Abstract
β-elemene, a Curcuma wenyujin plant extract, has been used widely as a tumor adjuvant therapeutic agent. However, how to obtain optimum therapeutic effects by combining this compound with other agents remain unclear. In this study, we found that β-elemene, which alone had little effect on hepatocellular carcinoma (HCC) cell proliferation, exerted a synergistic anti-proliferative effect in HCC cells when dosed in combination with oxaliplatin, which increased the amounts of platinum accumulation and platinum-DNA adduct significantly and augmented the oxaliplatin-induced apoptosis. Western blot and laser scanning confocal microscopy studies indicated that β-elemene enhanced the sensitivity of HCC cells to oxaliplatin by upregulating copper transporter 1 (CTR1), a major controller of intracellular platinum accumulation. In an orthotopic transplantation HCC model in nude mice, HCC tumor growth was inhibited significantly by oxaliplatin combined with β-elemene, as compared with oxaliplatin alone. Notably, CTR1 protein expression in xenograft HCC was upregulated in mice who received β-elemene treatment. Taken together, our findings show that β-elemene can block the reduction of CTR1 resulting from oxaliplatin treatment, and therefore has a synergistic anti-HCC effect with oxaliplatin by enhancing cellular uptake of oxaliplatin. The synergistic effects of β-elemene and oxaliplatin deserve further evaluation in clinical settings.
Liver cancer is one of the most highly fatal forms of cancer around the world and is more common in less developed countries. Worldwide, an estimated 782,500 new cases of liver cancer and 745,500 deaths occurred during 2012, half of them in China1. According to the latest statistics, the five-year survival rate for liver cancer is usually very low (10–20%) in the majority of countries, both in the East and the West2. Approximately 70% of patients diagnosed with liver cancer are not suitable for curative surgery due to a history of cirrhosis, a huge primary lesion, multifocal lesions, major blood vessel invasion, and/or extra-hepatic spread3. Hence, it is urgent to identify more effective chemotherapy agents.
Although many cytotoxic chemotherapeutic agents have been reported in the last 30 years, so far all lack convincing benefits in phase II studies4,5. A randomized, multicenter, open-label study of oxaliplatin [(trans-R,R)1,2-diaminocyclohexaneoxalatoplatinum (II), C8H14N2O4Pt] (Fig. 1a) plus fluorouracil/leucovorin (FOLFOX4) as palliative chemotherapy in HCC patients from Asia found that this regimen showed a trend toward improved overall survival, along with increased progression-free survival, although the primary end point was not met6. A retrospective multicenter study confirmed that gemcitabine and oxaliplatin (GEMOX) is effective with an acceptable profile of safety for advanced HCC patients3. Oxaliplatin as one member of mixed solution containing epirubicin, oxaliplatin, mitomycin and lipiodol participate in transcatheter arterial chemoembolization (TACE) for hepatocellular carcinoma (HCC) in China7,8. From the economic perspect, oxaliplatin, with lower price compared to sorafenib, gives more patients easily get access to chemotherapy and consequent benefit from it. Thus, a promising approach may be to investigate how to further improve the effects with combinations of existing drugs. Agents that influence the distribution, intracellular concentration, and excretion of oxaliplatin9,10,11,12,13 may have the potential to further improve the anti-tumor effects of oxaliplatin with less cytotoxicity.
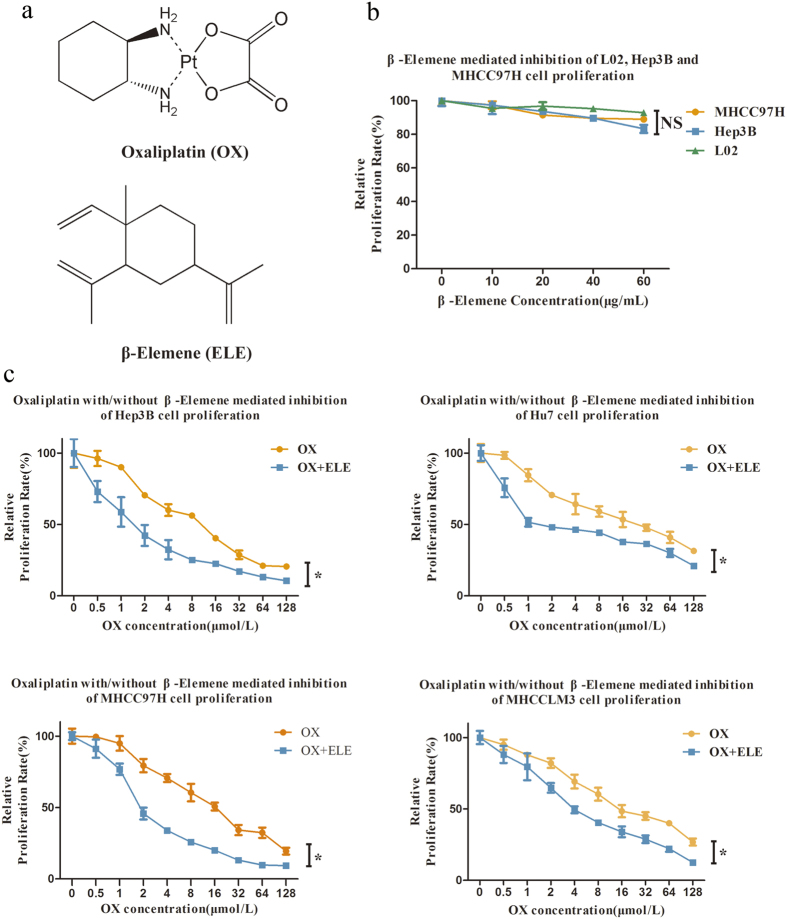
Figure 1. Antitumor activity of oxaliplatin (OX) and/or β-elemene (ELE) applied in human hepatocellular carcinoma cell lines.
(a) Chemical structures of oxaliplatin and β-elemene. (b) Effects of β-elemene on in vitro cytotoxicity in normal human liver cell line L02 and HCC cell lines MHCC97H and Hep3B. (c) β-elemene increases oxaliplatin cytotoxicity and enhances oxaliplatin sensitivity in human hepatocellular carcinoma cells, as determined by CCK8 assay. (*p < 0.05).
The anti-tumor effect of platinum-based drugs is directly related to the amount of the drug that enters the cell, and nearly all platinum-resistant cells have shown a reduction of platinum entering the cell. Copper transporter 1 (CTR1), the major copper influx transporter, has been shown to control the intracellular accumulation of platinum and therefore also the cytotoxic effects of platinum-based chemotherapy drugs, such as cisplatin and oxaliplatin14. Several studies suggested that CTR1 transports platinum drugs via chaperones and a series of transchelation reactions in a manner similar to copper transport15,16. Therefore, the agents acting on the platinum processing pathway should be explored as combination agents17.
β-elemene (1-methyl-1-vinyl-2, 4-diisopropenyl-cyclohexane, C15H24) (Fig. 1a), extracted from the Chinese herb Curcuma wenyujin, has been proven to be effective against tumors18,19,20,21,22 and is approved by China’s State Food and Drug Administration for treating tumors23. As a natural plant extract with fewer side effects and strong bone marrow protection, β-elemene is widely applied in the field of cancer chemotherapy20,24,25,26. However, the mechanism underlying its anti-tumor effects and how to obtain optimum therapeutic effects of combinational β-elemene and chemotherapy are not well understood.
In our previous research, we found that β-elemene could enhance the anti-HCC effect of oxaliplatin in vitro (not published), but how β-elemene enhances this effect remains unclear. The aim of this study was to address the issue of synergism between β-elemene and oxaliplatin in their anti-HCC effect and to elucidate the underlying molecular mechanism for the enhancement of the anti-cancer effect of oxaliplatin. On the basis of our preliminary findings, the effects of β-elemene or oxaliplatin alone and the synergistic effects of the two drugs in vitro and in vivo were evaluated. We found that β-elemene dramatically reinforced oxaliplatin activity by increasing the amount of intercellular platinum through CTR1 stabilization. Our findings indicate that a new oxaliplatin-based regimen in combination with β-elemene may provide clinical efficacy.
