Abstract
Juxtarenal aortic aneurysms (JAA) account for approximately 15% of abdominal aortic aneurysms. Fenestrated endovascular aneurysm repair (FEVAR) and chimney endovascular aneurysm repair (CH-EVAR) are both effective methods to treat JAAs, but the comparative effectiveness of these treatment modalities is unclear. We searched the PubMed, Medline, Embase, and Cochrane databases to identify English language articles published between January 2005 and September 2013 on management of JAA with fenestrated and chimney techniques to conduct a systematic review to compare outcomes of patients with juxtarenal aortic aneurysm (JAA) treated with the two techniques. We compared nine F-EVAR cohort studies including 542 JAA patients and 8 CH-EVAR cohorts with 158 JAA patients regarding techniques success rates, 30-day mortality, late mortality, endoleak events and secondary intervention rates. The results of this systematic review indicate that both fenestrated and chimney techniques are attractive options for JAAs treatment with encouraging early and mid-term outcomes.
Endovascular techniques are less invasive methods of treating infrarenal abdominal aortic aneurysms (AAAs)1,2, especially for patients with severe comorbidities3,4. However, 30 to 50% of AAA patients are not suitable for elective conventional endovascular repair due to anatomic constraints around the proximal neck5,6. The term juxtarenal aortic aneurysm (JAA) is routinely used to describe complex AAAs with very short proximal necks. They represent almost 15% of all AAAs7,8,9,10,11,12. These proximal neck adequacy and endograft seal zones have also been identified as key predictors of long term outcomes and success after EVAR13,14,15.
Fenestrated endografts were developed to treat patients with aneurysms with short proximal necks. This technique was first introduced in 199916. Fenestrated grafts extend the proximal sealing zone from the infrarenal segment to the juxtarenal aorta using fenestrations (holes) in the graft or scallops (gaps in the upper graft fabric margin) to permit perfusion of the visceral vessels. This procedure can be performed with or without bridging stents. Greenberg and colleagues first described the use of chimney or snorkel grafts in the endovascular repair of juxtarenal AAA17. This procedure involves placement of additional off-the-shelf stents parallel to the main body graft (between the aortic stent and the aortic wall) to facilitate branch vessel perfusion.
Although fenestrated endovascular aneurysm repair (FEVAR) and chimney endovascular aneurysm repair (CH-EVAR) are both effective modalities for treatment of JAAs, their comparative effectiveness is unclear. We conducted a systematic review to compare the outcomes of fenestrated and chimney techniques to traditional methods in the treatment of patients with JAAS. The advantages and limitations of each technique are discussed.
Methods
Search strategy
Three independent investigators performed a comprehensive search of PubMed, Medline Embase and the Cochrane Database. The search included all published articles in which patients were diagnosed with JAAAs and treated with F/CH/SN techniques between January 2005 and July 2013. The literature search for relevant articles was performed using the following key words alone and in combination: “juxtarenal aortic aneurysm,” “snorkel grafts,” “chimney graft,” “fenestrated graft,” and “zenith graft.” The search was restricted to articles published in English and human studies. Relevant articles in the reference lists of retrieved articles were searched manually to maximize our search scope. The two authors conducted literature searches independently using the same search terms then discussed which studies fit the inclusion criteria to produce the final list of studies.
Article selection
The inclusion criteria for this review included original articles reporting more than 5 patients with JAAs treated with F-EVAR or CH/SN-EVAR. Published articles with patients were treated between January 2005 and September 2013. Any studies describing patients treated for endovascular repair of juxtarenal aneurysms were considered. The terms “juxtarenal aneurysm,” “fenestrated,” and “chimney” or “snorkel” were considered sufficient to warrant inclusion. Studies describing elective F-EVAR of juxtarenal aneurysms using any currently licensed stent grafts were included. The basic characteristic of patients, clinical outcomes of complications, graft patency, endoprosthesis-related complications, primary technical success rate, and total mortality were stated. Studies that met any of the follow criteria were excluded: 1) patients treated with a hybrid procedure and multi-branched stent-graft; 2) fewer than 5 patients included; 3) case reports, comments, editorials, review articles, and letters; 4) report of fenestrated or chimney/snorkel technique for pararenal aneurysm repair were excluded.
Data extraction
Each article was reviewed carefully, and the data were extracted. Patient demographics, including number of patients, gender, mean age (years), aneurysm diameter, aneurysm neck length, date of publication, country of publication, preoperative comorbidity, operative time, fluoroscopy time, contrast dose, estimated blood loss, reconstructed vessels, main stent and fenestrated, chimney/snorkel stent graft involved in the procedural characteristics, were considered. The following clinical outcomes were recorded: success rate, 30-day mortality and cause, over-30-day mortality and cause, patency, duration of follow-up period (months), renal events, endoleak type, number of endoleaks, and major adverse events (MAEs). Data extraction and conversion to the desired format followed the Cochrane Handbook for Systematic Reviews of Interventions guidelines [http://handbook.cochrane.org/ Part 2: General methods for Cochrane reviews >7 studies selected and data collected >7.7 study results extracted and converted to the desired format].
Statistical analysis
The data are presented as the mean ± standard deviation (SD) or proportions. Comparisons between groups were made using chi-square tests or Fisher’s exact tests for categorical variables. A P-value ≤0.05 was considered statistically significant. All analyses were performed using R software version 2.15.1 (http://www.R-project.org/).
Results
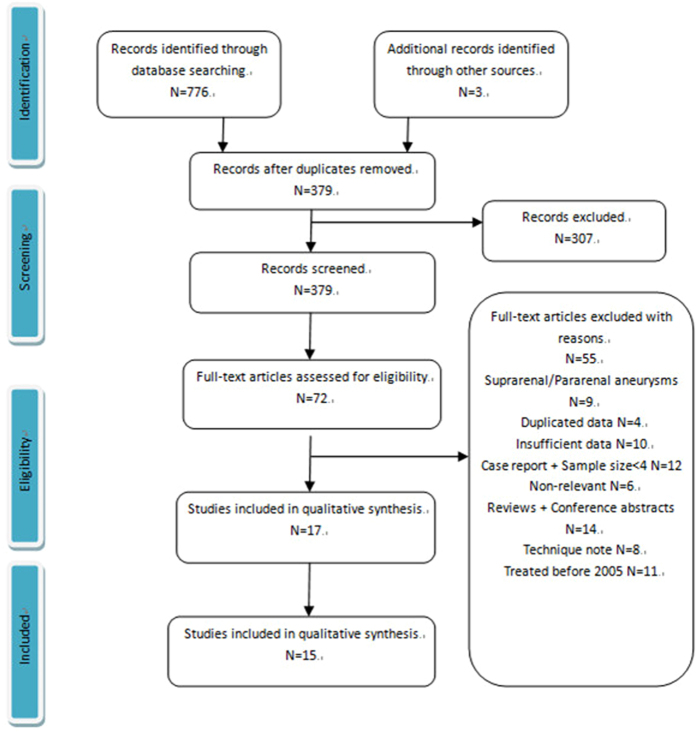
In a total, 776 articles were identified through electronic and manual searching (Fig. 1). Of these, 15 articles met the inclusion criteria and were eligible for analysis, including 8 CH/SN-EVAR case series and 9 F-EVAR case series. Only 2 articles included both CH/SN and F-EVAR cohorts.
Figure 1. PRISMA flow chart for article selection.
Patient characteristics
All of these patients were treated for juxtarenal aortic aneurysm (JAA) between January 2005 and July 2013. The studies included 700 patients, of whom 542 underwent F-EVAR and 158 underwent CH/SN-EVAR. Patient characteristics are summarized in Tables 1 and 2. The largest series (318 patients) was in the Globalstar study in F-EVAR group. Male patients predominated. In 8 CH/SN-EVAR studies and 9 F-EVAR studies, men comprised 82.9% and 85.7% of the study population, respectively. The mean age was 75 (59–88) years in the CH/SN-EVAR series and 74 (47–86) years in the F-EVAR series. The mean aneurysm diameter was 64.0 mm (47–112) in the F-EVAR group and 64.5 mm (33–110) in the CH/SN-EVAR group. The mean/median aneurysm neck length was 6.7 mm (0–14) in five F-EVAR studies and 2.3 mm (0–10) in six CH/SN-EVAR studies.
