734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risbud MV, Schoepflin ZR, Mwale F, Kandel RA, Grad S, Iatridis JC, Sakai D, Hoyland JA. Defining the phenotype of young healthy nucleus pulposus cells: Recommendations of the Spine Research Interest Group at the 2014 annual ORS meeting. J Orthop Res. 2015;33:283–293. doi: 10.1002/jor.22789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risbud MV, Shapiro IM. Notochordal cells in the adult intervertebral disc: new perspective on an old question. Crit Rev Eukaryot Gene Expr. 2011;21:29–41. doi: 10.1615/critreveukargeneexpr.v21.i1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risbud MV, Shapiro IM. Role of cytokines in intervertebral disc degeneration: pain and disc content. Nat Rev Rheumatol. 2014;10:44–56. doi: 10.1038/nrrheum.2013.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roughley PJ. Biology of intervertebral disc aging and degeneration: involvement of the extracellular matrix. Spine (Phila Pa 1976) 2004;29:2691–2699. doi: 10.1097/01.brs.0000146101.53784.b1. [DOI] [PubMed] [Google Scholar]
- Ruel N, Markova DZ, Adams SL, Scanzello C, Cs-Szabo G, Gerard D, Shi P, Anderson DG, Zack M, An HS, Chen D, Zhang Y. Fibronectin Fragments and the Cleaving Enzyme ADAM-8 in the Degenerative Human Intervertebral Disc. Spine (Phila Pa 1976) 2014;39:1274–1279. doi: 10.1097/BRS.0000000000000397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato S, Sugiyama M, Yamamoto M, Watanabe Y, Kawai T, Takeda K, Akira S. Toll/IL-1 receptor domain-containing adaptor inducing IFN-beta (TRIF) associates with TNF receptor-associated factor 6 and TANK-binding kinase 1, and activates two distinct transcription factors, NF-kappa B and IFN-regulatory factor-3, in the Toll-like receptor signaling. J Immunol. 2003;171:4304–4310. doi: 10.4049/jimmunol.171.8.4304. [DOI] [PubMed] [Google Scholar]
- Schaefer L, Babelova A, Kiss E, Hausser HJ, Baliova M, Krzyzankova M, Marsche G, Young MF, Mihalik D, Götte M, Malle E, Schaefer RM, Gröne HJ. The matrix component biglycan is proinflammatory and signals through Toll-like receptors 4 and 2 in macrophages. J Clin Invest. 2005;115:2223–2233. doi: 10.1172/JCI23755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Séguin CA, Pilliar RM, Madri JA, Kandel RA. TNF-alpha induces MMP2 gelatinase activity and MT1-MMP expression in an in vitro model of nucleus pulposus tissue degeneration. Spine (Phila Pa 1976) 2008;33:356–365. doi: 10.1097/BRS.0b013e3181642a5e. [DOI] [PubMed] [Google Scholar]
- Séguin CA, Pilliar RM, Roughley PJ, Kandel RA. Tumor necrosis factor-alpha modulates matrix production and catabolism in nucleus pulposus tissue. Spine (Phila Pa 1976) 2005;30:1940–1948. doi: 10.1097/01.brs.0000176188.40263.f9. [DOI] [PubMed] [Google Scholar]
- Shamji MF, Betre H, Kraus VB, Chen J, Chilkoti A, Pichika R, Masuda K, Setton LA. Development and characterization of a fusion protein between thermally responsive elastin-like polypeptide and interleukin-1 receptor antagonist: Sustained release of a local antiinflammatory therapeutic. Arthritis Rheum. 2007;56:3650–3661. doi: 10.1002/art.22952. [DOI] [PubMed] [Google Scholar]
- Shen B, Melrose J, Ghosh P, Taylor F. Induction of matrix metalloproteinase-2 and -3 activity in ovine nucleus pulposus cells grown in three-dimensional agarose gel culture by interleukin-1beta: a potential pathway of disc degeneration. Eur Spine J. 2003;12:66–75. doi: 10.1007/s00586-002-0454-2. [DOI] [PubMed] [Google Scholar]
- Sinclair SM, Shamji MF, Chen J, Jing L, Richardson WJ, Brown CR, Fitch RD, Setton LA. Attenuation of inflammatory events in human intervertebral disc cells with a tumor necrosis factor antagonist. Spine (Phila Pa 1976) 2011;36:1190–6. doi: 10.1097/BRS.0b013e3181ebdb43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivan SS, Hayes AJ, Wachtel E, Caterson B, Merkher Y, Maroudas A, Brown S, Roberts S. Biochemical composition and turnover of the extracellular matrix of the normal and degenerate intervertebral disc. Eur Spine J. 2014;23:S344–353. doi: 10.1007/s00586-013-2767-8. [DOI] [PubMed] [Google Scholar]
