Abstract
Synthetic pyrethroids (SPs) are the most common pesticides which are recently used for indoor pest control. The widespread use of SPs has resulted in the increased exposure to wild animals and humans. Recently, some SPs are suspected as endocrine disrupting chemicals (EDCs) and have been assessed for their potential estrogenicity by adopting various analyzing assays. In this study, we examined the estrogenic effects of lambda-cyhalothrin (LC) and cypermethrin (CP), the most commonly used pesticides in Korea, using BG-1 ovarian cancer cells expressing estrogen receptors (ERs). To evaluate the estrogenic activities of two SPs, LC and CP, we employed MTT assay and reverse-transcription polymerase chain reaction (RT-PCR) in LC or CP treated BG-1 ovarian cancer cells. In MTT assay, LC (10−6 M) and CP (10−5 M) significantly induced the growth of BG-1 cancer cells. LC or CP-induced cell growth was antagonized by addition of ICI 182,720 (10−8 M), an ER antagonist, suggesting that this effect appears to be mediated by an ER-dependent manner. Moreover, RT-PCR results showed that transcriptional level of cyclin D1, a cell cycle-regulating gene, was significantly up-regulated by LC and CP, while these effects were reversed by co-treatment of ICI 182,780. However, p21, a cyclin D-ckd-4 inhibitor gene, was not altered by LC or CP. Moreover, ERα expression was not significantly changed by LC and CP, while downregulated by E2. Finally, in xenografted mouse model transplanted with human BG-1 ovarian cancer cells, E2 significantly increased the tumor volume compare to a negative control, but LC did not. Taken together, these results suggest that LC and CP may possess estrogenic potentials by stimulating the growth of BG-1 ovarian cancer cells via partially ER signaling pathway associated with cell cycle as did E2, but this estrogenic effect was not found in in vivo mouse model.
Keywords: Endocrine disruption, Pyrethroids, Lambda-cyhalothrin, Cyperemethrin, Ovarian cancer, Xenograft models
INTRODUCTION
Endocrine disrupting chemicals (EDCs) are environmentally persistent chemicals that interfere with the physiological endocrine systems and abnormally regulated the activation by naturally circulating steroid hormones via those receptors (1-3). In general, EDCs have various chemical structures similar to that of 17β-estradiol (E2), an endogenous estrogen and they mimic estrogenic or androgenic effects on steroid receptors such as estrogen receptor (ER) or androgen receptor (AR), interfere with the actions of endogenous steroid hormones (1,4,5).Therefore, increasing concern about the environmental effects of EDCs, diverse xenoestrogens has found in natural or artificial environments.
Synthetic pyrethroids (SPs) are the most common pesticides in recent use, which are used as indoor pest control (6,7), especially, lambda-cyhalothrin (LC) and cypermethrin (CP) are the most commonly used in Republic of Korea. The widespread use of SPs has resulted in an increased presence in the wild animals and extensive human exposure (6,8-11). SPs are known to exert their pesticidal actions by altering the sodium permeability of insect neural membranes by adjusting voltage-sensitive sodium channels (12). They were regarded as safe pesticides because of their highly selectivity of favorable insect : mammalian toxicity ratio (13).
Although the use of SPs has been promoted by their low toxicities in mammals and birds (14), possible adverse effects on the endocrine system have been observed with several SPs (15). Moreover, some researchers have already included SPs in their lists of possible EDCs (16). Recently, many studies have estimated the estrogenic potential of SPs, LC or CP, by adopting several different analyzing assays such as cell proliferation assay or E-screen assay (11,15,17), reporter gene or binding assay (18,19), ER competitive-binding assay (17), animal reproductive system (20,21). However, yet there is no research on the relationship between the SPs and cell cycle-related genes or as regards in estrogen-responsive cells such as BG-1 human ovarian cancer cell line.
In this study, we examined the estrogenic effects of two pyrethroids, LC and CP, in the mechanism of cell cycle related via ER signaling pathway of BG-1 human ovarian cancer cells expressing ER in cellular levels and xenograft mouse model.
