Abstract
Study Objectives:
To identify cross-sectional and seasonal patterns of sleep and physical activity (PA) in community-dwelling, older Icelandic adults using accelerometers.
Methods:
A seven-day free-living protocol of 244 (110 female) adults aged 79.7 ± 4.9 years was conducted as part of a larger population-based longitudinal observational-cohort study in the greater Reykjavik area of Iceland. A subpopulation (n = 72) repeated the 7-day measurement during seasonal periods with greater (13.4 ± 1.4 h) and lesser (7.7 ± 1.8 h) daylight.
Results:
Cross-sectional analyses using multiple linear regression models revealed that day length was a significant independent predictor of sleep duration, mid-sleep, and rise time (all p < 0.05). However, the actual within-individual differences in sleep patterns of the repeaters were rather subtle between periods of longer and shorter day-lengths. Compared to women, men had a shorter sleep duration (462 ± 80 vs. 487 ± 68 minutes, p = 0.008), earlier rise time, and a greater number of awakenings per night (46.5 ± 18.3 vs. 40.2 ± 15.7, p = 0.007), but sleep efficiency and onset latency were similar between the two sexes. Daily PA was also similar between men and women and between periods of longer and shorter day-lengths. BMI, age, gender, and overall PA all contributed to the variations in sleep parameters using multiple regression analysis.
Conclusions:
The sleep and PA characteristics of this unique population revealed some gender differences, but there was limited variation in response to significant daylight changes which may be due to long-term adaptation.
Citation:
Brychta RJ, Arnardottir NY, Johannsson E, Wright EC, Eiriksdottir G, Gudnason V, Marinac CR, Davis M, Koster A, Caserotti P, Sveinsson T, Harris T, Chen KY. Influence of day length and physical activity on sleep patterns in older Icelandic men and women. J Clin Sleep Med 2016;12(2):203–213.
Keywords: aging, seasonal, total sleep time, physical activity, accelerometer
INTRODUCTION
The importance of sleep to physical and metabolic health is well documented.1,2 Although sleep needs are thought to be independent of age, older people often have more sleep problems, such as premature awakening, fragmented sleep patterns, and reduced depth of sleep.3–5 Poor sleep quality among older adults has been associated with declines in both physical and mental function and increased risk of all-cause-mortality.2,6–9 Thus, it is important to identify factors that may influence both the duration and the quality of sleep in older adults.
BRIEF SUMMARY
Current Knowledge/Study Rationale: Both physical activity and changes in day length are thought to influence sleep patterns, but the combined effect on older adults is not well understood. We sought to identify seasonal patterns of sleep and physical activity in a large group of community-dwelling, older Icelandic adults using objective actigraphy-based measurements.
Study Impact: We found that while day length and activity both had a significant influence on the pattern of sleep timing, the actual within-individual differences of the repeaters were rather subtle between periods of longer and shorter day-lengths. We conclude that the limited variation in sleep patterns and quality in response to significant changes in daylight may be due to long-term adaptation in this group of older Icelandic adults.
The causes for reduced sleep duration and quality in older people are not clear. There is some evidence to suggest that circadian regulation of sleep weakens with age,3 resulting in reduced sleep consolidation and changes in sleep-wake timing.10 However, additional factors, such as the response to various environmental cues could also play a role. For example, external environmental factors, particularly variations in artificial light exposure and day length, have been shown to influence sleep patterns and quality.11–15 Increased exposure to artificial light has been shown to increase sleep disturbances11 and prolong sleep onset latency in an older population.12 Similarly, increased light exposure in laboratory settings has been show to elicit similar circadian shifts in younger and older adult populations,13 but older adults appear to have blunted responses to lower light levels13 and blue light exposure.16,17 Reduced daylight exposure due to change of season has also been shown to prolong the onset of sleep14 and cause shifts in bed- and rise-times14 in younger adult populations. But day length has not been shown to have a dramatic effect on the quality of sleep,14 and observations of its influence on sleep duration have been mixed.14,15
It is also plausible that changes in physical activity (PA) patterns may contribute to reduced quality and duration of sleep in older adults. Increased PA is thought to lead to reduced sleep disturbances18, but PA is known to decline with age,19–21 and this trend may contribute to the reduced sleep quality reported by older individuals. Very few studies have simultaneously captured objectively measured sleep and PA data in older populations,22 and little is known about the interaction of day length and physical activity on sleep patterns in older individuals.
The primary goal of this study was to delineate the potential effects of day length, objectively measured PA, and other subject characteristics on sleep quality and patterns measured by wrist actigraphy in a group of generally healthy, community-dwelling, elderly Icelandic population. Iceland is known to have one of the world's highest life expectancies—currently 82 years—as well as one of the highest healthy life expectancies, or the number of years a person can expect to live in full health, as defined by the World Health Organization (WHO).23 Iceland's unique geographical location (latitude 64–66°North) results in a wide variation in daylight hours, 4–21 hours between winter and summer months. This large seasonal variation in daylight hours offered an ideal opportunity to study the impact of daylight length on the objectively measured sleep and PA in a population of older adults.
METHODS
Study Population
The Age, Gene/Environment Susceptibility (AGES)-Reykjavik Study was designed to assess the influence of environment, genetic factors, and gene-environment interaction on various health topics related to aging in a historically healthy, aging population with high life expectancy.24 The study was initiated with a first wave of data collection from 2002–2006 with 5,764 participants who were recruited from the original Reykjavik Study which has collected data on age-related topics since 1967.24 The second wave of data collection (AGESII-Reykjavik) involving 3,411 participants took place between 2007 and 2011. A sub-study involving objectively measured, free-living PA and sleep using accelerometers occurred between April 2009 and June 2010. Further details of the AGES-Reykjavik study design and assessment can be found elsewhere.24
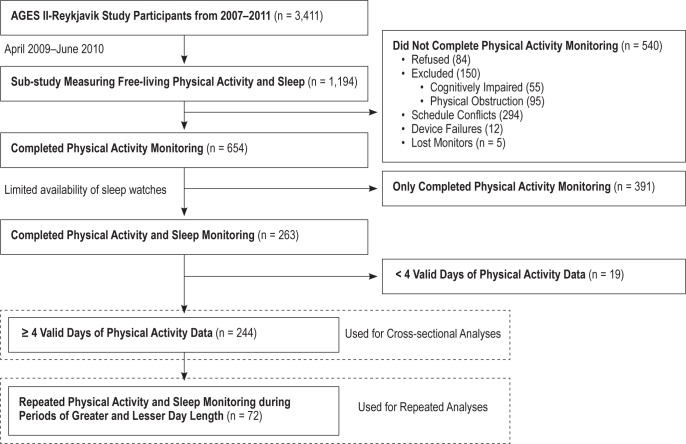
From a total of 1,194 people participated in the AGESII-Reykjavik study over the period in which PA were measured, completed data was successfully collected from 654 participants, all of whom did not have cognitive impairment (Mini Mental State Examination score > 20) or physical obstructions (e.g., blindness). A flow chart for this study population is shown in Figure 1. A detailed analysis of the PA patterns in this population has been previously described.25
Figure 1. Flow chart describing participants recruited from the AGES II-Reykjavik Study for cross-sectional and repeated visit analysis of sleep and activity patterns.
