Abstract
Study Objectives:
Both periodic limb movements during sleep (PLMS) and obstructive sleep apnea (OSA) have been associated with increased risk of cardiovascular disease (CVD). OSA has also been linked to increased large arterial stiffness, which is considered an independent risk factor for CVD. We utilized a previously validated index of large artery stiffness (SIDVP) derived from the digital volume pulse (DVP) to seek comparison in patients with PLMS and OSA.
Methods:
Forty-nine adult male subjects, without known comorbidities that could affect arterial stiffness or on vasoactive medication, were retrospectively identified and categorized into controls (n = 8), PLMS (n = 13), OSA (n = 17), and OSA/PLMS (n = 11). The cutoff for PLMS was a periodic limb movement index (PLMI) > 15 events/h, and for OSA an apnea-hypopnea index (AHI) > 10 events/h. SIDVP was derived from the raw data of photoplethysmography of the nocturnal polysomnography, averaged for 2 min prior to sleep study initiation (baseline), after completion in the morning, and every half hour after sleep onset.
Results:
The groups were age/body mass index-matched. Controls showed lower baseline, morning, and overall SIDVP compared to the other groups (p < 0.01). Patients with PLMS (PLMI: 50.69 ± 9.7 events/h) and the OSA group (AHI: 29.7 ± 2 events/h) demonstrated similar overall SIDVP (6.78 ± 0.08 versus 6.94 ± 0.04, respectively, p = 0.5), whereas the OSA/PLMS (AHI: 29.35 ± 8, PLMI: 50.63 ± 7.2) group demonstrated the highest (7.40 ± 0.06, p < 0.001).
Conclusions:
Based on an easily reproducible and applicable marker of large arterial stiffness, patients with significant PLMS had higher SIDVP when compared to controls and comparable to those with moderate/severe OSA. The OSA/PLMS group had the highest SIDVP, implying a possible additive effect of OSA and PLMS on arterial stiffness.
Citation:
Drakatos P, Higgins S, Pengo MF, Kent BD, Muza R, Karkoulias K, Leschziner G, Williams A. Derived arterial stiffness is increased in patients with obstructive sleep apnea and periodic limb movements during sleep. J Clin Sleep Med 2016;12(2):195–202.
Keywords: arterial stiffness, cardiovascular risk, digital pulse volume, periodic limb movements during sleep, stiffness index
INTRODUCTION
Periodic limb movements during sleep (PLMS) are characterized by highly stereotyped repetitive movements of the limbs, typically the legs, during sleep. Patients may complain of excessive daytime sleepiness (EDS), unrefreshing sleep, nocturnal awakenings and/or insomnia, a condition defined as periodic limb movement disorder (PLMD) according to International Classification of Sleep Disorders, Third Edition (ICSD-3) criteria.1 The prevalence of PLMS is estimated between 4% to 11% in adults.2 PLMS are present in nocturnal polysomnography (NPSG) in up to 90% of patients with restless legs syndrome (RLS) and in up to 25% in patients without RLS.3 PLMS also frequently occur in patients with obstructive sleep apnea (OSA),4 narcolepsy,5 rapid eye movement (REM) behavior disorder,6 and other medical and neurological disorders.2
BRIEF SUMMARY
Current Knowledge/Study Rationale: Periodic limb movements during sleep are a relatively common finding in sleep studies and even though not yet established, increasing evidence supports their possible effect on the development of cardiovascular disease. Several markers of large artery stiffness have been linked to cardiovascular disease and have been found to be increased in patients with obstructive sleep apnea.
Study Impact: An easily derived marker of the large artery stiffness, produced from the oximetry and accessible to the most sleep centers, was found increased in patients with significant periodic limb movements during sleep and comparable to patients with moderate/ severe obstructive sleep apnea patients and higher in patients with a combination of these two conditions. The SIDVP could potentially serve as an easily derived and widely available in clinical practice marker of large arteries stiffness and further research on the effect of periodic limb movements during sleep on cardiovascular disease through increased arterial stiffness should be undertaken.
Published data suggest that the magnitude of increments of blood pressure during PLMS, especially those associated with electroencephalographic (EEG) arousals, could be on average 22 mmHg for systolic blood pressure and 11 mmHg for diastolic blood pressure,7 and they have also been associated independently with diastolic blood pressure.8 Although the relationship between PLMS and hypertension remains unclear, prior large-scale, community-based studies have suggested an association between PLMS and prevalent hypertension, particularly in black men.9 More convincing evidence exists for a relationship between PLMS and incident cardiovascular disease in community-dwelling elderly men.9,10 Even though causality is far from established, sympathetic overactivity seems the most likely pathophysiological mechanism for CVD development in patients with PLMS.11
Elevated aortic pulse wave velocity (PWV) is considered an independent and major risk factor for cardiovascular disease.12,13 Several studies have validated more easily measured markers of large arterial stiffness derived from the digital volume pulse (DVP),14,15 either with the utilization of commercial devices or algorithms and in particular the stiffness index derived from DVP (SIDVP), which has been proposed as a simple, reproducible, and widely available in clinical practice noninvasive measurement of large artery stiffness.14,16
OSA is a well-recognized risk factor for CVD and the applicability of noninvasive assessment of large artery stiffness in OSA cohorts has shown great promise.15,17 PWV, which is considered a marker of large artery stiffness, has been found to be increased in patients with OSA when compared to age-, body mass index (BMI)- and sex-matched controls.18–20 More recently, a number of authors have demonstrated the effectiveness of continuous positive airway pressure (CPAP) treatment in decreasing the arterial stiffness derived either from PWV or DVP in subjects with OSA.15,17,21,22
In this retrospective study, we aimed to generate from oximetry recordings and measure the nocturnal SIDVP in patients with PLMS and compare with age- and BMI-matched controls. As a secondary objective, we compared the SIDVP between patients with OSA or PLMS or a combination of these conditions.
