Abstract
Microbial fuel cell (MFC) is a promising technology for direct electricity generation from organics by microorganisms. The type of electron donors fed into MFCs affects the electrical performance, and mechanistic understanding of such effects is important to optimize the MFC performance. In this study, we used a model organism in MFCs, Shewanella oneidensis MR-1, and 13C pathway analysis to investigate the role of formate in electricity generation and the related microbial metabolism. Our results indicated a synergistic effect of formate and lactate on electricity generation, and extra formate addition on the original lactate resulted in more electrical output than using formate or lactate as a sole electron donor. Based on the 13C tracer analysis, we discovered decoupled cell growth and electricity generation in S. oneidensis MR-1 during co-utilization of lactate and formate (i.e., while the lactate was mainly metabolized to support the cell growth, the formate was oxidized to release electrons for higher electricity generation). To our best knowledge, this is the first time that 13C tracer analysis was applied to study microbial metabolism in MFCs and it was demonstrated to be a valuable tool to understand the metabolic pathways affected by electron donors in the selected electrochemically-active microorganisms.
Microbial fuel cells (MFCs) are an emerging technology to convert organics in wastewater to electrical energy1,2. Electrochemically active bacteria (EAB) serve as effective microbial catalysts to transfer electrons from substrates to solid electron acceptors (e.g., an anode electrode) under anaerobic conditions3,4,5,6. Electron transfer from EAB to the electrode is one of the most important steps in electricity generation by MFCs, and can be critically influenced by the type of the electron donors that will result in difference in the composition, structure, and metabolism of microorganisms on the anode electrode7,8,9. Therefore, understanding the impact of electron donors on electricity generation in MFCs is important to improve the efficiency of energy recovery10,11,12.
Shewanellaceae is a typical family including several model bacterial species such as Shewanella oneidensis MR-1 that can generate electricity in MFCs9,13,14. S. oneidensis MR-1 can transport electrons via intracellular metabolic reactions to complete extracellular electron transfer (EET) pathways15, or via multiple electron-transferring mechanisms including direct contact with the electron acceptor16, redox reactions of mediators (e.g., flavin) to transfer electrons17,18,19, and/or conductive nanowire extension to contact the electron acceptor20. Versatile electron transferring abilities allow S. oneidensis MR-1 to use multiple organics as electron donors (e.g., lactate, pyruvate, acetate, formate) to process dissimilatory reactions for reducing insoluble metals (e.g. Fe(III), Mn(IV)) and some inorganic ions (e.g., nitrate, nitrite) as electron acceptors13,21,22,23,24. Among those electron donors, lactate has been extensively studied. It was found that S. oneidensis MR-1 prefers to catabolize lactate because of a series of internally competent enzymatic reactions that utilize the electron donors to obtain energy for growth and electricity generation15,25,26. Under anaerobic conditions, such as the anaerobic anode of an MFC, lactate can be utilized to release electrons with the involvement of NADH, specific cytochromes, as well as various complexes, and it is further oxidized to acetyl-CoA, and CO2 or formate14,15,27. To assess electricity generation efficiency, Coulombic efficiency (CE) and Coulombic recovery (CR) are quantitative indicators to show the fraction of collected electrons through EET versus total ideal electron production28,29,30. It was reported that the lactate-fed MFC inoculated with S. oneidensis MR-1 achieved CE at the range of about 15–20%16,31; thus, there is still a lot of room to improve electricity generation efficiency.
In addition to lactate, formate is another potentially important electron donor to serve energy source for S. oneidensis MR-113,15,32. S. oneidensis MR-1 has NAD+-dependent formate dehydrogenase (FDH) to catalyze the formate oxidation to CO2, and release electrons to complete EET pathway14. On the basis of EET started from lactate and pyruvate utilization, the catabolic metabolism of formate with the help of FDH can release and transfer more electrons to specific electron acceptors; such a mechanism is possibly helpful to improve CE/CR of S. oneidensis MR-1 in MFCs14,27. Thus, we hypothesized that extra formate addition on lactate utilization can boost the electrical output and improve CE/CR. However, formate is seldom studied for its effect on electricity generation of S. oneidensis MR-1 in MFCs.
This study aims to investigate the role of formate for the catabolic metabolism and electricity generation of S. oneidensis MR-1 in MFCs. Particularly, we applied 13C tracer experiment, which is a method widely used to facilitate the elucidation of metabolic rewiring in various non-model environmental microorganisms33,34,35. Normally, by feeding the 13C labeled substrate into a culture system, the carbon flow in the intracellular metabolism is traced to produce labeled metabolites (e.g., proteinogenic amino acids). By analyzing these metabolites using gas chromatography-mass spectrometry (GC-MS) and the following natural isotopic correction of the raw mass spectra, the isotopic labeling patterns (e.g., mass distribution vector) would be obtained and could be directly analyzed to reveal the metabolic rewiring of the target strains. Our previous study has applied the 13C tracer experiments to a well-designed anaerobic system for revealing the distinct intracellular metabolic behaviors between the biofilm and planktonic S. oneidensis MR-1 cells36. The 13C tracer experiment successfully revealed the cell metabolisms of S. oneidensis MR-1, and a conclusion was made that C1 metabolism such as formate oxidation is strongly related to the electron transferring to the extracellular environment. Therefore, in this study, 13C tracer experiment was applied to help us rigorously analyze the effects of formate on microbial metabolism of S. oneidensis MR-1 using lactate for electricity generation in an MFC. The results indicated, for the first time, that the addition of formate in MFCs might synergize with the lactate utilization to improve the electricity generation efficiency, which was achieved by a unique metabolism in S. oneidensis MR-1 to decouple cell growth from electricity generation during co-utilization of lactate and formate.