Two hundred sixty-three of the remaining 654 participants were given wrist-worn accelerometer for sleep assessment, due to the limited availability of the devices. The 244 participants with ≥ 4 valid days (≥ 10 h wear time)21 of hip-worn PA data were used in the final cross-sectional analysis. Four of the subjects failed to answer a questionnaire related to health status and were excluded from any multivariate analyses which used this as a covariate. This group of subjects formed the basis for our cross-sectional data analyses to explore the between-individual differences of sleep quality and patterns, and their associations to subject characteristics, environmental factors, and PA measures.
Understanding the limitations of cross-sectional analysis, we designed a further examination to study the influence of day length on within-individual changes in patterns of sleep and PA. Thus, we asked a subsample of 72 subjects whose measurement period began between August 1, 2009, and October 1, 2009, to repeat the measurements of sleep and PA during a period with fewer daylight hours occurring between January 1, 2010, and March 18, 2010. Two hip worn PA monitor device failures occurred during the repeat measurement, leaving 70 subjects with valid sleep assessment data and at least 1 day of valid hip-worn PA data during seasonal periods of greater and lesser hours of daylight.
All participants provided informed consent and the study was approved by the Icelandic National Bioethics Committee (VSN: 00–063), the Icelandic Data Protection Authority, and the institutional review board of the US National Institute on Aging, National Institutes of Health.
Demographic and Environmental Parameters
Participants came to the Icelandic Heart Association in Kópavogur, Iceland for assessment of cognitive and physical function as part of the AGESII-Reykjavik study. Height and weight were taken using standardized procedures and body mass index (BMI) was computed as the ratio weight/height2 (kg/m2). Participants reported overall health status on a discrete scale (1-excellent, 2-very good, 3-good, 4-fair, 5-poor) and whether or not they had been diagnosed with depression in the past 5 years (0-no, 1-yes, 8-not sure) as part of a comprehensive series of health history questionnaires administered to all AGESII participants.24 Subjects were also asked to report all medication used in the two weeks prior to their visit. The hours of daylight and over each participant's week of free-living measurement were obtained using the Sunrise/Sunset calculator provided by the Earth System Research Laboratory of the National Oceanic and Atmospheric Administration (http://www.esrl.noaa.gov/gmd/grad/solcalc/calcdetails.html). The average daily outdoor temperature over the same period was obtained from the Weather Underground (www.wunderground.com) historical weather data.
Sleep Measurements
Participants were given actigraphy-based sleep watches (Actiwatch Spectrum, Phillips-Respironics, Bend OR) to wear on the non-dominant wrist for 7-day free living sleep assessment. The watches contain motion-sensitive accelerometers which have been previously validated for objective sleep measurement.26 Each watch was programmed to record wrist-activity and white-light intensity in 15-sec epochs. Rest and sleep periods were automatically identified using the manufacturer software27 (Actiware version 4.0) and visually inspected and compared to the hip PA monitor non-wear events and the hand-written sleep diary that each subject kept. For sleep analysis, the wake threshold of the actiwatch data was set medium (40 activity counts), sleep onset and end were both set to 10 minutes, and white light threshold was 1000 lux. The following parameters were reported for each sleep event over the 7-day period using the Actiware software27: bedtime (start of the rest period, time subject gets in bed), rise time (end of the rest period, time subject gets out of bed), rest duration (rise time minus bed time), sleep duration (time within the rest period that was scored as sleep), onset latency (sleep onset time minus bed time), number of awakenings during sleep period, minutes of waking after sleep onset (WASO), mid-sleep time (midpoint between bed time and rise time), and sleep efficiency (percent of rest period that participant was scored as sleep). Further, the white light intensity was averaged over each complete day and for each rest and sleep period. Fifty-three different sleep watches were used over the course of the study.
Physical Activity Measurement
Accelerometry-based PA monitors (Actigraph GT3X, Acti-graph LLC, Pensacola, FL) were worn on the right hip throughout the 7-day free-living period. They were used to record PA intensity, computed as manufacturer specific activity counts in the vertical plane of motion, on a second-by-second basis and accumulated over each valid day of PA-accelerometer wear. The daily PA activity count totals provide similar PA intensity information as those found in other large epidemiologic studies of free-living PA, including the National Health and Nutrition Examination Survey (NHANES)21 and the larger cross-sectional analysis of PA in the AGES II cohort.25 Participants were told to remove the device before going to bed at night and before showering, bathing, or other water activities. Periods of non-wear were also automatically detected using a previously described method, 60 minutes or more of consecutive zero activity counts, allowing 1–2 minutes < 100 activity counts.21 A technician reviewed the diary and all detected non-wear periods with each participant using customized visualization software (Matlab version 2006, The Mathworks Inc, Natick, MA). Days with < 10 h of wear-time were considered invalid.21 Ninety-two different Actigraph accelerometers were used over the course of the study.
Statistical Methods
All descriptive data were reported in mean and standard deviation. Data were organized by a customized Matlab program, and statistical analyses were performed using R (version 3.1.0; http://www.r-project.org/). The significance level was set at p < 0.05 and two-sided. Normality of each variable was checked and confirmed using Q-Q plots before analyses.
Cross-Sectional Analysis
Spearman correlations were used to identify potential relationships between sleep measures, subject demographics, and PA measures. To preserve sufficient power, only those measures achieving Spearman correlation coefficients with the significance (p < 0.05) were selected to enter the stepwise, multivariate regression to further explore the relationship between sleep parameters, gender, PA, and day length. Separate multiple linear regression models were evaluated using sleep duration, rest duration, sleep efficiency, onset latency, WASO, number awakenings, and sleep midpoint time as independent response variables while age, sex, BMI, self-reported health status, total activity counts per wear time minute, outdoor temperature, diagnosed depression, and day length were used as covariates in each of the regression models. The use of antidepressants, benzodiazepines, and all other sleep medications were combined into one variable and used as a covariate in the regression models. Backward-elimination regression analysis was used to identify significant, independent predictors for each of the sleep parameters listed. Covariates with significance at or below 0.10 were retained during each step of the analysis.
Within-Individual Visits
Comparisons between the environmental and physical activity variables acquired during periods of long and short day lengths were performed using paired t-tests. Linear mixed models with random intercepts were used to compare sleep parameters collected over 2 measurement periods separated by 147 ± 18 days on average. Each sleep parameter served as a response variable in separate models. Subject identification was the random effects variable in each model. Other covariates of BMI, age, gender, self-reported health status, diagnosis of depression, sleep medication usage, and day length were included as fixed effects variables in each model.