METHODS
Patient Selection
Male patients older than 18 y who underwent NPSG between January 2011 and July 2014 at Guy's and St Thomas' Sleep Disorders Centre were retrospectively assessed. Subjects with an apnea-hypopnea index (AHI) > 10 events/h, and those with a periodic limb movement index (PLMI) > 15 events/h were considered for inclusion. To avoid known sex-related effects on arterial stiffness, only male subjects were eventually included in the study.23 Exclusion criteria were the presence of CVD diagnosis, diabetes mellitus, hypercholesterolemia, arrhythmias, or administration of vasoactive medications (e.g., antihypertensive drugs, hormone replacement therapy, antidiabetes drugs) until the date of the sleep study and based on patients' clinical records. All patients had recent blood pressure measurements within normal range (systolic blood pressure: 110 ± 8 mmHg [range: 129–99 mmHg], diastolic blood pressure: 71 ± 6 mmHg [range: 68–80 mmHg]. Smokers or ex-smokers and patients who had previously received a diagnosis or treatment for sleep disorders, including RLS, were also excluded from the study. A group of age-matched subjects with no exclusion criteria and who were initially assessed for slow wave sleep arousal disorder and who finally did not show any evidence of sleep disorders on NPSG, served as the control group.
Sleep Study Methodology
The NPSG was performed using the standard EEG montage and the sleep stages were scored using 30-sec epochs according to standard criteria by the American Academy of Sleep Medicine. Continuous recordings included electro-oculography, electrocardiography, submental and leg electromyography, pulse oximetry, and nasal cannula and respiratory inductance plethysmography with chest and abdominal belts.24 Patients were subsequently reviewed with the results of the NPSG and the diagnoses of OSA, PLMS, OSA/PLMS were made in accordance with ICSD-3 criteria1 using as cutoffs for OSA an AHI > 10/h and for PLMS a PLMI > 15/h. The PLMI was defined as the total number of periodic leg movements per hour of sleep. Leg movements after respiratory events were excluded. An obstructive apneic event was scored if at least 90% reduction of the airflow signal compared to that preceding was observed lasting ≥ 10 sec with current thoracoabdominal movements, and hypopnea was scored when a reduction of the airflow ≥ 50% was present lasting ≥ 10 sec associated with either an arousal or a desaturation of ≥ 3%.24
All NPSGs were analyzed and several variables were derived for further examination: AHI, PLMI, SIDVP, and total arousal index (AI) composed of respiratory-related arousal index, periodic limb movement AI, spontaneous AI, and major body movements.
The SIDVP was measured by transmission of the infrared light through the finger pulp (photoplethysmography) of the index finger, generated from the conventional pulse oximeter trace of the already recorded NPSGs. The contour of the DVP exhibits an early systolic peak and a later peak or point of inflection, as a result of a complex interaction between the left ventricle and the systemic circulation. The first peak is considered to be a result of pressure transmitted along a direct path from the left ventricle to the finger, and the second peak is formed partly by pressure transmitted along the aorta and large arteries to sites of increased resistance in the lower body, where it is reflected back up the aorta. The equation used for SIDVP equals the height of the patient (h) divided by the transit time taken for pressure to propagate from the aorta to the lower body and then reflected back to the root of the subclavian artery (ΔΤDVP); which is the difference between the first and second peak (SIDVP = h / ΔΤDVP).14,25 The SIDVP for all patients was measured blindly to clinical diagnosis, for 2 min prior to sleep onset, for 2 min every half an hour after sleep onset, and in the morning after completion of the study while the patient lay relaxed without external stimuli.
The protocol was approved by Guy's and St. Thomas' Hospital Research Ethics Committee.
Statistical Analysis
Statistical analysis was performed using the SPSS statistical analysis program (IBM, SPSS 20.0). Data are reported as mean ± standard deviation (SD) if not otherwise indicated. Comparison of demographics between groups was made using one-way analysis of variance with Bonferroni multiple comparison test, whereas for sleep parameters Kruskal-Wallis with Dunn multiple comparison test was used. Differences in SIDVP between groups during the night and for each sleep stage were analyzed by nonparametric Kruskal-Wallis with Dunn multiple comparison test and changes of SIDVP within groups overnight by nonparametric Friedman test with Dunn multiple comparison post hoc test or Wilcoxon signed rank test.