MATERIALS AND METHODS
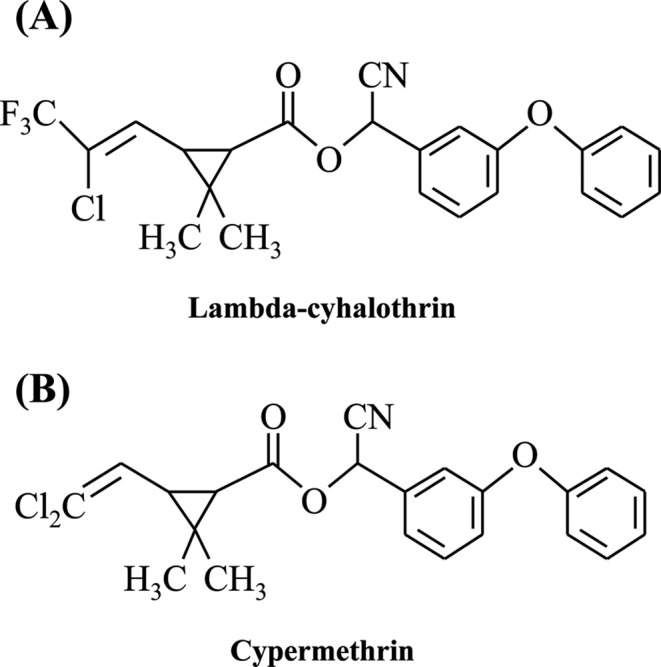
Chemicals. 17β-estradiol (E2), fluvestrant (ICI 182,780), lambda-cyhalothrin and cyperemethrin were purchased from Sigma-Aldrich Inc. (St. Louis, MO, USA). Chemical structures of the pyrethroids tested in this study were shown in Fig. 1. A final concentration of DMSO that used for vehicle was 0.1% in the culture media.
Fig. 1. Chemical structure of two synthetic pyrethroid insecticides.
Cell culture and media. BG-1 human ovarian cancer cells were obtained from Dr. K. S. Korach (National Institute of Environmental Health Sciences, NIH, Research Triangle Park, NC, USA). The cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM; Hyclone Laboratories, Inc. Logan, UT, USA) supplemented with 10% heat inactivated fetal bovine serum (FBS; Hyclone Laboratories), 1% penicillin-streptomycin (A&E Scientific, Rue de Lekernay, Belgium) at 37℃ in a humidified atmosphere of 5% CO2 containing air. To prevent the estrogenic effects of some components in DMEM and FBS, phenol red-free DMEM (Sigma-Aldrich Inc.) supplemented with 5% charcoal-dextran treated FBS was used for detection of the estrogenicity of EDCs in BG-1 cells. The cells were detached with 0.05% Trypsin-EDTA (Life Technologies Inc., Grand Island, NY, USA).
Cell viability assays (MTT assay). BG-1 cells were seeded at a density of 3 × 103 cells per well of 96-well plates. After 2-day incubation, the culture medium was replaced with new medium containing E2 or two-pesticides and changed every three days, and the cells were incubated for further 9 days. Next, the culture medium was removed and then 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT; Sigma-Aldrich Inc.) solution was added for 4 hr. After the culture medium was removed, 200 μL of DMSO (Junsei Chemical Co., Ltd., Tokyo, Japan) was added per well. The absorbance was measured at 540 nm, using an ELISA reader (Epoch, BioTek Instruments, Inc., VT, USA).
Total RNA extraction and semi-quantitative reverse transcription (RT)-PCR. Total RNA was isolated from cells using TRIzol reagent (Invitrogen Life Technologies, Carlsbad, CA, USA) following the manufacturer’s instructions. The total RNA concentration was measured with a microreader (BioTek Instruments) at 260/280 nm. One mg of total RNA was dissolved in 60 mL diethyl pyrocarbonate treated-deionized water (DEPC-DW) for cDNA synthesis. To synthesize cDNA from total RNA for RT-PCR, the PCR mixture consisted of 200 U murine leukemia virus reverse transcriptase (M-MLV RT), 200 pM nonamer random primers, 2.5 mM dNTPs, 0.4 μL RNA inhibitor, and 5x RT buffer (all from iNtRON Biotechnology, Seongnam, Republic of Korea). cDNA synthesis was performed at 37℃ for 1 hr and the enzyme was inactivated at 95℃ for 5 min.