Results
Effects of oxaliplatin with or without β-elemene on human HCC cell line proliferation
As shown in Fig. 1B, the anti-cancer activity of β-elemene was first measured in normal human liver cell line L02 and HCC cell lines MHCC97H and Hep3B. With increasing β-elemene concentration or incubation time, the percentage of cell viability showed no significant decrease in these cell lines. β-elemene displayed no cytotoxicity toward normal human liver cells. For HCC cells, at concentrations less than 60 μg/mL, β-elemene resulted in no apparent anti-proliferative effects in L02, MHCC97H and Hep3B(Fig. 1b), and similar results were found in two other common HCC cell lines, Huh7 and MHCCLM3 (Supplementary Fig. S1a).
To investigate whether β-elemene could enhance the anti-proliferative effect of oxaliplatin, several HCC cell lines with different malignant potentials were simultaneously treated with oxaliplatin and β-elemene and assessed in vitro by CCK8 assay (Fig. 1c). The results showed that oxaliplatin treatment alone (from 0.5 to 128 μm) resulted in a dose-dependent decrease of proliferation in adoptive HCC cell lines (the IC50 values for Hep3B, Huh7, MHCC97H, and MHCCLM3 cells at 96 h were 10.00 ± 0.67 μM, 23.24 ± 3.09 μM, 16.41 ± 1.11 μM, and 21.32 ± 1.67 μM, respectively). When combined with β-elemene at 60 μg/mL, the anti-proliferative effects of oxaliplatin increased significantly, showing a striking decrease of IC50 to 1.79 ± 0.84 μM, 3.27 ± 0.72 μM, 2.54 ± 0.27 μM, and 5.73 ± 0.74 μM , respectively (Table 1; p < 0.01). The values of the dose modifying factor [DMF = IC50 (oxaliplatin alone)/IC50 (60 μg/mL β-elemene + oxaliplatin)]27,28 expressing the intensity of enhancement were 5.59, 7.11, 6.46, and 3.72 in Hep3B, Huh7, MHCC97H, and MHCCLM3 cells, respectively. These results suggest that β-elemene and oxaliplatin may act synergistically to enhance cytotoxicity in HCC cell lines.
Table 1. β-elemene (60 μg/mL) increases oxaliplatin cytotoxicity and enhances oxaliplatin sensitivity in human hepatocellular carcinoma cells, as determined by CCK8 assay.
| Cell lines | Drug | IC50(96 h) | P-value | Dose-modifying factor |
|---|---|---|---|---|
| Hep3B | oxaliplatin (μM) | 10.00 ± 0.67 | 0.0016 | 5.59 |
| β-elemene+oxaliplatin (μM) | 1.79 ± 0.84 | |||
| Huh7 | oxaliplatin (μM) | 23.24 ± 3.09 | 0.0033 | 7.11 |
| β-elemene+oxaliplatin (μM) | 3.27 ± 0.72 | |||
| MHCC97H | oxaliplatin (μM) | 16.41 ± 1.11 | 0.0003 | 6.46 |
| β-elemene+oxaliplatin (μM) | 2.54 ± 0.27 | |||
| MHCCLM3 | oxaliplatin (μM) | 21.32 ± 1.67 | 0.0010 | 3.72 |
| β-elemene+oxaliplatin (μM) | 5.73 ± 0.74 |
IC50 value is the concentration of drug required to inhibit cell growth by 50% relative to dilute controls; presented as the mean of eight simultaneous replicates repeated three times. Data are mean ± SD of 3 biological replicates.
Evaluation of synergistic anti-tumor effect between oxaliplatin and β-elemene
To test the combination effect, the HCC cell lines were simultaneously treated with β-elemene and oxaliplatin and the coefficient of drug interaction (CDI) was calculated according to the equation: CDI = AB/(A × B)29,30,31. As expected from the CCK8 assay results, CDI indicated the combination of oxaliplatin and β-elemene yielded synergistic interactions across a wide concentration range (CDI < 1; Table 2). Moreover, for the concentrations applied to the MHCC97H cell line, the CDI value was consistently <0.7, which reveals a significant synergistic effect; the synergistic effect was most prominent (CDI = 0.45) when 60 μg/mL β-elemene was combined with 16 μm oxaliplatin.
Table 2. Synergistic effects of oxaliplatin and β-elemene in human hepatocellular carcinoma cells.
| Cell lines | β-elemene (60 μg/mL) |
||||
|---|---|---|---|---|---|
| Oxaliplatin(2 μm) | Oxaliplatin(4 μm) | Oxaliplatin(8 μm) | Oxaliplatin(16 μm) | ||
| CDI | Hep3B | 0.78 | 0.83 | 0.86 | 0.81 |
| Huh7 | 0.83 | 0.83 | 0.75 | 0.81 | |
| MHCC97H | 0.65 | 0.54 | 0.48 | 0.45 | |
| MHCCLM3 | 0.89 | 0.80 | 0.75 | 0.79 | |
Coefficient of drug interaction (CDI) was calculated with the equation CDI = AB/(A × B) (AB, relative cell viability of the combination; A or B, relative cell viability of the single agent groups). CDI < 1 indicates a synergistic effect; CDI = 1 indicates an additive effect; CDI > 1 indicates an antagonistic effect.
Platinum accumulation and platinum-DNA binding
To determine whether the presence of β-elemene at normal levels resulted in an enhancement in the uptake of oxaliplatin, the level of intracellular platinum in MHCC97H cells was determined after 24-h treatment with different oxaliplatin concentrations with or without β-elemene (Fig. 2a). The total cellular platinum accumulation and DNA-bound platinum was measured by ICP-MS. Exposure to 60 μg/mL β-elemene increased the intracellular accumulation of platinum at all concentrations tested. The 2.25-fold (25.50 ± 6.64 vs 57.50 ± 7.83 ng/mL 5 × 106 cells), 2.13-fold (65.10 ± 12.85 vs 138.70 ± 20.20 ng/mL 5 × 106 cells), and 1.38-fold (234.80 ± 23.90 vs 323.50 ± 14.95 ng/mL 5 × 106 cells) increases observed with 4, 8, and 16 μm oxaliplatin, respectively, were statistically significant. Similar results were found in Huh7 and Hep3B (data not shown).
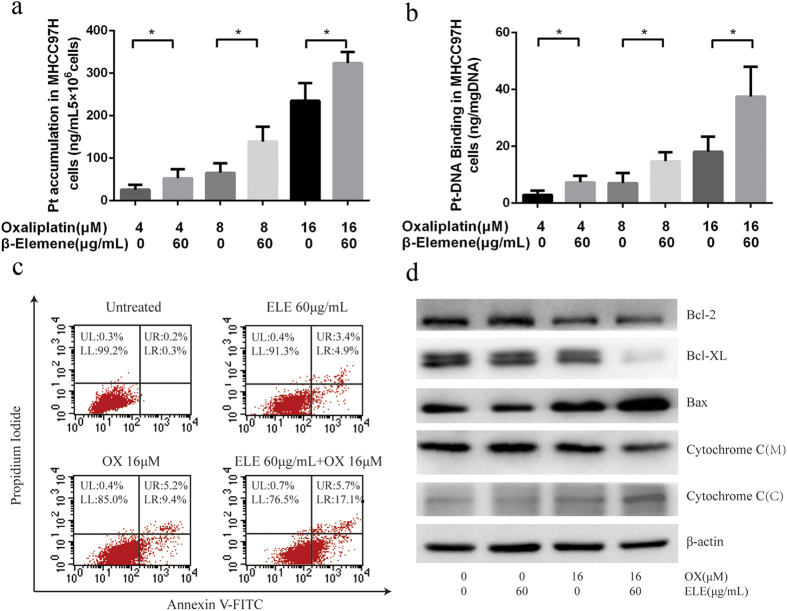
Figure 2. Effect of β-elemene on platinum intake and oxaliplatin-induced apoptosis of MHCC97H cells.