- Smith LJ, Chiaro JA, Nerurkar NL, Cortes DH, Horava SD, Hebela NM, Mauck RL, Dodge GR, Elliott DM. Nucleus pulposus cells synthesize a functional extracellular matrix and respond to inflammatory cytokine challenge following long-term agarose culture. Eur Cell Mater. 2011;22:291–301. doi: 10.22203/ecm.v022a22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solovieva S, Leino-Arjas P, Saarela J, Luoma K, Raininko R, Riihimäki H. Possible association of interleukin 1 gene locus polymorphisms with low back pain. Pain. 2004;109:8–19. doi: 10.1016/j.pain.2003.10.020. [DOI] [PubMed] [Google Scholar]
- Stirling A, Worthington T, Rafiq M, Lambert PA, Elliott TSJ. Association between sciatica and Propionibacterium acnes. Lancet. 2001;357:2024–2025. doi: 10.1016/S0140-6736(00)05109-6. [DOI] [PubMed] [Google Scholar]
- Stirling AJ, Rafiq M, Mathur K, Elliott TSJ, Worthington T, Lambert PA. Association between sciatica and skin commensals. J Bone Jt Surgery, Br. 2002;84B:147. [Google Scholar]
- Takahashi H, Suguro T, Okazima Y, Motegi M, Okada Y, Kakiuchi T. Inflammatory cytokines in the herniated disc of the lumbar spine. Spine (Phila Pa 1976) 1996;21:218–224. doi: 10.1097/00007632-199601150-00011. [DOI] [PubMed] [Google Scholar]
- Tian Y, Yuan W, Fujita N, Wang J, Wang H, Shapiro IM, Risbud MV. Inflammatory cytokines associated with degenerative disc disease control aggrecanase-1 (ADAMTS-4) expression in nucleus pulposus cells through MAPK and NF-κB. Am J Pathol. 2013;182:2310–2321. doi: 10.1016/j.ajpath.2013.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran CM, Markova D, Smith HE, Susarla B, Ponnappan RK, Anderson DG, Symes A, Shapiro IM, Risbud MV. Regulation of CCN2/connective tissue growth factor expression in the nucleus pulposus of the intervertebral disc: role of Smad and activator protein 1 signaling. Arthritis Rheum. 2010;62:1983–92. doi: 10.1002/art.27445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran CM, Schoepflin ZR, Markova DZ, Kepler CK, Anderson DG, Shapiro IM, Risbud MV. CCN2 suppresses catabolic effects of interleukin-1β through α5β1 and αVβ3 Integrins in nucleus pulposus cells: Implications in intervertebral disc degeneration. J Biol Chem. 2014;289:7374–7387. doi: 10.1074/jbc.M113.526111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergroesen P-PA, Kingma I, Emanuel KS, Hoogendoorn RJW, Welting TJ, van Royen BJ, van Dieën JH, Smit TH. Mechanics and biology in intervertebral disc degeneration: a vicious circle. Osteoarthr Cartil. 2015 doi: 10.1016/j.joca.2015.03.028. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Walter BA, Likhitpanichkul M, Illien-Junger S, Roughley PJ, Hecht AC, Iatridis JC. TNFα transport induced by dynamic loading alters biomechanics of intact intervertebral discs. PLoS One. 2015a;10:e0118358. doi: 10.1371/journal.pone.0118358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter BA, Purmessur D, Likhitpanichkul M, Weinberg A, Cho SK, Qureshi SA, Hecht AC, Iatridis JC. Inflammatory Kinetics and Efficacy of Anti-inflammatory Treatments on Human Nucleus Pulposus Cells. Spine (Phila Pa 1976) 2015b doi: 10.1097/BRS.0000000000000932. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Tian Y, Wang J, Phillips KLE, Binch ALA, Dunn S, Cross A, Chiverton N, Zheng Z, Shapiro IM, Le Maitre CL, Risbud MV. Inflammatory Cytokines Induce NOTCH Signaling in Nucleus Pulposus Cells: implications in intervertebral disc degeneration. J Biol Chem. 2013a;288:16761–16774. doi: 10.1074/jbc.M112.446633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Markova D, Anderson DG, Zheng Z, Shapiro IM, Risbud MV. TNF-α and IL-1β promote a disintegrin-like and metalloprotease with thrombospondin type I motif-5-mediated aggrecan degradation through syndecan-4 in intervertebral disc. J Biol Chem. 2011;286:39738–39749. doi: 10.1074/jbc.M111.264549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Tian Y, Phillips KLE, Chiverton N, Haddock G, Bunning RA, Cross AK, Shapiro IM, Le Maitre CL, Risbud MV. Tumor necrosis factor α- And interleukin-1β-dependent induction of CCL3 expression by nucleus pulposus cells promotes macrophage migration through CCR1. Arthritis Rheum. 