Abstract
The Red River is the second largest river in Viet Nam and constitutes the main water source for a large percentage of the population of North Viet Nam. Here we present the results of an annual survey of Escherichia coli (EC) and Total Coliforms (TC) in the Red River basin, North Viet Nam. The objective of this work was to obtain information on faecal indicator bacteria (FIB) numbers over an annual cycle and, secondly, to determine the die-off rates of these bacterial indicators. Monthly observations at 10 stations from July 2013–June 2014 showed that TC and EC reached as high as 39100 cfu (colony forming units) 100 ml−1 and 15300 colonies 100 ml−1, respectively. We observed a significant seasonal difference for TC (p < 0.05) with numbers being higher during the wet season. In contrast, no significant seasonal difference was found for EC. The FIB die-off rates ranged from 0.01 d−1 to a maximum of 1.13 d−1 for EC and from 0.17 d−1 to 1.33 d−1 for TC. Die-off rates were significantly higher for free bacteria than for total (free + particle attached) bacteria, suggesting that particle attachment provided a certain level of protection to FIB in this system.
Biological contamination of aquatic systems by water borne pathogens from untreated wastewater and agricultural effluent is a globally important water quality problem1. However, it is particularly problematic in tropical regions where a large proportion of the developing world is located. On a global scale, it is estimated that 88% of diarrheal diseases are due to the use of unclean water sources, leading to the deaths of 1.8 million people annually, most of whom are children in developing countries2. Indeed, in many developing countries, surface water (e.g. rivers and stagnant ponds) subject to wastewater contamination is often used for domestic purposes as access to uncontaminated water is limited3. Therefore, considering the high death rates as well as the large economic burden associated with the construction and maintenance of water treatment plants, having an understanding of the spatial distribution and temporal variability of the microbial pathogens responsible for these diseases is essential. Furthermore, understanding the factors that control their distribution is a prerequisite for reducing the human health risks associated with the use of unclean water. This is particularly important in tropical areas where there is a paucity of data, where population growth is high, and where populations are the most exposed to these contaminants1,3.
Rivers are a major source of fresh water for irrigation, industry and domestic water requirements. However, many tropical rivers have been adversely affected by human activities, such as industrialization, urbanization and agricultural intensification4,5. Although the chemical contamination of water bodies has been documented in many tropical systems6,7, the extent of biological contamination from untreated wastewater and animal husbandry is often unknown. This is despite the fact that detailed knowledge on the range and origin of microbial pollution is required for watershed management in order to provide safe water for human demands.
The Red River is the second largest river in Viet Nam, after the Mekong River, and one of the five largest rivers in East Asia8. Over 24 million inhabitants live in the Red River basin, including over 17 million people in its delta. This area is also characterized by several large industrial zones and by a large number of craft villages that are considered hotspots of biological and chemical contamination9. The Red River Delta (RRD) is the second most important rice-producing area in Viet Nam and accounts for 20% of the national production. It is also the main freshwater source for the surrounding areas as well as being the major outlet for wastewater10,11. According to the Viet Nam Environment Administration Report 2012, the urban area of the RRD concentrates 24% of the national production of domestic wastewater. It also receives the second largest proportion of industrial wastewater in the country after that of the South East region around Ho Chi Minh City. Despite the high proportions of wastewater that are released into the Red River on a daily basis, little information exists in the published literature on microbial or faecal contamination levels in this semi-tropical region.
Faecal indicator bacteria (FIB) are used to monitor faecal contamination levels and hence the possibility of pathogens of faecal origin in soils and water in both tropical and temperate systems12,13. FIB is a generic term for a range of bacteria that inhabit the gastrointestinal tract of homoeothermic animals. This group includes Escherichia coli, Salmonella spp., Enterococcus spp., and the coliforms. We hypothesized that FIB numbers would increase along the river length as a consequence of the increasing industrialization and urbanization in the downstream sections. Here we present the results of an annual survey of FIB at ten stations along the Red River, North Viet Nam. The objective of this work was to obtain information on FIB concentrations over one annual cycle and to identify the environmental factors controlling FIB numbers and to determine their die-off rates.
Materials and Methods
Study site
The Red River basin has an area of about 156 451 km2 of which 51.2% is in Viet Nam, 47.9% in China and 0.9% in Laos14. The basin is subject to a semi-tropical climate with two clear seasons. The wet season persists from May to October during which 80–90% of the total annual rainfall of 1900 mm occurs15. The cooler, dry season persists from November to April. Mean monthly temperatures are lowest in January, with June-August being generally the hottest. In general, temperature is relatively uniform across the basin and the mean relative humidity is greater than 80%16. Concomitant with the highest rainfall, discharge and suspended load peak during August in the middle of the wet season14.
Samples were collected monthly from July 2013 to June 2014 at 10 stations (total of 120 samples) in the Red River Basin. The sample sites are located on different river branches (distributaries) of the Red River system and include, from upstream to downstream, Yen Bai (Thao River), Hoa Binh (Da River), Vu Quang (Lo River), Gian Khau (Day river), Truc Phuong (Ninh Co River), Quyet Chien (Tra Ly River), Nam Dinh (Dao River) and Son Tay, Ha Noi and Ba Lat on the main axe of the Red River (Table 1).
Table 1. Location and characteristics of the surrounding areas at the 10 stations.
| Station | Sampling location | River | Characteristics | |
|---|---|---|---|---|
| Latitude | Longitude | |||
| Yen Bai | 21°42' | 104°53' | Thao river (upstream Red River) | Opposite a traditional brick factory that uses coal and mud and upstream of the agro-industrial processing zones of Van Yen (40 km) and the Tran Yen urban district (14 km). The sample was collected at 80 m from the bank. Water depth at this site is 5 m. |
| Vu Quang | 21°34' | 105°15' | Lo river | Low population area. The sample was collected at 130 m from the bank. Water depth is 17 m. |
| Hoa Binh | 20°49' | 105°19' | Da river | Site is 5.5 km upstream of the Hoa Binh hydroelectric dam. Sample was collected at 300 m from the bank. Water depth at this site is 10 m and the river banks are formed of weathered rock. |
| Son Tay | 21°09' | 105°52' | Red river | Site is 300 m upstream of the Son Tay coal ports. Sample was collected at 60 m from the bank, water depth 12 m. |
| Ha Noi | 21°02' | 105°51' | Red river | Site is 50 m downstream of the Chuong Duong bridge in Hanoi city. Sample was collected at 10m from the bank, water depth was 1m. This station is downstream of the confluence of the Da, Thao and Lo rivers. |
| Gian Khau | 20°19' | 105°55' | Day river | Site is 2 km from the Gian Khau industrial zone and Visai coal clinker port. Sample was collected at 38 m from the bank. This station is in a peri-urban area of the Red River delta. |
| Quyet Chien | 20°30' | 106°15' | Tra Ly river | Site is 2 km upstream from poultry and fish farms. Sample was collected at 35 m from the left bank, water depth was 7 m. |
| Nam Dinh | 20°25' | 106°10' | Dao river | Site is opposite a factory that produces construction materials. Sample was collected at 100 m from the left bank, water depth was 5 m. |
| Truc Phuong | 20°19' | 106°16' | Ninh Co river | Site is 7 km upstream of Nam Dinh city from which it receives sewage. Traditional silk spinning villages near the river release effluent containing silk chemicals and silkworm cocoon waste. Sample was collected at 100 m from the bank. |
| Ba Lat | 19°30' | 106°00' | Red river | Site is 7 km downstream of the Ba Lat seaport. Sample was collected at 300 m from the bank, water depth was 8 m. Site is under tidal influence. |
River depth (m) at the sampling site is also provided. All samples were collected from the surface layer as grab samples.