(a) Cellular platinum accumulation in the MHCC97H cell line with treatment of oxaliplatin alone and oxaliplatin with β-elemene. (*p < 0.05) (b) Platinum-DNA binding levels in the MHCC97H cell line due to treatment with oxaliplatin alone and in combination with β-elemene. (*p < 0.05) (c) β-elemene promotes Oxaliplatin-induced apoptosis in MHCC-97H cells as assessed by flow cytometric analysis. MHCC97H cells were exposed to oxaliplatin alone or in combination with β-elemene (60 μg/mL) for 48 h. UL upper left, represents necrotic cells (%); UR upper right, represents late apoptotic cells (%); LL lower left, represents viable cells (%); LR lower right, represents early apoptotic cells (%). (d) β-elemene increases oxaliplatin-induced changes in protein levels of apoptotic-related molecules in human HCC cells. MHCC97H cells were treated with β-elemene (60 μg/mL), or 16 μm oxaliplatin alone or in combination with β-elemene (60 μg/mL) for 48 hr. Total protein extracts were prepared from cells, separated by SDS-PAGE, and transferred to nitrocellulose membranes. β-actin was used as an internal control. M: mitochondria; C: cytosol.
Because the action of platinum drugs is associated with their binding with DNA and exerting cytotoxic effect mostly through DNA damage, the DNA-bound platinum levels in MHCC97H were also measured. β-elemene significantly increased oxaliplatin-generated platinum-DNA adduct in MHCC97H cells. As compared to oxaliplatin alone, the combination of β-elemene with oxaliplatin generated approximately 2.50-, 2.10-, and 2.08-fold more DNA adducts in MHCC97H cells with 4, 8, and 16 μm oxaliplatin, respectively (Fig. 2b). Similar results were found in Huh7 and Hep3B (data not shown).
β-elemene promotes oxaliplatin-induced apoptosis in MHCC97H cells
Platinum-based agents including oxaliplatin exert their anti-tumor effect by stimulating apoptosis. To investigate the effect of β-elemene on oxaliplatin-induced apoptosis, two-color flow cytometry of MHCC97H cells stained with Annexin V-FITC and propidium iodide (PI) was performed. MHCC97H cells staining with Annexin V-FITC resolved early apoptotic cells, whereas MHCC97H cells staining with Annexin V-FITC and PI resolved late apoptotic and necrotic cells. The percentages of early apoptotic cells after treatment with oxaliplatin plus 60 μg/mL β-elemene for 48 hr was about 2-fold the number after treatment with oxaliplatin alone (Fig. 2c).
Apoptosis triggered by oxaliplatin plus β-elemene was further confirmed by Western blot analysis in MHCC97H cells. The protein levels of apoptotic-related molecules in human HCC cells cells after exposure to oxaliplatin alone or oxaliplatin plus β-elemene for 48 hr were analyzed by Western blotting. Western blot analysis revealed that the oxaliplatin/β-elemene combination greatly reduced the expression of anti-apoptotic protein Bcl-2 and Bcl-XL and upregulated the expression of pro-apoptotic protein BAX. The oxaliplatin/β-elemene combination, but not β-elemene treatment alone, promoted cytochrome c release from the mitochondria into the cytosol (Fig. 2d).
β-elemene enhances anti-HCC effect of oxaliplatin by preventing oxaliplatin-induced degradation of hCTR1
The mechanism that allows β-elemene to enhance oxaliplatin’s anti-HCC effect was further studied by focusing on the oxaliplatin processing pathway. Western blot analysis was performed to measure the expression levels of copper transporters in MHCC97H cells. The expression level of the copper influx transporter CTR1 decreased with the increasing concentration of oxaliplatin32. In addition, an increase in CTR1 expression was found for oxaliplatin plus β-elemene relative to oxaliplatin alone. We applied real-time reverse-transcription PCR to perform comparative analysis of CTR1 differential expressions of different treatment groups. The data showed that oxaliplatin-induced down-regulation of CTR1 was independent of transcriptional and translational events (Fig. 3a). Confocal microscopic images suggested that CTR1 decreased sharply upon exposure to oxaliplatin and that this trend could be reversed by simultaneous treatment with β-elemene (Fig. 3b).
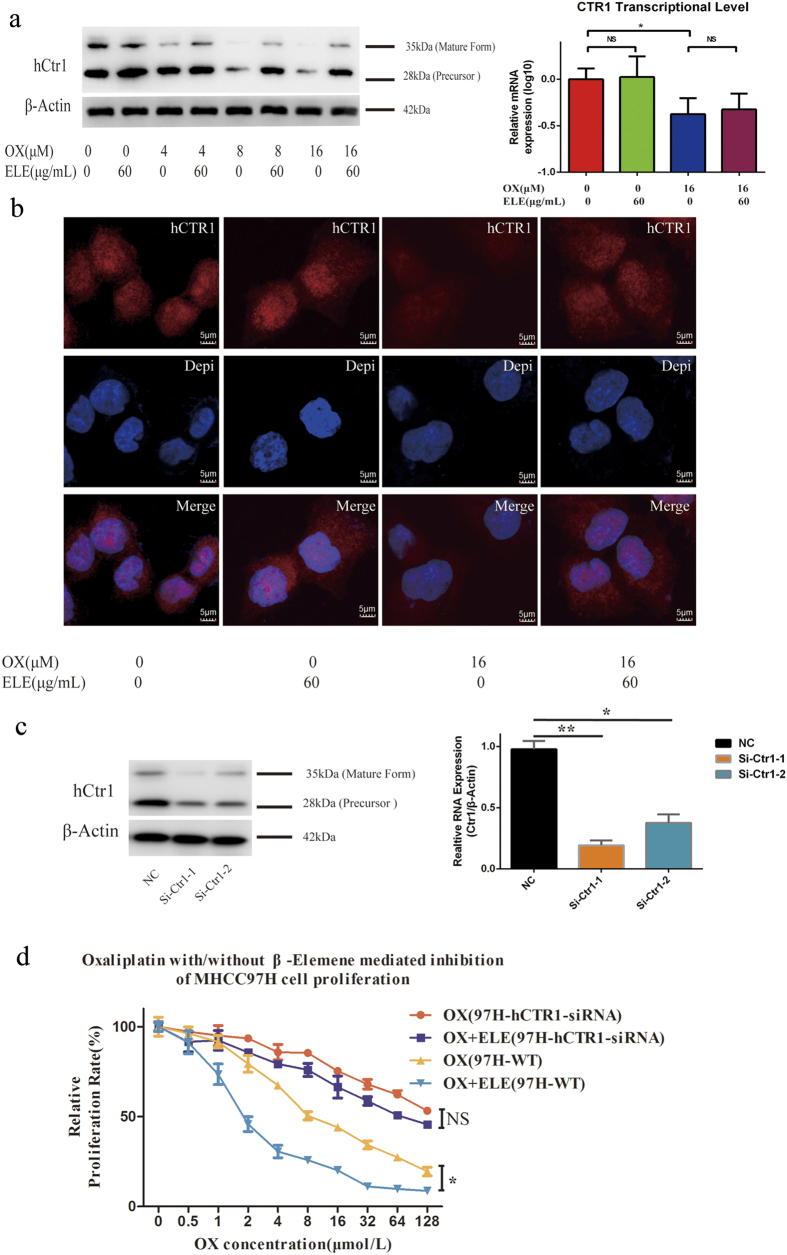
Figure 3. Effect of β-elemene (ELE) on the level of hCTR1 in the presence of oxaliplatin (OX).