Table 1. Patient demographics of all chimney/snorkel endovascular aneurysm repair (CH/SN-EVAR) case series.
| References | Type of study | Patients | Sex (M/F) | Age | Major Comorbidities | Aneurysm diameter (mm) | Length of aneurysm neck (mm) | Treatment period | Country |
|---|---|---|---|---|---|---|---|---|---|
| Donas et al. 201231 | 2 arms | 30 | 27:3 | 74.8 ± 7.3 | 22 CAD; 7 RI; 10 respiratory disease; | 62 | N.D. | 2008.1–2010.12 | Germany |
| 10 MI; 11 previous aortic intervention; | |||||||||
| 10 previous aortocoronary bypass or intervention; | |||||||||
| Suominen et al. 201332 | Single arm | 7 | 5:2 | 79 | 1 DM; 1 Hyperlipidemia; 4 HTN; 6 CAD; 3 respiratory; 6 renal failure; 2 smokers; | 65 ± 7 | 2.5 (0-10) | 2007.12–2011.8 | Finland |
| Lee et al. 201433 | 2 arms | 43 | 30:13 | 75(59–88) | 40 smoker; 43 HTN; 41 Hyperlipidemia; 30 CAD; 13 CHF; 18COPD; 5 DM; | 66 ± 11.9 (51–105) | 1.6 ± 2 | 2009–2012 | U.S. |
| 10 prior AAA repair | |||||||||
| Schiro et al. 201319 | Single arm | 9 | 6:3 | 77(65-88) | 8 HTN; 2 DM; 5 CAD; 6 Hyperlipidemia; 4 COPD; 5 smokers; 3 RI; 3 CVD; | 73 (58–110) | N.D. | 2008.7–2012.2 | U.K. |
| Ducasse et al. 201334 | Single arm | 22 | 21:1 | 73(63-88) | 17 CAD; 6MI; 3 CHF; 4 ejection fraction; | 58.5 (45–100) | 4.5 (1-9) | 2010.7–2012.11 | Multiple Center |
| 13 previous interventions; 18 HTN; | |||||||||
| 17 hyperlipidaemia; 26 smoker; 4 COPD; 2 DM; 4 RI; 4 hostile abdomen; 8 PAD; | |||||||||
| 2 CVD; | |||||||||
| Tolenaar et al. 201335 | Single arm | 5 | 4:1 | 75.9(68-85) | 3 MI; 3 COPD; 1 ICD 2 arrhythmia; 1 RI; | 64.6 (54–72) | 4 (0-7) | 2009.10–2011.7 | Netherland |
| Lgari et al. 201436 | Single arm | 5 | 4:1 | 78.4(76-84) | 5 HTN; 4 COPD; 2 CHD; 2 CVD; 1 CAD; 1 Hostile abdomen; 2 malignant disease | 60 (33–85) | 5.7 (3–10) | 2010.1–2013.7 | Tokyo |
| Banno et al. 201437 | Single arm | 37 | 34:3 | 74.3 ± 8.7 | 15 CAD; 14 CHF; 10 Arrhythmia; 9 RI; | 65.9 ± 15.3 | 2.3 ± 3.1 | 2006.1–2013.4 | France |
| 11 COPD; 30 HTN; 22 Hyperlipidemia; | |||||||||
| 10 DM; 4 CVD; 11 PAD; 1 dialysis; | |||||||||
| 9 prior aortic surgery 5 smokers; |
N.D. not documented; CAD, coronary artery disease; RI, renal impairment; MI, myocardial infarction arrhythmia; DM, diabetes mellitus; HTN, hypertension; CHF, congestive heart failure; PAD, peripheral arterial disease; CVD, cerebrovascular disease; CRF, Chronic renal failure; CKD, Chronic kidney disease; ICD, implantable cardioverter defibrillator;
Table 2. Patient demographics of all Fenestrated endovascular aneurysm repair (F-EVAR) case series included.
| References | Type of study | Patients | Sex (M/F) | Age | Major Comorbidities | Aneurysm diameter (mm) | Length of aneurysm neck (mm) | Treatment period | Country |
|---|---|---|---|---|---|---|---|---|---|
| Lee et al. 201438 | Single arm | 15 | 10:5 | 77.4 | 11 smokers; 12 CAD; 7 CHF; 14 HTN; | 61.6 (47–105) | 4.5 (2–8) | 2012–2013 | U.S. |
| 2 COPD; 3 DM; 1 Prior AAA repair; | |||||||||
| Globalstar. 201218 | Single arm | 318 | 274:44 | 74 (47–86) | 44 DM; 196 HTN; 149 CAD; 19 CHF; | 65 (46–112) | N.D. | 2007.1–2010.12 | U.K. |
| 44 Renal Failure; 27 CVD; | |||||||||
| 3 Previous aortic surgery; | |||||||||
| Liao et al. 201439 | Single arms | 8 | 4:4 | 75 (64–85) | 3 CAD; 5 COPD | 58 (52–63) | 6 (4–12) | 2012.8–2013.5 | U.S. |
| Dijkstra et al. 201440 | Single arm | 25 | 22:3 | 73 ± 7.1 | 3 DM; 15 HTN; 9 Hyperlipidemia; | 61 (55–88) | N.D. | 2011.5–2013.9 | Duth |
| 9 smoker; 18 cardiac; 8 renal disease; | |||||||||
| 3 pulmonary disease; | |||||||||
| Donas et al. 201231 | 2 arms | 29 | 29:0 | 73.7 ± 6.1 | 24 cardiac; 5 RI; 11 respiratory; 7 MI; | 65 | N.D. | 2008.1–2010.12 | Germany |
| 8 previous aortic intervention; | |||||||||
| 12 aortocoronary surgery; | |||||||||
| Suominen et al. 201332 | 2 arms | 21 | 21:0 | 73 | 4 DM; 9 hyperlipidemia; 13 HTN; 14 CAD; 6 respiratory disease; 19 renal failure; | 65 ± 7 | 2.5 (0–2.5) | 2007.12–2011.8 | Finland |
| 4 smokers; | |||||||||
| Greenberg et al. 200941 | Single arm | 30 | 24:6 | 75 (59–86) | 8 MI; 3 CHF; 15 CAD; 14 arrhythmia; | 61.4 ± 9.7 (48.8–88) | 9.2 ± 2.9 (2.4–14.4) | 2005.1–2006.1 | Global |
| 26 HTN; 5 thromboembolic event; 7 PAD; | |||||||||
| 9 COPD; 2 RI; 7 DM; 5 CVD; 27 smokers; | |||||||||
| Tambyraja et al. 201142 | Single arm | 29 | 27:2 | 74 (54–86) | 21 smoker/COPD; 15 HTN; 13 MI; 3 CRF ; | 68 ± 7 | N.D. | 2005.10–2010.3 | U.K. |
| 4 hostile abdomen;;2 CVD; 3 cardic failure | |||||||||
| Oderich et al. 201443 | Single arm | 67 | 54:13 | 74 ± 8 | 60 HTN; 59 smokers; 36 CAD; 20 MI; | 60 ± 10 (47–100) | 7.5 ± 2.3 (4–12) | 2005–2012 | U.S. |
| 21 Arrhythmia; 24 COPD; 16 CKD; 16 DM; 15 PAD; 11 CVD; 7 CHF; | |||||||||
| 7 history of thromboembolic event; |
N.D. not documented; CAD, coronary artery disease; RI, renal impairment; MI, myocardial infarction arrhythmia; DM, diabetes mellitus; HTN, hypertension; CHF, congestive heart failure; PAD, peripheral arterial disease; CVD, cerebrovascular disease; CRF, Chronic renal failure; CKD, Chronic kidney disease.