RESULTS
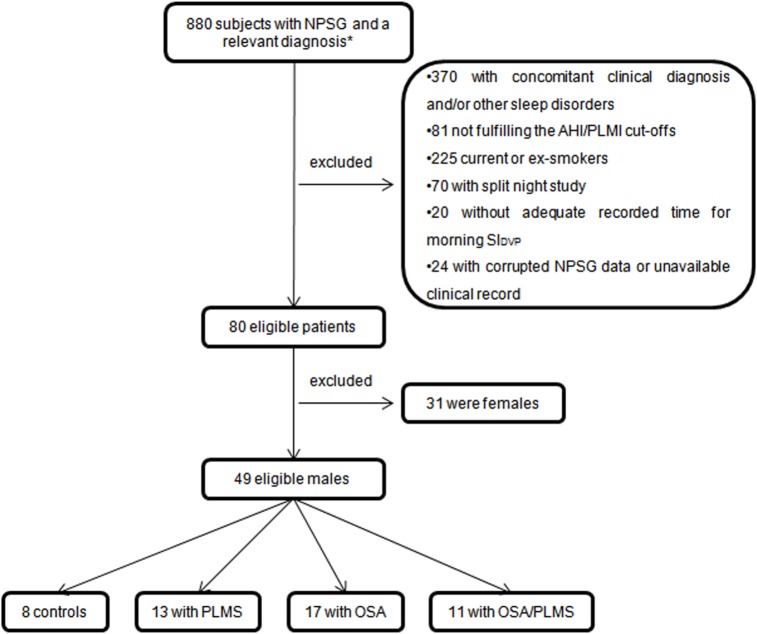
From the 880 subjects who underwent NPSG at Guy's and St. Thomas' Sleep Disorders Centre between January 2011 and July 2014 and received a diagnosis of one of the four included diagnoses in this study, only 49 male subjects were finally eligible for further analysis and generation of the oximetry derived SIDVP. The majority of the excluded patients had a concomitant clinical diagnosis and/or other sleep disorders included in the exclusion criteria (46%) or were current or ex-smokers (28%) (Figure 1). To avoid known sex influence on arterial stiffness, 31 female subjects were excluded from the remaining 80 eligible patients, and the males were categorized as follows: 8 were included in the control group, 13 in the PLMS, 17 in the OSA and another 11 in the OSA/PLMS group. Demographics and sleep variables of these subjects are presented in Table 1.
Figure 1. Flow chart summary of the sampling methods.
*Controls, PLMS, OSA, OSA/PLMS. AHI, apnea-hypopnea index; NPSG, nocturnal polysomnography; OSA, obstructive sleep apnea; PLMI, periodic limb movement index; PLMS, periodic limb movements during sleep.
Table 1.
Demographics and sleep parameters of the groups.
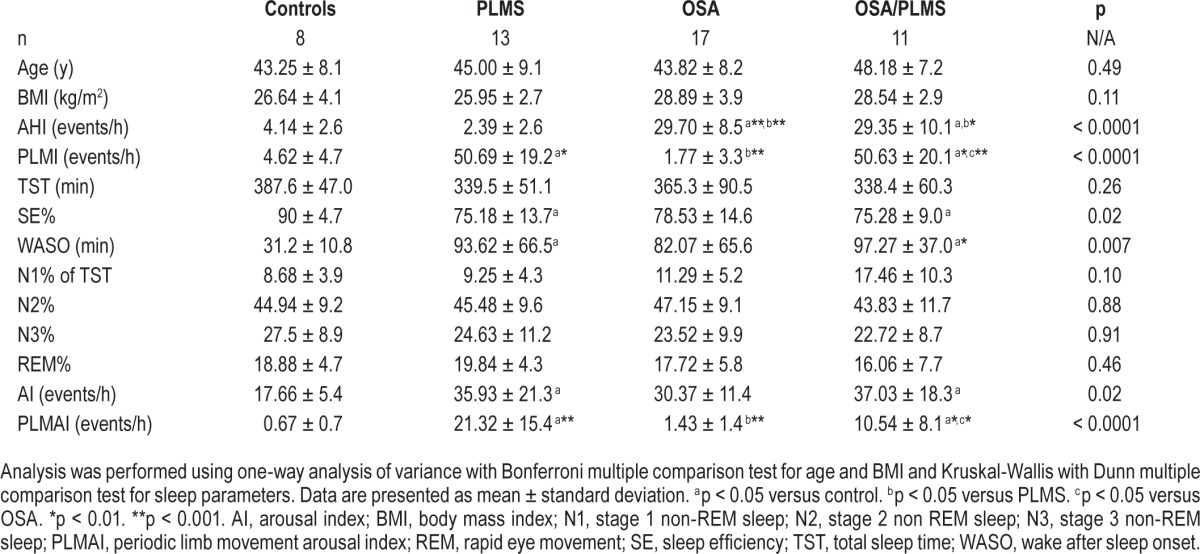
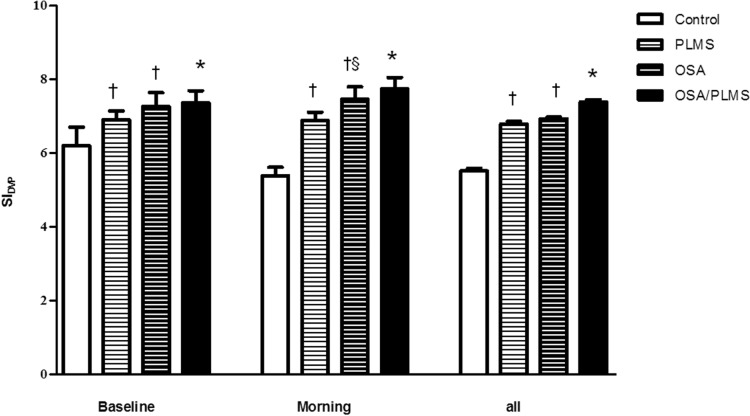
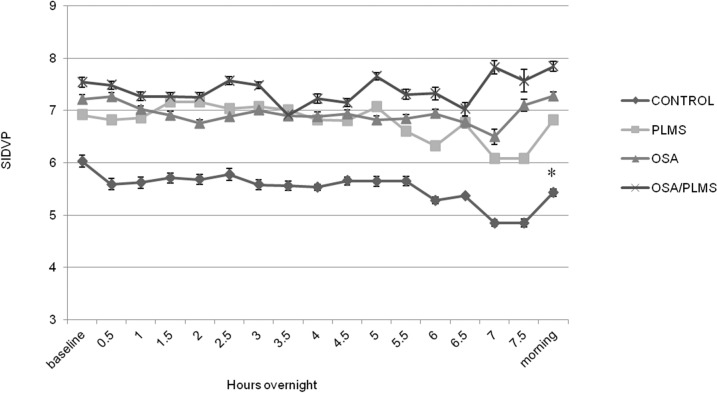
Groups were similar in terms of age and BMI, and PLMI and AHI indices did not differ significantly, where appropriate, between OSA/PLMS, PLMS and OSA groups (p < 0.05, Table 1). Control subjects, as expected, showed the lowest mean for all the predetermined overnight measurements of SIDVP (5.52 ± 0.3 m/sec) compared to PLMS, OSA and OSA/PLMS (6.78 ± 0.3, 6.94 ± 0.1, 7.40 ± 0.2 m/sec, p < 0.001), whereas the OSA/PLMS group demonstrated the highest (p < 0.001) (Figure 2). Patients with PLMS and OSA showed comparable overall