2013b;65:832–842. doi: 10.1002/art.37819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Wang H, Yang H, Li J, Cai Q, Shapiro IM, Risbud MV. Tumor Necrosis Factor-α- and Interleukin-1β-Dependent Matrix Metalloproteinase-3 Expression in Nucleus Pulposus Cells Requires Cooperative Signaling via Syndecan 4 and Mitogen-Activated Protein Kinase-Nuclear Factor κB Axis: Implications in Inflammatory Disc Disease. Am J Pathol. 2014a;184:2560–2572. doi: 10.1016/j.ajpath.2014.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Hutton WC, Yoon ST. Bone morphogenetic protein-7 antagonizes tumor necrosis factor-α-induced activation of nuclear factor κb and up-regulation of the ADAMTS, leading to decreased degradation of disc matrix macromolecules aggrecan and collagen II. Spine J. 2014b;14:505–512. doi: 10.1016/j.spinee.2013.08.016. [DOI] [PubMed] [Google Scholar]
- Weber A, Wasiliew P, Kracht M. Interleukin-1 (IL-1) pathway. Sci Signal. 2010;3:cm1. doi: 10.1126/scisignal.3105cm1. [DOI] [PubMed] [Google Scholar]
- Wedderkopp N, Thomsen K, Manniche C, Kolmos HJ, Secher Jensen T, de Leboeuf YC. No evidence for presence of bacteria in modic type I changes. Acta Radiol. 2009;50:65–70. doi: 10.1080/02841850802524485. [DOI] [PubMed] [Google Scholar]
- Williams CM, Maher CG, Latimer J, McLachlan AJ, Hancock MJ, Day RO, Lin C-WC. Efficacy of paracetamol for acute low-back pain: A double-blind, randomised controlled trial. Lancet. 2014;384:1586–1596. doi: 10.1016/S0140-6736(14)60805-9. [DOI] [PubMed] [Google Scholar]
- Xie M, Yang S, Win HL, Xiong L, Huang J, Zhou J. Rabbit annulus fibrosus cell apoptosis induced by mechanical overload via a mitochondrial apoptotic pathway. J Huazhong Univ Sci Technolog Med Sci. 2010;30:379–84. doi: 10.1007/s11596-010-0361-4. [DOI] [PubMed] [Google Scholar]
- Yamada K, Sudo H, Iwasaki K, Sasaki N, Higashi H, Kameda Y, Ito M, Takahata M, Abumi K, Minami A, Iwasaki N. Caspase 3 silencing inhibits biomechanical overload-induced intervertebral disk degeneration. Am J Pathol. 2014;184:753–64. doi: 10.1016/j.ajpath.2013.11.010. [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Sato S, Hemmi H, Hoshino K, Kaisho T, Sanjo H, Takeuchi O, Sugiyama M, Okabe M, Takeda K, Akira S. Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science. 2003;301:640–643. doi: 10.1126/science.1087262. [DOI] [PubMed] [Google Scholar]
- Ye S, Wang J, Yang S, Xu W, Xie M, Han K, Zhang B, Wu Z. Specific inhibitory protein Dkk-1 blocking Wnt/β-catenin signaling pathway improve protectives effect on the extracellular matrix. J Huazhong Univ Sci Technol - Med Sci. 2011;31:657–662. doi: 10.1007/s11596-011-0577-y. [DOI] [PubMed] [Google Scholar]
- Ye W, Zhou J, Markova DZ, Tian Y, Li J, Anderson DG, Shapiro IM, Risbud MV. Xylosyltransferase-1 Expression Is Refractory to Inhibition by the Inflammatory Cytokines Tumor Necrosis Factor α and IL-1β in Nucleus Pulposus Cells. Am J Pathol. 2015;185:485–495. doi: 10.1016/j.ajpath.2014.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida M, Nakamura T, Kikuchi T, Takagi K, Matsukawa A. Expression of monocyte chemoattractant protein-1 in primary cultures of rabbit intervertebral disc cells. J Orthop Res. 2002;20:1298–1304. doi: 10.1016/S0736-0266(02)00060-8. [DOI] [PubMed] [Google Scholar]
- Yoshida M, Nakamura T, Sei A, Kikuchi T, Takagi K, Matsukawa A. Intervertebral disc cells produce tumor necrosis factor alpha, interleukin-1beta, and monocyte chemoattractant protein-1 immediately after herniation: an experimental study using a new hernia model. Spine (Phila Pa 1976) 2005;30:55–61. doi: 10.1097/01.brs.0000149194.17891.bf. [DOI] [PubMed] [Google Scholar]
- You C, Zhu K, Liu X, Xi C, Zhang Z, Xu G, Yan J. Tumor Necrosis Factor-α Dependent Infiltration of Macrophages Into the Dorsal Root Ganglion in a Rat Disc Herniation Model. Spine (Phila Pa 1976) 2013;38:2003–2007. doi: 10.1097/BRS.0b013e3182a84701. [DOI] [PubMed] [Google Scholar]
- Zähringer U, Lindner B, Inamura S, Heine H, Alexander C. TLR2 - promiscuous or specific? A critical re-evaluation of a receptor expressing apparent broad specificity. Immunobiology. 2008;213:205–224. doi: 10.1016/j.imbio.2008.02.005. [DOI] [PubMed] [Google Scholar]