Results & Discussion
Combined supply of formate and lactate enhanced current generation
The MFC was operated under three conditions, each of which was for at least three repeated cycles and the current profile was recorded (Fig. 1). The added formate and lactate in the anode chamber was completely consumed in each cycle based on the HPLC results, indicating that the current drop at the end of each cycle was caused by the complete consumption of electron donors (Fig. 1). In the control reactor that did not have electricity generation (electrodes were disconnected under an open circuit condition), the electron donors were also consumed, possibly because of oxygen intrusion from the cathode (with continuous aeration) into the control chamber acting as the electron acceptor37. Though the oxygen diffusion through cation exchange membrane (CEM) was very limited38,39, the electron donor for each cycle could still be consumed because of its small amount (~0.8 mM) added into the control and anode chambers. Electricity generation in the MFC clearly competed for electron donors, and the comparable CR (Coulombic recovery) with other MFC studies of S. oneidensis MR-1 indicates that the anode electrode has strong ability to compete with oxygen for the electron donors to collect electrons (the data of CR were shown in the following section)16,31.
Figure 1. Current generation in the MFC supplied with various electron donors.
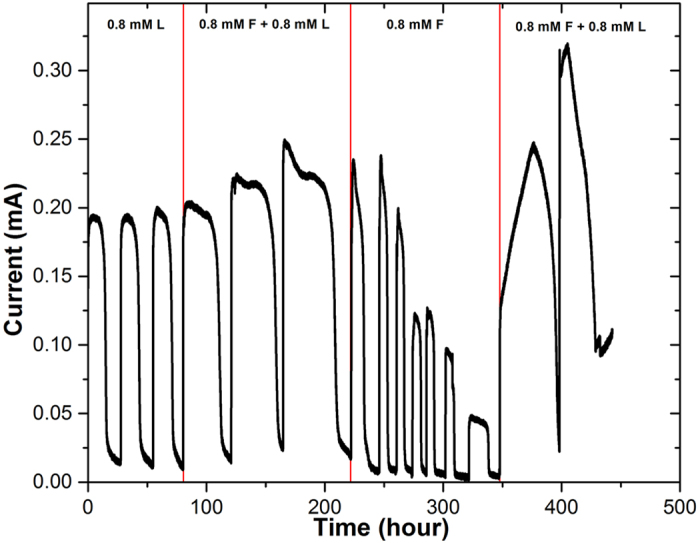
Note: “L” means lactate; “F” means formate; “(F + L)” means addition of both substrate together; “0.8 mM” means 0.8 mM of each substrate added each cycle.
The peak current of the MFC was increased from 0.194 ± 0.005 mA using lactate alone to 0.231 ± 0.019 mA with additional formate as the extra electron donor (p < 0.05, one-tailed two-sample t-test with unequal variance at α = 0.05 for all the following statistical tests). Sole supply of formate significantly decreased the peak current to 0.147 ± 0.067 mA, lower than the current of other conditions (p < 0.05). When extra lactate was added with formate at the last two cycles, the current was boosted up again, to 0.290 ± 0.056 mA, significantly higher than the current production with sole formate supply (p < 0.05). The catholyte pH was stable at 7.2–7.4 due to generally low current output and strong buffering capacity of phosphate buffer saline (PBS)40, which eliminated the limiting effects of pH. The current generation with the combination of formate and lactate is higher than that with only lactate or formate, indicating that formate and lactate could have achieved synergistic effect when metabolized by S. oneidensis MR-1 to benefit the electricity generation in the MFC.
Formate addition resulted in higher conversion efficiency
To further understand the impact of formate on the MFC performance containing S. oneidensis MR-1, CR (Coulombic recovery) and TC (total Coulombs) were used to reflect the conversion efficiency from electron donors to electrical output, with the equations below to calculate both parameters for each cycle (eqs 1, 2, 3)28,29,30:
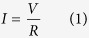 |
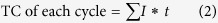 |
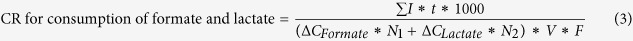 |
where in Eq. 1, V represents the voltage (V) and R is the external resistance (R = 8.2 Ω). In Eq. 2, t represents the voltage measurement interval (t = 120 s) of the digital multimeter, and I represents the current (A) measured for each measurement interval with the assumption that the current is the same within a time interval. In Eq. 3, ∆CFormate and ∆CLactate (mM) represent the moles of formate and lactate added into the reactor at the beginning of each cycle, respectively; N1 and N2 represent the mole of electrons transferring per mole substrate consumption, respectively, where N1 = 2 (formate → CO2 + 2H+ +2e−)41,42,43, and N2 = 4 (lactate + 2H2O → Acetate−+ HCO3− + 5H+ + 4e−, incomplete oxidation in anaerobic condition)16,31,44,45; V represents the reactor volume (0.14 L); and F represents the Faraday constant (96485 Coulombs per mole electrons).