SIDVP (6.78 ± 0.3 versus 6.94 ± 0.1 m/sec, p = 0.5). Similar results were seen when the mean SIDVP at baseline and in the morning was compared between groups, except that morning measurements in the OSA group was higher compared to PLMS (7.27 ± 1.5 versus 6.82 ± 0.9, p = 0.032). (Figure 2) Only the control group demonstrated a lower morning arterial stiffness compared to its baseline measurements (5.39 ± 0.6 versus 6.20 ± 0.50, p = 0.008); the PLMS showed no significant decrement (6.88 ± 0.9 versus 6.90 ± 0.8). The rest of the two groups showed a tendency for an increased SIDVP in the morning compared to baseline but not statistical significant (OSA, 7.46 ± 1.4 versus 7.26 ± 1.5; OSA/PLMS, 7.74 ± 1.1 versus 7.36 ± 1.1) (Figure 3).
Figure 2. Comparison of mean SIDVP between groups, at baseline, morning, and all measurements.
Data are presented as mean ± standard error of the mean. †p < 0.05 versus control, §p < 0.05 versus PLMS, *p < 0.05 versus all the rest of the groups. OSA, obstructive sleep apnea; PLMS, periodic limb movements of sleep; all = baseline, nocturnal, morning.
Figure 3. SIDVP for the four groups averaged for 2 min before (baseline), at the end (morning) and every half hour after sleep onset during the sleep study.
Data are presented as mean ± standard error of the mean. *p < 0.05 for SIDVP morning versus SIDVP baseline.
To better understand the influence of sleep stage on arterial stiffness, the SIDVP per sleep stage for each group was calculated. Within-group analysis revealed that in the control group the mean SIDVP in sleep stage 1 (N1) was lower compared to other sleep stages (p < 0.001) (Table 2), whereas in patient groups the mean SIDVP during N1 was higher compared to REM (p < 0.05) (Table 3, Figure 4).
Table 2.
Mean SIDVP per sleep stage and within-groups comparison.
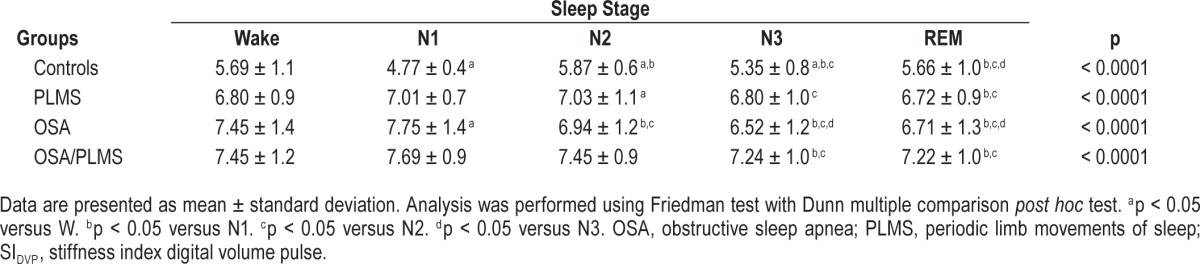
Table 3.