To measure the mRNA expression of p21, cyclin D1 and ERα, the genes were amplified using 10 pM of each forward and reverse primer (Bioneer Co., Daejeon, Republic of Korea), 2 units of Taq polymerase (iNtRON Biotechnology), MMLV-RT buffer (iNtRON Biotechnology), 5 pM dNTP mixture, and 1 μL cDNA as the template. PCR was carried out for 30 cycles of 95℃ for 30 s, 58℃ for 30 s, and 72℃ for 30 s. The following primers were used for this experiment: 5'-AGGCA CCGAG GCACT CAGAG-3' (forward) and 5'-TGACA GGTCC ACATG GTCTT CC-3' (reverse) for p21 (370 bp), 5'-TCTAA GATGA AGGAG ACCATC-3' (forward) and 5'-GCGGT AGTAG GACAG GAAGT TGTT-3' (reverse) for cyclin D1 (354 bp), 5'-AGACA TGAGA GCTGC CAACC-3' (forward) and 5'-GCCAG GCACA TTCTA GAAGG-3' (reverse) for ERα, and 5'-ATGTT CGTCA TGGGT GTGAA CCA-3' and 5'-TGGCA GGTT TTTCT AGACG GCAG-3' for human GAPDH (351 bp). The housekeeping gene GAPDH was used as the control. The PCR products were separated in a 1.5% agarose gel and size of the bands were estimated relative to SiZerTM-100 bp DNA marker (iNtRON Biotechnology). The gels were scanned and optical density of the bands was quantified using Gel Doc 2000 (Bio-Rad, Laboratories, Inc., Berkeley, CA, USA). Each experiment was carried out in triplicate.
Animal experiments. BALB/c Nude female mice (4 weeks old) were purchased from DAEHAN BioLink (Eumseong, Korea) and the experiments were conducted according to the protocols approved by the Animal Care Committee of Chungbuk National University. The room was kept at 24℃ under a 12-hr light-dark cycle. The mice were acclimatized for at least one week prior to the start of experiments. To manufacture animal models xenografted with BG-1 ovarian cancer cells, BG-1 cells (3 × 107) were mixed with Matrigel (BD Biosciences, Bedford, MA, USA) at 1 : 1 volume ratio of Matrigel to PBS in 100 μL and injected subcutaneously into the back of the mice. Mice were monitored for tumor growth every 10 days and the tumor volumes were measured using a vernier calipers and calculated by length × width × height × 0.5236 (mm3). Once the tumor reached over 60 mm3, the mice were surgically ovariectomized under anesthesia using avertin (diluted in PBS). The mice were divided into three groups and each mouse was injected intraperitoneal every 3-4 days for a period of 80 days with one of the followings: 1% DMSO in PBS as a negative control (n = 3), E2 (n = 3; 20 μg/kg body weight/day), and lambda-cyhalothrin (n = 3; 40 mg/kgbody weight/day). Injection concentration of pesticide was two thousands of acceptable daily intake (ADI) as provided by the Joint FAO/WHO Meeting on Pesticides Residues (JMPR) in 2005 or 2003.
Data analysis. Data are expressed as the mean ± standard error of the mean. Results were analyzed using a one-way analysis of variance (ANOVA) followed by post hoc Dunnett’s. P-values < 0.05 were considered statistically significant.