RESULTS
Cross-Sectional Analysis
Demographics
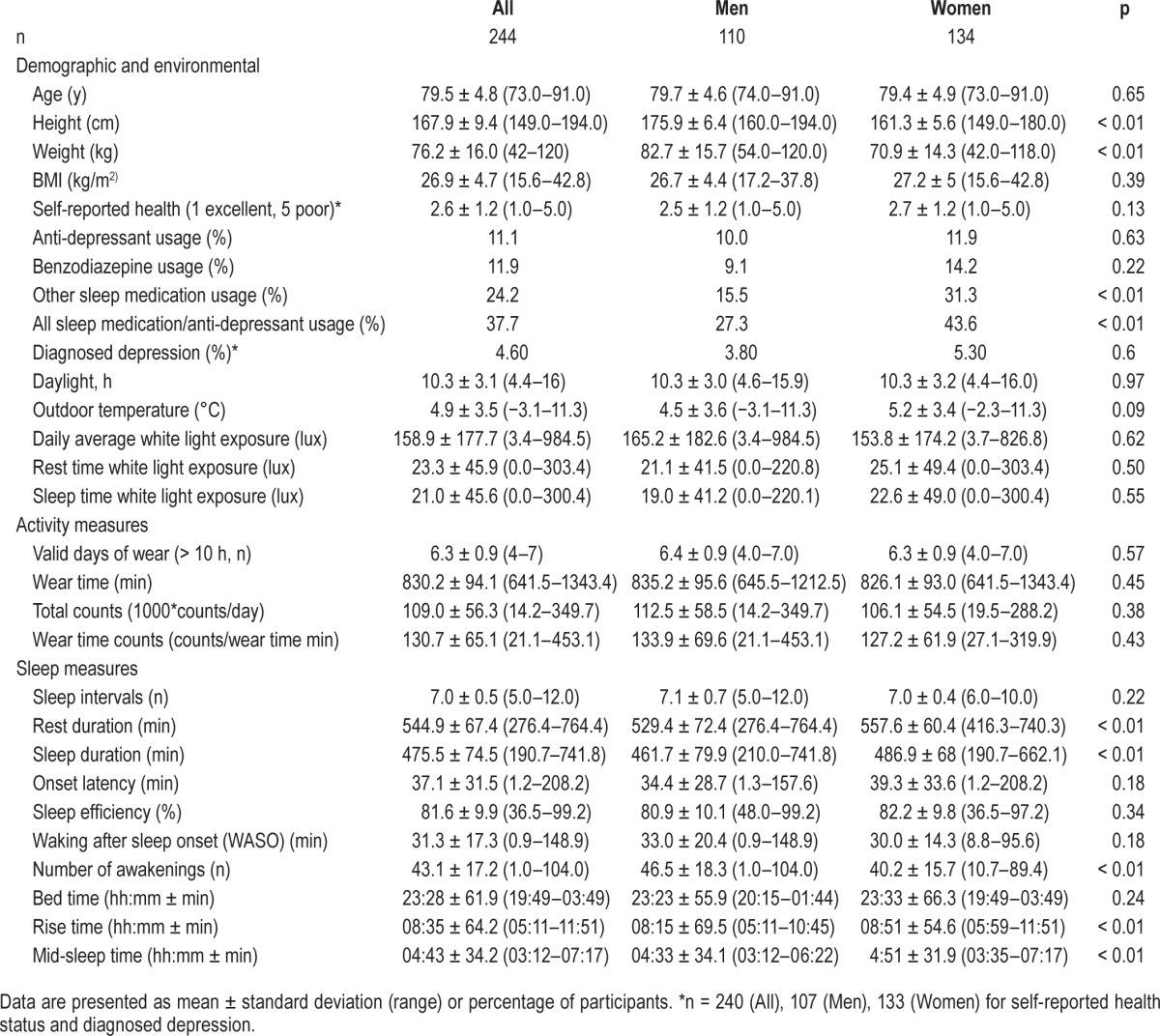
Characteristics of the study participants were presented in Table 1. The study population had an average age of 79.5 ± 4.8 years and BMI of 26.9 ± 4.7 kg/m2. Over 70% of the participants self-reported to be in good health or better, while less than 3% of participants reported to have poor health status. The daily hours of daylight over the study period varied widely, from 4.4 h (December) to 16 h (August) and the average outdoor temperature varied from −3.1°C to 11.3°C. Over a third of the subject (38%) reported using medications known to induce sleep including antidepressants, benzodiazepines, and other sleep medications, and usage was higher in women (44%) than in men (27%). However, no relationship was found between the use of sleep medication and hours of daylight during the study.
Table 1.
Demographic, environmental, activity, and sleep parameter for cross-sectional population of older Icelandic adults.
Sleep
Patterns of sleep are presented in Table 1. Both men and women went to bed at around the same time (23:28 ± 61.9 min), but men arose earlier (08:15 ± 69.5 min vs. 08:51 ± 54.6 min, p < 0.01), leading to significantly shorter rest (529.4 ± 72.4 vs. 557.6 ± 60.4 min/night, p < 0.01) and sleep durations (461.7 ± 79.9 vs. 486.9 ± 68.0 min/night, p < 0.01). Men awoke more often during sleep than women (46.5 ± 18.3 vs. 40.2 ± 15.7 awakenings/night, p < 0.01). There were no gender differences in sleep efficiency, WASO, or onset latency.
Physical Activity
Cross-sectional PA data is presented in Table 1. Most subjects (> 85%) acquired ≥ 6 valid days of PA measurement. There were no significant differences between men and women in terms of valid days of wear, daily wear-time, daily activity counts, or counts per wear-time minute.
Predictors of Sleep Measures
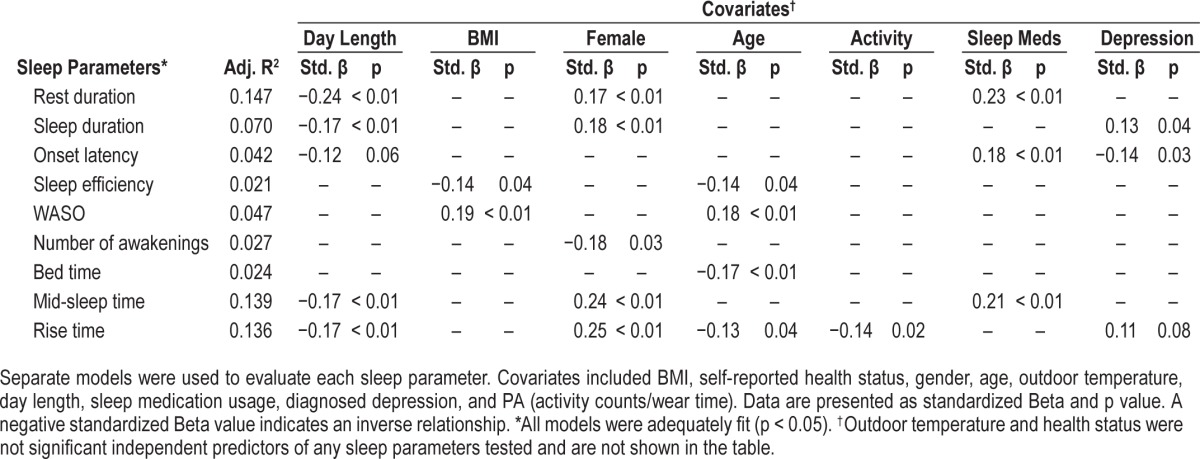
The results of the backward-elimination multiple regression analyses are presented in Table 2. Both increases in age and in BMI were independently associated with a decrease in sleep efficiency and an increase in WASO. Age was also negatively associated with both bedtime and rise time, suggesting that, as age advances, individuals go to bed and rise earlier. The multiple regression analysis also confirmed the independent association between genders and rest and sleep duration, with men having shorter durations as a result of earlier rise times compared to women. The analysis also confirmed that men had a greater number of awakenings during the night. Sleep medication usage was found to independently predict longer rest duration and onset latency and later rise and mid-sleep times, while diagnosed depression was found to significantly predict longer sleep duration and shorter onset latency. Higher daily PA was only found to be independently associated with earlier rise times. Interestingly, length of daylight was also found to independently predict rest and sleep duration and mid-sleep and rise times. On days with greater number of daylight hours, participants tended to have significantly shorter rest durations (β = −5.2 min/daylight h) and sleep durations (β = −4.1 min/daylight h) and earlier rise times (β = −3.5 min/daylight h) and mid-sleep times (β = −1.9 min/daylight h). Additionally, models pertaining to sleep timing and duration, including the mid-sleep and rise times and rest and sleep durations, had the highest adjusted R2 values and included both day length and gender as significant independent predictors. Neither self-reported health status nor average outdoor temperature was found to independently predict any of the sleep outcomes.