(a) Western blot analysis of hCTR1 expression. MHCC97H cells exposed to various oxaliplatin concentrations with or without 60 μg/mL β-elemene for 12-h exposure. Graph shows ratio between hCTR1 and β-actin expression. CTR1 transcriptional levels was measured by real-time PCR after MHCC97H cells were treated with and without β-Elemene for 48 h. Data shown are mean (± S.D.) from three independent experiments.*p < 0.05, compared with control cells. (b) Confocal digital microscopic analyses of hCTR1 levels in MHCC97H cells exposed to oxaliplatin (0, 16 μm) with or without 60 μg/mL β-elemene for 2 h. Red, hCTR1; blue, nucleus stained with 4,6-diamidino-2-phenylindole. Results are representative of three independent experiments; image intensity was normalized to cells stained only with secondary antibody. (c) MHCC97H cells were transfected with hCTR1-siRNA for 24 h. Cell lysates were then harvested and subjected to Western blot and RT-PCR analysis. (d) Transfected MHCC97H cells were more resistant to oxaliplatin and displayed no cytotoxicity difference between oxaliplatin plus β-elemene and oxaliplatin alone. (*p < 0.05, ** p < 0.01).
To further confirm the key role of CTR1 in the synergistic effect of β-elemene with oxaliplatin, we generated two CTR1-specific siRNAs to silence the endogenous CTR1 (si-hCTR1) of HCC cells to observe the different response of HCC cells to the regimen. Si-CTR1#1, inducing a >80% decrease in CTR1, was adopted for further study (Fig. 3c). As shown in Fig. 3d, transfected MHCC97H cells were more resistant to oxaliplatin and displayed no cytotoxicity difference between oxaliplatin combined with β-elemene and oxaliplatin alone (P = 0.8135), indicating the important role of CTR1stabilization induced by β-elemene on the anti-HCC effect of oxaliplatin. A similar result was found in the HCC cell line Hep3B (Supplementary Fig. S1).Thus, as documented by three different analytic techniques, β-elemene was able to prevent oxaliplatin-induced degradation of CTR1 in a manner similar to some proteasome inhibitors like bortezomib33.
β-elemene enhanced the anti-tumor activity of oxaliplatin in a HCC xenograft model
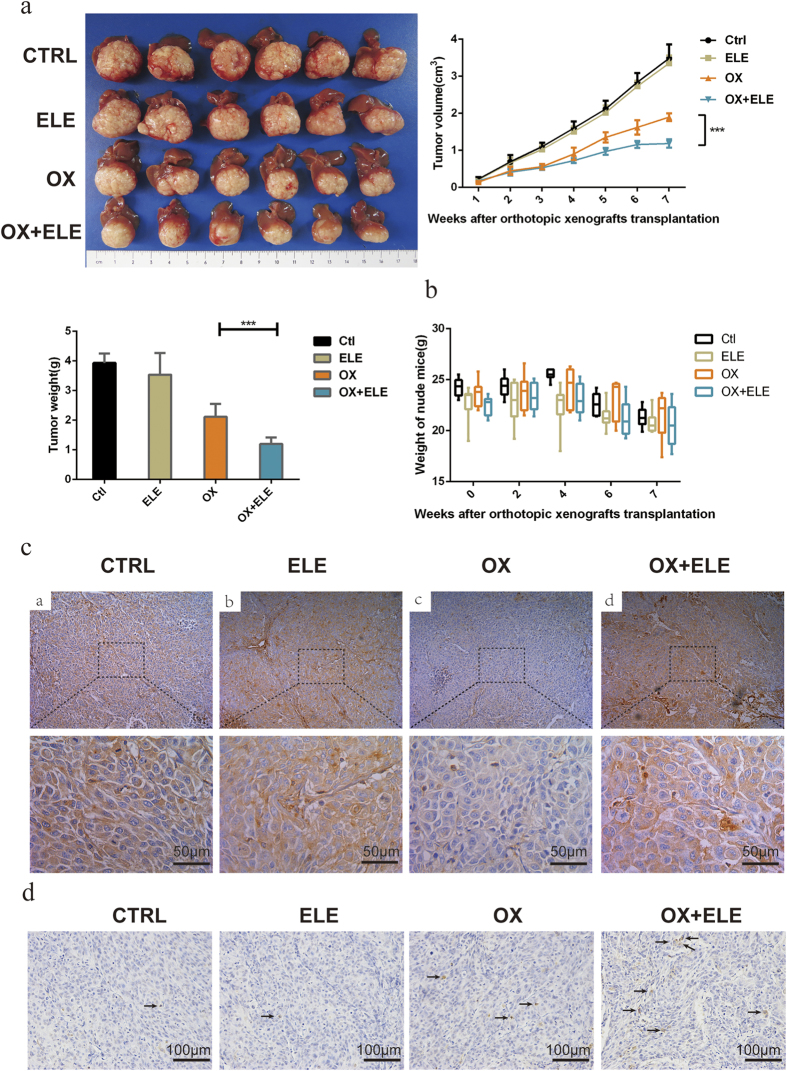
To confirm these findings, we further examined the influence of β-elemene on the anti-HCC effect of oxaliplatin on in vivo HCC growth. Compared with tumor growth in the PBS treatment group, β-elemene alone made no difference, but the enhancement of anti-tumor activity was observed when mice were treated with oxaliplatin plus β-elemene relative to oxaliplatin alone (Fig. 4a, Supplementary Fig. S2). Concurrently, β-elemene alone showed no significant influence on weight and morbidity of nude mice (Fig. 4b). Immunohistochemical analysis of HCC samples further verified the molecular mechanism underlying β-elemene’s ability to prevent oxaliplatin-induced degradation of hCTR1: the expression of hCTR1 in the oxaliplatin treatment group decreased significantly, but in the combined β-elemene treatment group the expression of hCTR1 reversed to the level of PBS treatment (Fig. 4c). To further validate β-elemene promoting oxaliplatin-induced apoptosis in vivo, we examined the HCC samples by TUNEL (Fig. 4d). The distribution of apoptotic cells was evaluated by counting positively labeled cells in randomly selected five microscopic fields. The apoptotic index was presented as the percentage of positive cells for each zone. The untreated group (0.60 ± 0.24) and the β-elemene treatment group(0.80 ± 0.37) showed lower apoptotic indices, but the oxaliplatin treatment group(2.6 ± 0.51) and the combined β-elemene treatment group(6.0 ± 0.32) showed higher apoptotic indices, as well as statistically significant difference between the latter two groups(p < 0.001). Taken together, these results indicate that β-elemene, via the major platinum influx transporter, potently enhanced oxaliplatin’s anti-HCC effect in vitro and in vivo by preventing oxaliplatin-induced degradation of hCTR1.
Figure 4. The effects of oxaliplatin (OX) with or without β-elemene (ELE) on in vivo tumor growth in an orthotopic implantation mouse model of MHCC97H cells and on CTR1 protein expression in tumor tissues.
Oxaliplatin with β-elemene suppressed tumor growth in orthotopic implantation mouse models of MHCC97H cells. (a) Tumor volumes and weight in the orthotopic implantation model mice at week 7. (b) The weight in the orthotopic implantation model mice at the indicated time points. (***p < 0.001) (c) Representative examples of immunohistochemistry staining for CTR1 protein from MHCC97H xenografts (400 × magnification; scale bars, 50 μm). (d) Representative examples of tumor xenografts stained with apoptosis by TUNEL assay (positive cell labelled with arrows; 400 × magnification; scale bars, 50 μm).
