Abstract
Root-knot nematodes (RKN; Meloidogyne sp.) are a major threat to crops in tropical and subtropical regions worldwide. The use of resistant crop varieties is the preferred method of control because nematicides are expensive, and hazardous to humans and the environment. Peanut (Arachis hypogaea) is infected by four species of RKN, the most damaging being M. arenaria, and commercial cultivars rely on a single source of resistance. In this study, we genetically characterize RKN resistance of the wild Arachis species A. stenosperma using a population of 93 recombinant inbred lines developed from a cross between A. duranensis and A. stenosperma. Four quantitative trait loci (QTL) located on linkage groups 02, 04, and 09 strongly influenced nematode root galling and egg production. Drought-related, domestication and agronomically relevant traits were also evaluated, revealing several QTL. Using the newly available Arachis genome sequence, easy-to-use KASP (kompetitive allele specific PCR) markers linked to the newly identified RKN resistance loci were developed and validated in a tetraploid context. Therefore, we consider that A. stenosperma has high potential as a new source of RKN resistance in peanut breeding programs.
Keywords: Arachis, peanut, QTL, root-knot nematode resistance, marker-assisted selection, drought, yield, introgression
Nematodes of the genus Meloidogyne, or root-knot nematodes (RKN) cause significant economic losses in agricultural crops worldwide. RKNs are sedentary obligate plant endoparasites, and, as a result of nematode feeding, large galls or “knots” are formed throughout the root system of infected plants. Severe infections reduce yields in numerous crops and can also affect consumer acceptance of many plants, especially vegetables. RKNs establish a complex biotrophic relationship with their hosts. Second-stage juveniles invade root tip cells, migrate through the root cortex, and, after electing suitable root cells, induce redifferentiation into specialized feeding cells. Feeding cells enlarge and are converted into multinucleate giant cells through synchronous nuclear divisions without cell division. Hyperplasia and hypertrophy of the surrounding cortical cells lead to the formation of the typical root gall, the primary visible symptom of infection. Plant nutrient and water uptake is substantially reduced by the resulting damage to the root system, and infested plants are therefore weak and low yielding (Caillaud et al. 2008). Management of nematodes typically includes use of chemicals, crop rotation, and use of resistant cultivars. Most chemical control agents against RKNs have been prohibited for environmental and health reasons (De Waele et al. 1989), and crop rotation is limited because of the wide range of hosts of RKN (Dong et al. 2007). Therefore, utilization of resistant cultivars is considered the best alternative for production in nematode-infested areas.
Peanut (Arachis hypogaea L.), an important oilseed and food crop worldwide, is affected by four RKN species, M. hapla Chitwood, M. javanica (Treub) Chitwood, M. haplanaria n. sp., and M. arenaria (Neal) Chitwood, with the latter being the most destructive (Carneiro et al. 2003; Eisenback et al. 2003). Cultivated peanut has only moderate levels of resistance to RKN (Holbrook and Stalker 2003), whereas wild relatives of peanut harbor much greater levels of resistance (Nelson et al. 1989; Holbrook and Noe 1990). Resistance to RKN has been introgressed into peanut from its wild relative A. cardenasii Krapov. & W.C. Greg. through the tetraploid (Simpson et al. 1993), and the hexaploid introgression pathways (Garcia et al. 1995; Stalker et al. 2002). In infested regions, the use of resistant cultivars that harbor resistance from this wild species, such as COAN, NemaTAM, Tifguard, Webb, Tifguard High O/L is essential for production and profitability (Simpson and Starr 2001; Simpson et al. 2003, 2013; Holbrook et al. 2008).
The resistance to RKN in these modern varieties is derived from a single chromosome segment from A. cardenasii (Burow et al. 2001; Nagy et al. 2010). However, since only a single source of resistance is used, there is a clear possibility that the resistance will be broken. New sources of resistances are very likely to be needed. Wild relatives of peanut are a rich source of alleles for resistance to biotic and abiotic stresses because they have been selected during evolution in a range of environments (Stalker and Moss 1987; Leal-Bertioli et al. 2012). In particular, A. stenosperma Krapov. & W. C. Greg has been shown to be highly resistant to M. arenaria (Proite et al. 2008; Leal-Bertioli et al. 2010).