Sample collection
At each sample site, 1500 ml of river water was collected with a plastic bottle that had been acid washed (10% HCl), rinsed copiously with distilled water and dried. The samples were stored in a cooler and returned to the laboratory for processing. The sample was used to measure dissolved oxygen (DO), pH, conductivity, temperature, salinity, total suspended solids (TSS), total phosphorus (TP), dissolved inorganic phosphate (PO4), ammonia-nitrogen (NH3-N) and free and attached FIB.
Die-off rates
At four stations, a second series of samples was collected in the same way for the determination of FIB die-off rate over time. These stations were (1) Yen Bai, located in the upstream main branch of the Red River known as the Thao River; (2) Ha Noi, after the confluence of three major upstream tributaries of the Da, Thao and Lo rivers; (3) Gian Khau, a peri-urban river system located in the Red River Delta and, (4) Truc Phuong, located in the downstream Red River on the Ninh Co River. These four stations were chosen to give a good representation of the land uses and population densities in the basin and to provide a good geographical separation over the area. For each station, duplicate 750 ml samples were incubated for five days in glass bottles in the dark and at the in situ temperature of the Hanoi station (17–29 °C). This temperature was selected for two reasons. The first being that only one incubator was available for the experiment and the second being that temperature at the Hanoi station was close to the average of the temperatures at the other stations. For the estimation of die-off rates, samples were collected from the incubations every day during 5 days (T0, T1, T2, T3, T4, T5) to determine the decrease in FIB numbers for both total and free bacteria using the method described below.
Analytical methods
Temperature, dissolved oxygen (DO), pH, and total suspended solids (TSS) were measured using a water quality probe WQC-22 A (TOA, Japan) and conductivity (Cond) was determined using a conductivity meter (Hach, USA) immediately upon sampling. Nutrients (N, P, Si) were spectrophotometrically determined on a Drell 2800 (HACH, USA) in the laboratory according to APHA17 methods.
FIB abundance (free and attached) was measured by a direct count method using 3 M Petrifilm™ E.coli/Coliform Count Plate (Petrifilm EC plate), which contain Violet Red Bile (VRB) nutrients. E.coli (EC) produces beta-glucuronidase, which produces a blue precipitate and Total coliform (TC) colonies growing on the Petrifilm EC plate produce acid and the colonies are denoted by dark red points. This method has been validated by the APHA and is a technique commonly used for coliform and EC counts18,19.
For the total counts (free + attached bacteria; ECtot and TCtot), 1 ml was removed from each sample (or incubation) after shaking to ensure an even distribution of bacteria, the sample was then aseptically delivered to the center of a Petrifilm EC plate. The water sample was then left to stand for 1 h and a second 1 ml aliquot was inoculated onto a second Petrifilm EC plate to estimate the number of free EC (ECfree or TCfree; i.e. non-sedimented). The Petrifilm EC plates were then incubated at 37 °C for 24 hours, using a Fukusima incubator (Japan). The number of colonies (EC and TC) was determined using a Colony Counter CL-560 (Sibata, Japan). To facilitate the comparison of our data with that of previously published data and with water quality limits, we express our data as the number of colonies per 100 ml of sample.
The number of attached EC or TC (ECatt or TCatt) is determined from the difference between ECtot (or TCtot) and ECfree (or TCfree) as:
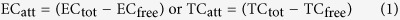 |
and the percent of attached EC or TC (%ECatt or %TCatt) calculated as:
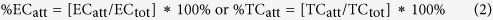 |
The die-off rates of TC and EC were estimated by fitting an exponential equation to bacterial abundances measured over time. The equations were expressed as first order decay in the general form of:
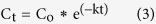 |
where Ct = is the number of EC or TC at elapsed time t, Co is the initial number of EC or TC per ml, k is the decay constant in day−1 and t is the elapsed time in days. As in several incubations complete die-off was observed by day 4, k is calculated for the first 4 days for all incubations to ensure comparability between dates and stations. The k value was determined for both the free and total fractions (e.g. ECfree, TCfree, ECtot, and TCtot).
All statistical analyses were performed with XLSTAT (v. 2014). Pearson’s correlation was used to test the relationships between environmental variables and FIB. Wilcoxon’s non-parametric test was used to test for significant differences between variables and the Kruskal-Wallace test was used to test differences between stations and season as the data were non-normally distributed even after normalization. When statistical relationships concerning FIB number were tested, log(EC) or log(TC) was used. When a significant difference was observed, an a posteriori Dunn’s all-pairwise test was used and significance is determined as p < 0.05.
Results
Physico-chemical variables
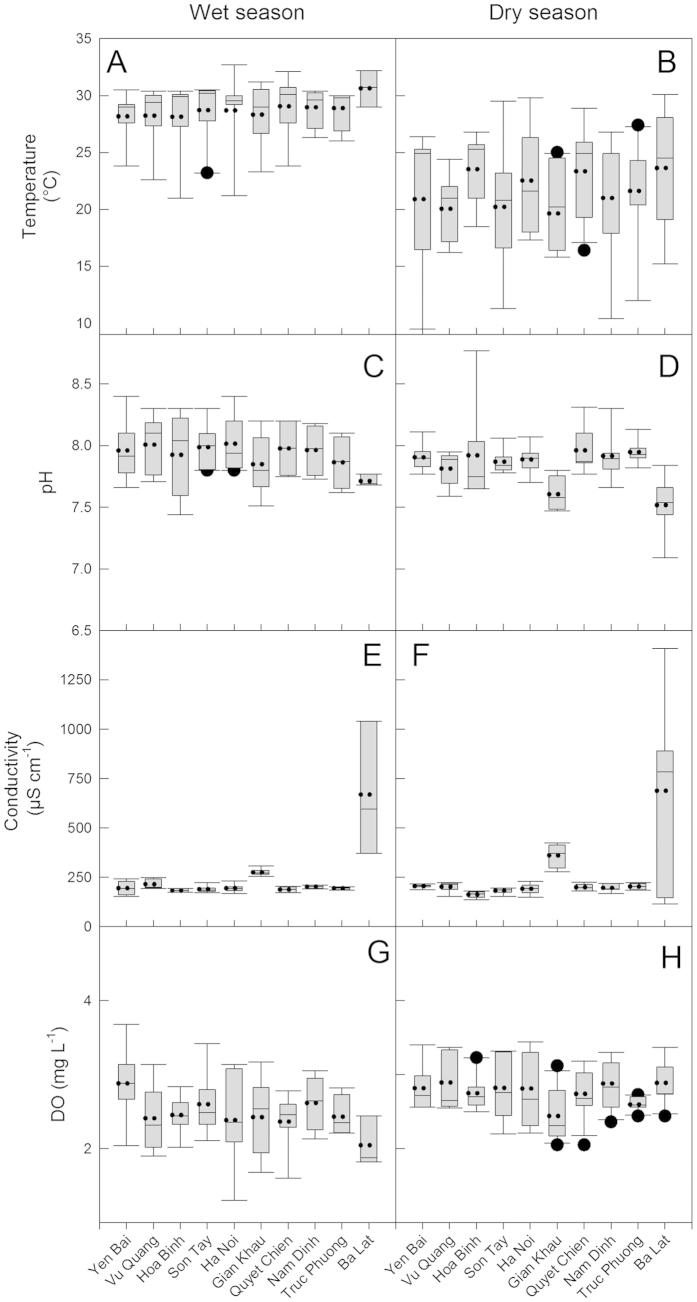
The variability of the physico-chemical parameters observed in the ten stations for the wet and dry seasons are presented in Figs 1 and 2. Temperature varied between 9.5 and 35° C and was significantly higher during the wet season (p < 0.0001). Temperature was generally highest at Ba Lat and lowest at Gian Khau.