Discussion
Due to the poor liver function and chemotherapy tolerance, no successful chemotherapy regimen to prolong the survival of HCC patients has been widely accepted34,35. The efficacy of oxaliplatin in treating otherwise fatal diseases has been established based on 30 years of experience36. Recently, this agent has gained increasing interest when used in combination with resistance modulators or new molecularly targeted drugs for the purposes of lowering doses of chemotherapeutic agents, reducing side effects, and increasing treatment efficacy15. Our institute has explored the role of autophagy as a resistance mechanism of oxaliplatin treatment and the potential therapeutic value of combining oxaliplatin and autophagy inhibitors to treat HCC37. In the present study, we assessed the combination effect of oxaliplatin and the botanical extract β-elemene on human HCC cell lines. The synergism of oxaliplatin and β-elemene was determined in vivo and in vitro, suggesting greater cellular accumulation of oxaliplatin and a possible interaction between β-elemene and the influx transporter of oxaliplatin. The results also indicated the pharmacologic advantage of β-elemene prompting more oxaliplatin accumulation than when oxaliplatin is used alone. These insights into the interaction between oxaliplatin and β-elemene will help in the development of a novel strategy to improve the efficacy of HCC chemotherapy and overcome acquired oxaliplatin resistance.
Many preclinical studies have suggested that HCC is chemotherapy-resistant, with an objective response rate to systemic chemotherapy that is normally less than 10%38,39. Hence, it is imperative to identify and validate novel therapeutic strategies for improving chemo-resistance in HCC research. Recently, the promising anti-cancer activity of many plant extracts has aroused researchers’ interests. β-elemene has been shown to have broad-spectrum anti-tumor effects on many types of cancer and low toxicity in patients19,20,21,40,41,42,43, although the precise mechanism of β-elemene remains to be elucidated. Previous studies showed β-elemene could induce G2/M phase arrest and promote apoptosis in ovarian cancer cells and non-small cell lung cancer cells20,21. Another study demonstrated that β-elemene could inhibit breast cancer cell growth via an inhibitory effect on mTOR and by inducing autophagy44. In lung carcinoma β-elemene may induce the alteration of cell membrane permeability, which has the potential to result in enhanced cellular uptake of taxanes26. In ovarian cancer β-elemene may reduce DNA repair activity and enhance cisplatin cytotoxicity by blocking the activation of PI3K/JNK signaling pathway43, and in prostate cancer β-elemene has been shown to enhance the cisplatin-induced apoptosis via mitochondrial activation of the caspase-mediated apoptotic pathway24.
We investigated the anti-tumor effect of β-elemene in human HCC in vivo and in vitro, but found little effect of β-elemene on HCC. However, we showed for the first time that β-elemene could dramatically enhance oxaliplatin-induced cytotoxicity in HCC cells. Furthermore, we explored the mechanisms of β-elemene increasing oxaliplatin sensitivity in human HCC and found that β-elemene promoted oxaliplatin importation by upregulating expression of the copper influx transporter CTR1, which was degraded significantly upon oxaliplatin treatment45,46. This is the first demonstration that β-elemene could act on CTR1 to deliver a sufficient amount of platinum to reach the target DNA (Fig. 5).
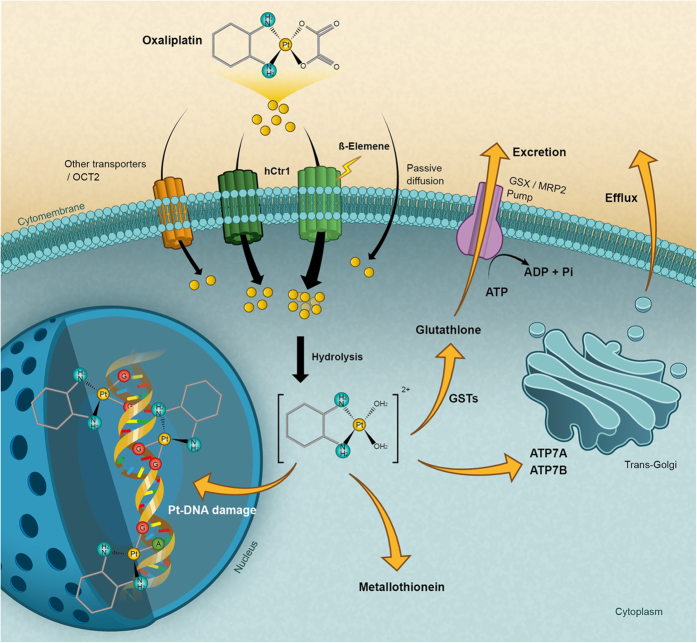
Figure 5. A hypothetical schematic of the contribution of β-elemene to oxaliplatin inhibition of HCC via activation of the CTR1.
Platinum drugs enter cells either by transporters, such as CTR1, or by passive diffusion. β-elemene increases the intercellular platinum amount by stabilizing CTR1, counteracting the degradation of CTR1 upon oxaliplatin treatment. Once inside cells, oxaliplatin is hydrolyzed by the addition of water molecules to form a chemically reactive aqua species, which reacts with species containing high sulfur levels from many cysteine or methionine amino acids including tripeptide glutathione or metallothioneins. Oxaliplatin can be exported from cells through the copper exporters ATP7A and ATP7B or through the glutathione S-conjugate export GS-X pump. Once the activated aqua oxaliplatin species has entered the nucleus, prior binding to the nitrogen at position 7 of guanine occurs and causes DNA damage.
CTR1 was originally recognized as a multispanning plasma membrane protein that was closely related to copper transport into yeast cells47 and was proven to be elementary for stimulating copper accumulation in mammalian cells48,49,50. Subsequent research found that CTR1 markedly influenced the cellular accumulation of platinum-containing drugs, which strengthened the value of CTR1 in platinum-based chemotherapy51,52. Emerging evidence indicated that the degradation of the CTR1 protein localized in plasma membrane and cytoplasm was triggered by platinum drugs45,46. Consistent with previous research, in this study the loss of CTR1 protein upon oxaliplatin treatment was observed both by Western blot and immunofluorescence confocal microscopy analysis in MHCC97H cells. β-elemene could counteract the loss of CTR1 expression and enhance the activity of platinum drugs, as demonstrated by the fact that β-elemene had no effect on CTR1 expression and platinum influx when MHCC97H cells were transfected by CTR1 siRNA, indicating the key role of CTR1 in the synergistic effects of β-elemene plus oxaliplatin. Unexpectedly, the ability of β-elemene to regulate CTR1 expression depends on oxaliplatin, as β-elemene alone had little effect on the expression of CTR1. CTR1 shows to control the tumor cell accumulation and cytotoxic effect of cisplatin, carboplatin, and oxaliplatin and cisplatin can trigger the down-regulation of CTR1 via a process that involves ubiquitination and proteosomal degradation14. Our data shows that oxaliplatin-induced down-regulation of CTR1 are independent of transcriptional and translational events. So we hereby hypothesize that oxaliplatin can influence the processes of ubiquitination of CTR1. In addition, structure-function analysis of CTR1 indicated that N-terminal Met-rich motifs were critical for copper and platinum transport binding to the N terminus53,54,55. Whether β-elemene regulates CTR1-mediated oxaliplatin transport by targeting the N terminus remains to be clarified and deserves further study.
We found this powerful combination is therapeutically effective, providing a novel, safe and practical form of chemotherapy. However, the mechanism of β-elemene stabilizing CTR1 need further study. We plan to study on weather β-elemene act on the ubiquitination of CTR1 in the next step. We also design a dose response study in vivo including the different doses of β-elemene to testify if there is the dose response relationship between β-elemene and the efficacy.
In summary, we found that β-elemene dramatically reinforced the anti-proliferative effect of oxaliplatin on HCC both in vitro and in vivo. This synergism results from platinum accumulation and increasing of platinum-DNA adduct. Moreover, the CTR1 siRNA-based analysis showed that β-elemene increases the intercellular platinum amount by stabilizing CTR1, counteracting the degradation of CTR1 upon oxaliplatin treatment. These data support the concept that an oxaliplatin-based regimen in combination with β-elemene can result in a more powerful anti-HCC effect, and it deserves further evaluation in future clinical settings.