A. stenosperma is also resistant to several fungal pathogens of peanut, including rust (Puccinia arachidis Speg.), late leaf spot (Cercosporidium personatum Berk. & M.A. Curtis), web blotch (Phoma arachidicola Marasas, Pauer & Boerema), and scab (Sphaceloma arachidis Bitanc. & Jenkins) (Leal-Bertioli et al. 2010; Michelotto et al. 2015). Furthermore, A. stenosperma is relatively conservative in terms of water use under limited availability, showing a higher soil moisture threshold for transpiration decline than the cultivated peanut (Leal-Bertioli et al. 2012, and unpublished data). To study the genetics of these potentially valuable traits we have previously developed and characterized a diploid mapping population from a cross of A. duranensis (the A-subgenome ancestor of cultivated peanut) and A. stenosperma (Moretzsohn et al. 2005; Leal-Bertioli et al. 2009; Shirasawa et al. 2013). To enable the introgression of its wild alleles into cultivated peanut we have developed A. stenosperma-derived induced allotetraploids that are sexually compatible with A. hypogaea (Leal-Bertioli et al. 2015c).
In this work, we identified genomic regions that control two main components of nematode infection: gall and egg production. Quantitative trait loci (QTL) were identified in positions distinct from the genetic location of the A. cardenasii chromosomal segment introgressed previously, indicating that the genes involved are different. QTL were also identified for drought-related, domestication, and agronomically important traits. KASP (kompetitive allele specific PCR) markers were designed for the genome regions that confer strongest nematode resistance and validated in tetraploid backgrounds. We envisage that these markers will be useful for marker-assisted selection in breeding programs.
Materials and Methods
Plant material
Arachis species seeds were obtained from the Brazilian Arachis germplasm collection, maintained at Embrapa Genetic Resources and Biotechnology (Brasília-DF, Brazil). A. monticola seeds were obtained from the United States Department of Agriculture collection (USDA; http://www.ars-grin.gov/). The parental accessions for the recombinant inbred lines (RILs) were two A-genome accessions that contrast for nematode resistance (Proite et al. 2008): A. duranensis Krapov. & W. C. Greg. K7988 and A. stenosperma Krapov. & W. C. Greg. V10309 (USDA PI666100), used as the female and male parents, respectively. The F2 population derived from this cross was used in the genetic studies described in Moretzsohn et al. 2005, Bertioli et al. 2009 and Leal-Bertioli et al. 2009. The F6 RIL population used for this study composed of 93 individuals was obtained by single seed descent from this F2 population. Previous genetic studies of this population are described in Shirasawa et al. (2013) and Bertioli et al. (2014).
Phenotyping
Nematode resistance:
The parents of the population, A. duranensis K7988 and A stenosperma V10309, and the wild resistant accession A. cardenasii GKP10017 (PI648354), were evaluated for resistance to four nematode species: M. hapla, M. arenaria race 1, M. arenaria race 2, M. javanica race 4 (Carneiro et al. 2003), and the peanut pod nematode Ditylenchus africanus Wendt, Swart, Vrain & Webster (Wendt et al. 1995). The susceptible peanut cultivar IAC-Tatu (A. hypogaea subsp. fastigiata var. fastigiata) was used as susceptible control. All Meloidogyne populations were maintained on the susceptible tomato variety ‘Santa Cruz’ at Embrapa Genetic Resources and Biotechnology. D. africanus was obtained from South Africa (De Waele et al. 1989), and multiplied in alfalfa plants in vitro. All assays were performed in a greenhouse under quarantine conditions. Plants (10- to 12-wk-old) were inoculated with 5000–10,000 eggs; 10 wk after inoculation, eggs were extracted from roots using 0.5% NaOCl (Hussey and Barker 1973), stained with acid fuchsin and counted using a Peters slide under the microscope. For D. africanus, nematodes were extracted from whole plants 35 d after inoculation. The nematode reproductive factor (RF) was calculated as RF = Pf/Pi (Oostenbrink 1966), where Pf = final nematode population, and Pi = initial nematode population. Average reproduction factors, log (x + 1) transformed, were compared by the Tukey test with significance at the 5% probability level. Treatments with RF < 1.00 were considered resistant to the nematode species and, those with RF > 1.0 as susceptible (Oostenbrink 1966).