All 15 studies reported information on patient co-morbidities (Tables 1 and 2). Nine studies documented smoking status; patients with any history of smoking comprised 69.6% of the patients in the F-EVAR group and 66.1% of those in the CH/SN-EVAR group. Diabetes mellitus was reported in 16.9% and 16.2% of patients in 5 CH/SN-EVAR studies and 6 F-EVAR studies, respectively. The hypertension rates were high in each EVAR group (67% in the F-EVAR group and 87.8% in the CH/SN-EVAR group). Hyperlipidemia was not widely reported in the F-EVAR group. Cardiovascular disease rates were retrieved from all 15 studies. CAD, CHF, MI, arrhythmia, or any combination of these was noted in 33.1% of patients in the F-EVAR series and 44.6% of patients in the CH/SN-EVAR series. In total, 36% and 29.7% of patients in the F-EVAR group and CH//SN-EVAR group, respectively, reported respiratory diseases. Here, 18.6% patients in the F-EVAR series had renal diseases, and 28% of the patients in the CH/SN-EVAR had renal diseases. Previous major abdominal surgery was reported in 4 studies of the CH/SN-EVAR group, which covered a total of 30 patients and 3 studies with 12 patients in the F-EVAR group. Regarding American Society of Anesthesiologists (ASA) grade, the factor was not widely reported, and groups were often combined, making it difficult to extrapolate exact numbers.
Intra-operative and stent grafts
Here, 7 articles provided procedure time for the CH/SN-EVAR group, which averaged 178 (75–810) minutes. In addition, 6 studies documented a mean of 54.6 (15–290) minutes of fluoroscopy time. According to 7 articles, the median volume of contrast used was 146 (45–465) ml. Blood loss was reported in 5 series. The estimated mean volume was 332 ml and ranged from 30 to 2204 ml Table 3. A great variety of aortic stent grafts were utilized as the main body in all 7 studies (121 patients 76.6%). The main aortic stent grafts implanted included the Zenith (Cook Inc., Bloomington, IN, U.S.) (53 patients, 43.8%); Endurant (Medtronic Inc., Minneapolis, MN, U.S.) (40 patients, 33.1%); Excluder (W. L. Gore and Associates, Newark, DE, U.S.) (14 patients, 11.6%); Renu (Cook Inc., Bloomington, IN, U.S.); TX2 (Cook Inc., Bloomington, IN, U.S.); Powerlink (Endologix Inc., Irvine, CA, U.S.); Talent (Medtronic Inc., Minneapolis, MN); TAG (W. L. Gore and Associates, Newark, DE, U.S.); and Trivascular Ovation (Ovation; TriVascular Inc., Santa Rosa, CA, U.S.) (Table 4). A total of 229 visceral vessels were treated with stents. An additional 7 studies listed the specific stent type (121 patients, 76.6%). The most commonly used chimney grafts included Advanta (Atrium Medical Corporation, Hudson, NH U.S.) (47, 26.6%); iCAST stents (Atrium Medical Corporation, Hudson, NH, U.S.) (46, 26%); Viabahn (W. L. Gore and Associates, Newark, DE, U.S.) (39, 22%); Lifestent (C.R. Bard, Murray Hill, NJ, U.S.); and Luminexx and Fluency stents (both from C.R. Bard, Murray Hill, NJ, U.S.) (7.34% each) (Table 4).
Table 3. Procedural characteristics of CH/SN-EVAR cohort.
| References | Operative time (min) | Fluoroscopy time (min) | Contrast dose (ml) | Estimated Blood loss (ml) | Technique success rate |
|---|---|---|---|---|---|
| Donas et al. 201231 | 89 ± 21 | 44.8 ± 13.2 | 112 ± 23 | N.D. | 97.70% |
| Suominen et al. 201332 | 213 ± 67 (118–351) | 71 (43–189) | 267 ± 80 (120–465) | 425 (100–2200) | 93% |
| Lee et al. 201433 | 237 (110–810) | 77.8 ± 48.1 (30–290) | 180.5 ± 66.2(66–400) | 428 (100–2000) | N.D. |
| Schiro et al. 201319 | 187 ± 30 | 41 ± 11 | 194 ± 52 | 212 ± 102 | N.D. |
| Ducasse et al. 201334 | 105 (75–290) | 23 (15–55) | 65 (45–120) | 55 (30–550) | 100% |
| Tolenaar et al. 201335 | N.D. | N.D. | N.D. | N.D. | 92.3% |
| Lgari et al. 201436 | 171 (107–511) | N.D. | 105 (100–200) | 235 (100–2204) | 100% |
| Banno et al. 201437 | 183 ± 69 | 43 | 139 ± 102 | N.D. | N.D. |
N.D. not documented.
Table 4. Data on aortic stent grafts and chimney/snorkel stent graft utility.
| References | Stented vessels: RRA/LRA/SMA/CA | Main stent | Chimney/snorkel stent grafts |
|---|---|---|---|
| Donas et al. 201231 | Stented vessels: 38 | 30 Endurant stent grafte2 | Covered balloon expandable |
| RRA/LRA/SMA | 38 Advantac | ||
| 19/16/3 | |||
| Suominen et al. 201332 | Stented vessels: 9 | 7 Excludera1 | 9 Advantac |
| 3 RRA/6 LRA | |||
| Lee et al. 201433 | Stented vessels: 74 RA | 27 Zenith bifurcated EVAR systemb1 | Balloon-expandable |
| 6 Endurante2 | 46 iCAST covered stentsc (5, 6, or 7 mm _59 mm) | ||
| 1 Talente2 | Self-expanding | ||
| 5 Renub1 | 27 Viabahn covered stentsa2 | ||
| 2 TX2b1 | (5, 6, or 7 mm _ 50 mm) | ||
| 1 TAGa1 | Bare stent | ||
| 2 Excludera1 | 1 Omnilink Elitei | ||
| Schiro et al. 201319 | Stented vessels: 9 | 6 Zenithb1 | 9 Fluencyd1 |
| 1PowerlinkEndologixg | 3 Luminexxd2 | ||
| 1 Talente1 | |||
| 1 Trivascular Ovationl | |||
| Ducasse et al. 201334 | Stented vessels: 22 | 12 Zenith LPb1 | 13 Lifestentd1 |
| 6 Zenith Flexb1 | 3 Absolutei | ||
| 2 Zenith AUIb1 | 2 Astronj | ||
| 1 Endurante1 | 2 Epicf1 | ||
| 1 Powerlinkg | 1 S.M.A.R.Th1 | ||
| 1 Everflexk1 | |||
| Tolenaar et al. 201335 | Stented vessels: 8 | 3 Endurante1 | 12 Viabahna1 |
| RRA/LRA/SMA | 2 Excludera1 | 1 Fluencyd1 | |
| 4/4/0 | |||
| Lgari et al. 201436 | Stented vessels: 9 | 3 Excludera1 | 7 Express SDf2 |
| 4 RRA/5 LRA | 2 Endologic Powerlink bifurcated graftg | 1 Coyote | |
| 1 SHIDEN | |||
| Banno et al. 201437 | Stented vessels: 60 | N.D. | N.D. |
| RRA/LRA/SMA: | |||
| 24/26/10 |
a1. W. L. Gore and Associates, Newark, DE, U.S. a2. W. L. Gore, Flagstaff, AZ, U.S. b1 .Cook Inc, Bloomington, IN. U.S. b2. Cook Australia Ltd., Australia. b3. William A. Cook Australia, Ltd., Brisbane, Australia b4. Cook Medical, Canvey Island, U.K. c Atrium Medical Corporation, Hudson, NH, U.S. d1. C.R. Bard, Murray Hill, NJ, U.S. d2. Bard Peripheral Vascular, Inc. e1. Medtronic, Inc, Minneapolis, MN, U.S. e2. Medtronic Vascular, Santa Rosa, CA, U.S. f1. Boston Scientific, Natick, MA, U.S. f2. Boston Scientific, Bloomington, MN, U.S. g Endologix, Inc, Irvine, CA,U.S. h1 Cordis Corporation, Johnson & Johnson Company, Miami, FL, U.S. h2. Cordis, Warren, NJ, U.S. i Abbott Vascular, Temecula, CA, U.S. j Biltronic, Bulach, Switzerland. k1 ev3Endovascular Inc, Plymouth, MN. U.S. k2. Covidien, Plymouth, CA, U.S. l Ovation; TriVascular Inc., Santa Rosa, CA, U.S > m. Vascutek, Renfrewshire, Scotland, U.K.