Combining non-labeled formate and lactate resulted in higher CR of 34.9 ± 6.0% than that of lactate alone (19.2 ± 0.9%) (p < 0.05), indicating that extra formate addition on lactate supply could enhance the efficiency of substrate conversion to electrons, which can be ultimately harvested by the solid electron acceptor in the anode chamber (Fig. 2). Supplying formate as the sole electron donor was found to have a lower CR of 19.3 ± 6.8% than that condition of combined formate and lactate (p < 0.05), suggesting that lactate was also needed for S. oneidensis MR-1 to achieve good electron transferring efficiency. The TC with formate addition to lactate was 30.8 ± 5.4 C, much higher than the sum of TC (16.5 ± 2.1 C) of both solely supplying formate (5.6 ± 1.9 C) and lactate (10.9 ± 0.2 C) (p < 0.05) (Fig. 2). These results demonstrate that the increased electricity generation with combined formate with lactate was not simply “1 + 1 = 2”; instead, the combination achieved “1 + 1 > 2”. This further confirms the pivotal role of formate in the electricity generation by S. oneidensis MR-1. Lactate is a commonly acknowledged as an electron donor in MFCs, being involved in the central metabolism of S. oneidensis MR-116,23,31,46,47. Formate is more likely an electron transferring driving stimulus, which is related to the existence of FDH to convert the formate in periplasmic region to CO2 with the electrons being released extracellularly14,27,48. Therefore, to explain the synergistic effects of lactate and formate in electricity generation in MFCs, we hypothesize that lactate may be more involved in central metabolism while formate may be more related to EET.
Figure 2. CR and TC obtained in the MFC under different supplies of formate and lactate.
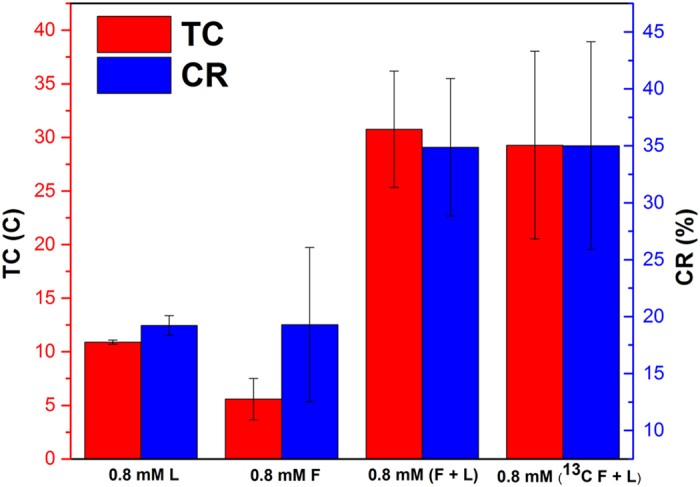
Note: “L” means lactate; “F” means formate; “(F + L)” means addition of both substrate together; “13C” represents the isotopomer addition; “0.8 mM” means 0.8 mM of each substrate added each cycle.
13C tracer experiments facilitated the elucidation of the role for formate
To examine our hypothesis, we conducted the 13C tracer experiment to investigate the intracellular metabolism of S. oneidensis MR-1 when metabolizing formate and lactate in the MFC. The addition of 13C formate to non-labeled lactate also improved MFC electricity generation with the peak current of 0.308 ± 0.084 mA (Fig. 3), suggesting that the labeled formate was also favored by S. oneidensis MR-1 to produce electricity. When the labeled formate was used with non-label lactate, similar improvements in both CR and TC were obtained (Fig. 2). By examining the corrected mass distribution vectors from the isotopic analysis, no labeled carbon was found in the proteinogenic amino acids (Table 1), which are the building blocks for the cell growth of both planktonic and biofilm cells. This indicates that formate did not participate in the central metabolism to produce the building blocks for the cell growth. Instead, non-labeled lactate was mainly used for growth of the S. oneidensis MR-1. In other words, the cell growth and electricity generation might be decoupled when culturing S. oneidensis MR-1 with combined formate and lactate in MFCs. Indeed, it has been reported that lactate was a more preferential carbon substrate than formate for cell growth of S. oneidensis MR-1. The biomass yield of S. oneidensis MR-1 cultivating under an oxygen limited condition could reach 0.22 g/g when using lactate as the carbon substrate, much higher than 0.11 g/g with formate as the carbon substrate49. In addition, the expression levels of FDH genes in S. oneidensis MR-1 have been found to be up-regulated in MFCs48. Recently, a recombinant S. oneidensis MR-1 that harbored additional copies of FDH genes was found to generate a higher current density14, indicating the important role of formate in supplying electrons through FDH in MFCs. Considering the importance of lactate and formate for cell growth and electricity generation, respectively, it is interesting but unsurprising to find in this study that these two carbon substrates could synergize with each other to improve current generation via a decoupled metabolism of cell growth and electricity generation.
Figure 3. Current generation in the MFC supplied with 0.8 mM 13C isotopic formate and 0.8 mM non-labelled lactate.