Methods
Chemicals and reagents
β-elemene (99.2% purity) was purchased from the Chinese National Institutes for Food and Drug Control, with a molecular formula of C15H24 and molecular weight of 204.35. Injectable solution of β-elemene (#081152) was purchased from Dalian Holley Kingkong Pharmaceutical Co., Ltd (Liaoning, China). Oxaliplatin was purchased from Sigma Chemical Company (#O9512, St. Louis, MO, USA). For Western blotting and/or immunochemistry, anti-CTR1/SLC31A1 antibody was purchased from Abcam (#ab129067, San Francisco, CA, USA), anti-β-actin was purchased from Santa Cruz Biotechnology (#sc-47778, Paso Robles, CA, USA), and goat-anti-rabbit IgG (#cw0103A) and goat-anti-mouse IgG (#cw0102) conjugated to horseradish peroxidase were purchased from Cwbiotech (Beijing, China). Primary antibodies of apoptotic-related molecules were purchased from Proteintech (Bcl-2(#12789-1-AP, Chicago, IL, USA), Bcl-XL (#12783-1-AP, Chicago, IL, USA), BAX (#50599-2-AP, Chicago, IL, USA), Cytochrome C(#10993-1-AP, Chicago, IL, USA)). Cell proliferation was measured using the Cell Counting Kit-8 assay (#ck04, Dojindo, Kumamoto, Japan).
HCC cell lines
Two HCC cell lines (MHCC97H and HCCLM3) that were established from the same parent human HCC cell line were obtained from the Liver Cancer Institute, Fudan University, Shanghai, China. The HCC cell lines Hep3B and Huh7 were purchased from Shanghai Institute of Cell Biology, Shanghai, China. All cell lines were maintained and cultured in high-glucose Dulbecco’s modified Eagle’s medium (DMEM) (Gibco BRL, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (Gibco BRL), which were incubated at 37 °C in a humidified incubator containing 5% CO2.
Cell transfections
MHCC97H cells were seeded at a density of 5 × 105 cells/well in 6-well polystyrene microplates and incubated until they reached 50% confluence. Small interfering RNA (siRNA) was purchased from Biomics (Nantong, China), including hs-CTR1-si-1,2 and a negative control (si-NC) (Supplementary Table S1). Cells were transiently transfected with siRNA for 6 h at a final concentration of 50 nM with Lipofectamine 2000 transfection reagent (Life Technologies-Invitrogen, Shanghai, China) according to the manufacturer’s instructions. After the transfection in serum-free DMEM for 6 h, the cells were cultured with DMEM containing 10% fetal bovine serum for 48 h, followed by quantification of mRNA using real-time qPCR analysis and Western blot analysis.
CCK8 assay and combination studies
A CCK8 assay was used to detect the absorbance at a wavelength of 450 nm as the OD value. Inhibition concentration 50 (IC50) values were calculated and converted into dose modifying factors [DMF = IC50 (oxaliplatin alone)/IC50 (60 μg/mL β-elemene + oxaliplatin)]. The nature of drug interaction was analyzed by the coefficient of drug interaction (CDI)29,30,31, which was calculated as CDI = AB / (A × B). According to the absorbance of each group, AB is the ratio of the two-drug combination group to the control group, and A or B is the ratio of the single agent group to the control group. Thus, a CDI value of <1, = 1, or >1 indicates that the drugs are synergistic, additive, or antagonistic, respectively.
Platinum cellular accumulation and platinum-DNA binding studies
MHCC97H cells were seeded in 100-mm culture dishes at a density of 5 × 106 cells per well in aliquots of 10 mL complete culture medium (see HCC Cell lines). Cells in the exponential growth phase were exposed to different concentrations (for details see Results) of oxaliplatin with or without β-elemene at 37 °C in a humidified incubator containing 5% CO2 for 24 h. After incubation, cells were washed with ice-cold PBS, harvested, and pelleted by centrifugation at 400 × g and at 4 °C for 15 min. The cell pellets were dissolved in 70% nitric acid at 65 °C for at least 2.5 h. Distilled water containing 10 ppb of iridium (Sigma) and 0.1% Triton X-100 was added to the samples to dilute the nitric acid to 7%. The platinum content was measured by inductively coupled plasma mass spectrometry (ICP-MS) in the State Key Laboratory of Estuarine and Coastal Research at the East China Normal University at Shanghai.
Genomic DNA was isolated from the cell pellets using a Wizard Genomic DNA Purification kit (Promega, Madison, WI, USA) following the manufacturer’s instructions. The platinum-binding genomic DNA was analyzed by ICP-MS.
Flow cytometric analysis of apoptosis
To quantify apoptosis, MHCC97H cells were treated with oxaliplatin (16 μm), either alone or in combination with β-elemene (60 μg ⁄ml), for 48 hr. After treatment, the cells were harvested by trypsinization, washed twice with PBS and stained with Annexin V: FITC Apoptosis Detection Kit (#556547, BD Bioscience, Eugene, NJ, USA), following the manufacturer’s protocol. Flow cytometric analysis of the stained cells was performed by BD FACSCalibur with CellQuest software (BD Bioscience, San Jose, CA).
Animal treatment
BALB/c nu/nu mice (aged 4 weeks and weighing approximately 20 g) were purchased from the Shanghai Institute of Materia Medica and maintained under standard pathogen-free conditions in the experimental animal center of Zhongshan Hospital, Fudan University. The animal care and experimental protocols were in accordance with the guidelines established by the Shanghai Medical Experimental Animal Care Commission. Ethical approval was obtained from the Zhongshan Hospital Research Ethics Committee.
Orthotopic implantation of the human HCC cell line MHCC97H in the BALB/c mice was carried out as described previously56. Seven days after orthotopic implantation, 24 BALB/c nu/nu mice bearing orthotopic xenografts were randomly divided into a control group, which was treated by intraperitoneal injection with 0.1 mL 5% glucose solution twice a week; β-elemene group, which was treated by intraperitoneal injection with 0.1 mL β-elemene (45 mg/kg) twice a week; oxaliplatin group, which received intraperitoneal injection with 0.1 mL oxaliplatin (5 mg/kg) twice a week; and oxaliplatin plus β-elemene group, which was treated by intraperitoneal injection with 0.1 mL oxaliplatin (5 mg/kg) plus 0.1 mL β-elemene (45 mg/kg) twice a week. Tumor growth and mice weight were evaluated twice a week, and mice were sacrificed after 7 weeks.
Tumors, livers, and lungs were removed, fixed in 4% formalin fixative overnight at 4 °C, and embedded in paraffin. Consecutive 5-μm thick sections of tumor and liver were made for immunohistochemistry. The expression of CTR1 in tumors was measured in serial tissue sections of each group by immunohistochemistry.
Immunohistochemistry
Immunohistochemistry staining of paraffin sections was performed using a two-step protocol, using Novolink Polymer Detection System (NovoLink. TM. Detection System, Leica, UK) and the GTVision II Detection Kit with peroxidase/DAB, rabbit/mouse (#gk500506A, Gene Tech, Shanghai, China). After antigen retrieval, the slices were incubated with the primary antibody (#sc-66847, CTR1, Santa Cruz, CA, USA) overnight at 4 °C, followed by incubation with the secondary antibody (#gk500506A, Gene Tech) at 37 °C for 30 min. The sections were stained with DAB and counterstained with hematoxylin (Dako, Glostrup, Denmark), dehydrated in ethanol, mounted in dimethyl benzene, and placed under a coverslip. Apoptotic cells were visualized with an enzymatic reaction using the TUNEL method. TUNEL staining with an in situ cell death detection kit (#11684817910, Roche Molecular Biochemicals, Indianapolis, IN, USA), following the manufacturer’s protocol.