Seven articles of the F-EVAR group reported a mean procedure time of 261 (80–554) minutes. Six studies documented the mean volume of contrast. The average volume was 166, ranging from 90 to 465 ml. The mean duration of fluoroscopy was 64 (5–223) minutes, and estimates of blood loss ranged from 50 to 7000 ml (mean 534 ml) (Table 5). Custom-made Zenith grafts (Cook Medical) were the most widely implanted as the main graft (517 patients, 95.3%). In the study reported by Dijkstra, 25 patients received Anaconda stents (Vascutek, Renfrewshire, Scotland, U.K.) as the main body graft (4.7%). A total of 1082 visceral vessels described in 9 articles were implanted with stent grafts. Advanta (Atrium Medical Corporation, Hudson, NH U.S.) was the most commonly used visceral stent (603, 55.7%) followed by Zenith (Cook Inc., Bloomington, IN, U.S.) (58, 5.4%); iCAST (Atrium Medical Corporation, Hudson, NH U.S.) (53, 4.9%); Plamaz (Cordis Corporation, Johnson & Johnson Company, Miami, FL, U.S.) (49 vessels, 4.5%); EV3 (ev3Endovascular Inc., Plymouth, MN, U.S.) (Table 6). In addition, the stent type used (cover or bare, balloon or self-expansion) was not specified for 134 visceral vessels. The technical success rate of the chimney operation was 97.4% and 98.8% for fenestrated operations.
Table 5. F-EVAR procedural characteristics of F-EVAR cohort.
| References | Operative time (min) | Fluoroscopy time (min) | Contrast dose (ml) | Estimated blood loss (ml) | Technique success rate |
|---|---|---|---|---|---|
| Lee et al. 201438 | 282 | 99 | 123.04 | 650 | 96% |
| Globalstar. 201218 | 271 (80–720) | N.D. | N.D. | 807 ± 500 (50–7000) | 99% |
| Liao et al. 201439 | N.D. | 55 (17–85) | 90 (42–122) | N.D. | 100% |
| Dijkstra et al. 201440 | 240 (190–356) | 67 (53–107) | 194 (103–320) | N.D. | 94.6% |
| Donas et al. 201231 | 290 ± 122 | 54.3 ± 12.2 | 156 ± 56 | N.D. | N.D. |
| Suominen et al. 201332 | 213 ± 67 (118–351) | 71 (43–189) | 267 ± 80 (120–465) | 425 (100–2200) | 93% |
| Greenberg et al. 200941 | 234 (170–554) | N.D. | N.D. | 601 (50–2400) | 100% |
| Tambyraja et al. 201142 | N.D. | N.D. | N.D. | 200 (50–3000) | 100% |
| Oderich et al. 201443 | 236 ± 81 (104–554) | 60 ± 34 (5–223) | N.D. | 526 (50–2400) | 100% |
N.D. not documented.
Table 6. Data on aortic stent grafts and fenestrated stent graft utility.
| References | Stented vessels: RRA/LRA/SMA/CA | Main Stents | Fenestrated stent grafts |
|---|---|---|---|
| Lee et al. 201438 | Stented vessels: 25 | 15 ZFENb1 | Covered stents: |
| 25 iCASTc | |||
| Globalstar 201218 | Target vessels: 889 | 318 Zenithb4 | Bare metal stents: 63 vessels |
| Stented vessels: 670. | 35 Palmaz Genesish1. | ||
| RRA/LRA/SMA/CA | 13 EV3k | ||
| 269/278/113/10 | 7 Luminexxd2 | ||
| 2 AVEe1 | |||
| 1Expressf1 | |||
| 5 unspecified bare stents | |||
| Covered stents :529 vessels | |||
| 522 Advantac | |||
| 4 Jostenti | |||
| 3 Fluencyd2 | |||
| 78 unspecified covered stents. | |||
| Liao et al. 201439 | Target vessels: 21 | 8 Zenithb1 | Covered balloon-expandable stents: |
| Stented vessels: 8 | 8 iCASTc | ||
| Dijkstra et al. 201440 | Stented vessels: 56 | 25 Anacondam | Covered stents for all renal artery |
| 54 Advanta V12c | |||
| 2 unspecified bare stents | |||
| Donas et al. 201231 | Stented vessels: 44 | 29 Zenithb1 | Covered balloon expandable |
| 32 Advantac | |||
| Bare balloon expandable | |||
| 12 Palmazh1 | |||
| Suominen et al. 201332 | Target vessels: 54 | 21 Zenithb2 | Covered stents : |
| Stented vessels: 49 | 49 Advanta V12c | ||
| RRA/LRA/SMA | |||
| 17/16/21 | |||
| Greenberg et al. 200941 | Target vessels: 77 | 30 Zenithb1 | N.D. |
| Stented vessels: 54 | |||
| Tambyraja et al. 201142 | Target vessels: 79 | 29 Cook Zenithb3 | 29 unspecified covered stent |
| 18 unspecified bare stent | |||
| 2 unspecified stent | |||
| Stented vessels: 49 | |||
| Oderich et al. 201443 | Target vessels: 178 | 67 Zenithb1 | 58 Zenith alignment stentb1 |
| Stented vessels: 127 | |||
| 29 Express LD stentf1 | |||
| 25 eV3 IntraTherapeutics stentk2 | |||
| 20 iCAST Covered stentc | |||
| 2 Palmaz Genesis stenth2 | |||
| 1 Bridge Assurant stent e1 |
a1. W. L. Gore and Associates, Newark, DE, U.S. a2. W. L. Gore, Flagstaff, AZ, U.S. b1 .Cook Inc, Bloomington, IN, U.S. b2. Cook Australia Ltd, Australia. b3. William A. Cook Australia, Ltd., Brisbane, Australia b4. Cook Medical, Canvey Island, U.K. c Atrium Medical Corporation, Hudson, NH, U.S. d1. C.R. Bard, Murray Hill, NJ, U.S. d2. Bard Peripheral Vascular, Inc.US e1. Medtronic, Inc, Minneapolis, MN, U.S. e2. Medtronic Vascular, Santa Rosa, CA, U.S. f1. Boston Scientific, Natick, MA, U.S. f2. Boston Scientific, Bloomington, MN, U.S. g Endologix, Inc, Irvine, CA,U.S. h1 Cordis Corporation, Johnson & Johnson Company, Miami, FL, U.S. h2. Cordis, Warren, NJ, U.S. i Abbott Vascular, Temecula, CA, U.S. j Biltronic, Bulach, Switzerland. k1 ev3Endovascular Inc, Plymouth, MN, U.S. k2. Covidien, Plymouth, CA, U.S. l Ovation; TriVascular Inc., Santa Rosa, CA, U.S. m. Vascutek, Renfrewshire, Scotland, U.K.
Postoperative
The length of the hospital stay was reported for the F-EVAR group in 7 studies (mean, 7 (1–100) days). This information was not widely reported for the CH/SN group. Only 3 articles provided this information. The average length of hospital stay was 4.4 (2–50) days, which is likely an underrepresentation. The patency rate was 95.9% in the F-EVAR group and 97% in the CH-EVAR group (Tables 7 and 8).