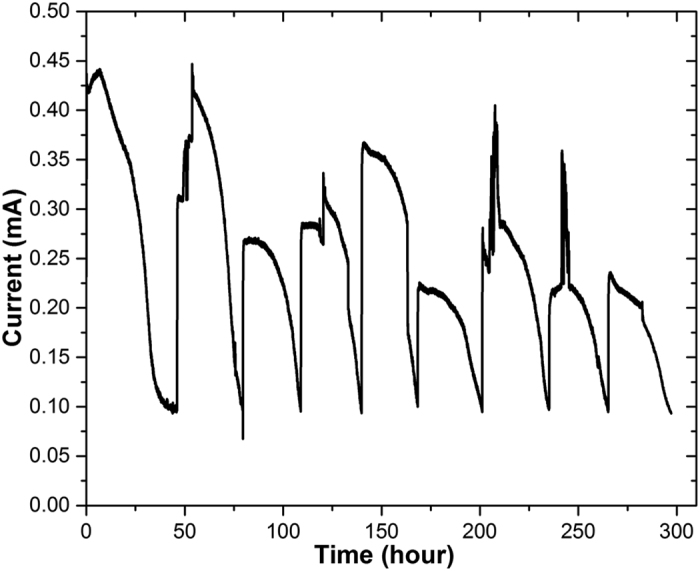
Table 1. Mass distribution vectors of the key proteiogenic amino acids.
| Cell sources | Anode-biofilm |
Anode-planktonic |
Control-biofilm |
Control-planktonic |
||||
|---|---|---|---|---|---|---|---|---|
| Fragments | M-57 | M-159 | M-57 | M-159 | M-57 | M-159 | M-57 | M-159 |
| Ala | ||||||||
| M0 | 0.93 | 0.95 | 0.96 | 0.95 | 0.95 | 0.95 | 0.94 | 0.94 |
| M1 | 0.06 | 0.03 | 0.04 | 0.02 | 0.05 | 0.03 | 0.05 | 0.03 |
| M2 | 0.01 | 0.03 | 0.00 | 0.03 | 0.00 | 0.03 | 0.00 | 0.03 |
| M3 | 0.00 | 0.00 | 0.00 | 0.01 | ||||
| Gly | ||||||||
| M0 | 1.00 | 1.00 | 0.98 | 0.98 | 0.95 | 1.00 | 0.95 | 0.97 |
| M1 | 0.00 | 0.00 | 0.02 | 0.02 | 0.05 | 0.00 | 0.05 | 0.03 |
| M2 | 0.00 | 0.00 | 0.00 | 0.00 | ||||
| Ser | ||||||||
| M0 | 0.98 | 0.99 | 0.97 | 0.98 | 0.88 | 0.94 | 0.93 | 0.96 |
| M1 | 0.04 | 0.02 | 0.03 | 0.02 | 0.17 | 0.06 | 0.06 | 0.04 |
| M2 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 | 0.00 |
| M3 | 0.00 | 0.00 | 0.00 | 0.01 | ||||
| Asp | ||||||||
| M0 | 0.96 | 0.94 | 0.92 | 0.94 | 0.84 | 0.91 | 0.88 | 0.93 |
| M1 | 0.04 | 0.05 | 0.09 | 0.04 | 0.16 | 0.05 | 0.10 | 0.06 |
| M2 | 0.00 | 0.01 | 0.00 | 0.00 | 0.00 | 0.02 | 0.03 | 0.00 |
| M3 | 0.00 | 0.01 | 0.00 | 0.01 | 0.00 | 0.01 | 0.00 | 0.01 |
| M4 | 0.00 | 0.00 | 0.00 | 0.00 | ||||
| Glu | ||||||||
| M0 | 0.94 | 0.98 | 0.96 | 0.95 | 0.92 | 0.96 | 0.92 | 0.89 |
| M1 | 0.06 | 0.01 | 0.03 | 0.05 | 0.10 | 0.03 | 0.08 | 0.10 |
| M2 | 0.00 | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| M3 | 0.00 | 0.00 | 0.01 | 0.00 | 0.01 | 0.00 | 0.00 | 0.00 |
| M4 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 | 0.00 |
| M5 | 0.00 | 0.00 | 0.00 | 0.00 | ||||
A metabolic pathway was proposed (Fig. 4), in which lactate is metabolized in the central metabolism of S. oneidensis MR-1 as its favorable substrate for cell growth13,14,16,23,27,31,46,47,50, while formate is more likely used as an electron-transfer driving stimulus from either extracellular exchange or the central metabolism to release electrons in periplasm by FDH14,27,48. In this scheme, the unlabeled lactate would be directly metabolized via central metabolism to produce various unlabeled building block (e.g., unlabeled amino acids) as we observed, and the labeled formate would be transported into periplasm and oxidized by inner membrane FDH to CO2 with the release of electrons, and eventually the labeled carbon was lost in the form of CO2 in the MFC. The released electrons from formate would be further transported by EET to the anode electrode or riboflavin intermediate to enhance electricity generation. Therefore, such decoupled cell growth from electricity generation could be responsible for the synergistic improvement of electricity generation with the addition of formate during the lactate uptake by S. oneidensis MR-1 in MFCs.
Figure 4. Proposed pathway of formate in the metabolism of S. oneidensis MR-1 after 13C formate experimental analysis.