Table 2.
Results of backward-elimination, multiple regression analysis of cross-sectional sleep parameters for AGES II cohort.
Repeated Measures in a Subgroup
Demographic and Environmental Measures
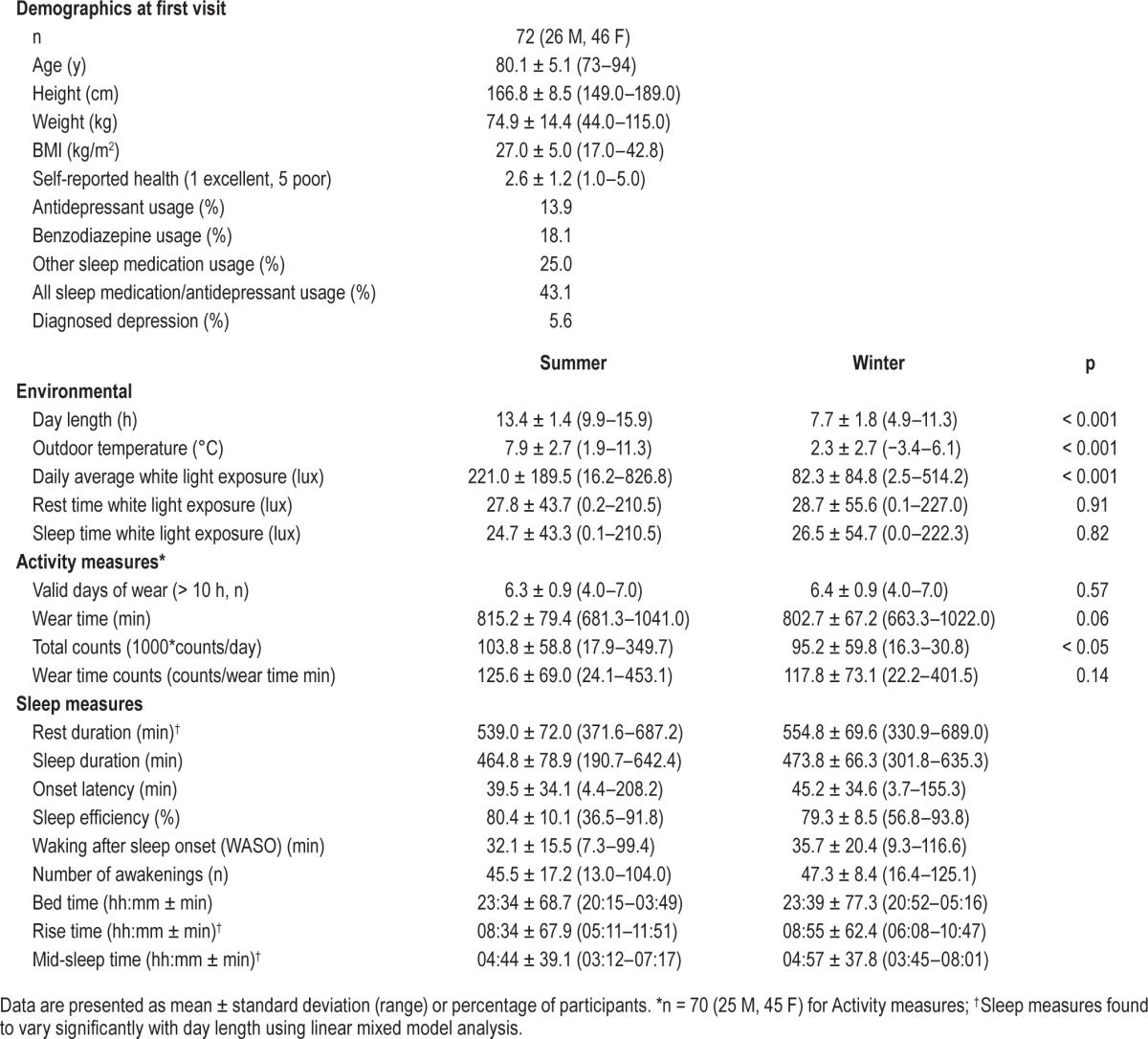
Mean results for the subpopulation of 72 individuals who repeated the week long PA and sleep measurements during periods of longer (13.4 ± 1.4 h) and shorter day length (7.7 ± 1.8 h, p < 0.001) are displayed in Table 3. Sleep watch light sensor data indicated that participants were exposed to a greater daily amount of white light during periods of longer day length compared to those with shorter day length (221.0 ± 189.5 vs. 82.3 ± 84.8 lux, respectively, p < 0.001). However, there were no differences in the white light exposure recorded by the Actiwatches during rest or sleep time between the 2 measurement periods.
Table 3.
Demographic, environmental, activity, and sleep parameters for sub-population of participants with repeat visits during periods of longer and shorter day length.
Physical Activity
When days were longer, participants had higher total PA counts (103,781 ± 58,777 vs. 95,152 ± 59,786 counts, p < 0.05) but also trended toward longer device wear-times (815.2 ± 79.4 vs. 802.7 ± 67.2 min, p = 0.06). Consequently, there was no difference between periods of longer and shorter day lengths when PA was normalized for wear-time (125.6 ± 69.0 vs. 117.8 ± 73.1 counts/wear-time min, respectively, p = 0.14).