Eighty-two lines of the recombinant inbred F6 population (A. duranensis K7988 × A. stenosperma V10309), the parents and controls were evaluated for resistance to M. arenaria race 1. Bioassays were performed essentially as described in Morgante et al. (2013). Briefly, 4-wk-old plantlets were inoculated with 50,000 eggs of M. arenaria extracted from tomato cv. UC82 plants. Five replicate plants of each genotype were tested; the five sets of replicates were arranged on greenhouse benches in a randomized complete block design. Bioassays were performed in each of two years (2011 and 2013). The peanut cultivar Florunner (Norden et al. 1969) was used as a susceptible control. Temperature in the greenhouse was maintained between 28° and 35° in the day, and 24° at night. Root systems were washed free of soil and scored for phenotype 9 wk (experiment I—2011) or 11 wk (experiment II—2013) after inoculation. A 0–10 root-gall rating scale (Bridge and Page 1980) was used to evaluate resistance reaction to nematodes [root galling index (GI)] (Wang et al. 2012). Nematode reproduction was evaluated as another phenotypic component of resistance. Eggs were extracted in NaOCl from weighed root systems and counted to provide numbers of eggs per gram of root (EGR).
Agronomic, domestication, and drought-related traits:
Plants were grown in long trays (1 m × 30 cm × 30 cm), with enough space for lateral branch trailing and seed set. Branches were regularly trailed back to the pots to ensure that pegs would get to the soil. Between 40 and 60 d after planting, height of main stem (MSH) and lateral branches were counted (NLB), and measured (LBL). At harvest (about 120 d after planting), peg length (PL) was measured on six pods. Harvested seeds were counted (SN), dried at 20° at 15% RH for 15 d, and then weighed. Pod isthmus was measured (Pod_constr). Plants were oven-dried for 96 hr at 80°. Aerial parts and roots were weighed separately (ADW, RDW), and the total weight, including that of seeds was added, and comprised total biomass (TB). The weight of 10 seeds (10-SW), randomly selected, was used for QTL analyses. Evaluations were conducted in each of 2 years. Pollen viability (PV) was estimated by the staining method with acetic carmine (Linsley and Cazier 1963). For each genotype, 1000 pollen grains were analyzed from oblong anthers as follows: 100 pollen grains per anther, two anthers per flower, and five flowers per plant.
Drought-related traits SPAD chlorophyll meter reading (SCMR), and specific leaf area (SLA), were evaluated on the first expanded leaves of four lateral branches of each F6 plant and parents, as described in Leal-Bertioli et al. (2012). All SCMR and SLA evaluations were performed in the morning, at three stages: 40, 60, and 120 d after germination. Transpiration per total leaf area (TR/LA, proxy for stomatal conductance) was evaluated on the parents. Transpiration was measured gravimetrically on well-watered plants over three subsequent days. TR/LA was expressed as g/cm.
Statistical analysis
Phenotypic data were analyzed using the Statistical package R (R team). Data normality was tested using the Shapiro test. Tukey HSD test (normally distributed data) and Kruskal-Wallis one-way analysis of variance by ranks (non-normally distributed data) were used for comparison of averages at P = 5%. For QTL identification, non-normal data were transformed to Log10 (x + 1).
Marker development and genotyping
Total genomic DNA extraction and quantification were performed essentially as described in Leal-Bertioli et al. (2015b). Single nucleotide polymorphisms (SNPs) were identified using transcriptome of roots of young seedlings and developing seeds of A. duranensis PI 475887, and A. duranensis Grif 15036 (Nagy et al. 2012). SNPs were also identified between A. duranensis K7988 and A. stenosperma V10309 ESTs (Guimarães et al. 2012). SNP genotyping was performed using the GoldenGate Illumina array described by Nagy et al. (2012), and calling of genotypes was using GenomeStudio 2011.1. Scores to each data point were assigned using the software GenCall. The GenCall score is a value between zero and one, and is primarily designed to filter out failed genotypes, DNAs, and/or loci (Oliphant et al. 2002). Scores less than 0.2 usually indicate failed assays, and more than 0.7 usually report high-quality genotypes. All markers used for map construction are described in Supporting Information, File S1.