Figure 1. Box plots of temperature, pH, conductivity and DO concentrations for each station for the wet (May to October) and the dry (November to April) seasons for the study period (July 2013 to June 2014).
Left side panels are for the wet season and the right hand side panels are for the dry season. Panels (A,B): Temperature, panels (C,D): pH, panels (E,F): conductivity and panels (G,H): DO. Mean (dotted line), median (solid line) and whiskers (error bars) above and below the box indicating the 90th and 10th percentiles are shown.
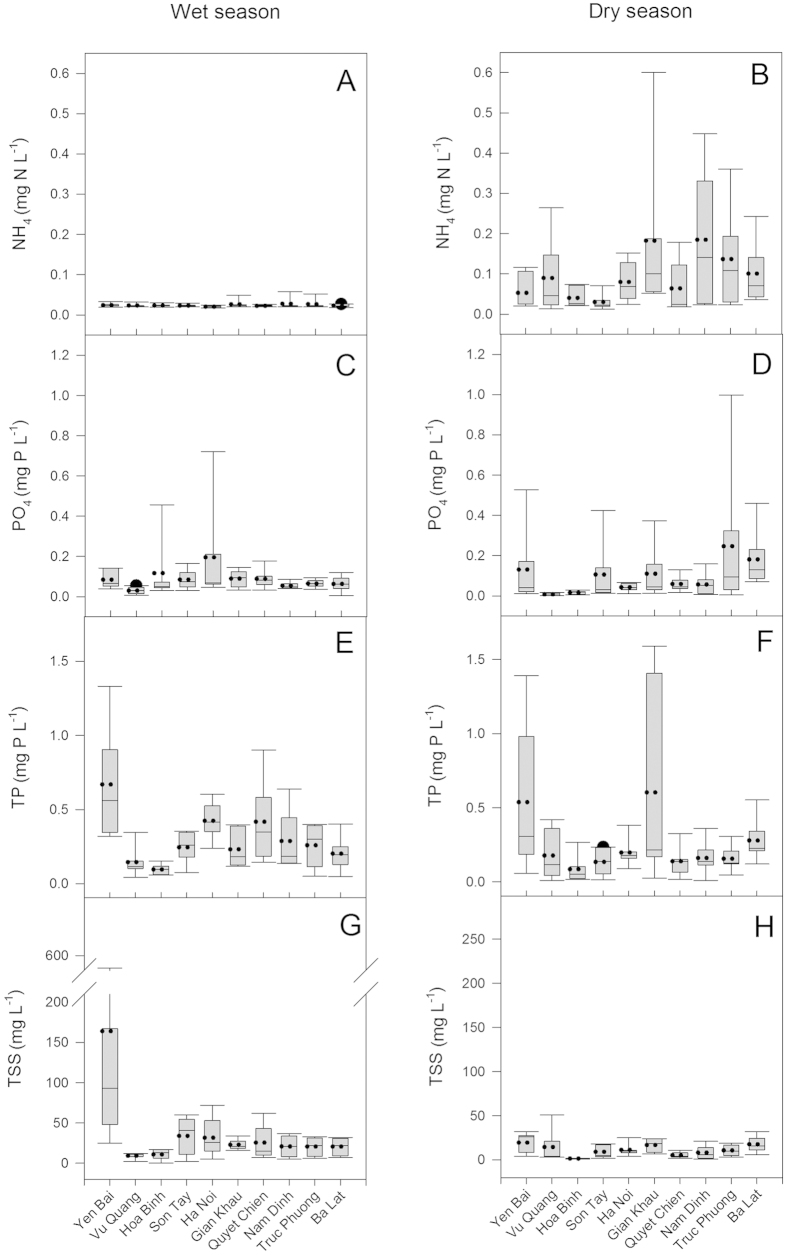
Figure 2. Box plots of NH4, PO4, TP and TSS concentrations for each station for the wet (May to October) and the dry (November to April) seasons for the study period (July 2013 to June 2014).
Left side panels are for the wet season and the right hand side panels are for the dry season. Panels (A,B): NH4, panels (C,D): PO4, panels (E,F): TP and panels (G,H): TSS. Mean (dotted line), median (solid line) and whiskers (error bars) above and below the box indicating the 90th and 10th percentiles are shown.
The pH ranged from ranged 7.1 to 8.8, with the lowest values observed at the downstream Ba Lat station and the highest at upstream Hoa Binh station, however, no seasonal differences were observed. Salinity was almost 0 at all stations during the entire year; the only exception was during March at the most downstream station (Ba Lat, located at the river mouth and under tidal influence) where a salinity of 8.5 was observed (data not shown). The highest conductivities were also observed at this station at this time (1410 μScm−1). At the other stations, conductivity varied between 136–423 μScm−1 (Fig. 1) and had no significant seasonal pattern.
Concentrations of NH4, PO4 and TP are shown in Fig. 2. Ammonium varied from 0.01 to 0.6 mg N l−1 and was significantly higher during the dry season (November – April) at low dilution and in the downstream stations (Gian Khau, Nam Dinh, Truc Phuong and Ba Lat) of the highly populated delta area. Similar to NH4, the PO4 tended to be higher during the dry season however the difference was not significant. TSS was significantly higher during the wet season, when particle load is higher (p < 0.0001). Overall, TSS concentrations were significantly higher at the upstream Yen Bai station (p < 0.03) than at the other stations. As for TSS, TP was significantly higher during the wet season (p < 0.012). High concentrations of TP were found at Yen Bai and Gian Khau during the dry season (January) when values of up to 1.39 and 1.59 mg PO4 l−1 were observed.