Table 7. CH/SN-EVAR cohort clinical outcome.
| Authors | MAE (major adverse events) | 30-day mortality | Cause of death | Over 30day mortality | Cause of death | Patency (6 months) | Follow-up (months) | Length of stay (days) | Secondary intervention rate |
|---|---|---|---|---|---|---|---|---|---|
| Donas et al. 201231 | 1 MI; 2 Type II endoleaks; 1 RA occlusion; | 0 | N.D. | N.D. | 97.4% | 15.2 ± 6.2 | 3.5 | 3.3% | |
| Suominen et al. 201332 | 1 MI; 4 wound Infection; 1 common ilac artery embolism; 1 Type II endoleak; 2 RFI;1 Renal stent twist | 0 | 3 | 2 M.I. (5 and 7 months) 1 lower limb ischemia | N.D. | 22 (1–46) | N.D. | 25% | |
| Lee et al. 201433 | 3 Type I, 6 Type IIand 1 Type III endoleaks; 19 RFI; | 2 | 2 M.I. | 4 | 4 M.I. | 95% (24 months) | 21.1 (2.6–40.4) | N.D. | 4.7% |
| Schiro et al. 201319 | 1 MI; 1 arrhythmia; 5Type I endoleaks; 1 ARF(need dialysis) | 0 | 2 | 2 AAA rupture (11 and 16 months, caused by type I endoleak) | N.D. | 12 (5–24) | N.D. | 0 | |
| Ducasse et al.201334 | 1 stroke; 1 lower limb embolism; 1Type I; 4Type IIendoleaks; 2 ARF;2 accessory renal artery occlusion | 1 | 1 acute heart disease | 0 | N.D. | 18 (7–35) | 6.5 (4–50) | 9% | |
| Tolenaar et al. 201335 | 1Type I endoleak; 1 RA occlusion | 0 | 2 | 1tumor 1 M.I. (26 months) | 90.9% | 10.87 (m4–19.4) | 4 (3–9.5) | 0 | |
| Lgari et al. 201436 | 1 pneumonia; 1Type II endoleak; | 0 | 0 | 100% | 11 (2–22) | N.D. | 0 | ||
| Banno et al. 201437 | 1 arrhythmia; 1 COPD; 2 bowel ischemia; 1 colitis; 2 cerebral infarction; 8 wound complications; 3 intra-abdominal or retroperitoneal hemorrhage; 1 urinary tract infection; 2Type I; 2Type IIendoleaks; 7 RFI;1 dialysis;2 Renal infract | 3 | 3 bowel ischemia M.O.F. | 4 | Not related to AAA | 95.2% (12 months) | 12 (0–48) | N.D. | 28% |
N.D. not documented; M.I. myocardial infarction; COPD, chronic obstructive pulmonary disease; M.O.F. multiple organ failure; RFI, renal function impairment; ARF, acute renal failure; RF, renal failure; RA, renal artery; SMA, superior mesenteric artery.
Table 8. F-EVAR cohort clinical outcomes.
| Authors | MAE (major adverse events) | 30-day mortality | Cause of death | Over-30-day mortality | Cause of death | Patency (6 months) | Follow-up (months) | Length of stay (days) | Secondary intervention rate |
|---|---|---|---|---|---|---|---|---|---|
| Lee et al. 201438 | 2 MI; 1 stroke; | 0 | 2 | Not related to AAA | 96% | 6 | 4 (2–23) | 13.30% | |
| 3 Type IIand 1 Type III endoleaks; | |||||||||
| 1 RA occlusion | |||||||||
| Globalstar 201218 | 8 MI; 5 cardiac failure; 7 arrhythmia; 8 pneumonia; | 2 | Not related to AAA | 11 | Not related to AAA | 98% | 6 | 9 (1–100) | 10% (12 months) |
| 3 COPD; 5 GI ischemia;6 sepsis or septicemia; | |||||||||
| 9 wound complications; 3 TIA; 5 spinal ischemia; 3 lower limb ischemia; | |||||||||
| 17 Type I;22 Type II;5 Type III endoleaks; | |||||||||
| 2 RA perforation;1 RA stenosis; 4 RFI; 1 ARF; 11 RF;1 RA occlusion;3 Renal branch Bleeding; | |||||||||
| Liao et al. 201439 | 1 splenic embolization; | 0 | 2 | 1 C.O.P.D. + heart failure 1 bowel ischemia + M.O.F. | N.D. | 6.1 (2.7–8.3) | 3 (1–9) | 0 | |
| 2 Type IIendoleaks; 1 renal hematoma; | |||||||||
| Dijkstra et al. 201440 | 1 compartment syndrome left lower leg; 1 rupture of common iliac artery; 1 occluded SMA; 1 cutaneous bleeding; 1 hemorrhagic CVA; | 1 | M.O.F. | 1 | 1 stroke (6 months) | 96% (1 month) | 11 (1–29) | N.D. | 0 |
| 5 Type I, 12 Type II and 4 Type III endoleaks; | |||||||||
| 1 RFI; 1 RA occlusion | |||||||||
| Donas et al. 201231 | 1 occluded SMA; | 0 | N.D. | 97.7% | 13.2 ± 4.2 | 3.5 ± 1.1 | 3.4% | ||
| 3 Type I and 7 Type II endoleaks; | |||||||||
| 1 LRA occlusion | |||||||||
| Suominen et al. 201332 | 3 wound infection; 1 MI; 1 occluded common iliac artery; | 2 | 1 pneumonia 1 MI | 3 | 1 stroke (51 months) | N.D. | 22 (1–46) | N.D. | 10% (12 month) |
| 1 Type II endoleak; | 1 gastrointestinal bleeding (12 months) | ||||||||
| 1 RFI; 1 stent twist | 1 tumor (41 months) | ||||||||
| Greenberg et al. 200941 | 2 arrhythmia; 7 transfusions; 1 low extremite embolus; | 0 | 2 | not related to AAA (677 days) | 89% | 24 | 3.7 (1–8) | 17% | |
| 1 supplemental O2 ; 1 paralytic ileus; 1 wound infection 3 CHF; 1 arrhythmia; 1 pneumonia; 2 incisional hernia; | 1 MI (754 days) | 51/57 | |||||||
| 1 Type I, 1 Type II and 1 Type III endoleaks; | |||||||||
| 2 RFI; 4 RA stenosis; 2 RA occlusion | |||||||||
| Tambyraja et al. 201142 | 3 Iliac limb stenosis/occlusion; 1 SMA occlusion; | 0 | 4 | 1 stroke (22 months) | N.D. | 20 (7–62) | 3 (1–12) | 38% | |
| 2 Type I, 5 Type II and 2 Type III endoleaks; | 1 M.O.F. (18 months) | ||||||||
| 1 RA perforation; 9 RA stenosis; 2 RA occlusion; 3 stent migration | 1 pneumonia (15 months) | ||||||||
| 1 renal failure. (18 months) | |||||||||
| Oderich et al. 201443 | 3 bowel obstruction; 1 bowel obstruction; 1 stroke; 1 MI; 3 CHF; 2 cardiac ischemia; | 1 | Bowel ischemia (related to AAA) | 4 | 1 M.O.F. | 95% | 37 (3–65) | 3.3 ± 2.1 | 22% |
| 1 Type I and 16 Type IIendoleaks; | 2 M.I. | ||||||||
| 4 RA occlusion;12 RA stenosis; 8 RFI; 3 Renal failure | 2 unknown cause |
N.D. not documented; M.I. myocardial infarction; COPD, chronic obstructive pulmonary disease; M.O.F. multiple organ failure; RFI, renal function impairment; ARF, acute renal failure; RF, renal failure; RA, renal artery; SMA, superior mesenteric artery.
Post-operative major adverse events (MAE) of F-EVAR included 42 cardiac events (7.7%), 25 respiratory events (4.6%), 12 gastrointestinal events (2.2%), 11 neurological problems (2%), and 11 ischemic problem (2%). Here, 5 patients suffered from spinal ischemia18. One patient eventually made a full recovery, two made a partial recovery, and two did not recover. Cardiac events were also commonly reported after chimney/snorkel operations as noted in 4.5% of cases (5 of 110 patients from 6 studies). The wound complication rate was 6.36%.
The 30-day mortality rate was 3.8% (6/158) in the CH/SN-EVAR group and 1.1% (6/542) in the F-EVAR group. Six deaths occurred after CH/SN-EVAR, including three cases of MOF (multiple organ failure) caused by bowel ischemia and three cardiac events with no intraoperative deaths. One patient in the fenestrated group died due to myocardial infarction (MI), one died from pneumonia, one died of multisystem organ failure, and one patient died of bowel ischemia related to AAA. The reasons for the last two deaths were not specifically listed but were not related to AAA.