Sleep
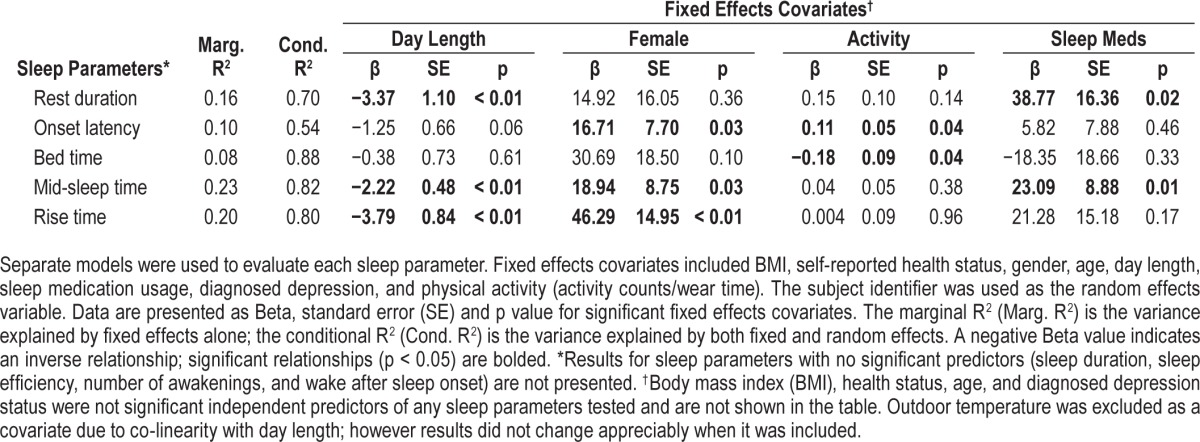
Results of the linear mixed effects model performed on the repeat subpopulation are summarized in Table 4. After controlling for demographic variables and environmental conditions in the linear mixed model, participants with repeat visits tended to rise earlier in summer months (β = −3.8 min/daylight hour, p < 0.01) but go to bed at approximately the same time (p > 0.05), leading to a shift toward earlier mid-sleep times (β = −2.2 min/ daylight hour, p < 0.01) and a reduced rest duration (β = −3.4 min/daylight hour, p < 0.05). Sleep duration, sleep efficiency, sleep onset latency, WASO, and number of awakes were not found to vary significantly with hours of daylight. Similar to the cross-sectional results, gender also had a significant, independent influence on sleep with women having longer onset latency (β = 16.7 min, p < 0.05) and later rise (β = 46.3 min, p < 0.01) and mid-sleep times (β = 18.9 min, p < 0.05) than men. Participants who used sleep medications were found to have a significantly longer rest duration (β = 38.8 min, p < 0.05) and later mid-sleep time (β = 23.1 min, p < 0.05). Physical activity was also found to have a small, but significant impact onset latency (β = 0.11 min/wear-time count, p < 0.05) and bed time (β = −0.18 min/wear-time count, p < 0.05). None of the other covariates such as age, BMI, health status, and diagnosed depression, were found to influence sleep patterns or quality in the repeat subpopulation. Outdoor temperature was excluded as a covariate due to co-linearity with day length; however results did not change appreciably when it was included.
Table 4.
Results of a linear mixed models regression analysis of sleep parameters for the sub-population of participants with repeat visits during periods of longer and shorter day length.
DISCUSSION
To our knowledge, this is the first study that used objective measurements of sleep and physical activity in a group of free-living older men and women in a region where there is considerable daylight variability throughout the year. We observed that this healthy Icelandic older population slept about 8 hours per night, with around 80% efficiency, and approximately 43 awakenings per night. We also found that, on average, men rose about 35 minutes earlier than women, although bedtimes were similar across sexes (around 23:30), resulting in a significantly shorter sleep duration for men (p < 0.01). The cross-sectional analyses revealed some associations between sleep parameters and age, gender, BMI, PA levels, sleep medication use, and length of daylight. Using a subpopulation of 72 participants who repeated the measurements during months with longer and shorter days, we were able to confirm the cross-sectional finding that participants rose later in winter resulting in a longer time spent in bed.
In other studies where objective sleep monitors were used in generally healthy, older, free living populations, Blackwell, et al. reported similar sleep efficiency (78.1% ± 12.0%) in about 3,000 community-dwelling men (76 ± 6 years, 90% Caucasian) in the US (6 sites).28 However, the total sleep time as measured by actigraphy was only 6.4 ± 1.2 hours in their sample, which is more than one hour less than the 7.7 ± 1.3 hours that we measured in Icelandic men. The WASO time in their study was 78.4 ± 44.3 min, more than double the 33.0 ± 20.4 min found in our study. In studies focusing on older women, Spira et al. found the total sleep time was 409.2 ± 66.0 min (6.8 ± 1.1 h), sleep efficiency of 79.9% ± 9.9%, and WASO of 65.9 ± 40.4 min in over 800 women with an average age of 82.4 years living at their own homes in the US (4 different sites).8 Again, the Icelandic older women appeared to have longer total sleep times (487 ± 68 min or 8.1 ± 1.1 h) and about half of the WASO (30.0 ± 14.3 min). With differences between our Icelandic older population and the US studies being quite large, we could not discount the fact that both US studies used a different wrist-worn actigraphy sleep watch (Sleepwatch-O by Ambulatory Monitoring Inc., Ardsley NY) than ours (Actiwatch Spectrum by Respironics). Weiss et al. performed a validation study comparing the Sleepwatch and Actiwatch simultaneous in a group of 30 adolescent participants and found near equivalent detections of total sleep time and sleep efficiency with significant correlation coefficient values.29 However, while the Blackwell and Spira studies used the proportional integration mode (PIM) for the Sleepwatch-O sleep/wake scoring given its higher correlation to polysomnography measurements in older adults,30 Weiss used the time-above-threshold (TAT) algorithm. In a more recent study, Lambiase et al. (2014) used an Actiwatch-64m (also by Respironics) which uses the same accelerometer sensor and same data analysis software (Actiware), and found the total sleeping time in 121 healthy women (73.3 ± 1.7 years) in Pittsburgh PA to be 397 ± 53 min.22 This is closer to the previous US study, conducted by Spira8 than to our current results in Iceland, but the authors did not specify the sleep/wake detection parameters which may have influenced the results. Our results suggest that the total sleep time in these healthy, older Icelandic men and women were more than one hour longer than the US older people. Although sleep parameter definitions seem to be similar in all cases (e.g. total sleep time, WASO), it is still unclear what influence the monitor choice and various sleep detection algorithms may have had on these findings To the best of our knowledge, no similar data exist from other countries and regions to which we could further compare.
Another factor to consider in the interpretation of the results is the difference in the use of sleep medications amongst the populations studied. Over a third (38%) of the Icelandic participants studied reported using either a sleep-inducing medication or antidepressant (including 11% antidepressant use, 12% benzodiazepine use, and 24% other sleep medication use), which is a high percentage compared to the other studies mentioned (Lambiase22 reported 9% using sleep medication; Spira8 reported 7% antidepressant use and 4.8% benzodiazepine use; Blackwell28 reported 7.9% antidepressant use, 4.5% benzodiazepine use, and 2.0% other sleep medication use). The participants in our study who reported use of these medications had longer sleep duration (488 ± 71 min with sleep medication vs. 468 ± 76 min without, p < 0.05), with a similar bedtime but a later rise time (08:50 vs. 08:27, p < 0.01) than participants who did not use sleep medication. Consequently, the high prevalence of sleep medication use may have contributed to the long sleep duration seen in this cohort. However, the average sleep duration in those participants not taking sleep medication (468 ± 76 min) was still longer than those reported in other studies. Additionally, sleep medication use had little influence on the measured quality of sleep, as users and non-users did not differ in sleep efficiency, number of awakenings, or WASO. When sleep medication use was included as a covariate in the multiple linear regression analysis of the cross-sectional data, it was found to be a significant predictor of rest duration, onset latency, and rise time but had little influence on the contribution of the other independent predictors of sleep patterns and quality, such as day length, gender, age, or PA.