RESULTS
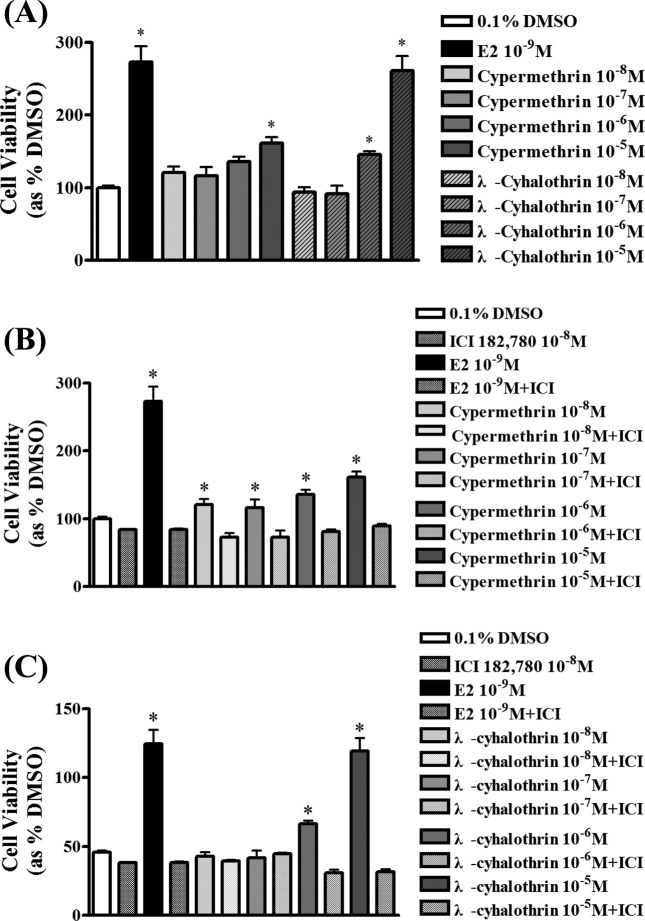
Effects of pyrethroids on the cell viability of BG-1 cells. The estimate the effect of pyrethroids on BG-1 cell growth, an estrogen responsive cells, these cells were cultured with a vehicle (0.1% DMSO), E2 (10−9 M), CP (10−5-10−8 M), LC (10−5-10−8 M). Each treatment was cultured for 9 days. E2 as a positive control markedly increased BG-1 cell proliferation about 2.5 times more compared to 0.1% DMSO (negative control) as shown in Fig. 2A. Two pyrethroids significantly increased BG-1 cell proliferation the most at 10−5 M in a dose-dependent manner but CP and LC did not induce BG-1 cell proliferation at 10−6 M and 10−7 M, respectively, indicating that two pyrethroids have an estrogenic potential even at a much higher concentration. Then, to identify the relevance of ER signaling pathway, ICI 182,780 was used by an ER-antagonist and treated with two pyrethroids. In the co-treatment of ICI 182,780, E2 or two pyrethroids-induced cell growth was reversed to a level of a negative control regardless of the concentration of pyrethroids (Fig. 2B and C). These findings indicate that two pyrethroids may have estrogenic potential via an ER signaling pathway.
Fig. 2. Cell proliferation of BG-1 ovarian cancer cells following the treatment with E2 or two-pyrethroids. To evaluate the effect of E2 and two-pesticides on cell viability, BG-1 cells were seeded at 96-well plate with 3,000 cells per well in 200 μL of phenol red-free DMEM with 5% CD–FBS medium. Cells were cultured in phenol red-free DMEM with 0.1% DMSO (control), E2 (10−8 M), cyperemethrin (10−5-10−8 M) and lambda-cyhalothrin (10−5-10−8 M) in the present (B,C) or absence (A) of ICI 182,780 (10−8 M) for 9 days. The cell viability was measured using an MTT assay. Values are the means ± SD. *: Mean values were significantly different from 0.1% DMSO (control), p< 0.05 (Dunnett’s multiple comparison test).
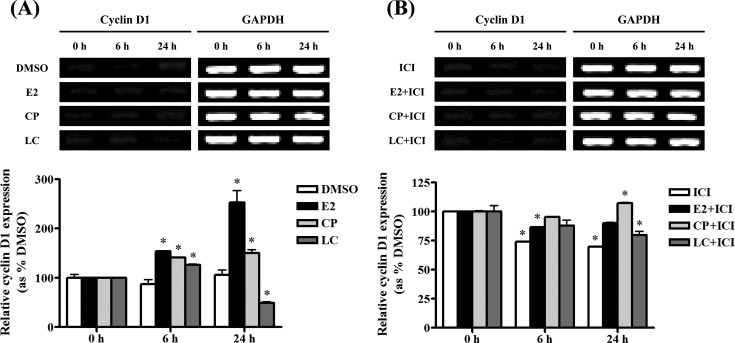
Cyclin D1 gene expression in BG-1 cells treated with pyrethroids. Since the cell growth of BG-1 cells was induced by two pyrethroids, we investigated their effects on the expression of genes associated with cyclin D1, a cell cycle-regulating gene, using by RT-PCR. We treated the cells with E2, two pyrethroids (10−5 M) for 6 and 24 hr, based on results of the cell viability assay. RT-PCR results showed that the expression of cyclin D1 was significantly up-regulated by treatment with E2 and two pyrethroids at each time point, but it was decreased by LC at 24 hr when normalized as GAPDH (Fig. 3A). In parallel, the expression of E2 or pyrethroids induced-cyclin D1 gene was down-regulated by the co-treatment of ICI 182,780 at 6 hr or 24 hr compared to the negative control level (Fig. 3B). These results indicate that cyclin D1 expression may be induced by E2 or pyrethroids via an ER dependent signaling pathway in BG-1 cells.