FIB abundance: monthly observation
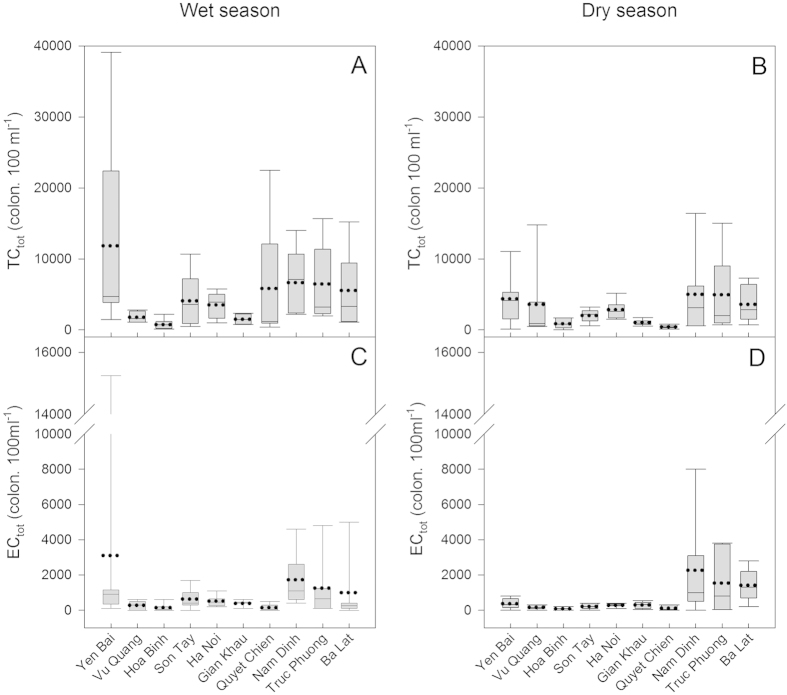
The ECtot, and TCtot numbers for each season at the ten different stations are shown in Fig. 3 and Table S1 (Supplementary Materials). The number of TCtot varied between 0 colonies 100 ml−1 at Hoa Binh in January to over 39100 colonies per 100 ml−1 at Yen Bai in September. During the wet season, TCtot at Yen Bai, the most upstream station, was 9783 ± 14431 (mean ± standard deviation) colonies 100 ml−1 as compared to 4383 ± 3797 colonies 100 ml−1 for this station during the dry season, with no significant difference between these two seasons. For the other upstream tributaries, relatively low mean TCtot numbers were observed during the wet season (1850 ± 715 and 800 ± 789 colonies 100 ml−1 for Hoa Binh and Vu Quang, respectively. Mean values in the downstream delta stations were 6066 ± 4506, 5408 ± 5379 and 6050 ± 5469 colonies 100 ml−1 for Nam Dinh, Truc Phuong and Ba Lat, respectively. The overall pattern was similar for the dry season, i.e. higher mean values at Yen Bai and in downstream delta stations such as the peri-urban Nam Dinh station where a seasonal average of 5016 ± 5840 colonies 100 ml−1 was found. When the data from all of the stations were combined TCtot exhibited a significant seasonal difference with lower numbers during the dry season as compared to the wet season (Fig. 3; p = 0.042).
Figure 3. Box plots of the number colonies of TCtot and ECtot for the wet season (left hand side) and dry season (right hand side) for the ten stations.
Panels (A,B): TCtot, panels (C,D): ECtot. Mean (dotted line), median (solid line) and whiskers (error bars) above and below the box indicating the 90th and 10th percentiles are shown.
ECtot numbers were significantly lower than TCtot (p < 0.05) during both seasons at all of the stations. As for TCtot, low numbers of ECtot were found in the upstream tributaries during wet season (283 ± 248 and 150 ± 234 colonies 100 ml−1 at Vu Quang and Hoa Binh, respectively), and high numbers were observed at Yen Bai (3108 ± 5960 colonies 100 ml−1). The more anthropogenically impacted downstream stations of the delta had high values during wet season (e.g. 1733 ± 1632 colonies 100 ml−1 at Nam Dinh). During the dry season, a similar pattern was observed with high mean ECtot numbers at Nam Dinh (2266 ± 3007 colonies 100 ml−1) and low values in the upstream stations (167 ± 122, 83 ± 75.7 colonies 100 ml−1 for Vu Quang and Hoa Binh, respectively). Regarding the dataset as a whole, there was no significant difference between the wet and dry seasons for ECtot.
Particle attached FIB
The percentage of ECatt and TCatt varied greatly between station (Fig. 4, Table S1). For example, the percentage of ECatt varied between 9.7% and 100% with a mean ( ± se) of 46.5 ± 2.9% (Table S1). The percentage of TCatt was also highly variable (2.7–100%) with a mean 34.8 ± 1.8% for the whole data set combined. The number of ECatt or TCatt was significantly lower than for ECfree or TCfree when the entire dataset was examined (p < 0.05).
Figure 4. Percentage TCatt and ECatt for each of the 10 stations.
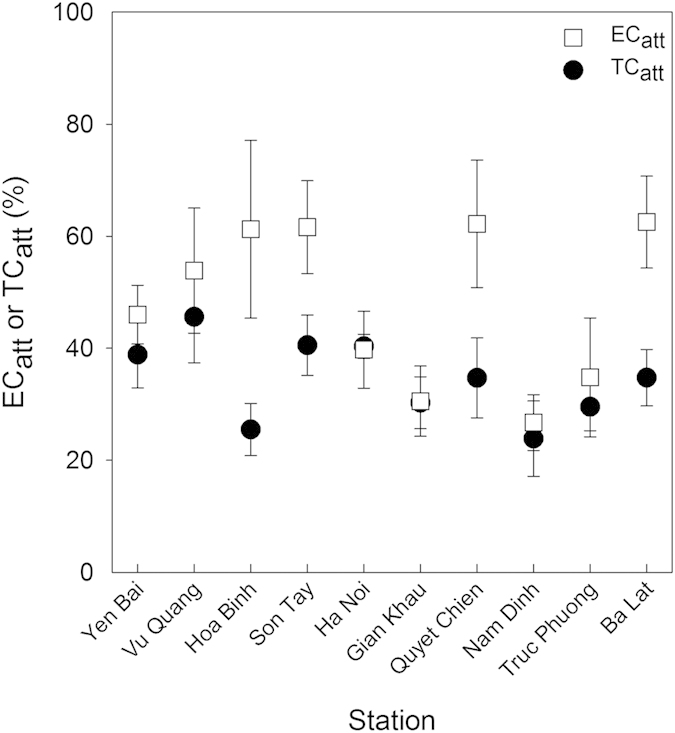
The mean and standard error for each station are given. Filled circles: TCatt, open squares: ECatt.
The number of ECatt differed significantly between station (p < 0.05) and was highest at Yen Bai (4900 cells 100 ml−1) and lowest at Hoa Binh (100 colonies 100 ml−1; Table S1), similar to what was observed for the total number (free + attached). As with ECatt, the number of TCatt differed significantly between station. TCatt was highest at Yen Bai (13050 colonies 100 ml−1) and the lowest at Hoa Binh (0 colonies 100 ml−1). The numbers of free and attached TC and EC were positively correlated with each other and had correlation coefficients of 0.73 and 0.81, respectively. This indicates that attached and free TC and EC increased concomitantly even though the actual numbers were different.
Die-off rates
The die-off rates of EC and TC calculated over 4 days are shown in Table 2. In general, die-off rates for TCtot were significantly higher than for ECtot. For example, at Yen Bai in August, the die-off rate for TCtot was 1.33 d−1 compared to 0.50 d−1 for ECtot and 1.03 d−1 and 0.43 d−1 TCtot and ECtot respectively, at Truc Phuong during February. It is worth mentioning again the specific behavior of FIB at Yen Bai. Along with the high TC and EC numbers at this station, TCtot die-off rates were higher than at the other three stations examined e.g. 1.33 d−1 as compared to 0.70 d−1, 1.03 and 0.42 d−1 for Hanoi, Truc Phuong and Gian Khau, respectively. Similarly, ECtot die-off rates at Yen Bai were also significantly higher (p < 0.05) than those observed for the three other stations. However, and in contrast to TCfree, we observed a significant station effect for ECfree with the rates found at Yen Bai and Truc Phuong being significantly higher than those found at the two other stations, Hanoi and Gian Khau. Overall, die-off rates were significantly higher for TCfree than for TCtot (p < 0.05) when the whole data set was examined. In contrast, the opposite was true for ECtot and ECfree (p < 0.05).