If this was indeed a healthier study population than those from the US, the PA measured by the hip-worn accelerometer on the Icelandic women (127 ± 62 ct/min/day) contradictorily appeared to be much less than that measured from the group studied by Lambiase22 from the Healthy Women Study (HWS) cohort in Pittsburgh, PA, USA,31 where the average daily activity was reported to be 190 ct/min/day. One explanation for this PA discrepancy is the difference in the age of the subjects. The women enrolled in the Pittsburgh HWS were on average about 6 years younger than those in the current study, and age-related declines in PA have been documented in similar older populations.32,33 However, in a similar study of older adults living in urban areas in the UK with a closer mean age to the participants in our study (77.7 ± 5.8 years for men and 78.6 ± 5.7 years for women), Davis33 also reported higher daily wear-time activity counts than those observed in our population (198 ± 117 vs. 134 ± 70 ct/min/day for men and 163 ± 116 ct/min/day vs. 127 ± 62 ct/min/day for women). Other factors that may influence the reported physical activity may be the activity monitor type and the data processing methods used in each of the studies; however, the older Actigraph GT1M monitor used in both the Lambiase22 and Davis33 studies has been shown to be comparable to the newer GT3X monitors used in the current study.34 Each of the studies used different methods to determine monitor wear-time, which may have a small impact on the results. However, the wear time reported by Davis33 (13.9 h for women and 14.4 h for men) was similar to what we have reported (13.8 h for women and 13.9 h for men); wear time in the Lambiase study22 was not reported. It is possible that subjects in our Icelandic cohort achieved more activity than the other groups during non-wear periods by performing water sports such as swimming, given that roughly one-quarter of the AGES cohort who received an accelerometer reported swimming for exercise both in summer and winter25; however, we do not have sufficient information from other studies to confirm this conjecture.
We also found that total hours of daylight were statistically significantly related to sleep timing and duration but were not associated with sleep quality or physical activity patterns. Specifically, shorter days were associated with the later mid-sleep and rise times leading to longer rest and sleep durations in both the cross-sectional analysis of 244 participants and in the 72 participant subgroup which repeated the week long sleep assessments. However, the absolute sleep duration differences between the two measurement periods were only about 20 minutes (4.3 min/daylight hour). Furthermore, no differences were observed in the total sleep time, WASO, sleep efficiency, or wear-time PA level during the winter, suggesting that the older population studied here is able to adapt sleeping and physical activity patterns to accommodate the change in daylight across seasons. In fact, the comparable Actiwatch Spectrum white-light sensor readings during sleep- or rest-time between darker and lighter months seem to support this rationale (Table 3). However, data from the light sensor should be interpreted with caution, however, since the sensor may have become obstructed by heavy blankets or clothing35 and the variability of light sensor measurements between devices has been shown to be significant36 and they were not calibrated prior to use, as in other studies using the Spectrum.37 Further, due to device memory and data use constraints, we were limited to collecting broad spectrum white light rather than the red, green, and blue spectral components which may have been helpful in differentiating the contributions of natural and artificial light sources to the daily patterns of light exposure.38 It has also been shown that, after a bout of increased light exposure, older adults have a diminished cognitive response,16 reduced subjective changes in alertness and sleepiness,17 an absence of increased PER2 clock-gene expression,39 and reduced circadian responsiveness13 as compared to younger subjects. These blunted responses in older subjects may be due to reduced corneal light exposure as a result of changes in pupil size40 and lens opacity13 that occur with advancing age, which may also help explain the small seasonal differences in sleep patterns observed in this study.
We cannot rule out the idea that the seasonal difference may be due to a difference in the outdoor temperature or precipitation rather than the difference in daylight hours, as the difference in outdoor temperature was found to differ significantly between the two study visits. However, when outdoor temperature was included as a covariate in the cross-sectional regression model and the repeat participant mixed model analysis it was not found to independently predict any of the sleep parameters. Also, the variation in outdoor temperature and precipitation from summer to winter in Reykjavik, Iceland is known to be slight relative to other locations of similar latitude. For instance, the variation in temperature in Reykjavik (average winter month low of −3°C and average summer month high of 13.3°C, a range of 16.3°C) is closer to that of San Francisco (37.8°N with a low of 7.6°C to a high of 21.2°C high, range of 13.6°C, one of the lowest in the continental US) than to Stockholm, Sweden (59°N, −5°C low to 22°C high, a 27°C difference).41
Our findings regarding sleep patterns and the length of daylight was similar to a Norwegian population study conducted by Sivertsen,42 where the latitude was equal to that of Iceland. In that population study of over 43,000 participants, their self-reported time in bed was not related to the length of daylight (or month). Conversely, a self-report study conducted by Fri-borg14 examined summer and winter sleep patterns in 150 Norwegian (69°N) and 180 Ghanese (5°N) men and women and found that the Norwegians rose 32 min later, had longer onset latency, and slightly reduced sleep efficiency but no change in total sleep time in winter, while sleep patterns of the Ghanese were unchanged from winter to summer. The results of the Friborg study, as well as our own, reflect some of the shifts in circadian profile found in laboratory-based light exposure studies.13 It is worth mentioning, however, the average age of the study participants in the Sivertsen42 study was 44.6 years and in the Friborg14 study was 25.4 years for the Ghanese and 22.7 years for Norweigans, and we could not ascertain any subgroups that have a comparable age to our subjects (about 80 years). Additionally our assessments of free-living sleep patterns and quality used an objective monitor, which often differ from subjective self-reports.43–45
One of the strengths of the current study was the inclusion of a generally healthy population of older men and women living in Iceland who were part of a longitudinal study which included detailed assessments of their health status over the past 45 years. Additionally, we were able to simultaneously measure one week of free-living sleep and PA using objective wrist- and hip-worn accelerometers, respectively, with high compliance rate (nearly 100% with 6+ days of sleep and 79% with 6+ days for PA). Concordant measurement of objective sleep and hip-worn PA is rare, particularly in older populations.22 And, unlike previous studies, we were able to compare the sleep patterns and qualities between men and women, and explore multiple factors that are thought to influence sleep. Due to Iceland's unique geographical location, which provided a relatively large variation in daylight, we were able to investigate the relationship between day length and free-living sleep-patterns in older adults more extensively. We were also able to use within-individual comparisons of sleep and PA patterns gained by repeating measurements in an opposite daylight condition to confirm cross-sectional findings that season had a statistically significant, although practically minor impact on sleep patterns and quality in this population.
A limitation of the current study is that while age, gender, length of daylight, BMI, and PA levels were independently associated with cross-sectional differences in sleep patterns (bed time, rise time, rest and sleep durations) and sleep quality (total sleep time, efficiency, WASO, and number of awakenings), we could not state with certainty that any causal relationships exist. Studying a mostly healthy population may have limited our ability to draw conclusions that relate health status to sleep patterns and it does not permit us to investigate other important relationships between sleep and health in patients with clinical conditions common to older individuals. Thus, the question of whether sleep patterns and quality impact future health remains to be answered with further follow-up in this cohort and in future studies that are designed to address these questions. Moreover, the cohort that we studied consisted of subjects between 73 and 91 years of age. The older age and healthy status could partly explain the longer sleep time and limited influence by outdoor daylight variations. The usage of sleep medication was high in the Icelandic population we studied and, along with the health status, was assessed using self-report during the participants' first visit. Therefore, it is not known whether the participants changed medication usage, or health status, between visits. However, in the cross-sectional sample, neither sleep medication use nor health status was related to day length, suggesting that it may be consistent throughout the year. Additionally, subjects who repeated the one-week measurements did not necessarily wear the same sleep-watch or hip-worn PA monitor, due to logistical limitations. This may have contributed to some variability in sleep, PA, and light measurements across study periods. Lastly, we only analyzed the night sleeping patterns and excluded naps.