Mean SIDVP per sleep stage and between-groups comparison.
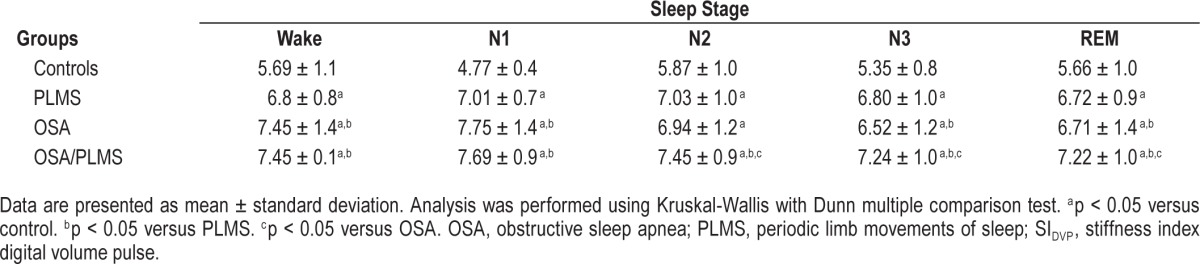
Figure 4. Mean SIDVP per sleep stage for the four groups.
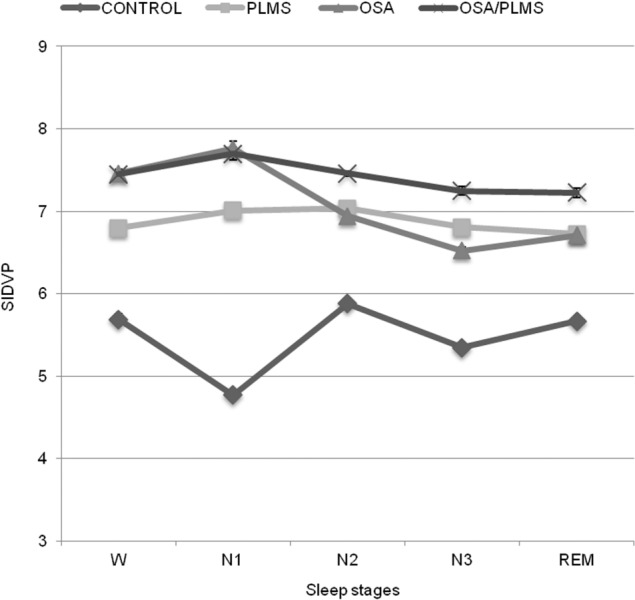
Data are presented as mean ± standard error of the mean.
The control group also had the lowest SIDVP per sleep stage when compared to the patient groups (p < 0.001). The OSA/PLMS group had the highest arterial stiffness in sleep stage 2 (N2), sleep stage 3 (N3) and REM (p < 0.001) and comparable with the OSA group in wake (W) and N1 (p = 0.09 and p = 0.5 respectively) (Table 3, Figure 4).
DISCUSSION
Although the hypothesis that PLMS may be a possible risk factor for CVD was raised several years ago,26 only recently have larger studies demonstrated a positive association.8,9,11,27 Although conventional methods of measuring large artery stiffness would not be applicable during an overnight sleep study, new validated methods provide this option. SIDVP derived from photoplethysmography shares the same principles as pulse oximetry, allowing for overnight analysis in patients with various sleep conditions.14,28 OSA is already considered an important contributory factor in CVD,29 and increased arterial stiffness in patients with OSA has already been demonstrated.9,30 To our knowledge, this is the first study to examine arterial stiffness in patients with PLMS, and to compare this relationship with arterial stiffness in OSA. In this study, patients with significant PLMS exhibited an increased SIDVP compared to controls, comparable to patients with moderate to severe OSA, whereas the OSA/PLMS group exhibited the highest SIDVP, implying a possible additive role of the two conditions in driving endothelial dysfunction.
Sympathetic activation remains the most likely pathophysiological mechanism through which PLMS lead to CVD.11 PLMS are associated with significant rises in pulse rate and transient increases in blood pressure which, in the long term, may lead to hypertension, heart disease, and stroke.7,27 A 10- to 20-mmHg elevation in blood pressure and 10 beats per minute (bpm) increase in heart rate have been associated with limb movements comprising PLMS.7,27 A recent observational study by Koo et al.9 demonstrated that the frequency of PLMS is associated with incident cardiovascular disease in elderly men. The Multi-Ethnic Study of Atherosclerosis (MESA) concluded that OSA is a risk marker for increased blood pressure, whereas limb movements with arousals were independently associated with increased diastolic blood pressure.8 In the current study, patients with significant PLMS had higher arterial stiffness at baseline, overnight, and in the morning, compared to ageand BMI-matched controls, and were generally comparable to those of patients with moderate/severe OSA. These findings may reflect the importance of PLMS in the development of CVD but also the applicability of using a validated marker of large artery stiffness, SIDVP, in large-scale studies through conventional pulse oximetry recordings, even without the utilization of commercial devices or sophisticated algorithms.