Fig. 3. Effects of pyrethroids on the expression of cyclinD1 mRNA in BG-1 ovarian cancer cells. BG-1 cells were seed in 6-well plate and treated with 0.1% DMSO, cypermethrin (10−5 M) and lambda-cyhalothrin (10−5 M) in the present (B) or absence (A) of ICI 182,780 in time-dependent manner. Cyclin D1 mRNA levels were determined by RT-PCR using GAPDH (a housekeeping gene) as an internal control. Quantification of gene of cyclin D1 was conducted by scanning the densities of bands on agarose gels using Gel Doc 2000. *: Mean values were significantly different from control (0.1% DMSO), p< 0.05 (Dunnett’s multiple comparison test).
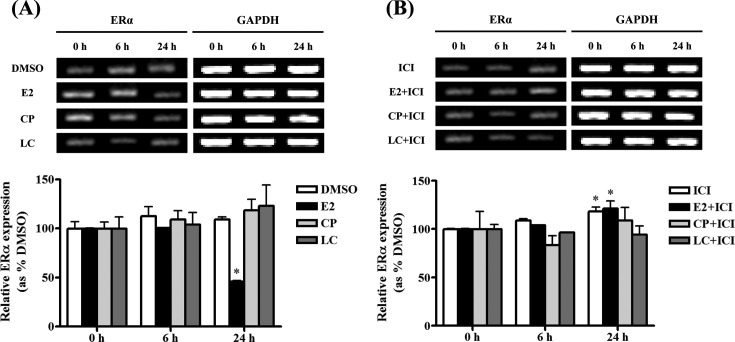
ERα gene expression in BG-1 cells treated with pyrethroids. In mRNA level, to identify the potential of relevance of ER-dependent signaling pathway as shown by MTT assay (Fig. 2), we further investigated relationship of ERα and pyrethroids in terms of ER-related cell proliferation using RT-PCR. However, the expression of ERα was not altered by treatment with pyrethroids, while it was reduced by E2 compared to a negative control at all time points (Fig. 4). These results suggested that pyrethroids-induced cell growth may be derived via other signaling pathways that partially involve an ER.
Fig. 4. Effects of pyrethroids on the expression of ERα mRNA in BG-1 ovarian cancer cells. BG-1 cells were seed in 6-well plate and treated with 0.1% DMSO, cypermethrin (10−5 M) and lambda-cyhalothrin (10−5 M) in the present (B) or absence (A) of ICI 182,780 in time-dependent manner. ERα mRNA levels were determined by RT-PCR using GAPDH (a housekeeping gene) as an internal control. Quantification of gene of ERα was conducted by scanning the densities of bands on agarose gels using Gel Doc 2000. *: Mean values were significantly different from control (0.1% DMSO), p< 0.05 (Dunnett’s multiple comparison test).
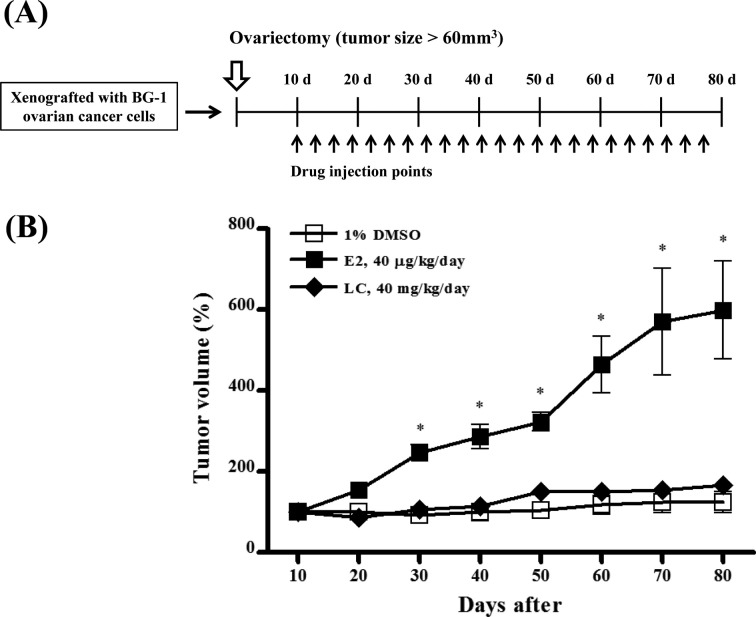
Effects of lambda-cyhalothrin on tumor growth in xenograft mouse model. To assess the potential of ability of lambda-cyhalothrin to stimulate estrogen-responsive tumor growth, the xenograft mice transplanted with BG-1 ovarian cancer cells were injected subcutaneously with DMSO, E2 or LC about every 3-4 days and tumor volumes were measured every 10 days during the experiments period (Fig. 5A). Since LC significantly increased the BG-1 cell proliferation at the higher concentrations of 1,000-10,000 times than that of E2 in vitro, we treated the mice with LC at a dose of 40 mg/kg/day, which was also 1,000 times higher than that of E2 (40 μg/kg/day). The mice injected with 1% DMSO in PBS as a negative control showed slight change, but the mice injected with E2 as a positive control showed increased tumor growth compared to a negative control as shown in Fig. 5B. Although LC increased significantly BG-1 cell growth at 10−6 M in in vitro study, the tumor growth in xenograft mice model was not significantly increased by LC compared to a negative control. Unlike in vitro results, these results indicate that LC may not promote the growth of BG-1 ovarian cancer in vivo as shown in Fig. 5.