In conclusion, our study of objectively measured free-living sleep and physical activity in older Icelandic men and women revealed that this population had a relatively long sleep time (about 8 h in women, and 7.7 h for men), lower average nightly awakenings, but higher use of sleep medications than most previous reports from similarly older populations. Age and BMI were found to have the greatest association with the quality of sleep and men were found to rise earlier and sleep fewer minutes per night than women. The statistically significant but small changes in sleep patterns and quality observed during periods of disparate daylight length suggest that this population is well adapted to the seasonal variation of daylight in Iceland.
DISCLOSURE STATEMENT
This was not an industry supported study. This study was funded by National Institutes of Health (NIH) contract N01-AG-1-2100, the National Institute on Aging (NIA) Intramural Research Program, Hjartavernd (the Icelandic Heart Association), and the Althingi (the Icelandic Parliament). This study was also supported by National Institutes of Health Intramural Research Program contract number: Z01 DK071013 and Z01 DK071014 for R.J.B. and K.Y.C. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The researchers are indebted to the participants for their willingness to participate in the study. We would also like to recognize and extend our appreciation to Elin Sandra Skuladottir and Nina Dora Oskardottir for their support in data collection and preliminary data analysis, Gregory C. McMahon for his role in inspecting and cleaning of the Actiwatch sleep data, and Xiongce Zhao for his statistical advice.
ABBREVIATIONS
- AGES
age, gene/environment susceptibility study
- BMI
body mass index
- HWS
Healthy Women Study
- PA
physical activity
- PIM
proportional integration mode
- TAT
time-above-threshold
- WASO
wake after sleep onset
- WHO
World Health Organization
REFERENCES
- 1.Depner CM, Stothard ER, Wright KP., Jr Metabolic consequences of sleep and circadian disorders. Curr Diab Rep. 2014;14:507. doi: 10.1007/s11892-014-0507-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Driscoll HC, Serody L, Patrick S, et al. Sleeping well, aging well: a descriptive and cross-sectional study of sleep in “successful agers” 75 and older. Am J Geriatr Psychiatry. 2008;16:74–82. doi: 10.1097/JGP.0b013e3181557b69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Czeisler CA, Dumont M, Duffy JF, et al. Association of sleep-wake habits in older people with changes in output of circadian pacemaker. Lancet. 1992;340:933–6. doi: 10.1016/0140-6736(92)92817-y. [DOI] [PubMed] [Google Scholar]
- 4.Foley DJ, Monjan AA, Brown SL, Simonsick EM, Wallace RB, Blazer DG. Sleep complaints among elderly persons: an epidemiologic study of three communities. Sleep. 1995;18:425–32. doi: 10.1093/sleep/18.6.425. [DOI] [PubMed] [Google Scholar]
- 5.Ohayon MM, Carskadon MA, Guilleminault C, Vitiello MV. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: developing normative sleep values across the human lifespan. Sleep. 2004;27:1255–73. doi: 10.1093/sleep/27.7.1255. [DOI] [PubMed] [Google Scholar]
- 6.Dam TT, Ewing S, Ancoli-Israel S, Ensrud K, Redline S, Stone K. Association between sleep and physical function in older men: the osteoporotic fractures in men sleep study. J Am Geriatr Soc. 2008;56:1665–73. doi: 10.1111/j.1532-5415.2008.01846.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goldman SE, Stone KL, Ancoli-Israel S, et al. Poor sleep is associated with poorer physical performance and greater functional limitations in older women. Sleep. 2007;30:1317–24. doi: 10.1093/sleep/30.10.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spira AP, Covinsky K, Rebok GW, et al. Poor sleep quality and functional decline in older women. J Am Geriatr Soc. 2012;60:1092–8. doi: 10.1111/j.1532-5415.2012.03968.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dew MA, Hoch CC, Buysse DJ, et al. Healthy older adults' sleep predicts all-cause mortality at 4 to 19 years of follow-up. Psychosom Med. 2003;65:63–73. doi: 10.1097/01.psy.0000039756.23250.7c. [DOI] [PubMed] [Google Scholar]
- 10.Duffy JF, Czeisler CA. Age-related change in the relationship between circadian period, circadian phase, and diurnal preference in humans. Neurosci Lett. 2002;318:117–20. doi: 10.1016/s0304-3940(01)02427-2. [DOI] [PubMed] [Google Scholar]
- 11.Cho JR, Joo EY, Koo DL, Hong SB. Let there be no light: the effect of bedside light on sleep quality and background electroencephalographic rhythms. Sleep Med. 2013;14:1422–5. doi: 10.1016/j.sleep.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 12.Obayashi K, Saeki K, Iwamoto J, et al. Effect of exposure to evening light on sleep initiation in the elderly: a longitudinal analysis for repeated measurements in home settings. Chronobiol Int. 2014;31:461–7. doi: 10.3109/07420528.2013.840647. [DOI] [PubMed] [Google Scholar]
- 13.Duffy JF, Zeitzer JM, Czeisler CA. Decreased sensitivity to phase-delaying effects of moderate intensity light in older subjects. Neurobiol Aging. 2007;28:799–807. doi: 10.1016/j.neurobiolaging.2006.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Friborg O, Bjorvatn B, Amponsah B, Pallesen S. Associations between seasonal variations in day length (photoperiod), sleep timing, sleep quality and mood: a comparison between Ghana (5 degrees) and Norway (69 degrees) J Sleep Res. 2012;21:176–84. doi: 10.1111/j.1365-2869.2011.00982.x. [DOI] [PubMed] [Google Scholar]
- 15.Lehnkering H, Siegmund R. Influence of chronotype, season, and sex of subject on sleep behavior of young adults. Chronobiol Int. 2007;24:875–88. doi: 10.1080/07420520701648259. [DOI] [PubMed] [Google Scholar]
- 16.Daneault V, Hebert M, Albouy G, et al. Aging reduces the stimulating effect of blue light on cognitive brain functions. Sleep. 2014;37:85–96. doi: 10.5665/sleep.3314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sletten TL, Revell VL, Middleton B, Lederle KA, Skene DJ. Age-related changes in acute and phase-advancing responses to monochromatic light. J Biol Rhythms. 2009;24:73–84. doi: 10.1177/0748730408328973. [DOI] [PubMed] [Google Scholar]
- 18.Sherrill DL, Kotchou K, Quan SF. Association of physical activity and human sleep disorders. Arch Intern Med. 1998;158:1894–8. doi: 10.1001/archinte.158.17.1894. [DOI] [PubMed] [Google Scholar]