The comparable results of SIDVP between PLMS and OSA raises the question of whether PLMS are as important as OSA from a CVD perspective. Data are emerging that assess PLMS and hypertension but still research of greater scale is required. Furthermore, large prospective studies have demonstrated that nocturnal blood pressure is a better predictor of CVD risk than daytime blood pressure,31 emphasizing the need for further studies to be undertaken regarding PLMS as a possible risk factor for CVD.
The OSA/PLMS group demonstrated the highest SIDVP compared to PLMS and OSA groups. An additive role of the two conditions for the increased arterial stiffness is conceivable, with, a recent study demonstrating that PLMS in patients with OSA is associated with increased systemic inflammation and fibrinogen levels, both predictive factors for future cardiovascular events.32 As PLMS tend to be frequent in patients with OSA, a more detailed analysis of this hypothesized additive effect on CVD should be considered.
We found a significant influence of sleep stage on the SIDVP, both in controls and in patient groups. Although in controls the lowest arterial stiffness was in N1, in the patient groups N3 and REM demonstrated the lowest arterial stiffness. Previous studies have shown that there is a downregulation of the sympathoadrenal and noradrenergic branches of the sympathetic nervous system with significant decrease of catecholamine concentration from N1 to REM sleep.33,34 However, increased muscle sympathetic activity, blood pressure, and heart rate during REM have also been demonstrated implying a complex orchestration of autonomic nervous system functionality.35 Our patients' results are in line with previous analysis in patients with OSA,15 and suggest a complicated dysregulation of the sympathetic nervous system as a result of these sleep disorders.
Limitations
By its retrospective nature, and the strict exclusion criteria applied, we recognize the major limitation of this study as small sample size, and clearly the findings of this pilot study need to be replicated in larger prospective cohorts, which would allow further correlation analysis of arousals (respiratory and leg related) with SIDVP. Excluding female subjects due to known effects on arterial stiffness is another limitation and future matched for sex studies would be recommended. That was a sleep laboratory-based study and future community-based enrollment would be advisable. This analysis is cross-sectional in nature and as such, inferences to causality cannot be made.
CONCLUSION
In this study, utilizing a validated contour analysis of the DVP, which is easily derived from the oximetry recordings and widely available in clinical practice, we have demonstrated that the arterial stiffness index in patients with significant PLMS is increased compared to controls, comparable to patients with moderate to severe OSA, whereas a possible additive impact of PLMS on arterial stiffness in patients with OSA was also identified. Establishing the clinical relevance of these findings will require larger, prospective studies.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr Drakatos was supported by the Greek State Scholarships Foundation (SSF) resources from the Operational Program “Education and Lifelong Learning,” the European Social Fund (ESF) of the National Strategic Reference Framework (NSRF) 2007-2013. Dr Leschziner has received honorarium from UCB Pharma and Somnomed as an advisory board member. Dr. Williams has participated in speaking engagements for UCB Pharma. The other authors have indicated no financial conflicts of interest. The study was conducted at Guy's Hospital Sleep Disorders Centre, 3rd floor, Nuffield House Great Maze Pond London SE1 9RT. United Kingdom. There was no off-label or investigational use.
ABBREVIATIONS
- AHI
apnea-hypopnea index
- AI
arousal index
- BP
blood pressure
- bpm
beats per minute
- CPAP
continues positive airway pressure
- CVD
cardiovascular disease
- DVP
digital volume pulse
- EDS
excessive daytime sleepiness
- EEG
electroencephalographic
- h
height
- ICSD
International Classification of Sleep Disorders
- MESA
Multi-Ethnic Study of Atherosclerosis
- N1
sleep stage 1
- N2
sleep stage 2
- N3
sleep stage 3
- NPSG
nocturnal polysomnography
- OSA
obstructive sleep apnea
- PLMAI
periodic limb movement arousal index
- PLMD
periodic limb movement disorder
- PLMI
periodic limb movement index
- PLMS
periodic limb movements during sleep
- PWV
pulse wave velocity
- REM
rapid eye movement
- RLS
restless legs syndrome
- SD
standard deviation
- SEM
standard error of the mean
- SIDVP
stiffness index derived from DVP