Western blot analysis and immunofluorescence
Total protein was extracted by cell lysis buffer supplemented with protease inhibitor. Protein samples were separated using 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and electrotransferred onto polyvinylidene fluoride membranes (Millipore, Billerica, MA, USA). After blocking with 5% non-fat milk in TBS-T, membranes were incubated with the primary antibody (#ab129067, CTR1, Abcam, San Francisco, CA, USA). Protein was detected with Image Acquisition using ImageQuant LAS 4000 (Pittsburg, PA, USA).
For immunofluorescence assays, cells were cultured on glass slides with cell abundance of 30% in 6-well polystyrene microplates. The cells on glass slides were fixed with 4% formaldehyde and then permeabilized with PBS containing 0.2% Triton X-100 after washing three times in PBST. After blocking with 1% BSA in PBS, the cells were incubated with primary antibody (#sc-66847, CTR1, Santa Cruz, CA, USA) overnight at 4 °C. Cells were washed three times in PBS, followed by incubation with 2 μg/mL Alexa Fluor 594 phalloidin (Invitrogen, Carlsbad, CA, USA) for 1 h at room temperature. Cells were washed with PBS three times, nuclear stained with 5 μg/mL DAPI, and analyzed with a confocal laser scanning microscope (Olympus FluoView FV1000 confocal microscope, Melville, NY, USA).
Statistical analyses
Statistical analyses were performed with SPSS 19.0 for Windows (SPSS Inc., Chicago, IL, USA). Values of continuous variables are expressed as the mean ± SD. The difference between groups was analyzed using a Student’s t-test when comparing only two groups or one-way analysis of variance when comparing more than two groups. Quantitative variable comparisons were analyzed using χ2 test or Fisher’s exact test. Two-tailed P values < 0.05 were considered statistically significant.
Additional Information
How to cite this article: Li, X. et al. β-elemene sensitizes hepatocellular carcinoma cells to oxaliplatin by preventing oxaliplatin-induced degradation of copper transporter 1. Sci. Rep. 6, 21010; doi: 10.1038/srep21010 (2016).
Supplementary Material
Acknowledgments
We thank Professor Jian-Guo Qu of the State Key Laboratory of Estuarine and Coastal Research at the East China Normal University for platinum analyses, and Ying Shi of the Institute of Brain Science at Fudan University for confocal laser scanning microscopy. This work was supported in part by grants from the State Key Basic Research Program of China (2013CB910500), the National Key Project for Infectious Disease of China (2012ZX10002012-003), and the National Natural Science Foundation of China (81071993, 81372654).
Footnotes
Author Contributions X.L., Z.L. and B.Z. contributed equally to the manuscript. X.L. and J.Z. designed, performed experiments and analyzed data. Z.L. and B.Z. performed RNA-interfere in gene expression and contributed to the experiments. L.G., S.L. and H.L. performed the HCC xenograft experiment. X.L. drafted the manuscript. Q.Y. and J.Z. supervised the study the project and edited the manuscript. All authors read and approved the final manuscript.
References
- Torre L. A. et al. Global cancer statistics, 2012. CA Cancer J Clin 65, 87–108 (2015). [DOI] [PubMed] [Google Scholar]
- Harlan L. C. & Warren J. L. Global survival patterns: potential for cancer control. The Lancet 385, 926–928 (2015). [DOI] [PubMed] [Google Scholar]
- Zaanan A. et al. Gemcitabine plus oxaliplatin in advanced hepatocellular carcinoma: a large multicenter AGEO study. J Hepatol 58, 81–88 (2013). [DOI] [PubMed] [Google Scholar]
- Abdel-Rahman O. Revisiting oxaliplatin-based regimens for advanced hepatocellular carcinoma. Curr Oncol Rep 16, 394 (2014). [DOI] [PubMed] [Google Scholar]
- Chan S. L. Drug development for hepatocellular carcinoma: knowing the past helps to understand the future. Oncologist 19, 1115–1117 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin S. et al. Randomized, multicenter, open-label study of oxaliplatin plus fluorouracil/leucovorin versus doxorubicin as palliative chemotherapy in patients with advanced hepatocellular carcinoma from Asia. J Clin Oncol 31, 3501–3508 (2013). [DOI] [PubMed] [Google Scholar]
- Li J. H. et al. Oxaliplatin and 5-fluorouracil hepatic infusion with lipiodolized chemoembolization in large hepatocellular carcinoma. World J Gastroenterol 21, 3970–3977 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F. Y. et al. Hepatocellular carcinoma associated with Budd-Chiari syndrome: imaging features and transcatheter arterial chemoembolization. BMC Gastroenterol 13, 105 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A. et al. Neuropilin-1-targeted gold nanoparticles enhance therapeutic efficacy of platinum(IV) drug for prostate cancer treatment. ACS Nano 8, 4205–4220 (2014). [DOI] [PubMed] [Google Scholar]
- Samimi G. et al. Increased expression of the copper efflux transporter ATP7A mediates resistance to cisplatin, carboplatin, and oxaliplatin in ovarian cancer cells. Clin Cancer Res 10, 4661–4669 (2004). [DOI] [PubMed] [Google Scholar]
- Al-Eisawi Z., Beale P., Chan C., Yu J. Q. & Huq F. Carboplatin and oxaliplatin in sequenced combination with bortezomib in ovarian tumour models. J Ovarian Res 6, 78 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiragami R. et al. Enhanced antitumor activity of cerulenin combined with oxaliplatin in human colon cancer cells. Int J Oncol 43, 431–438 (2013). [DOI] [PubMed] [Google Scholar]
- Alcindor T. & Beauger N. Oxaliplatin: a review in the era of molecularly targeted therapy. Curr Oncol 18, 18–25 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell S. B., Safaei R., Larson C. A. & Sailor M. J. Copper transporters and the cellular pharmacology of the platinum-containing cancer drugs. Mol Pharmacol 77, 887–894 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelland L. The resurgence of platinum-based cancer chemotherapy. Nat Rev Cancer 7, 573–584 (2007). [DOI] [PubMed] [Google Scholar]
- Larson C. A., Blair B. G., Safaei R. & Howell S. B. The role of the mammalian copper transporter 1 in the cellular accumulation of platinum-based drugs. Mol Pharmacol 75, 324–330 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D. & Lippard S. J. Cellular processing of platinum anticancer drugs. Nat Rev Drug Discov 4, 307–320 (2005). [DOI] [PubMed] [Google Scholar]
- Yao Y. Q. et al. Anti-tumor effect of beta-elemene in glioblastoma cells depends on p38 MAPK activation. Cancer Lett 264, 127–134 (2008). [DOI] [PubMed] [Google Scholar]
- Liu J. et al. beta-Elemene-induced autophagy protects human gastric cancer cells from undergoing apoptosis. BMC Cancer 11, 183 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X. et al. Antiproliferative effect of beta-elemene in chemoresistant ovarian carcinoma cells is mediated through arrest of the cell cycle at the G2-M phase. Cell Mol Life Sci 62, 894–904 (2005). [DOI] [PubMed] [Google Scholar]
- Wang G. et al. Antitumor effect of beta-elemene in non-small-cell lung cancer cells is mediated via induction of cell cycle arrest and apoptotic cell death. Cell Mol Life Sci 62, 881–893 (2005). [DOI] [PubMed] [Google Scholar]
- Yu Z. et al. beta-Elemene piperazine derivatives induce apoptosis in human leukemia cells through downregulation of c-FLIP and generation of ROS. PLoS One 6, e15843 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrero A. F. et al. Efficient synthesis of the anticancer beta-elemene and other bioactive elemanes from sustainable germacrone. Org Biomol Chem 9, 1118–1125 (2011). [DOI] [PubMed] [Google Scholar]