Fig. 5. Effects of lambda-cyhalothrin on the tumor growth in the absence of endogenous estrogen. The mice were injected intraperitoneally with DMSO, E2 and lambda-cyhalothrinevere 3-4 days (A) and tumor volumes were measured by length × width × height × 0.5236 (mm3) using a vernier calipers every 10 days during the experiment period of 80 days (B). *: Mean values were significantly different from DMSO (control), p< 0.05 (One-way ANOVA).
DISCUSSION
Previous studies reported that EDCs have a significant impact on ER expressing cancer cell proliferation such as BG-1 ovarian cancer via ER-dependent pathways (22-24). Despite many previous studies showed that SPs has week estrogenicity in various assays, but it still is required to discover estrogenicity of SPs because these caused the estrogen dependent disease such as estrogen responsible cancer of reproductive system in humans or mammalians (25).
In the cell viability assay, E2 and two pyrethroids significantly cantly increased BG-1 cell proliferation than a negative control (0.1% DMSO). And co-treatment of ICI 182,780 with E2 or two pyrethroids reversed this effect to negative control levels. These results suggest that these two-pyrethroids may have estrogenicity related with an ER dependent signaling pathway. Then, based on above cell viability results, we further investigated the expression of cyclin D1, a cell proliferation-inducing gene. RT-PCR results showed that the treatment of E2 or two-pesticides induced the increased expression of cyclin D1, while this effect was reversed by the addition of ICI 182,780, suggesting that two pyrethroids may induce the increased gene expression of cyclin D1 via an ER-dependent signaling pathway in BG-1 ovarian cells.
In the previous study, the cell growth of BG-1 cells was induced by E2 or EDCs mediated by ERs, particularly ERα than ERβ (26). Thus, we identified relationship of ERα and pyrethroids that induced estrogen-responsive BG-1 cell proliferation by RT-PCR. In this study ERα was not altered by treatment of CP or LC while E2 down-regulated the expression of this gene in BG-1 cells. These results indicate that two pyrethroids-induced BG-1 cell growth may be not related with ERα expression level.
Unlike cell viability assay results in vitro, LC did not significantly induce BG-1 tumor growth compared to a negative control (1% DMSO) in xenograft mouse model. This lack of estrogenic activity of pyrethroids in vivo was occasionally shown in some previous studies (7). It is considered that these non-estrogenic effect of LC in in vivo be caused by properties that SPs are readily metabolized in vivo system (13). Therefore, it is difficult to expect identical results obtained in in vivo compared to in vitro studies. In addition, the immune system to interfere with the tumor growth in live body is even more systematical controlled than in vitro model. Since we focused on the growth of estrogen-responsive tumor following the treatment with LC, biological toxicities involved were not considered. According to these previous reports, the reason why LC did not induce BG-1 ovarian tumor growth in vivo may be explained by lack of estrogenic activity of pyrethroids in vivo due to their easy breakdown in the body. Furthermore, rapid metabolic activity and immunity in the mouse body may contribute to the inhibition of tumor growth by LC. Since there are a lot of systematical factors to be considered in in vivo test, it appears to be actually difficult to correlate both in vitro and in vivo results.
In conclusion, two synthetic pyrethroids, LC and CP, may possess estrogenic potentials by stimulating the proliferation of BG-1 ovarian cancer cells via partially ER signaling pathway associated with cell cycle as E2 did, but these estrogenic effects were not found in in vivo xenografted mouse model. In our experiments, it is difficult to fully conclude that these two pyrethroids are EDCs. Based on these results, further studies need to be performed in diverse pathways and histochemical analysis of tumor tissues such as protein expression of proliferating cell nuclear antigen (PNCA), 5-bromo-2-deoxyuridine (BrdUrd), cyclin D1 as well as monitoring of tumor size change by treatment of these pyrethroids in BG-1 ovarian cancer cells.