- W
wake
- ΔT
transit time
- ΔΤDVP
transit time taken for pressure to propagate from aorta to the lower body and then reflected back to the root of the subclavian artery
REFERENCES
- 1.American Academy of Sleep Medicine. International classification of sleep disorders. 3rd ed. Darien, IL: American Academy of Sleep Medicine; 2014. [Google Scholar]
- 2.Hornyak M, Feige B, Riemann D, Voderholzer U. Periodic leg movements in sleep and periodic limb movement disorder: prevalence, clinical significance and treatment. Sleep Med Rev. 2006;10:169–77. doi: 10.1016/j.smrv.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 3.Allen RP, Picchietti D, Hening WA, Trenkwalder C, Walters AS, Montplaisi J. Restless legs syndrome: diagnostic criteria, special considerations, and epidemiology. A report from the restless legs syndrome diagnosis and epidemiology workshop at the National Institutes of Health. Sleep Med. 2003;4:101–19. doi: 10.1016/s1389-9457(03)00010-8. [DOI] [PubMed] [Google Scholar]
- 4.Al-Alawi A, Mulgrew A, Tench E, Ryan CF. Prevalence, risk factors and impact on daytime sleepiness and hypertension of periodic leg movements with arousals in patients with obstructive sleep apnea. J Clin Sleep Med. 2006;2:281–7. [PubMed] [Google Scholar]
- 5.Dauvilliers Y, Pennestri MH, Petit D, Dang-Vu T, Lavigne G, Montplaisir J. Periodic leg movements during sleep and wakefulness in narcolepsy. J Sleep Res. 2007;16:333–9. doi: 10.1111/j.1365-2869.2007.00601.x. [DOI] [PubMed] [Google Scholar]
- 6.Fantini ML, Michaud M, Gosselin N, Lavigne G, Montplaisir J. Periodic leg movements in REM sleep behavior disorder and related autonomic and EEG activation. Neurology. 2002;59:1889–94. doi: 10.1212/01.wnl.0000038348.94399.f6. [DOI] [PubMed] [Google Scholar]
- 7.Pennestri MH, Montplaisir J, Colombo R, Lavigne G, Lanfranchi PA. Nocturnal blood pressure changes in patients with restless legs syndrome. Neurology. 2007;68:1213–8. doi: 10.1212/01.wnl.0000259036.89411.52. [DOI] [PubMed] [Google Scholar]
- 8.Dean DA, Wang R, Jacobs DR, et al. A systematic assessment of the association of polysomnographic indices with blood pressure: the Multi-Ethnic Study of Atherosclerosis (MESA) Sleep. 2015;38:587–96. doi: 10.5665/sleep.4576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koo BB, Sillau S, Dean DA, 2nd, Lutsey PL, Redline S. Periodic Limb Movements During Sleep and Prevalent Hypertension in the Multi-Ethnic Study of Atherosclerosis. Hypertension. 2015;65:70–7. doi: 10.1161/HYPERTENSIONAHA.114.04193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koo BB, Blackwell T, Ancoli-Israel S, Stone KL, Stefanick ML, Redline S. Association of incident cardiovascular disease with periodic limb movements during sleep in older men: outcomes of sleep disorders in older men (MrOS) study. Circulation. 2011;124:1223–31. doi: 10.1161/CIRCULATIONAHA.111.038968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walters AS, Rye DB. Review of the relationship of restless legs syndrome and periodic limb movements in sleep to hypertension, heart disease, and stroke. Sleep. 2009;32:589–97. doi: 10.1093/sleep/32.5.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laurent S, Boutouyrie P, Asmar R, et al. Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension. 2001;37:1236–41. doi: 10.1161/01.hyp.37.5.1236. [DOI] [PubMed] [Google Scholar]
- 13.Mattace-Raso FU, van der Cammen TJ, Hofman A, et al. Arterial stiffness and risk of coronary heart disease and stroke: the Rotterdam Study. Circulation. 2006;113:657–63. doi: 10.1161/CIRCULATIONAHA.105.555235. [DOI] [PubMed] [Google Scholar]
- 14.Millasseau SC, Kelly RP, Ritter JM, Chowienczyk PJ. Determination of age-related increases in large artery stiffness by digital pulse contour analysis. Clin Sci (Lond) 2002;103:371–7. doi: 10.1042/cs1030371. [DOI] [PubMed] [Google Scholar]
- 15.Scholze A, Lamwers S, Tepel M, Sanner BM. Nasal continuous positive airway pressure: influence on digital volume pulse in obstructive sleep apnoea patients. Eur Respir J. 2012;39:1127–35. doi: 10.1183/09031936.00052611. [DOI] [PubMed] [Google Scholar]
- 16.Millasseau SC, Guigui FG, Kelly RP, et al. Noninvasive assessment of the digital volume pulse. Comparison with the peripheral pressure pulse. Hypertension. 2000;36:952–6. doi: 10.1161/01.hyp.36.6.952. [DOI] [PubMed] [Google Scholar]
- 17.Kartali N, Daskalopoulou E, Geleris P, et al. The effect of continuous positive airway pressure therapy on blood pressure and arterial stiffness in hypertensive patients with obstructive sleep apnea. Sleep Breath. 2014;18:635–40. doi: 10.1007/s11325-013-0926-0. [DOI] [PubMed] [Google Scholar]