- 19.Caspersen CJ, Pereira MA, Curran KM. Changes in physical activity patterns in the United States, by sex and cross-sectional age. Med Sci Sports Exerc. 2000;32:1601–9. doi: 10.1097/00005768-200009000-00013. [DOI] [PubMed] [Google Scholar]
- 20.Sallis JF. Age-related decline in physical activity: a synthesis of human and animal studies. Med Sci Sports Exerc. 2000;32:1598–600. doi: 10.1097/00005768-200009000-00012. [DOI] [PubMed] [Google Scholar]
- 21.Troiano RP, Berrigan D, Dodd KW, Masse LC, Tilert T, McDowell M. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc. 2008;40:181–8. doi: 10.1249/mss.0b013e31815a51b3. [DOI] [PubMed] [Google Scholar]
- 22.Lambiase MJ, Gabriel KP, Kuller LH, Matthews KA. Temporal relationships between physical activity and sleep in older women. Med Sci Sports Exerc. 2013;45:2362–8. doi: 10.1249/MSS.0b013e31829e4cea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wolrd Health Organization. Geneva, Switzerland: WHO Press; 2014. World Health Statistics 2014: Part III, Global Health Indicators; pp. 59–69. [Google Scholar]
- 24.Harris TB, Launer LJ, Eiriksdottir G, et al. Age, Gene/Environment Susceptibility-Reykjavik Study: multidisciplinary applied phenomics. Am J Epidemiol. 2007;165:1076–87. doi: 10.1093/aje/kwk115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arnardottir NY, Koster A, Van Domelen DR, et al. Objective measurements of daily physical activity patterns and sedentary behaviour in older adults: Age, Gene/Environment Susceptibility-Reykjavik Study. Age Ageing. 2013;42:222–9. doi: 10.1093/ageing/afs160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kushida CA, Chang A, Gadkary C, Guilleminault C, Carrillo O, Dement WC. Comparison of actigraphic, polysomnographic, and subjective assessment of sleep parameters in sleep-disordered patients. Sleep Med. 2001;2:389–96. doi: 10.1016/s1389-9457(00)00098-8. [DOI] [PubMed] [Google Scholar]
- 27.Actiware Clinicians Guide. Murrysville, PA: Respironics, Inc.; 2008. [Google Scholar]
- 28.Blackwell T, Yaffe K, Ancoli-Israel S, et al. Associations between sleep architecture and sleep-disordered breathing and cognition in older community-dwelling men: the Osteoporotic Fractures in Men Sleep Study. J Am Geriatr Soc. 2011;59:2217–25. doi: 10.1111/j.1532-5415.2011.03731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weiss AR, Johnson NL, Berger NA, Redline S. Validity of activity-based devices to estimate sleep. J Clin Sleep Med. 2010;6:336–42. [PMC free article] [PubMed] [Google Scholar]
- 30.Blackwell T, Redline S, Ancoli-Israel S, et al. Comparison of sleep parameters from actigraphy and polysomnography in older women: the SOF study. Sleep. 2008;31:283–91. doi: 10.1093/sleep/31.2.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matthews KA, Meilahn E, Kuller LH, Kelsey SF, Caggiula AW, Wing RR. Menopause and risk factors for coronary heart disease. N Engl J Med. 1989;321:641–6. doi: 10.1056/NEJM198909073211004. [DOI] [PubMed] [Google Scholar]
- 32.Buman MP, Hekler EB, Haskell WL, et al. Objective light-intensity physical activity associations with rated health in older adults. Am J Epidemiol. 2010;172:1155–65. doi: 10.1093/aje/kwq249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Davis MG, Fox KR, Hillsdon M, Sharp DJ, Coulson JC, Thompson JL. Objectively measured physical activity in a diverse sample of older urban UK adults. Med Sci Sports Exerc. 2011;43:647–54. doi: 10.1249/MSS.0b013e3181f36196. [DOI] [PubMed] [Google Scholar]
- 34.Kaminsky LA, Ozemek C. A comparison of the Actigraph GT1M and GT3X accelerometers under standardized and free-living conditions. Physiol Meas. 2012;33:1869–76. doi: 10.1088/0967-3334/33/11/1869. [DOI] [PubMed] [Google Scholar]
- 35.Flynn JI, Coe DP, Larsen CA, Rider BC, Conger SA, Bassett DR., Jr Detecting indoor and outdoor environments using the ActiGraph GT3X+ light sensor in children. Med Sci Sports Exerc. 2014;46:201–6. doi: 10.1249/MSS.0b013e3182a388c0. [DOI] [PubMed] [Google Scholar]
- 36.Markvart J, Hansen AM, Christoffersen J. Comparison and correction of the light sensor output from 48 wearable light exposure devices by using a side-by-side field calibration method. Leukos. 2015;11:155–71. [Google Scholar]
- 37.Goulet G, Mongrain V, Desrosiers C, Paquet J, Dumont M. Daily light exposure in morning-type and evening-type individuals. J Biol Rhythms. 2007;22:151–8. doi: 10.1177/0748730406297780. [DOI] [PubMed] [Google Scholar]
- 38.Thorne HC, Jones KH, Peters SP, Archer SN, Dijk DJ. Daily and seasonal variation in the spectral composition of light exposure in humans. Chronobiol Int. 2009;26:854–66. doi: 10.1080/07420520903044315. [DOI] [PubMed] [Google Scholar]
- 39.Jud C, Chappuis S, Revell VL, et al. Age-dependent alterations in human PER2 levels after early morning blue light exposure. Chronobiol Int. 2009;26:1462–9. doi: 10.3109/07420520903385564. [DOI] [PubMed] [Google Scholar]
- 40.Daneault V, Vandewalle G, Hebert M, et al. Does pupil constriction under blue and green monochromatic light exposure change with age? J Biol Rhythms. 2012;27:257–64. doi: 10.1177/0748730412441172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.World Meteorological Organization. World Weather Information Service (WWIS) 2014. http://worldweather.wmo.int/
- 42.Sivertsen B, Overland S, Krokstad S, Mykletun A. Seasonal variations in sleep problems at latitude 63 degrees -65 degrees in Norway: the Nord-Trondelag Health Study, 1995-1997. Am J Epidemiol. 2011;174:147–53. doi: 10.1093/aje/kwr052. [DOI] [PubMed] [Google Scholar]
- 43.McCrae CS, Rowe MA, Tierney CG, Dautovich ND, Definis AL, McNamara JP. Sleep complaints, subjective and objective sleep patterns, health, psychological adjustment, and daytime functioning in community-dwelling older adults. J Gerontol B Psychol Sci Soc Sci. 2005;60:P182–9. doi: 10.1093/geronb/60.4.p182. [DOI] [PubMed] [Google Scholar]
- 44.Van Den Berg JF, Van Rooij FJ, Vos H, et al. Disagreement between subjective and actigraphic measures of sleep duration in a population-based study of elderly persons. J Sleep Res. 2008;17:295–302. doi: 10.1111/j.1365-2869.2008.00638.x. [DOI] [PubMed] [Google Scholar]
- 45.Young T, Rabago D, Zgierska A, Austin D, Laurel F. Objective and subjective sleep quality in premenopausal, perimenopausal, and postmenopausal women in the Wisconsin Sleep Cohort Study. Sleep. 2003;26:667–72. doi: 10.1093/sleep/26.6.667. [DOI] [PubMed] [Google Scholar]