Genetic mapping and QTL analyses
Two linkage maps for this same RIL population have been previously constructed (Bertioli et al. 2014; Shirasawa et al. 2013). We used all genotyped markers of these two studies plus SNP markers genotyped in the present work to construct a saturated map using JoinMap 4.0 (Van Ooijen 2006). Based on this map, genomic regions with no recombination or identical markers were identified, and all loci but one were removed from these regions. Remaining loci were used to construct a framework map using Mapmaker Macintosh 2.0 (Lander et al. 1987; Lincoln et al. 1992). A χ2 test was performed to test the null hypothesis of 1:1 segregation on all scored markers. A minimum LOD score of 9.0 and maximum recombination fraction of 0.35 were set as thresholds for linkage groups (LG) determination with the “group” command. The most likely marker order within each LG was estimated by the matrix correlation method using the “first order” command. Marker orders were confirmed by comparing the log-likelihood of the possible orders by permuting all adjacent triple orders (“ripple” command). After establishment of the group orders, the LOD score was set to 3.0 in order to include additional markers in the groups. The “try” command was then used to determine the exact position of the new markers within each group. The new marker orders were again confirmed with the “ripple” command. Recombination fractions were converted into map distances in centimorgans (cM) using the Kosambi’s mapping function (Lander et al. 1987; Lincoln et al. 1992).
This newly developed framework map was used for QTL analysis. Phenotyping data included: components of resistance to M. arenaria race 1 and drought-related, domestication and agronomic traits (File S1). Traits evaluated in different trials or years were analyzed separately. The normality of data distribution was evaluated by skewness and kurtosis values using WinQTL Cartographer, version 2.5 (Wang et al. 2006). QTL were mapped by using the composite interval mapping (CIM) method, proposed by Zeng (1993, 1994) also using WinQTL Cartographer. Some of the data sets were non-normally distributed and were log transformed. CIM analysis used the Standard Model (Model 6), scanning the genetic map, and estimating the likelihood of a QTL and its corresponding effects at every 1 cM, while using eight significant marker cofactors to adjust the phenotypic effects associated with other positions in the genetic map. A window size of 10 cM was used, and therefore cofactors within 10 cM on either side of the QTL test site were not included in the QTL model. Thresholds were determined for each trait by permutation tests (Churchill and Doerge 1994; Doerge and Churchill 1996), using 1,000 permutations and a significance level of 0.05. Graphic presentation of the LGs and the significant QTL was drawn with MapChart, version 2.1 (Voorrips 2002).
The effect of markers linked to QTL contributing to nematode resistance was analyzed individually and cumulatively. For the first analyses, the phenotypic average of the RILs with each of the positive alleles (presence of the marker closest linked to the QTL) was calculated and compared with the average of the RILs without the positive alleles. To analyze the cumulative effect of the alleles, phenotypic averages of the RILs with any combination of 0, 2, 4 or 6 positive alleles were compared. Class-specific means of GI and EGR and standard errors were calculated for each genotypic class.
KASP marker development and validation on tetraploid backgrounds
The longer-term aim of this research is the introgression of the A. stenosperma chromosomal segments that confer nematode resistance into cultivated peanut by marker-assisted backcrossing. For this, it is necessary that the markers function within a tetraploid genetic context. We tested a strategy that uses the genome sequence of A. duranensis V14167 (http://www.peanutbase.org). In principle this strategy allows the development of markers to directed chromosomal regions. Also because of the inclusion of A. hypogaea controls in the marker tests, the results of the test would give a measure of how well the genome sequence of A. duranensis V14167 serves as a proxy for the A-subgenome of A. hypogaea.
SNP discovery:
SNPs were discovered by aligning sequences from the nematode resistant A. stenosperma V10309 with the reference genome of A. duranensis using the Bowtie2 pipeline (Langmead & Salzberg 2012) by tagging the specific regions where the main QTL for nematode resistance were identified on pseudomolecules Adur.A02, Adur.A04 and Adur.A09, using default parameters. SNPs were called using SAMtools (Li et al., 2009).
Primer design and test:
Allele-specific forward primers and a common reverse primer were designed for use in KASP (Kompetitive Allele Specific PCR) assays (LGC Genomics Ltd. Hoddesdon, U.K.), using BatchPrimer3 (http://probes.pw.usda.gov/batchprimer3/) with the “Allele specific primers and allele flanking primers” option. The parameter used were 60–120 bp in size, Tm between 58–60° and GC content between 30 and 80%. The alternative alleles were marked with 6-FAM and reference alleles (A. duranensis V14167, http://www.peanutbase.org) with VIC. For each SNP, two allele-specific forward primers, and one common reverse primer were designed, essentially as described in (Leal-Bertioli et al. 2015b). All KASP primers are listed on Table 1.