Table 2. Average (±se) die-off rates for ECtot and ECfree and TCtot and TCfree (k, d−1) in the Red river basin.
| Decay rate(d–1) | Yen Bai |
Ha Noi |
Gian Khau |
Truc Phuong |
||||
|---|---|---|---|---|---|---|---|---|
| ave | se | ave | se | ave | se | ave | se | |
| k ECtot | 0.59Bab | 0.31 | 0.31Aa | 0.13 | 0.36Aab | 0.06 | 0.60Ba | 0.21 |
| k ECfree | 0.50BCa | 0.28 | 0.25Aa | 0.10 | 0.25ABa | 0.09 | 0.54Ca | 0.229 |
| k TCtot | 0.85Bb | 0.21 | 0.57ABab | 0.21 | 0.47Abc | 0.18 | 0.55Aa | 0.35 |
| k TCfree | 0.77Aab | 0.24 | 0.90Ab | 0.13 | 0.79Ac | 0.20 | 0.84Aa | 0.21 |
The values were calculated for the first 4 days of the incubation. Different capital letters indicate a significant difference between station and different lowercase letters indicate a significant difference between decay rates of ECtot, ECfree,TCtot and TCfree within the same station.
Relationships between FIB and environmental factors
When the data set was regarded either as a whole or by station, EC and TC (free, attached and total) numbers were only positively correlated with TSS and TP (p < 0.05; Table 3). No other significant relationship was found between EC or TC and the measured environmental variables (pH, DO, NH4, conductivity, or PO4).
Table 3. Pearson’s correlation matrix for the environmental variables and FIB.
| Variables | ECfree log(col.100 ml−1) | ECatt log(col.100 ml−1) | ECtot log(col.100 ml−1) | TCfree log(col.100 ml−1) | TCatt log(col.100 ml−1) | TCtot log(col.100 ml−1) |
|---|---|---|---|---|---|---|
| pH | −0.112 | −0.168 | −0.170 | −0.017 | 0.039 | 0.001 |
| T (°C) | 0.147 | 0.017 | 0.054 | 0.196 | 0.137 | 0.176 |
| DO (mg l−1) | 0.060 | 0.006 | 0.039 | 0.024 | 0.095 | 0.042 |
| Cond (μs cm−1) | 0.093 | 0.151 | 0.123 | 0.012 | 0.108 | 0.038 |
| TSS (mg l−1) | 0.238 | 0.224 | 0.216 | 0.247 | 0.236 | 0.293 |
| NH4 (mg l−1) | −0.008 | 0.026 | −0.016 | 0.100 | −0.090 | 0.098 |
| PO4 (mg l−1) | −0.081 | −0.260 | −0.169 | −0.314 | −0.011 | −0.251 |
| TP (mg l−1) | 0.252 | 0.230 | 0.222 | 0.270 | 0.262 | 0.322 |
The correlation was performed with the log(TC or EC) values.
Values in bold indicate significance at p < 0.05.
As for TCtot and ECtot numbers, the die-off rates of both TCfree and ECfree and TCtot and ECtot were uncorrelated with temperature, DO, NH4 or conductivity (Table 4). However, ECtot die-off rate was significantly correlated with TP and TSS. No significant correlations were observed for TCtot and TCfree and TSS.
Table 4. Pearson’s correlation matrix for the environmental variables and k(d−1) for the free and total (attached + free) TC and EC.
| Variables | ECtot die-off k (d−1) | ECfree die-off k (d−1) | TCtot die-off k (d−1) | TCfree die-off k (d−1) |
|---|---|---|---|---|
| pH | 0.078 | 0.078 | 0.043 | 0.096 |
| T (°C) | 0.079 | 0.070 | 0.046 | 0.256 |
| DO (mg l−1) | 0.181 | 0.160 | 0.014 | 0.082 |
| Cond (μs cm−1) | −0.060 | −0.213 | −0.158 | −0.086 |
| TSS (mg l−1) | 0.405 | 0.213 | 0.195 | 0.025 |
| NH4 (mg l−1) | −0.024 | −0.072 | 0.208 | −0.208 |
| PO4 (mg l−1) | −0.130 | −0.123 | −0.112 | 0.065 |
| TP (mg l−1) | 0.424 | 0.223 | 0.272 | −0.059 |
The k(d−1) was determined over 4 days.
Values in bold indicate significance at p < 0.05.
Discussion
Distribution of FIB
Land use affects the abundance and distribution of FIB in both tropical20,21 and temperate regions22,23. It is also probably the case in the Red River system. In general, mean FIB numbers (EC, TC) in the upstream stations (e.g. Vu Quang, Hoa Binh, Son Tay) were lower than the downstream stations (Nam Dinh, Truc Phuong and Ba Lat). Of the ten stations investigated, the upstream Hoa Binh station on the Da River had the lowest FIB levels. The Da River sub-basin is primarily forest (74% of total sub-basin area) and population density is sparse (<100 people per km−2) and this probably explains the low FIB numbers at this station. In contrast, Yen Bai on the Thao River has the highest and most variable levels of contamination, despite being upstream. The Thao River basin has a relatively high population density (190 people km−2) and a large proportion of the total sub-basin area (33%) is used for agriculture and livestock grazing. Livestock are known to be an important source of FIB contamination in streams and rivers20 and this along with non-treated human wastewater from the surrounding population may well explain the high and variable FIB numbers observed at this site during both seasons. Furthermore, the Van Yen industrial zone which processes agricultural products is located in the upstream Thao River and this is probably also a source of FIB. Indeed, the high and variable FIB numbers observed during the wet season also suggest that FIB originated mostly from diffuse runoff sources.
The higher FIB abundances observed in the downstream stations (Nam Dinh, Ba Lat and Truc Phuong) are also probably related to the surrounding land use. These three sites are in peri-urban areas of the delta with high population densities (1200 people km−2 compared to 100–150 people km−2 in the upstream basin14), agriculture and industry all of which release untreated wastewater into the system. Although the Hanoi station is located in the city and the FIB numbers are relatively low (Fig. 3), they are still above the acceptable limits for individual, non-commercial water supplies in Viet Nam (20 EC colonies 100 ml−1 and 150 TC colonies 100 ml−1)24. Domestic wastewater from Hanoi city is mostly discharged into small urban rivers as shown by the high ammonium concentrations in the To Lich and Nhue Rivers10,25,26 and other work from the urban rivers in Hanoi city has reported very high TC numbers. For example, TC numbers at Lien Mac, at Phu Van Bridge (Nhue River) and at Thanh Liet Dam (To Lich river) were 7820, 9820 and 148480 MPN 100 ml−1, respectively27 meaning that the water at these locations is considered as being unfit even for irrigation due to its high FIB load.