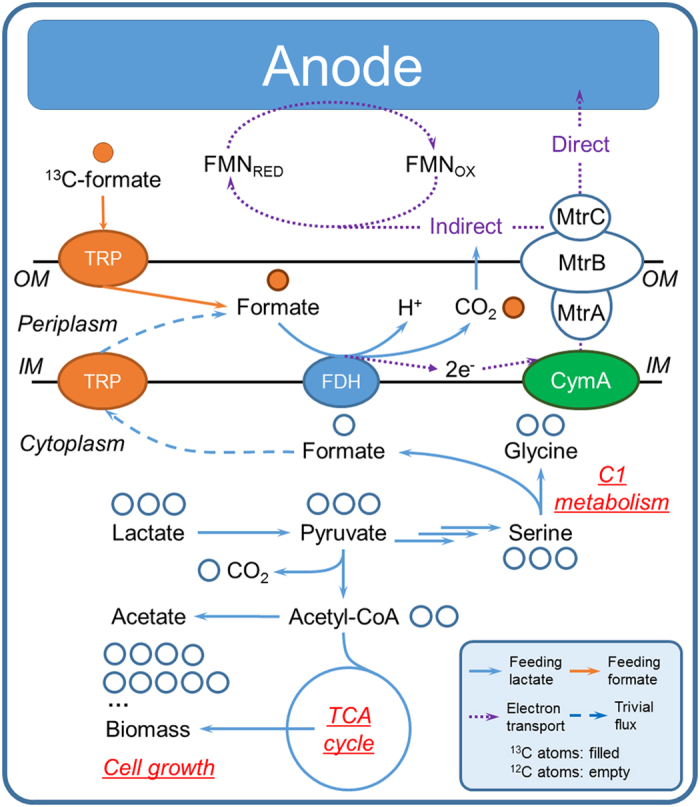
Designation: IM: inner membrane; OM: outer membrane; FDH: formate dehydrogenase; FMNRED: reduced flavin mononucleotide; FMNOX: oxidized flavin mononucleotide; CymA: tetraheme cytochrome anchored in inner membrane, accepts electrons from formate and transfers them to MtrA, MtrB and MtrC; MtrA, MtrB, MtrC: three kinds of periplasmic decaheme c-type cytochrome cytochromes anchored on outer membrane; TRP: formate transporter.
In summary, this study has contributed to an initial understanding of the role of formate in the electricity generation by S. oneidensis MR-1 using lactate in MFCs. The results show that the co-supply of two substrates would affect the EET behaviors, and ultimately the electricity generation in the MFC, implying the conception of synergy in substrates. Mutually complementary substrates may take advantage of substrate interaction in the cell metabolism, and generate a total effect greater than the sum of the individual contribution of single substrate for electricity generation. This may raise a question if other combinations of synergistic substrates also exist to enhance the MFC performance for pure or mixed culture. Moreover, 13C tracer experiment proves to be an effective technique to explore the influence of substrates on EET behavior of a selected species. Future studies can target what substrate has greater stimulating effect on EET behavior qualitatively and quantitatively to potentially improve the electricity generation. It will not only help reveal the pathway of EET and carbon flow, but also formulate a strategy for adjusting substrate combination to achieve optimal electricity generation under a certain special conditions (e.g., known composition of substrates being used for MFC application).
Methods
Bacterial strains and growing conditions in the MFC
S. oneidensis MR-1 (kindly provided by Dr. Y.J. Tang’s lab, Washington University, St. Louis, MO, USA) was initially grown in the shaking flasks with minimal medium containing 5 mM sodium L-lactate (Sigma-Aldrich, St. Louis, MO, USA) for two days at 100 rpm and 30 °C24,46. The culture medium was then transferred to the autoclaved anode chamber and the control chamber of the MFC system.
MFC setup and operation
A three-chamber MFC was constructed by connecting three glass bottles together with two CEMs as separators (UltexCMI7000, Membranes International, Inc., GlenRock, NJ, USA) between each set of two bottles (Fig. S1). The liquid volume of three chambers was 140 mL/each. The middle chamber served as a cathode chamber, while one bottle containing an anode electrode connected to the cathode electrode was the functioning anode and the other bottle (also containing an anode electrode but under the open circuit condition) was the control chamber. The anode and control chambers were sealed with creamy epoxy to create oxygen-limited environment. The anode electrode was a piece of rectangular carbon cloth (2.5 cm × 4.5 cm PANEX® 30PW06, Zoltek Corporation, St. Louis, MO, USA), and the cathode electrode using the same carbon cloth coated with platinum/carbon as the catalyst for oxygen reducing reaction (0.1 mg Pt cm−2). The cathode chamber was continually aerated to provide sufficient oxygen for cathodic reaction. The catholyte was 50 mM PBS (2.65 g L−1 KH2PO4 and 5.35 g L−1 K2HPO4), to buffer the catholyte pH.