CONFLICT OF INTEREST
Authors declare that there are no conflicts of interest.
Acknowledgments
This work was supported by the research grant of the Chungbuk National University in 2013.
References
- 1.Choi K.C., Jeung E.B. The biomarker and endocrine disruptors in mammals. J. Reprod. Dev. (2003);49:337–345. doi: 10.1262/jrd.49.337. [DOI] [PubMed] [Google Scholar]
- 2.Diamanti-Kandarakis E., Bourguignon J.P., Giudice L.C., Hauser R., Prins G.S., Soto A.M., Zoeller R.T., Gore A.C. Endocrine-disrupting chemicals: an Endocrine Society scientific statement. Endocr. Rev. (2009);30:293–342. doi: 10.1210/er.2009-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hwang K.A., Park S.H., Yi B.R., Choi K.C. Gene alterations of ovarian cancer cells expressing estrogen receptors by estrogen and bisphenol a using microarray analysis. Lab. Anim. Res. (2011);27:99–107. doi: 10.5625/lar.2011.27.2.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Korach K.S., Chae K., Gibson M., Curtis S. Estrogen receptor stereochemistry: ligand binding and hormonal responsiveness. Steroids. (1991);56:263–270. doi: 10.1016/0039-128X(91)90045-W. [DOI] [PubMed] [Google Scholar]
- 5.Sun H., Xu X.L., Xu L.C., Song L., Hong X., Chen J.F., Cui L.B., Wang X.R. Antiandrogenic activity of pyrethroid pesticides and their metabolite in reporter gene assay. Chemosphere. (2007);66:474–479. doi: 10.1016/j.chemosphere.2006.05.059. [DOI] [PubMed] [Google Scholar]
- 6.Garey J., Wolff M.S. Estrogenic and antiprogestagenic activities of pyrethroid insecticides. Biochem. Biophys. Res. Commun. (1998);251:855–859. doi: 10.1006/bbrc.1998.9569. [DOI] [PubMed] [Google Scholar]
- 7.Kunimatsu T., Yamada T., Ose K., Sunami O., Kamita Y., Okuno Y., Seki T., Nakatsuka I. Lack of (anti-) androgenic or estrogenic effects of three pyrethroids (esfenvalerate, fenvalerate, and permethrin) in the Hershberger and uterotrophic assays. Regul. Toxicol. Pharmacol. (2002);35:227–237. doi: 10.1006/rtph.2001.1527. [DOI] [PubMed] [Google Scholar]
- 8.Casida J.E. Pyrethrum flowers and pyrethroid insecticides. Environ. Health Perspect. (1980);34:189–202. doi: 10.1289/ehp.8034189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu H., Zhao M., Zhang C., Ma Y., Liu W. Enantioselective cytotoxicity of the insecticide bifenthrin on a human amnion epithelial (FL) cell line. Toxicology. (2008);253:89–96. doi: 10.1016/j.tox.2008.08.015. [DOI] [PubMed] [Google Scholar]
- 10.Liu H., Xu L., Zhao M., Liu W., Zhang C., Zhou S. Enantiomer-specific, bifenthrin-induced apoptosis mediated by MAPK signalling pathway in Hep G2 cells. Toxicology. (2009);261:119–125. doi: 10.1016/j.tox.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 11.Zhao M., Zhang Y., Liu W., Xu C., Wang L., Gan J. Estrogenic activity of lambda-cyhalothrin in the MCF-7 human breast carcinoma cell line. Environ. Toxicol. Chem. (2008);27:1194–1200. doi: 10.1897/07-482.1. [DOI] [PubMed] [Google Scholar]
- 12.Bloomquist J.R. Ion channels as targets for insecticides. Annu. Rev. Entomol. (1996);41:163–190. doi: 10.1146/annurev.en.41.010196.001115. [DOI] [PubMed] [Google Scholar]
- 13.McCarthy A.R., Thomson B.M., Shaw I.C., Abell A.D. Estrogenicity of pyrethroid insecticide metabolites. J. Environ. Monit. (2006);8:197–202. doi: 10.1039/B511209E. [DOI] [PubMed] [Google Scholar]
- 14.Glickman A.H., Lech J.J. Differential toxicity of trans-permethrin in rainbow trout and mice. II. Role of target organ sensitivity. Toxicol. Appl. Pharmacol. (1982);66:162–171. doi: 10.1016/0041-008X(82)90281-2. [DOI] [PubMed] [Google Scholar]