Free and attached FIB
Understanding the relative numbers of attached and free FIB and their differential fate allows us to better estimate the time these indicator organisms remain viable in the environment. In soils and sediments, bacteria tend to be associated with particles as opposed to in the free-state e.g.28 whereas in aquatic systems the percentages of particle associated bacteria are highly variable and can range from 10% in clear waters with very low organic particle loads to over 70% in estuaries with high particle loads29,30. Suter et al.31 have shown that high proportions of FIB are associated with particles (52.9% ± 20.9% and over 90% in some areas) and that these values were related to turbidity levels.
In our work, free-living bacteria generally predominated (only 36% ± 20% of TC were particle attached and 50% ± 26.9% of EC particle attached). However, the %ECatt and %TCatt were highly variable (7.8% to 100% and 2.7% to 80% for ECatt and TCatt, respectively). Previous studies from temperate systems have indicated that the attachment to particles by FIB in the aquatic environment is influenced by various factors, including temperature, bacterial genotype, soil particle size, organic matter, pH, ionic strength, dissolved nutrients and turbidity32,33. We also found significant correlations among TCatt, ECatt, TSS and TP. In one of the few articles investigating the factors controlling FIB concentrations in the tropics, Byamukama et al.34 working in Uganda, also found that FIB were correlated with TSS concentrations as we show here. The Red River system has high turbidity levels8,14 and this may explain the relatively high %ECatt and %TCatt observed in this system. Moreover, attachment to particles probably plays a strong role in controlling the transport of FIB in the system as well35. Particles in aquatic systems are often associated with nutrients and particulate carbon and phosphorus in particular, both of which can be limiting substrates for bacterial growth and in turn, can affect FIB survival in non-host environments36 and in systems with high particle loads, the TP pool is generally dominated by the particulate fraction, as is the case here. This, along with higher carbon concentrations in the particles probably confers a competitive advantage to the bacteria thereby enhancing their survival. However, for the moment, little other information exists on the proportions of attached and free FIB in other tropical water bodies and so it is difficult to compare our results with other tropical ecosystems. Nevertheless it also appears that TSS and TP plays an important role in determining the proportions of attached and free FIB in this highly turbid, tropical riverine system.
Die-off rates
Schumacher37 in an in-stream incubation in the Upper Shoal Creek Basin, Southwestern Missouri, found that fecal coliform and EC densities decreased more than 90% from initial densities over a 42 h period. Troussellier et al.38 also reported rapid die-offs with over 90% of FIB lost over a 128 h period. We also observed a rapid decrease in EC and TC over the 5 day incubation, with in many cases up 100% loss after 120 h. We therefore chose to calculate the decay constants over the first 4 days. Our TC and EC die-off rate decay constants (k) ranged from a minimum of 0.01 d−1 to a maximum of 1.13 d−1 for EC and from 0.17 d−1 to 1.33 d−1 for TC, with a mean of 0.36 ± 0.21 d−1 for ECfree and 0.44 ± 0.23 d−1 for ECtot and 0.83 ± 0.19 d−1 for TCfree and 0.61 ± 0.28 d−1 for TCtot. These rates are similar to other studies from temperate aquatic environments. For example, Menon et al.39, working in the Seine river, France observed die-off rates of 0.19 to 0.82 × 10−3 h−1 for EC and Blaustein et al.40, in a review on EC survival in a range of temperate aquatic environments reported an average rate of 0.725 ± 0.078 d−1. In one of the few articles dealing with die-off rates in sub-tropical systems, Chan et al.41 also found values of between 0.85 to 1.50 d−1 for the coastal water around Hong Kong, similar to the values we report in this work.
We observed higher die-off rates for TCfree than for TCtot, however, this was not the case for EC, where die-off was significantly higher for ECtot than for ECfree. Some authors42,43 have shown lower decay rates for fecal bacteria attached to sediments as compared to free fecal bacteria, as we show here. Attachment to particles may protect FIB against grazing by small heterotrophic nanoflagellates (the main grazers of bacteria in aquatic systems) as well as providing a micro-environment rich in nutrients. Although, according to Sinton et al.44 and Chan et al.41, exposure to solar radiation and predation are among the most important factors controlling die-off, although it seems from our work that attachment to particles can also be an important factor at least for TC.
We observed systematically higher die-off rates for TCtot than for ECtot. Why this might be the case in this system merits some reflection. The appropriateness of using TC and EC as indicators of faecal contamination in tropical systems has been questioned by several authors e.g.45,46. Indeed, it has been shown that E.coli may be able to persist and even proliferate for some time in tropical freshwaters47,48,49 particularly in those with high temperatures and elevated nutrient and organic matter concentrations. This may well explain the differences in die-off rates between EC and TC. It may also be why die-off in ECfree was lower than that for ECatt. Although the technique used in this work allowed us to determine the presence or absence of TC or EC, it provides no information on the sources of the TC or EC. Therefore, if the FIB are from different origins with different levels of adaptation to the environment, then this may explain some of the differences between the die-off rates of free and attached TC and EC. Nevertheless, this clearly is a hypothesis that merits further investigation in situ using some of the newer microbial source tracking techniques36.
Many studies have indicated that seasonal variations can influence FIB die-off rates through modifications of temperature, pH, nutrient concentration, and dissolved oxygen50,51. In this study, die-off rates were determined over the whole year with temperatures varying between 9.5 and 35 °C and we did not observe any significant relationship with in situ temperature. Moreover, we only observed a significant correlation between temperature and TCfree in contrast to what has been found by other investigators23,52. We did, however, find significant differences in FIB numbers and die-offs between the wet and dry seasons, emphasizing the dominant role of the hydrology. Why we did not find a clear temperature relationship for FIB number or die-off rate is not clear from the data, however, it is probably related to other environmental and source related factors not investigated in this work.
Conclusions
There have been many studies on the survival of EC and other indicators of faecal pollution in freshwater, marine and estuarine habitats in temperate ecosystems however less information exists for tropical aquatic environments. Crane and Moore53 observed that the identification of relationships between environmental and physical parameters and FIB survival was a fundamental topic for future research in the field. Although their work is dated, and much work has been published since on temperate ecosystems, it is still the case that very little information is available on die-off rates in tropical and sub-tropical ecosystems where the management of FIB concentration is most needed and where the risks to human populations are highest36. In our work from the sub-tropical Red River system in Viet Nam, we found that FIB numbers exceed Vietnamese water quality guidelines of 20 and 150 colonies 100 ml−1 for EC and TC, respectively, throughout the whole year at almost all of the 10 stations investigated. Moreover, many values exceeded 500 colonies 100 ml−1 above which the World Health Organization considers that there is a 10% risk of gastro-intestinal illness after one single exposure. Therefore, the use of water from sites with high FIB numbers such as those in the downstream sites pose a real risk to public health. We also found a significant correlation between particles (as TSS or TP) and FIB as has been observed in temperate riverine ecosystems. However, in contrast to other studies we did not find a significant correlation between FIB and temperature for this subtropical environment, but instead a correlation with discharge (wet vs. dry season). Indeed, the highest TSS concentrations and FIB numbers were found during the wet season at high discharge. It is therefore probable that in developing countries where sanitation facilities are deficient and where people and their livestock live in close proximity to the river, FIB contamination mostly originates from diffuse sources21. Nevertheless, the data presented here, notably that of FIB numbers and their respective die-off rates, provides a base for the application of a model that will allow the parameterization of FIB dynamics in Red River and delta and potentially in other large river basins of the sub-tropical and tropical belt.