- Li Q. Q., Wang G., Reed E., Huang L. & Cuff C. F. Evaluation of cisplatin in combination with beta-elemene as a regimen for prostate cancer chemotherapy. Basic Clin Pharmacol Toxicol 107, 868–876 (2010). [DOI] [PubMed] [Google Scholar]
- Li Q. Q. et al. beta-Elemene, a novel plant-derived antineoplastic agent, increases cisplatin chemosensitivity of lung tumor cells by triggering apoptosis. Oncol Rep 22, 161–170 (2009). [DOI] [PubMed] [Google Scholar]
- Zhao J. et al. In vitro combination characterization of the new anticancer plant drug beta-elemene with taxanes against human lung carcinoma. Int J Oncol 31, 241–252 (2007). [PubMed] [Google Scholar]
- Banuelos C. A. et al. Radiosensitization by the histone deacetylase inhibitor PCI-24781. Clin Cancer Res 13, 6816–6826 (2007). [DOI] [PubMed] [Google Scholar]
- Sedlar A. et al. Radiosensitizing effect of intratumoral interleukin-12 gene electrotransfer in murine sarcoma. BMC Cancer 13, 38 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L. et al. Autophagy inhibition contributes to the synergistic interaction between EGCG and doxorubicin to kill the hepatoma Hep3B cells. PLoS One 9, e85771 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prichard M. N. & Shipman C. Jr. A three-dimensional model to analyze drug-drug interactions. Antiviral Res 14, 181–205 (1990). [DOI] [PubMed] [Google Scholar]
- Soica C. et al. The synergistic biologic activity of oleanolic and ursolic acids in complex with hydroxypropyl-gamma-cyclodextrin. Molecules 19, 4924–4940 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S. J. et al. Mechanistic basis of a combination d-penicillamine and platinum drugs synergistically inhibits tumor growth in oxaliplatin-resistant human cervical cancer cells in vitro and in vivo. Biochem Pharmacol 95, 28–37 (2015). [DOI] [PubMed] [Google Scholar]
- Jandial D. D. et al. Enhanced delivery of cisplatin to intraperitoneal ovarian carcinomas mediated by the effects of bortezomib on the human copper transporter 1. Clin Cancer Res 15, 553–560 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo W. et al. A randomized phase III study of doxorubicin versus cisplatin/interferon alpha-2b/doxorubicin/fluorouracil (PIAF) combination chemotherapy for unresectable hepatocellular carcinoma. J Natl Cancer Inst 97, 1532–1538 (2005). [DOI] [PubMed] [Google Scholar]
- Lopez P. M., Villanueva A. & Llovet J. M. Systematic review: evidence-based management of hepatocellular carcinoma--an updated analysis of randomized controlled trials. Aliment Pharmacol Ther 23, 1535–1547 (2006). [DOI] [PubMed] [Google Scholar]
- Stein A. & Arnold D. Oxaliplatin: a review of approved uses. Expert Opin Pharmacother 13, 125–137 (2012). [DOI] [PubMed] [Google Scholar]
- Ding Z. B. et al. Autophagy activation in hepatocellular carcinoma contributes to the tolerance of oxaliplatin via reactive oxygen species modulation. Clin Cancer Res 17, 6229–6238 (2011). [DOI] [PubMed] [Google Scholar]
- Nowak A. K., Chow P. K. & Findlay M. Systemic therapy for advanced hepatocellular carcinoma: a review. Eur J Cancer 40, 1474–1484 (2004). [DOI] [PubMed] [Google Scholar]
- Gish R. G. et al. Phase III randomized controlled trial comparing the survival of patients with unresectable hepatocellular carcinoma treated with nolatrexed or doxorubicin. J Clin Oncol 25, 3069–3075 (2007). [DOI] [PubMed] [Google Scholar]
- Wang B. et al. Systematic review of beta-elemene injection as adjunctive treatment for lung cancer. Chin J Integr Med 18, 813–823 (2012). [DOI] [PubMed] [Google Scholar]
- Li Q. Q. et al. beta-Elemene promotes cisplatin-induced cell death in human bladder cancer and other carcinomas. Anticancer Res 33, 1421–1428 (2013). [PMC free article] [PubMed] [Google Scholar]
- Li Q. Q., Lee R. X., Liang H. & Zhong Y. Anticancer activity of beta-Elemene and its synthetic analogs in human malignant brain tumor cells. Anticancer Res 33, 65–76 (2013). [PMC free article] [PubMed] [Google Scholar]
- Li Q. Q. et al. beta-Elemene enhances susceptibility to cisplatin in resistant ovarian carcinoma cells via downregulation of ERCC-1 and XIAP and inactivation of JNK. Int J Oncol 43, 721–728 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding X. F., Shen M., Xu L. Y., Dong J. H. & Chen G. 13,14-bis(cis-3,5-dimethyl-1-piperazinyl)-beta-elemene, a novel beta-elemene derivative, shows potent antitumor activities via inhibition of mTOR in human breast cancer cells. Oncol Lett 5, 1554–1558 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzer A. K., Katano K., Klomp L. W. & Howell S. B. Cisplatin rapidly down-regulates its own influx transporter hCTR1 in cultured human ovarian carcinoma cells. Clin Cancer Res 10, 6744–6749 (2004). [DOI] [PubMed] [Google Scholar]
- Holzer A. K., Manorek G. H. & Howell S. B. Contribution of the major copper influx transporter CTR1 to the cellular accumulation of cisplatin, carboplatin, and oxaliplatin. Mol Pharmacol 70, 1390–1394 (2006). [DOI] [PubMed] [Google Scholar]
- Dancis A. et al. Molecular characterization of a copper transport protein in S. cerevisiae: an unexpected role for copper in iron transport. Cell 76, 393–402 (1994). [DOI] [PubMed] [Google Scholar]
- Moller L. B., Petersen C., Lund C. & Horn N. Characterization of the hCTR1 gene: genomic organization, functional expression, and identification of a highly homologous processed gene. Gene 257, 13–22 (2000). [DOI] [PubMed] [Google Scholar]
- Lee J., Prohaska J. R. & Thiele D. J. Essential role for mammalian copper transporter Ctr1 in copper homeostasis and embryonic development. Proc Natl Acad Sci USA 98, 6842–6847 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo Y. M., Zhou B., Cosco D. & Gitschier J. The copper transporter CTR1 provides an essential function in mammalian embryonic development. Proc Natl Acad Sci USA 98, 6836–6841 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X., Okuda T., Holzer A. & Howell S. B. The copper transporter CTR1 regulates cisplatin uptake in Saccharomyces cerevisiae. Mol Pharmacol 62, 1154–1159 (2002). [DOI] [PubMed] [Google Scholar]
- Song I. S. et al. Role of human copper transporter Ctr1 in the transport of platinum-based antitumor agents in cisplatin-sensitive and cisplatin-resistant cells. Mol Cancer Ther 3, 1543–1549 (2004). [PubMed] [Google Scholar]
- De Feo C. J., Aller S. G., Siluvai G. S., Blackburn N. J. & Unger V. M. Three-dimensional structure of the human copper transporter hCTR1. Proc Natl Acad Sci USA 106, 4237–4242 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puig S., Lee J., Lau M. & Thiele D. J. Biochemical and genetic analyses of yeast and human high affinity copper transporters suggest a conserved mechanism for copper uptake. J Biol Chem 277, 26021–26030 (2002). [DOI] [PubMed] [Google Scholar]
- Sinani D., Adle D. J., Kim H. & Lee J. Distinct mechanisms for Ctr1-mediated copper and cisplatin transport. J Biol Chem 282, 26775–26785 (2007). [DOI] [PubMed] [Google Scholar]
- Yang X. et al. MicroRNA-26a suppresses tumor growth and metastasis of human hepatocellular carcinoma by targeting interleukin-6-Stat3 pathway. Hepatology 58, 158–170 (2013). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.