- 18.Shiina K, Tomiyama H, Takata Y, et al. Concurrent presence of metabolic syndrome in obstructive sleep apnea syndrome exacerbates the cardiovascular risk: a sleep clinic cohort study. Hypertens Res. 2006;29:433–41. doi: 10.1291/hypres.29.433. [DOI] [PubMed] [Google Scholar]
- 19.Drager LF, Bortolotto LA, Maki-Nunes C, et al. The incremental role of obstructive sleep apnoea on markers of atherosclerosis in patients with metabolic syndrome. Atherosclerosis. 2010;208:490–5. doi: 10.1016/j.atherosclerosis.2009.08.016. [DOI] [PubMed] [Google Scholar]
- 20.Tsioufis C, Thomopoulos K, Dimitriadis K, et al. The incremental effect of obstructive sleep apnoea syndrome on arterial stiffness in newly diagnosed essential hypertensive subjects. J Hypertens. 2007;25:141–6. doi: 10.1097/HJH.0b013e32801092c1. [DOI] [PubMed] [Google Scholar]
- 21.Bakker JP, Balachandran JS, Tecilazich F, et al. Pilot study of the effects of bariatric surgery and continuous positive airway pressure treatment on vascular function in obese subjects with obstructive sleep apnoea. Intern Med J. 2013;43:993–8. doi: 10.1111/imj.12224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Litvin AY, Sukmarova ZN, Elfimova EM, et al. Effects of CPAP on “vascular” risk factors in patients with obstructive sleep apnea and arterial hypertension. Vasc Health Risk Manag. 2013;9:229–35. doi: 10.2147/VHRM.S40231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Benetos A, Waeber B, Izzo J, et al. Influence of age, risk factors, and cardiovascular and renal disease on arterial stiffness: clinical applications. Am J Hypertens. 2002;15:1101–8. doi: 10.1016/s0895-7061(02)03029-7. [DOI] [PubMed] [Google Scholar]
- 24.Iber C, Anconi-Israel S, Chesson A, et al. Westchester, IL: American Academy of Sleep Medicine; 2007. for the American Academy of Sleep Medicine. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications. [Google Scholar]
- 25.Chowienczyk PJ, Kelly RP, MacCallum H, et al. Photoplethysmographic assessment of pulse wave reflection: blunted response to endothelium-dependent beta2-adrenergic vasodilation in type II diabetes mellitus. J Am Coll Cardiol. 1999;34:2007–14. doi: 10.1016/s0735-1097(99)00441-6. [DOI] [PubMed] [Google Scholar]
- 26.Espinar-Sierra J, Vela-Bueno A, Luque-Otero M. Periodic leg movements in sleep in essential hypertension. Psychiatry Clin Neurosci. 1997;51:103–7. doi: 10.1111/j.1440-1819.1997.tb02370.x. [DOI] [PubMed] [Google Scholar]
- 27.Siddiqui F, Strus J, Ming X, Lee IA, Chokroverty S, Walters AS. Rise of blood pressure with periodic limb movements in sleep and wakefulness. Clin Neurophysiol. 2007;118:1923–30. doi: 10.1016/j.clinph.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 28.Padilla JM, Berjano EJ, Saiz J, Rodriguez R, Facila L. Pulse wave velocity and digital volume pulse as indirect estimators of blood pressure: pilot study on healthy volunteers. Cardiovasc Eng. 2009;9:104–12. doi: 10.1007/s10558-009-9080-5. [DOI] [PubMed] [Google Scholar]
- 29.Kent BD, Ryan S, McNicholas WT. Obstructive sleep apnea and inflammation: relationship to cardiovascular co-morbidity. Respir Physiol Neurobiol. 2011;178:475–81. doi: 10.1016/j.resp.2011.03.015. [DOI] [PubMed] [Google Scholar]
- 30.Phillips CL, Butlin M, Wong KK, Avolio AP. Is obstructive sleep apnoea causally related to arterial stiffness? A critical review of the experimental evidence. Sleep Med Rev. 2013;17:7–18. doi: 10.1016/j.smrv.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 31.Dolan E, Stanton A, Thijs L, et al. Superiority of ambulatory over clinic blood pressure measurement in predicting mortality: the Dublin outcome study. Hypertension. 2005;46:156–61. doi: 10.1161/01.HYP.0000170138.56903.7a. [DOI] [PubMed] [Google Scholar]
- 32.Murase K, Hitomi T, Hamada S, et al. The additive impact of periodic limb movements during sleep on inflammation in patients with obstructive sleep apnea. Ann Am Thorac Soc. 2014;11:375–82. doi: 10.1513/AnnalsATS.201306-144OC. [DOI] [PubMed] [Google Scholar]
- 33.Otter HP, Baust W. Experiments on cholinergic mechanisms for the control of tonic and phasic components of REM sleep. Electroencephalogr Clin Neurophysiol. 1970;29:218. [PubMed] [Google Scholar]
- 34.Dodt C, Breckling U, Derad I, Fehm HL, Born J. Plasma epinephrine and norepinephrine concentrations of healthy humans associated with nighttime sleep and morning arousal. Hypertension. 1997;30:71–6. doi: 10.1161/01.hyp.30.1.71. [DOI] [PubMed] [Google Scholar]
- 35.Somers VK, Dyken ME, Mark AL, Abboud FM. Sympathetic-nerve activity during sleep in normal subjects. N Engl J Med. 1993;328:303–7. doi: 10.1056/NEJM199302043280502. [DOI] [PubMed] [Google Scholar]