The mean follow-up was 14.7 months in the CH/SN-EVAR group (range 0–46 months) and 12.8 months in the F-EVAR group (range 1–65 months). During follow-up, 15 (8.46%) deaths occurred in the CH/SN-EVAR group (range 1–26 months) In addition, 29 patients (5.4%) died in the fenestrated group, and the last death occurred in the 51th month postoperation. After the chimney/snorkel operation, 7 (7/15 46.6%) patients died of cardiac causes. Two patients died of AAA rupture, and 4 died for reasons that were not specified but not related to AAA19. The 29 deaths that occurred after the fenestrated operation, including 4 deaths caused by cardiac and respiratory factors, 3 deaths due to MOF, 3 deaths due to stroke, 1 death due to CKD, 1 death due to gastrointestinal bleeding, 1 death due to tumor, and 16 deaths with non-specific causes but not related to AAA. A total of 21 deaths (13.3%) occurred in the CH/SN group, and 35 deaths (6.4%) occurred in the F-EVAR group. Cardiac disease was the most common cause of postoperative death (reported death cause).
Secondary interventions included the resolution of endoleaks, target vessel occlusion or stenosis, limb occlusion or stenosis, and excess bleeding or hematoma. A total of 58 secondary intervention events occurred after fenestrated operation, 15 of which were noted in the CH/SN-EVAR group.
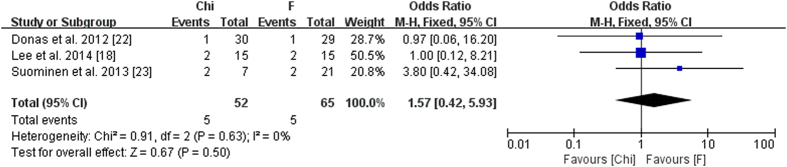
The comparison results were extracted from two groups of studies and provided in Table 5. We also performed a meta-analysis on two-arm studies (Fig. 2).
Figure 2. Meta-analysis on two-arm studies.
Endoleak events
The endoleaks were divided into groups according to leak site as described by Veith et al.20: type I, attachment site leak; type II, branch leak; type III, graft defect; and type IV, graft wall (fabric) porosity. Pooled analyses were performed by calculating the overall rates of events. All 15 articles reported endoleak events. Among a total of 106 patients, 19.6% of patients in the F-EVAR group (106/542) and 14.6% of patients in the CH/SN-EVAR group (29/158) experienced endoleak events. In the F-EVAR series, 29 type I (5.35%, 29/542), 69 type II (12.7%, 69/542), and 13 type III (2.4%, 13/542) endoleaks were detected. Of these, 9 type I endoleaks and 5 type III endoleaks were diagnosed intraoperatively and treated successfully using the kissing balloon technique, Palmaz stents, proximal coil embolization, or extender cuff placement. In addition, 4 type I, 9 type II, and 5 type III endoleaks required secondary intervention to seal the leak after the primary procedure. However, 2 type II endoleaks were not resolved by this treatment. These 2 patients required further observation. A total of 18 endoleaks disappeared during follow-up without any treatment. Here, 12 type I, 12 type II, and 12 type III became stable, making treatment unnecessary. No sac enlargement, complications, or deaths were observed during follow-up.
Post-procedural CT scans showed 12 type I (7.6%, 12/158), 16 type II (10.1%, 16/158) and 1 type III endoleaks in the CH/SN-EVAR group. Here, 6 patients (2 type I, 4 type II) required secondary intervention to resolve the endoleak. A total of 12 endoleaks resolved spontaneously during follow-up, ranging from 1 to 12 months. In addition, 7 type II endoleaks and one type I endoleak were under surveillance during follow-up. According to Schiro, two deaths were caused by aneurysm rupture related to a type I endoleak19.
Renal events
Here, 49 renal artery events were reported in F-EVAR group, including stenosis (26), occlusions (12), perforation/bleeding (7) and stent events (4). Of these, 5 events were resolved during the operation, and 32 events required secondary intervention. Four cases of renal artery occlusion were documented in the CH/SN-EVAR group.
Of the 15 studies included in the review, 10 reported renal events. These events were defined as an increase in serum creatinine to >2 mg/dl or by >30% relative to baseline during the peri-operative period. Of the 542 relevant patients, 30 (5.5%) developed renal impairment or failure following F-EVAR. In addition, 16 suffered from postoperative renal impairment. This complication was temporary for 7 patients, and only 2 patients required temporary or persistent postoperative dialysis. In contrast, 31 patients (19.6%) in the chimney group developed this complication, and 1 patient required persistent dialysis.
Discussion
The present review compared the clinical outcomes of patients who underwent F-EVAR and the chimney/snorkel technique for treatment of juxtarenal aortic aneurysms. The fenestrated technique exhibited advantages compared with the chimney/snorkel technique with respect to 30-day mortality, late mortality and renal adverse events. However, patients in the CH/SN-EVAR group experienced shorter operative and fluoroscopy procedures, required lower contrast doses, and suffered less blood loss during the operation. The present study aimed to evaluate the safety and efficacy of fenestrated and chimney techniques for JAAA.
The fundamental goal of fenestrated and chimney/snorkel techniques is to extend the sealed area and maintain flow to a branch vessel with or without the use of a stent-graft. F-EVAR is an expensive procedure that is tailored specifically to each individual patient’s anatomy. The design of each fenestrated device is very complicated and requires accurate calculations of the distances between the visceral vessels. This procedure can easily take 4 to 6 weeks or more in centers lacking staff experienced in this method, where measurements must be double-checked. This technique is costly, time-consuming and not suitable for urgent situations21,22,23,24. The chimney/snorkel technique is widely available and can be performed in smaller centers. The technique is less complex and can be performed with off-the-shelf endografts. The technique can be used to provide immediate treatment in acute cases25. The major concerns regarding chimney/snorkel are endoleaks and subsequent complications25,26.
With larger delivery systems, fenestrated grafts must use conduits to open the arteries to insert the transfer system. This procedure may increase the mean operative time and blood loss relative to chimney techniques (operative time: 261 min for F-EVAR vs 178 min for CH-EVAR; estimated blood loss: 534 ml for F-EVAR vs 332 ml for CH-EVAR). Fluoroscopy time and contrast dose were both slightly increased in the fenestrated series compared with the chimney series (64 min vs 54.6 min for fluoroscopy; 166 ml vs 146 ml for contrast dose). This finding is potentially attributed to the fact that the graft can be placed more accurately, and secondary procedures are often performed to verify that the new position is suitable.
Not surprisingly, 30-day mortality rates favor F-EVAR over CH/SN-EVAR (1% vs 3.8%) (Table 9). The increased 30-day mortality rate of CH/SN-EVAR may be attributed to the inclusion of acute patients (acute or semi-acute) and patients with more challenging anatomical structures. Late mortality was 5.35% in the F-EVAR group and 9.5% in the CH/SN-EVAR group. The all-cause death rate was 6.46% (35 patients) in the F-EVAR series and 13.3% (21 patients) in CH/SN-EVAR series. One possible explanation for the relatively increased mortality in the CH group is postprocedural renal dysfunction, which is a strong indicator of poor long-term survival27. In the current review, 30 (5.5%) renal events (renal impairment or failure) were reported in the F-EVAR series; 21.5% (34/158) patients suffered from postprocedural renal impairment or failure. Age is also a well-known predictor of mortality after AAA repair.