The MFC was operated under a batch mode and at 30 °C with the external resistance of 8.2 Ω. Non-labelled electron donors were first supplied, and three conditions were tested: 1) 0.8 mM lactate in 1st–3rd cycles; 2) 0.8 mM lactate +0.8 mM formate in 4th–6th and 14th–15th cycles; 3) 0.8 mM formate in 7th–13th cycles. Once the current dropped to the baseline (~0.04 mA) in each cycle, the medium in the anode chamber and the control chamber was refreshed by removing 10 mL medium with sterile syringe and then injecting 10 mL fresh filtered (0.22 μm pore size) medium into the chambers. Depending on the substrate concentration, each cycle time period was 16–60 hours. To accelerate electron transfer, 0.2 μM riboflavin was added as an electron mediator to the anode and the control chambers at the beginning of the entire experiment (there was no more addition until the end of the experiment).
13C Tracer Experiment
Isotopically-labelled formate as an electron donor was supplied after finishing the cycles with non-isotopic labeled electron donors in the MFC. The combination of 0.8 mM [13C] sodium formate (99% purity, Cambridge Isotope Laboratory, USA) and 0.8 mM non-labeled sodium L-lactate was supplied to the anode and the control chambers at the beginning of a new cycle. After nine-cycle tests, both the liquid culture and carbon cloth in the anode and the control chambers were extracted to collect the planktonic and the biofilm cells, respectively, followed by the protocol developed previously for the isotopic analysis of proteiogenic amino acids36,51,52. In general, the biomass was hydrolyzed using 6 M HCl (20 h at 100 °C). The amino acids were derivatized in 50 μl of tetrahydrofuran and 50 μL of N-(tert-butyldimethylsilyl)-N-methyl-trifluoroacetamide (Sigma-Aldrich). A gas chromatograph (GC2010, Shimadzu) equipped with a SH-Rxi-5Sil column (Shimadzu) and a mass spectrometer (QP2010, Shimadzu) was used for analyzing the labeling profiles of metabolites. Three types of charged fragments were detected by GC-MS for Ala, Gly, Ser, Asp and Glu: the [M-57]+ group (containing unfragmented amino acids); and the [M-159]+ or [M-85]+ group (containing amino acids that had lost an α-carboxyl group). For each type of fragments, the labeling patterns were represented by M0, M1, M2, etc., which were fractions of non-labeled, singly labeled, and doubly labeled amino acids. The effects of natural isotopes on isotopic labeling patterns were corrected by previously reported algorithms53.
Data Measurement
The voltage of the MFC was recorded by a digital multimeter (2700, Keithley Instruments, Inc., Cleveland, OH, USA) with measurement interval of 2 min. The concentrations of formate and lactate at the beginning and the end of each cycle were measured by high-performance liquid chromatorgraphy (HPLC) (Shimadzu, Columbia, MD, USA) equipped with an Aminex HPX-87H column (Bio-Rad, Hercules, CA, USA) and refractive index detector (RID, 10A, Shimadzu), with the following program: column temperature, 65 °C; mobile phase, 0.5 mM sulfuric acid solution; flow rate, 0.6 mL/min. The electrolyte pH was measured by using a pH meter (Oakton Instruments, Vernon Hills, IL, USA).
Additional Information
How to cite this article: Luo, S. et al.13C Pathway Analysis for the Role of Formate in Electricity Generation by Shewanella Oneidensis MR-1 Using Lactate in Microbial Fuel Cells. Sci. Rep. 6, 20941; doi: 10.1038/srep20941 (2016).
Supplementary Material
Acknowledgments
S.L. was supported by a fellowship from Water INTERface IGEP, Graduate School of Virginia Tech. The MFC and isotopic analysis work was financially supported by faculty startup fund from Virginia Tech. Publishing costs were provided by Virginia Tech’s Open Access Subvention Fund.
Footnotes
Author Contributions S.L. and Z.H. designed and operated the MFC experiment; W.G. and X.F. provided the strains and conducted 13C isotope analysis; Z.H., X.F. and K.N. contributed to the planning and coordination of the project; all the authors discussed the results, wrote and edited the manuscript.
References
- McCarty P. L., Bae J. & Kim J. Domestic wastewater treatment as a net energy producer–can this be achieved? Environ. Sci. Technol. 45, 7100–7106 (2011). [DOI] [PubMed] [Google Scholar]
- Logan B. E. et al. Microbial fuel cells: Methodology and Technology. Environ. Sci. Technol. 40, 5181–5192 (2006). [DOI] [PubMed] [Google Scholar]
- Phuc Thi Ha Beomseok Tae & Chang I. S. Performance and Bacterial Consortium of Microbial Fuel Cell Fed with Formate. Energy Fuels 22, 164–168 (2008). [Google Scholar]
- Li W., Yu H. & He Z. Towards sustainable wastewater treatment by using microbial fuel cells-centered technologies. Energy Environ. Sci. 7, 911–924 (2013). [Google Scholar]
- Rosenbaum M. A. & Henrich A. W. Engineering microbial electrocatalysis for chemical and fuel production. Curr. Opin. Biotechnol. 29, 93–98 (2014). [DOI] [PubMed] [Google Scholar]
- Pant D. et al. Bioelectrochemical systems (BES) for sustainable energy production and product recovery from organic wastes and industrial wastewaters. RSC Adv. 2, 1248–1263 (2012). [Google Scholar]
- He Z. Microbial fuel cells: now let us talk about energy. Environ Sci Technol 47, 332–333 (2013). [DOI] [PubMed] [Google Scholar]
- Schroder U. Anodic electron transfer mechanisms in microbial fuel cells and their energy efficiency. Phys. Chem. Chem. Phys. 9, 2619–2629 (2007). [DOI] [PubMed] [Google Scholar]
- Dhere N. G. et al. Application of acetate, lactate, and fumarate as electron donors in microbial fuel cell. 8825, 1–7 (2013).