Additional Information
How to cite this article: Nguyen, H. T. M. et al. Seasonal variability of faecal indicator bacteria numbers and die-off rates in the Red River basin, North Viet Nam. Sci. Rep. 6, 21644; doi: 10.1038/srep21644 (2016).
Supplementary Material
Acknowledgments
This work was financed by the ARCP2013_06CMY_Quynh project of the Asian Pacific Network, the UMR METIS and the UMR iEES-Paris. NTMH was financed by a PhD fellowship from the French Research Institute for Development (IRD). We are grateful to Drs. Gilles Billen and Olivier Ribolzi for helpful comments during this research. This work forms part of the PhD thesis requirements of NTMH.
Footnotes
Author Contributions E.J.R.N., Q.T.P.L. and J.G. designed the experiments; H.T.M.N., J.L.J. and Q.T.P.L. carried out the work; E.J.R.N., H.T.M.N., Q.T.P.L. and J.G. interpreted the results and H.T.M.N. and E.J.R.N. wrote the manuscript that was revised and improved by all the coauthors.
References
- Ashbolt N. J. Microbial contamination of drinking water and disease outcomes in developing regions. Toxicology 198, 229–238, doi: 10.1016/j.tox.2004.01.030 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO. World Health Organisation global data repository, http://apps.who.int/ghodata/ (2012). Access date: March 8, 2014.
- Bain R. et al. Fecal contamination of drinking-water in low- and middle-income countries: a systematic review and meta-analysis. PLoS Med 11, e1001644, doi: 10.1371/journal.pmed.1001644 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broussard W. & Turner R. E. A century of changing land-use and water-quality relationships in the continental US. Frontiers in Ecology and the Environment 7, 302–307, doi: 10.1890/080085 (2009). [DOI] [Google Scholar]
- Seitzinger S. P. et al. Global river nutrient export: a scenario analysis of past and future trends. Glob. Biogeochem. Cycl. 24, doi: 10.1029/2009gb003587 (2010). [DOI] [Google Scholar]
- Navarro P. et al. Fate and tidal transport of butyltin and mercury compounds in the waters of the tropical Bach Dang Estuary (Haiphong, Vietnam). Mar. Pollut. Bull. 64, 1789–1798, doi: 10.1016/j.marpolbul.2012.05.036 (2012). [DOI] [PubMed] [Google Scholar]
- Berg M. et al. Magnitude of arsenic pollution in the Mekong and Red River Deltas — Cambodia and Vietnam. Sci. Total Environ. 372, 413–425, doi: 10.1016/j.scitotenv.2006.09.010 (2007). [DOI] [PubMed] [Google Scholar]
- Vinh V. D., Ouillon S., Thanh T. D. & Chu L. V. Impact of the Hoa Binh Dam (Vietnam) on water and sediment budgets in the Red River basin and delta. Hydrol. Earth Syst. Sci. 18, 3987–4005, doi: 10.5194/hess-18-3987-2014 (2014). [DOI] [Google Scholar]
- Mahanty S., Dan T. & Hai P. Crafting sustainability: managing water pollution in Viet Nam’s craft villages. (Crawford School of Public Policy, The Australian National University, Canberra, 2012). [Google Scholar]
- Luu T. N. M. et al. Hydrological regime and water budget of the Red River Delta (Northern Vietnam). J. Asian Earth Sci. 37, 219–228, doi: 10.1016/j.jseaes.2009.08.004 (2010). [DOI] [Google Scholar]
- Luu T. N. M. et al. N, P, Si budgets for the Red River Delta (northern Vietnam): how the delta affects river nutrient delivery to the sea. Biogeochem. 107, 241–259, doi: 10.1007/s10533-010-9549-8 (2012). [DOI] [Google Scholar]
- Ishii S. & Sadowsky M. J. Escherichia coli in the environment: Implications for water quality and human health. Microbes Environ. 23, 101–108, doi: 10.1264/jsme2.23.101 (2008). [DOI] [PubMed] [Google Scholar]
- Pachepsky Y. A. & Shelton D. R. Escherichia coli and Fecal Coliforms in freshwater and estuarine Sediments. Crit. Rev. Env. Sci. Tech. 41, 1067–1110, doi: 10.1080/10643380903392718 (2011). [DOI] [Google Scholar]
- Le T. P. Q., Garnier J., Billen G., Thery S. & Chau V. M. The changing flow regime and sediment load of the Red River, Viet Nam. J. Hydrol. 334, 199- 214, doi: doi: 10.1016/j.jhydrol.2006.10.020. (2007). [DOI] [Google Scholar]
- Xuan T. T. Preliminary Assessment of rainwater resources: Statistical indicators of water resources (in Vietnamese). (Hanoi, Vietnam, 2010).
- IMH. Vietnamese Journal of Meteo-hydrology (in Vietnamese). (Institute of Meteo-Hydrology in Vietnam, Hanoi, 1997–2004).
- APHA. Standard methods for the examination of water and wastewater. 22nd edn, (American Public Health Association, 2012).
- Harmon S. M., West R. & Yates J. Identifying fecal pollution sources using 3M™ Petrifilm™ count plates and antibiotic resistance analysis in the Horse Creek Watershed in Aiken County, SC (USA). Environ. Monit. Assess., 1–13, doi: 10.1007/s10661-014-3999-8 (2014). [DOI] [PubMed] [Google Scholar]
- APHA. Compendium of methods for the microbiological examination of foods (4th ed.). (American Public Health Association, 2001). [Google Scholar]
- Crowther J., Kay D. & Wyer M. D. Faecal-indicator concentrations in waters draining lowland pastoral catchments in the UK: relationships with land use and farming practices. Water Res. 36, 1725–1734, doi: 10.1016/S0043-13540100394-3 (2002). [DOI] [PubMed] [Google Scholar]
- Causse J. et al. Field and modelling studies of Escherichia coli loads in tropical streams of montane agro-ecosystems J Hydro-Environ. Res. 9, 496–507, doi: 10.1016/j.jher.2015.03.003 (2015). [DOI] [Google Scholar]
- Boyer D. G. & Pasquarell G. C. Agricultural land use impacts on bacterial water quality in a karst groundwater aquifer. JAWRA Journal of the American Water Resources Association 35, 291–300, doi: 10.1111/j.1752-1688.1999.tb03590.x (1999). [DOI] [Google Scholar]
- Isobe K. O., Tarao M., Chiem N. H., Minh L. Y. & Takada H. Effect of environmental factors on the relationship between concentrations of coprostanol and fecal indicator bacteria in tropical (Mekong delta) and temperate (Tokyo) freshwaters. Appl. Environ. Microbiol. 70, 814–821, doi: 10.1128/aem.70.2.814-821.2004 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- MoH. National Technical Regulation on domestic water quality (QCVN 02:2009/BYT) Report No. Circular No. 05/2009/TT-BYT (Minstry of Health, Vietnam, Hanoi, 2009).
- Trinh A. D. et al. Experimental investigation and modelling approach of the impact of urban wastewater on a tropical river; a case study of the Nhue River, Hanoi, Viet Nam. J. Hydrol. 334, 347–358, doi: 10.1016/j.jhydrol.2006.10.022 (2007). [DOI] [Google Scholar]
- Trinh A. D., Meysman F., Rochelle-Newall E. J. & Bonnet M.-P. Quantification of sediment water interactions in a polluted tropical river through biogeochemical modeling. Glob. Biogeochem. Cycl. 26, GB3010, doi: 10.1029/2010GB003963 (2012). [DOI] [Google Scholar]
- Cuong T. K. Survey results of water quality of the Red River, 3/2012, (Hanoi, Vietnam, 2012).