Table 9. Preoperative patient demographics and main outcomes in F-EVAR and CH-EVAR cohorts.
| F-EVAR | CH-EVAR | P value | |
|---|---|---|---|
| Preoperative | |||
| Age | 74 (47–86) | 75 (59–88) | |
| Aneurysm diameter | 64 (47–112) | 64.5 (33–110) | |
| Length of aneurysm neck | 6.7 ± 3.6(0–14.4) | 2.3 ± 4.3 (0–10) | |
| Outcomes | |||
| Operative time (min) | 261 (80–554) | 178 (75–810) | |
| Fluoroscopy time (min) | 64 (5–223) | 54.6 (15–290) | |
| Contrast dose (ml) | 166 (90–465) | 146 (45–465) | |
| Estimated blood loss (ml) | 534 (50–7000) | 332 (30–2204) | |
| Technique success rate | 98.8% | 97.4% | 0.15 |
| 30-day mortality | 6 (1.1%) | 8 (3.8%) | 0.02 |
| Over-30-day mortality | 29 (5.35%) | 15 (9.5%) | 0.01 |
| All-cause mortality | 35 (6.46%) | 21 (13.3%) | 0.0002 |
| Patency | 95.9% | 97% | 0.34 |
| Follow-up (month) | 12.8 (1–65) | 14.7 (0–46) | |
| Length of stay (day) | 7 (1–100) | 4.4 (2–50) | |
| Secondary intervention rate | 58 (10.7%) | 17 (9.5%) | 0.98 |
Endoleak is the most common procedure-specific feature and complications of chimney/snorkel and fenestrated grafting. The postoperative rate of type I endoleak was 7.6% (12/158) in the CH/SN-EVAR group, which was increased compared with the F-EVAR group (3.7% (20/542)) in the current review, excluding nine endoleaks of F-EVAR and one endoleak of CH/SN-EVAR that was detected and treated intraoperatively. In contrast to F-EVAR, chimney grafts were positioned along the outside of the main abdominal endograft and rely on the close conformation of the endograft and the aortic wall around the chimney stent. The gaps that formed between the grafts and the aortic wall can be imagined as small cylinders and conduits (CGs and main graft) within a larger cylinder (the aorta). The gaps may have increased the risk of type I endoleakage in the CH-EVAR group. Oversizing was considered an effective method of narrowing the gaps. In this study series, 5 studies, including 2 F-EVAR studies, reported increases in the main stent size ranging from 10% to 30%. Lachat proposed an elliptical model for the estimation of the appropriate aortic stent graft diameter. Generally, to facilitate the formation of a good seal, the graft should increase in size by 30%. Some authors also recommend that the endograft should be up to 40% oversized to minimize the effects of the chimney gaps28. The ideal amount of oversizing remains undetermined29. Recent in vitro data demonstrated that increasing oversizing significantly decreased the sizes of gap areas, but main endograft in-folding was also detected in most oversized stentgrafts28. Interestingly, 8 type I endoleaks disappeared during follow-up. We hypothesize that the longer the gutters, the more resistance to blood flow and the more likely the gutters will thrombose. However, no evidence is provided to support these hypotheses. A high secondary intervention rate was noted after F-EVAR and CH/SN-EVAR. The reintervention rate was approximately 10.7% in the F-EVAR group and 9.95% of in the CH/SN-EVAR group during follow-up. Persistent endoleakage, renal artery stenosis, occlusion, and bleeding all require secondary intervention to relieve these procedures.
One limitation of this study is that some studies did not report all relevant information (i.e., the aneurysm neck length, information regarding stents, fluoroscopy time, and blood loss are not widely reported). Second, case studies and technical reports were excluded. The small number of patients included was insufficient for analysis, and this limitation may have led to underestimation of the rate of post-procedural complications. A number of acute and semi-acute procedures were performed in the CH/SN-EVAR group, whereas fenestrated stents required 4 to 6 weeks to measure and manufacture. Publication bias must also be acknowledged. Nevertheless, this review describes the current state of experience with fenestrated and chimney/snorkel techniques and provides considerable insight into the potential indications, technical considerations, and complications associated with these procedures. Juxtarenal aneurysm has no standard classification system that was applied throughout the current published works on EVAR; however, each of these reports cases of JAA. JAA was defined as cases in which the cross-clamp could not be placed above the infrarenal area safely during open surgery. In studies of EVAR, the term JAA typically refers to normal inter-renal aortic aneurysms without renal artery involvement. There are two situations in which it is unclear whether the term JAA should be applies: 1) extension of the AAA immediately above the inter-renal aorta and 2) aneurismal involvement of renal artery origins with an otherwise normal inter-renal aorta30. True comparisons of F-EVAR and CH/SN-EVAR can be made only when study participants are anatomically homogeneous. In the endovascular era, any new classification of JRA should include the location and diameter of the aneurysm and the length and angulation of the aneurysm neck.
Conclusion
F-EVAR and CH-EVAR techniques are both effective treatment for JAAs patients. The fenestrated technique was considered the priority treatment for JAAs, whereas CH-EVAR is frequently performed in patients with more complex anatomy and urgent cases. Although the early and mid-term outcomes are satisfactory, the long-term durability of these techniques requires further assessment.
Additional Information
How to cite this article: Li, Y. et al. Fenestrated and Chimney Technique for Juxtarenal Aortic Aneurysm: A Systematic Review and Pooled Data Analysis. Sci. Rep. 6, 20497; doi: 10.1038/srep20497 (2016).
Footnotes
Author Contributions Conception and design: W.G. and Y.L. Analysis and interpretation: Y.L., Z.Z.H., W.G. and T.Z. Data collection: Z.Z.H., J.L., C.J.B. and S.S.L. Writing the article: Y.L., Z.Z.H. and C.J.B. Critical revision of the article: Y.L., Z.Z.H., W.G. and Y.Y.G. Final approval of the article: Y.L., Z.Z.H., J.L. and Y.Y.G., Statistical analysis: Y.L., J.L., Z.Z.H., T.Z. and S.S.L. Obtained funding: Logistics Research Plan of the Chinese People’s Liberation Army BWS13C029. Overall responsibility: W.G.
References
- Greenhalgh R. M. et al. United Kingdom EVAR Trial Investigators: Endovascular versus open repair of abdominal aortic aneurysm. N Engl J Med. 362(20), 1863–1871 (2010). [DOI] [PubMed] [Google Scholar]
- De Bruin J. L. et al. Long-term outcome of open or endovascular repair of abdominal aortic aneurysm. N Engl J Med. 362(20), 1881–1889 (2010). [DOI] [PubMed] [Google Scholar]
- Prinssen M. et al. A randomized trial comparing conventional and endovascular repair of abdominal aortic aneurysms. N Engl J Med. 351(16), 1607–1618 (2004). [DOI] [PubMed] [Google Scholar]
- Nordon I. M. et al. Modern treatment of juxtarenal abdominal aortic aneurysms with fenestrated endografting and open repair – a systematic review. Eur J Vasc Endovasc Surg. 38(1), 35–41 (2009). [DOI] [PubMed] [Google Scholar]
- Ricotta J. J. & Oderich G. S. Fenestrated and branched stent grafts. Perspect Vasc Surg Endovasc Ther. 20(2), 174–187 (2008). [DOI] [PubMed] [Google Scholar]
- Bruen K. J. et al. Endovascular chimney technique versus open repair of juxtarenal and suprarenal aneurysms. J Vasc Surg. 53(4), 895–904 (2011). [DOI] [PubMed] [Google Scholar]
- Allen B. T. et al. Preservation of renal function in juxtarenal and suprarenal abdominal aortic aneurysm repair. J Vasc Surg. 17(5), 948–958 (1993). [DOI] [PubMed] [Google Scholar]