- Lovley D. R. The microbe electric: conversion of organic matter to electricity. Curr. Opin. Biotechnol. 19, 564–571 (2008). [DOI] [PubMed] [Google Scholar]
- Clauwaert P. et al. Minimizing losses in bio-electrochemical systems: the road to applications. Appl. Microbiol. Biotechnol. 79, 901–913 (2008). [DOI] [PubMed] [Google Scholar]
- Kim B. H., Chang I. S. & Gadd G. M. Challenges in microbial fuel cell development and operation. Appl. Microbiol. Biotechnol. 76, 485–494 (2007). [DOI] [PubMed] [Google Scholar]
- Fredrickson J. K. et al. Towards environmental systems biology of Shewanella. Nat. Rev. Microbiol. 6, 592–603 (2008). [DOI] [PubMed] [Google Scholar]
- Mordkovich N. N. et al. Effect of NAD+-dependent formate dehydrogenase on anaerobic respiration of Shewanella oneidensis MR-1. Microbiology 82, 404–409 (2013). [PubMed] [Google Scholar]
- Kouzuma A., Kasai T., Hirose A. & Watanabe K. Catabolic and regulatory systems in Shewanella oneidensis MR-1 involved in electricity generation in microbial fuel cells. Front Microbiol 6 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton G. J., Mori S., Nakamura R., Hashimoto K. & Watanabe K. Analyses of current-generating mechanisms of Shewanella loihica PV-4 and Shewanella oneidensis MR-1 in microbial fuel cells. Appl Environ Microbiol 75, 7674–7681 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsili E. et al. Shewanella secretes flavins that mediate extracellular electron transfer. Proceedings of the National Academy of Sciences 105, 3968–3973 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velasquez-Orta S. B. et al. The effect of flavin electron shuttles in microbial fuel cells current production. Appl. Microbiol. Biotechnol. 85, 1373–1381 (2010). [DOI] [PubMed] [Google Scholar]
- Roy J. N. et al. A study of the flavin response by Shewanella cultures in carbon-limited environments. RSC Advances 2, 10020–10027 (2012). [Google Scholar]
- Gorby Y. A. et al. Electrically conductive bacterial nanowires produced by Shewanella oneidensis strain MR-1 and other microorganisms. Proc Natl Acad Sci U S A 103, 11358–11363 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hau H. H. & Gralnick J. A. Ecology and biotechnology of the genus Shewanella. Annu. Rev. Microbiol. 61, 237–258 (2007). [DOI] [PubMed] [Google Scholar]
- Meshulam-Simon G., Behrens S., Choo A. D. & Spormann A. M. Hydrogen metabolism in Shewanella oneidensis MR-1. Appl. Environ. Microbiol. 73, 1153–1165 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretschger O. et al. Current production and metal oxide reduction by Shewanella oneidensis MR-1 wild type and mutants. Appl Environ Microbiol 73, 7003–7012 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y. J., Laidlaw D., Gani K. & Keasling J. D. Evaluation of the effects of various culture conditions on Cr(VI) reduction by Shewanella oneidensis MR-1 in a novel high-throughput mini-bioreactor. Biotechnol. Bioeng. 95, 176–184 (2006). [DOI] [PubMed] [Google Scholar]
- Hunt K. A., Flynn J. M., Naranjo B., Shikhare I. D. & Gralnick J. A. Substrate-level phosphorylation is the primary source of energy conservation during anaerobic respiration of Shewanella oneidensis strain MR-1. J Bacteriol 192, 3345–3351 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinchuk G. E. et al. Genomic reconstruction of Shewanella oneidensis MR-1 metabolism reveals a previously uncharacterized machinery for lactate utilization. Proceedings of the National Academy of Sciences 106, 2874–2879 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinchuk G. E. et al. Pyruvate and lactate metabolism by Shewanella oneidensis MR-1 under fermentation, oxygen limitation, and fumarate respiration conditions. Appl Environ Microbiol 77, 8234–8240 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin M., Molitor H., Brazil B., Novak J. T. & He Z. Recovery of nitrogen and water from landfill leachate by a microbial electrolysis cell-forward osmosis system. Bioresour Technol 200, 485–492 (2015). [DOI] [PubMed] [Google Scholar]
- Ge Z., Ping Q., Xiao L. & He Z. Reducing effluent discharge and recovering bioenergy in an osmotic microbial fuel cell treating domestic wastewater. Desalination 312, 52–59 (2013). [Google Scholar]
- Ge Z., Ping Q. & He Z. Hollow-fiber membrane bioelectrochemical reactor for domestic wastewater treatment. J. Chem. Technol. Biotechnol. 88, 1584–1590 (2013). [Google Scholar]
- Watson V. J. & Logan B. E. Power production in MFCs inoculated with Shewanella oneidensis MR-1 or mixed cultures. Biotechnol. Bioeng. 105, 489–498 (2010). [DOI] [PubMed] [Google Scholar]