- Oliver D. M., Clegg C. D., Heathwaite A. L. & Haygarth P. M. Preferential attachment of Escherichia coli to different particle size fractions of an agricultural grassland soil. Water. Air. Soil Pollut. 185, 369–375, doi: 10.1007/s11270-007-9451-8 (2007). [DOI] [Google Scholar]
- Crump B. C., Baross J. A. & Simenstad C. A. Dominance of particle-attached bacteria in the Columbia River estuary, USA. Aquat. Microb. Ecol. 14, 7–18 (1998). [Google Scholar]
- Lemee R. et al. Seasonal variation of bacterial production, respiration and growth efficiency in the open NW Mediterranean Sea. Aquat. Microb. Ecol. 29, 227–237 (2002). [Google Scholar]
- Suter E., Juhl A. & O’Mullan G. Particle association of Enterococcus and total Bacteria in the Lower Hudson River Estuary, USA. J. Water Resour. Prot. 3, 715–725 (2011). [Google Scholar]
- Pachepsky Y. A. et al. Transport and fate of manure-borne pathogens: Modeling perspective. Ag. Wat. Man. 86, 81–92, doi: 10.1016/j.agwat.2006.06.010 (2006). [DOI] [Google Scholar]
- Garcia-Armisen T. & Servais P. Partitioning and fate of particle-associated E. coli in river waters. Water Environment Research 81, 21–28 (2009). [PubMed] [Google Scholar]
- Byamukama D., Mach R. L., Kansiime F., Manafi M. & Farnleitner A. H. Discrimination efficacy of fecal pollution detection in different aquatic habitats of a high-altitude tropical country, using presumptive coliforms, Escherichia coli, and Clostridium perfringens spores. Appl. Environ. Microbiol. 71, 65–71, doi: 10.1128/aem.71.1.65-71.2005 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribolzi O. et al. Use of fallout radionuclides (7Be, 210Pb) to estimate resuspension of Escherichia coli from streambed sediments during floods in a tropical montane catchment. Environ. Sci. Pollut. Res. 10.1007/s11356-11015-15595-z (2015). [DOI] [PubMed] [Google Scholar]
- Rochelle-Newall E. J., Nguyen T. M. H., Le T. P. Q., Sengtaheuanghoung O. & Ribolzi O. A short review of faecal indicator bacteria in tropical aquatic ecosystems: knowledge gaps and future directions. Frontiers in Microbiology: Aquatic Microbiology 6, 308, doi: 10.3389/fmicb.2015.00308 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher J. G. Survival, transport, and sources of fecal bacteria in streams and survival in land-applied poultry litter in the upper Shoal Creek basin, southwestern Missouri, 2001–2002. 45 (USGS, Rolla, Missouri, 2003).
- Troussellier M. et al. Water quality and health status of the Senegal River estuary. Mar. Pollut. Bull. 48, 852–862, doi: 10.1016/j.marpolbul.2003.10.028 (2004). [DOI] [PubMed] [Google Scholar]
- Menon P., Billen G. & Servais P. Mortality rates of autochthonous and fecal bacteria in natural aquatic ecosystems. Water Res. 37, 4151–4158, doi: 10.1016/s0043-1354(03)00349-x (2003). [DOI] [PubMed] [Google Scholar]
- Blaustein R. A., Pachepsky Y., Hill R. L., Shelton D. R. & Whelan G. Escherichia coli survival in waters: Temperature dependence. Water Res. 47, 569–578, doi: 10.1016/j.watres.2012.10.027 (2013). [DOI] [PubMed] [Google Scholar]
- Chan Y. M., Thoe W. & Lee J. H. W. Field and laboratory studies of Escherichia coli decay rate in subtropical coastal water. J Hydro-Environ. Res. 9, 1–14, doi: 10.1016/j.jher.2014.08.002 (2015). [DOI] [Google Scholar]
- Craig D. L., Fallowfield H. J. & Cromar N. J. Use of microcosms to determine persistence of Escherichia coli in recreational coastal water and sediment and validation with in situ measurements. J. Appl. Microbiol. 96, 922–930, doi: 10.1111/j.1365-2672.2004.02243.x (2004). [DOI] [PubMed] [Google Scholar]
- Davies C. M., Long J. A., Donald M. & Ashbolt N. J. Survival of fecal microorganisms in marine and freshwater sediments. Appl. Environ. Microbiol. 61, 1888–1896 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinton L. W., Hall C. H., Lynch P. A. & Davies-Colley R. J. Sunlight inactivation of fecal indicator bacteria and bacteriophages from waste stabilization pond effluent in fresh and saline waters. Appl. Environ. Microbiol. 68, 1122–1131, doi: 10.1128/aem.68.3.1122-1131.2002 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byamukama D., Kansiime F., Mach R. L. & Farnleitner A. H. Determination of Escherichia coli contamination with Chromocult Coliform Agar showed a high level of discrimination efficiency for differing fecal pollution levels in tropical waters of Kampala, Uganda. Appl. Environ. Microbiol. 66, 864–868, doi: 10.1128/aem.66.2.864-868.2000 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nshimyimana J. P., Ekklesia E., Shanahan P., Chua L. H. C. & Thompson J. R. Distribution and abundance of human-specific Bacteroides and relation to traditional indicators in an urban tropical catchment. J. Appl. Microbiol. 116, 1369–1383, doi: 10.1111/jam.12455 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiminez L., Muniz I., Toranzos G. A. & Hazen T. C. Survival and activity of Salmonella typhimurium and Escherichia coli in tropical freshwater. J. Appl. Bacteriol. 67, 61–69 (1989). [DOI] [PubMed] [Google Scholar]
- Carillo M., Estrada E. & Hazen T. C. Survival and enumeration of the fecal indicators Bifidobacterium adolescentis and Escherichia coli in a tropical rain forest watershed. Appl. Environ. Microbiol. 50, 468–476 (1985). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winfield M. D. & Groisman E. A. Role of nonhost environments in the lifestyles of Salmonella and Escherichia coli. Appl. Environ. Microbiol. 69, 3687–3694, doi: 10.1128/aem.69.7.3687-3694.2003 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton G. A., Gunnison D. & Lanza G. R. Survival of pathogenic bacteria in various freshwater sediments. Appl. Environ. Microbiol. 53, 633–638 (1987). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flint K. P. The long-term survival of Escherichia coli in river water. J. Appl. Bacteriol. 63, 261–270 (1987). [DOI] [PubMed] [Google Scholar]
- Crowther J., Kay D. & Wyer M. D. Relationships between microbial water quality and environmental conditions in coastal recreational waters: the Fylde coast, UK. Water Res. 35, 4029–4038, doi: 10.1016/S0043-13540100123-3 (2001). [DOI] [PubMed] [Google Scholar]
- Crane S. R. & Moore J. A. Modeling enteric bacterial die-off: A review. Water. Air. Soil Pollut. 27, 411–439, doi: 10.1007/BF00649422 (1986). [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.