- Ayari R. et al. Juxtarenal aneurysm. Comparative study with infrarenal abdominal aortic aneurysm and proposition of a new classification. Eur J Vasc Endovasc Surg. 22(2), 169–174 (2001). [DOI] [PubMed] [Google Scholar]
- Knott A. W. et al. Open repair of juxtarenal aortic aneurysms (JAA) remains a safe option in the era of fenestrated endografts. J Vasc Surg. 47(4), 695–701 (2008). [DOI] [PubMed] [Google Scholar]
- Sarac T. P. et al. Contemporary results of juxtarenal aneurysm repair. J Vasc Surg. 36(6), 1104–1111 (2002). [DOI] [PubMed] [Google Scholar]
- Jongkind V. et al. Juxtarenal aortic aneurysm repair. J Vasc Surg. 52(3), 760–767 (2010). [DOI] [PubMed] [Google Scholar]
- Taylor S. M., Mills J. L. & Fujitani R. M. The juxtarenal abdominal aortic aneurysm. A more common problem than previously realized? Arch Surg. 129(7), 734–737 (1994). [DOI] [PubMed] [Google Scholar]
- Mohan I. V., Laheij R. J. & Harris P. L. Risk factors for endoleak and the evidence for stent-graft oversizing in patients undergoing endovascular aneurysm repair. Eur J Vasc Endovasc Surg. 21(4), 344–9 (2001). [DOI] [PubMed] [Google Scholar]
- Sternbergh W. C. et al. Yoselevitz M, Money SR. Aortic neck angulation predicts adverse outcome with endovascular abdominal aortic aneurysm repair. J Vasc Surg. 35(3), 482–6 (2002). [DOI] [PubMed] [Google Scholar]
- Chaikof E. L. et al. Identifying and grading factors that modify the outcome of endovascular aortic aneurysm repair. J Vasc Surg. 35(5), 1061–6 (2002). [DOI] [PubMed] [Google Scholar]
- Farqui R. M. et al. Endovascular repair of abdominal aortic aneurysm using pararenal fenestrated stent-graft. J Endovasc Surg. 6(4), 354–358 (1999). [DOI] [PubMed] [Google Scholar]
- Greenberg R. K. et al. Should patients with challenging anatomy be offered endovascular aneurysm repair? J Vasc Surg. 38(5), 990–996 (2003). [DOI] [PubMed] [Google Scholar]
- British Society for Endovascular Therapy and GLOBALSTAR. Early Results of Fenestrated Endovascular Repair of Juxtarenal Aortic Aneurysms in the United Kingdom. Circulation. 125(22), 2707–2715 (2012). [DOI] [PubMed] [Google Scholar]
- Schiro A. et al. The Chimney Technique in Endovascular Aortic Aneurysm Repair: Late Ruptures After Successful Single Renal Chimney Stent Grafts. Ann Vasc Surg. 27 (7), 835–843 (2013). [DOI] [PubMed] [Google Scholar]
- Veith F. J. et al. Nature and significance of endoleaks and endotension: summary of opinions expressed at an international conference. J Vasc Surg. 35(5), 1029–1035 (2002). [DOI] [PubMed] [Google Scholar]
- Ketelsen D. et al. Endovascular aneurysm repair using a reverse chimney technique in a patient with Marfan syndrome and contained ruptured chronic type B dissection. Cardiovasc Intervent Radiol. 34(5), 1080–1084 (2011). [DOI] [PubMed] [Google Scholar]
- Haulon S. et al. An analysis of the French multicentre experience of fenestrated aortic endografts: medium-term outcomes. Ann Surg. 251(2), 357–62 (2010). [DOI] [PubMed] [Google Scholar]
- Greenberg R. K. et al. Intermediate results of a United States multicenter trial of fenestrated endograft repair for juxtarenal abdominal aortic aneurysms. J Vasc Surg. 50(4), 730–737 (2009). [DOI] [PubMed] [Google Scholar]
- O’neill S. et al. A prospective analysis of fenestrated endovascular grafting: intermediate-term outcomes. Eur J Vasc Endovasc Surg. 32(2), 115–123(2006). [DOI] [PubMed] [Google Scholar]
- Banno H., Cochennec F., Marzelle J. & Becquemin J. P. Comparison of fenestrated endovascular aneurysm repair and chimney graft techniques for pararenal aortic aneurysm. J Vasc Surg. 60(1), 31–39 (2014). [DOI] [PubMed] [Google Scholar]
- Massmann A., Serracino-Inglott F. & Buecker A. Endovascular Aortic Repair With the Chimney Technique Using the Ultra Low–Profile Ovation Stent-Graft for Juxtarenal Aneurysms Having Small Iliac Access Vessels. Cardiovasc Intervent Radiol. 37(2), 488–492 (2014). [DOI] [PubMed] [Google Scholar]
- Haddad F. et al. Fenestrated endovascular grafting: the renal side of the story. J Vasc Surg. 41(2), 181–190 (2005). [DOI] [PubMed] [Google Scholar]
- Mestres G. et al. The best conditions for parallel stenting during EVAR: an in vitro study. Eur J Vasc Endovasc Surg. 44(5), 468–473 (2012). [DOI] [PubMed] [Google Scholar]
- Bruen K. J., Feezor R. J., Daniels M. J., Beck A. W. & Lee W. A. Endovascular chimney technique versus open repair of juxtarenal and suprarenal aneurysms. J Vasc Surg. 53(4), 895–904 (2011). [DOI] [PubMed] [Google Scholar]
- Ayari R., Paraskevas N., Rosset E., Ede B. & Branchereau A. Juxtarenal aneurysm. Comparative study with infrarenal abdominal aortic aneurysm and proposition of new classification. Eur J Vasc Endovasc Surg. 22(2), 169–174 (2001). [DOI] [PubMed] [Google Scholar]
- Donas K. P. et al. The role of open and endovascular treatment with fenestrated and chimney endografts for patients with juxtarenal aortic aneurysms. J Vasc Surg. 56(2), 285–90 (2012). [DOI] [PubMed] [Google Scholar]
- Suominen V. Pimenoff G. & Salenius J. Fenestrated and chimney endografts for juxtarenal aneurysms: early and midterm results. Scandinavian Journal of Surgery. 102(3), 182–188 (2013). [DOI] [PubMed] [Google Scholar]
- Lee J. T., Varu V. N., Tran K. & Dalman R. L. Renal function changes after snorkel/chimney repair of juxtarenal aneurysms. J Vasc Surg. 60(3), 563–570 (2014). [DOI] [PubMed] [Google Scholar]
- Ducasse E. et al. The “Open” Chimney Graft Technique for Juxtarenal Aortic Aneurysms with Discrepant Renal Arteries. Eur J Vasc Endovasc Surg. 47(7), 124–130 (2014). [DOI] [PubMed] [Google Scholar]
- Tolenaar J. L., Zandvoort H. J., Moll F.L. & van Herwaarden J. A. Technical considerations and results of chimney grafts for the treatment of juxtarenal aneursyms. J Vasc Surg. 58(3), 607–615 (2013). [DOI] [PubMed] [Google Scholar]
- Iqari K., Kudo T., Uchiyama H., Toyofuku T. & Inoue Y. Early Experience with the Endowedge Technique and Snorkel Technique for Endovascular Aneurysm Repair with Challenging Neck Anatomy. Ann Vasc Dis. 7(1), 46–51 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banno H., Cochennec F., Marzelle J. & Becquemin J. P. Comparison of fenestrated endovascular aneurysm repair and chimney graft techniques for pararenal aortic aneurysm. J Vasc Surg. 60(1), 31–39 (2014). [DOI] [PubMed] [Google Scholar]
- Lee J. T., Lee G. K., Chandra V. & Dalman R. L. Comparison of fenestrated endografts and the snorkel/chimney technique. J Vasc Surg. 60(4), 849–57 (2014). [DOI] [PubMed] [Google Scholar]
- Liao T. H. et al. Preliminary results of Zenith Fenestrated abdominal aortic aneurysm endovascular grafts. The American Journal of Surgery. 207(3), 417–421 (2014). [DOI] [PubMed] [Google Scholar]
- Martijn L. et al. Dutch experience with the fenestrated Anaconda endograft for short-neck infrarenal and juxtarenal abdominal aortic aneurysm repair. J Vasc Surg. 60(2), 301–7 (2014). [DOI] [PubMed] [Google Scholar]
- Greenberg R. K. et al. Charles Sternbergh et al. Intermediate results of a United States multicenter trial of fenestrated endograft repair for juxtarenal abdominal aortic aneurysms. J Vasc Surg. 50(4), 730–737 (2009). [DOI] [PubMed] [Google Scholar]
- Tambyraja A. L. et al. Fenestrated Aortic Endografts for Juxtarenal Aortic Aneurysm: Medium Term Outcomes. Eur J Vasc Endovasc Surg. 42(1), 54–58 (2011). [DOI] [PubMed] [Google Scholar]
- Oderich G. S. et al. Results of the United States multicenter prospective study evaluating the Zenith fenestrated endovascular graft for treatment of juxtarenal abdominal aortic aneurysms. J Vasc Surg. 60(6), 1420–1428 (2014). [DOI] [PubMed] [Google Scholar]