- Mao L. & Verwoerd W. S. Theoretical exploration of optimal metabolic flux distributions for extracellular electron transfer by Shewanella oneidensis MR-1. Biotechnology for biofuels 7, 1–20 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng X., Zhuang W. Q., Colletti P. & Tang Y. J. Metabolic pathway determination and flux analysis in nonmodel microorganisms through 13C-isotope labeling. Methods Mol Biol 881, 309–330 (2012). [DOI] [PubMed] [Google Scholar]
- Zhuang L., Guo W., Yoshida M., Feng X. & Goodell B. Investigating Oxalate Biosynthesis in Wood-decaying Fungus Gloeophyllum trabeum using 13C Metabolic Flux Analysis. RSC Advances. In press (2015). [Google Scholar]
- Tang Y. J. et al. Invariability of central metabolic flux distribution in Shewanella oneidensis MR-1 under environmental or genetic perturbations. Biotechnol. Progr. 25, 1254–1259 (2009). [DOI] [PubMed] [Google Scholar]
- Guo W., Luo S., He Z. & Feng X. 13C pathway analysis of biofilm metabolism of Shewanella oneidensis MR-1. RSC Adv. 5, 39840–39843 (2015). [Google Scholar]
- Kim J. R., Cheng S., Oh S.-E. & Logan B. E. Power generation using different cation, anion, and ultrafiltration membranes in microbial fuel cells. Environ. Sci. Technol. 41, 1004–1009 (2007). [DOI] [PubMed] [Google Scholar]
- Freguia S., Rabaey K., Yuan Z. & Keller J. Electron and carbon balances in microbial fuel cells reveal temporary bacterial storage behavior during electricity generation. Environ. Sci. Technol. 41, 2915–2921 (2007). [DOI] [PubMed] [Google Scholar]
- Freguia S., Rabaey K., Yuan Z. & Keller J. Non-catalyzed cathodic oxygen reduction at graphite granules in microbial fuel cells. Electrochim. Acta 53, 598–603 (2007). [Google Scholar]
- Ahn Y. & Logan B. E. Saline catholytes as alternatives to phosphate buffers in microbial fuel cells. Bioresour Technol 132, 436–439 (2013). [DOI] [PubMed] [Google Scholar]
- Bandarenka A. S., Ventosa E., Maljusch A., Masa J. & Schuhmann W. Techniques and methodologies in modern electrocatalysis: evaluation of activity, selectivity and stability of catalytic materials. Analyst 139, 1274–1291 (2014). [DOI] [PubMed] [Google Scholar]
- Neurock M., Janik M. & Wieckowski A. A first principles comparison of the mechanism and site requirements for the electrocatalytic oxidation of methanol and formic acid over Pt. Faraday Discuss. 140, 363–378 (2009). [DOI] [PubMed] [Google Scholar]
- Chen Y. X., Heinen M., Jusys Z. & Behm R. J. Kinetic isotope effects in complex reaction networks: formic acid electro-oxidation. Chemphyschem 8, 380–385 (2007). [DOI] [PubMed] [Google Scholar]
- Lanthier M., Gregory K. B. & Lovley D. R. Growth with high planktonic biomass in Shewanella oneidensis fuel cells. FEMS Microbiol. Lett. 278, 29–35 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y. et al. Physiological and electrochemical effects of different electron acceptors on bacterial anode respiration in bioelectrochemical systems. Bioresour Technol 164, 270–275 (2014). [DOI] [PubMed] [Google Scholar]
- Tang Y. J. et al. Invariability of central metabolic flux distribution in Shewanella oneidensis MR-1 under environmental or genetic perturbations. Biotechnol. Prog. 25, 1254–1259 (2009). [DOI] [PubMed] [Google Scholar]
- Tang Y. J., Meadows A. L., Kirby J. & Keasling J. D. Anaerobic central metabolic pathways in Shewanella oneidensis MR-1 reinterpreted in the light of isotopic metabolite labeling. J Bacteriol 189, 894–901 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang V. B. et al. Metabolite-enabled mutualistic interaction between Shewanella oneidensis and Escherichia coli in a co-culture using an electrode as electron acceptor. Sci. Rep. 5, 11222 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinchuk G. E. et al. Constraint-based model of Shewanella oneidensis MR-1 metabolism: a tool for data analysis and hypothesis generation. PLoS Comput. Biol. 6, e1000822 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brutinel E. D. & Gralnick J. A. Shuttling happens: soluble flavin mediators of extracellular electron transfer in Shewanella. Appl. Microbiol. Biotechnol. 93, 41–48, (2012). [DOI] [PubMed] [Google Scholar]
- Feng X. & Zhao H. Investigating xylose metabolism in recombinant Saccharomyces cerevisiae via 13C metabolic flux analysis. Microb Cell Fact 12, 1–12 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng X. et al. Characterization of the central metabolic pathways in Thermoanaerobacter sp. strain X514 via isotopomer-assisted metabolite analysis. Appl. Environ. Microbiol. 75, 5001–5008 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl S. A., Dauner M. & Wiechert W. New tools for mass isotopomer data evaluation in 13C flux analysis: mass isotope correction, data consistency checking, and precursor relationships. Biotechnol. Bioeng. 85, 259–268 (2004). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