- 15.Jin M., Li L., Xu C., Wen Y., Zhao M. Estrogenic activities of two synthetic pyrethroids and their metabolites. J. Environ. Sci. (China) (2010);22:290–296. doi: 10.1016/S1001-0742(09)60107-8. [DOI] [PubMed] [Google Scholar]
- 16.Colborn T., vom Saal F.S., Soto A.M. Developmental effects of endocrine-disrupting chemicals in wildlife and humans. Environ. Health Perspect. (1993);101:378–384. doi: 10.1289/ehp.93101378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim I.Y., Shin J.H., Kim H.S., Lee S.J., Kang I.H., Kim T.S., Moon H.J., Choi K.S., Moon A., Han S.Y. Assessing estrogenic activity of pyrethroid insecticides using in vitro combination assays. J. Reprod. Dev. (2004);50:245–255. doi: 10.1262/jrd.50.245. [DOI] [PubMed] [Google Scholar]
- 18.Du G., Shen O., Sun H., Fei J., Lu C., Song L., Xia Y., Wang S., Wang X. Assessing hormone receptor activities of pyrethroid insecticides and their metabolites in reporter gene assays. Toxicol. Sci. (2010);116:58–66. doi: 10.1093/toxsci/kfq120. [DOI] [PubMed] [Google Scholar]
- 19.Sun H., Chen W., Xu X., Ding Z., Chen X., Wang X. Pyrethroid and their metabolite, 3-phenoxybenzoic acid showed similar (anti)estrogenic activity in human and rat estrogen receptor alpha-mediated reporter gene assays. Environ. Toxicol. Pharmacol. (2014);37:371–377. doi: 10.1016/j.etap.2013.11.031. [DOI] [PubMed] [Google Scholar]
- 20.Li Y.F., Pan C., Hu J.X., Li J., Xu L.C. Effects of cypermethrin on male reproductive system in adult rats. Biomed. Environ. Sci. (2013);26:201–208. doi: 10.3967/0895-3988.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 21.Liu L., Hu J.X., Wang H., Chen B.J., He Z., Xu L.C. Effects of beta-cypermethrin on male rat reproductive system. Environ. Toxicol. Pharmacol. (2010);30:251–256. doi: 10.1016/j.etap.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 22.Kang N.H., Hwang K.A., Kim T.H., Hyun S.H., Jeung E.B., Choi K.C. Induced growth of BG-1 ovarian cancer cells by 17beta-estradiol or various endocrine disrupting chemicals was reversed by resveratrol via downregulation of cell cycle progression. Mol. Med. Rep. (2012);6:151–156. doi: 10.3892/mmr.2012.887. [DOI] [PubMed] [Google Scholar]
- 23.Park M.A., Hwang K.A., Lee H.R., Yi B.R., Jeung E.B., Choi K.C. Cell growth of BG-1 ovarian cancer cells is promoted by di-n-butyl phthalate and hexabromocyclododecane via upregulation of the cyclin D and cyclindependent kinase-4 genes. Mol. Med. Rep. (2012);5:761–766. doi: 10.3892/mmr.2011.712. [DOI] [PubMed] [Google Scholar]
- 24.Park M.A., Hwang K.A., Lee H.R., Yi B.R., Jeung E.B., Choi K.C. Benzophenone-1 stimulated the growthof BG-1 ovarian cancer cells by cell cycle regulation via an estrogen receptor alpha-mediated signaling pathway in cellular and xenograft mouse models. Toxicology. (2013);305:41–48. doi: 10.1016/j.tox.2012.12.021. [DOI] [PubMed] [Google Scholar]
- 25.Neubert D. Vulnerability of the endocrine system to xenobiotic influence. Regul. Toxicol. Pharmacol. (1997);26:9–29. doi: 10.1006/rtph.1997.1149. [DOI] [PubMed] [Google Scholar]
- 26.Hwang K.A., Kang N.H., Yi B.R., Lee H.R., Park M.A., Choi K.C. Genistein, a soy phytoestrogen, prevents the growth of BG-1 ovarian cancer cells induced by 17beta-estradiol or bisphenol A via the inhibition of cell cycle progression. Int. J. Oncol. (2013);42:733–740. doi: 10.3892/ijo.2012.1719. [DOI] [PubMed] [Google Scholar]