Table 1. Information about KASP assays, including primer name (with linkage group, position on A. duranensis pesudomolecule, orientation, and dye), primer sequence and type, melting temperature, GC content, and SNP type amplification pattern.
| Primer Name (LG, Position_Orientation_Dye)a | Sequence | Type | Tm | GC% | SNP | Amplification patternb |
|---|---|---|---|---|---|---|
| Nem_Aradu.A02_76738828_Fwd_ | CAACTAAGCAACAGGAAAGACG | AF | 58.93 | 47.62 | (As = BatSten = GregSten) ≠ Ad ≠ (Ah = Am)* | |
| Nem_Aradu.A02_76738828_Rev_FAM | GAAGGTGACCAAGTTCATGCTGATCATTGTTGCCGAATCTC | AS | 58.09 | 45 | A | |
| Nem_Aradu.A02_76738828_Rev_VIC | GAAGGTCGGAGTCAACGGATTGATCATTGTTGCCGAATCTT | AS | 57.61 | 40 | G | |
| Nem_Aradu.A02_83608917_Fwd_ | TTTGTGGCTGCAATAACTTCA | AF | 59.36 | 38.1 | Ad ≠ (As = BatSten = GregSten = Ah = Am) | |
| Nem_Aradu.A02_83608917_Rev_VIC | GAAGGTCGGAGTCAACGGATTCATGACATTGTAAGTGGCAAAAAC | AS | 60.66 | 37.5 | G | |
| Nem_Aradu.A02_83608917_Rev_FAM | GAAGGTGACCAAGTTCATGCTCATGACATTGTAAGTGGCAAAAAT | AS | 60.17 | 33.33 | A | |
| Nem_Aradu.A02_84440546_Rev_ | GCGATTAATACATTCAACAACCA | AF | 58.93 | 34.78 | (As = BatSten = GregSten) ≠ (Ad = Ah = Am)* | |
| Nem_Aradu.A02_84440546_Fwd_FAM | GAAGGTGACCAAGTTCATGCTGCTCTCCTTCTTGGTGGTTTG | AS | 61.17 | 52.38 | A | |
| Nem_Aradu.A02_84440546_Fwd_VIC | GAAGGTCGGAGTCAACGGATTGCTCTCCTTCTTGGTGGTTTA | AS | 58.45 | 47.62 | G | |
| Nem_Aradu.A02_84440594_Rev_ | GGAAGCGGATTCCACTCA | AF | 59.72 | 55.56 | (As = BatSten = GregSten) ≠ Ad ≠ (Ah = Am)* | |
| Nem_Aradu.A02_84440594_Fwd_FAM | GAAGGTGACCAAGTTCATGCTGAAGTGTGTCATAATCTCCAAAGTG | AS | 59.12 | 40 | A | |
| Nem_Aradu.A02_84440594_Fwd_VIC | GAAGGTCGGAGTCAACGGATTCTGAAGTGTGTCATAATCTCCAAAGTA | AS | 60 | 37.04 | G | |
| DS_c1614_886_A02_88903581_Rev_ | AGCTGAGGAGAACCCCTTTT | AF | 59.32 | 50 | Ad ≠ (As = BatSten = GregSten = Ah = Am) | |
| DS_c1614_886_A02_88903581_Fwd_FAM | GAAGGTGACCAAGTTCATGCTCAGATACAGTGACAGATATGAATGGTG | AS | 61.02 | 40.74 | G | |
| DS_c1614_886_A02_88903581_Fwd_VIC | GAAGGTCGGAGTCAACGGATTTCAGATACAGTGACAGATATGAATGGTA | AS | 60.2 | 35.71 | A | |
| TOG894171_695_A02_92486807_Rev_ | CTTCTGTTGGGGTGTTGGAT | AF | 59.82 | 50 | (As = BatSten = GregSten) ≠ (Ad = Ah = Am)* | |
| TOG894171_695_A02_92486807_Fwd_VIC | GAAGGTCGGAGTCAACGGATTYATTAATCAGGCAATAGCAACG | AS | 59.65 | 36.36 | G | |
| TOG894171_695_A02_92486807_Fwd_FAM | GAAGGTGACCAAGTTCATGCTCTAYATTAATCAGGCAATAGCAACA | AS | 59.62 | 32 | A | |
| Nem_Aradu.A02_92631394_Fwd_ | AAGAAATTGGGCGTTTTCAG | AF | 68 | 118 | (As = Ah = Am) ≠ Ad ≠ (BatSten = GregSten)* | |
| Nem_Aradu.A02_92631394_Rev_FAM | GAAGGTGACCAAGTTCATGCTATCCCCATATCTAGTGTCTTCTGC | AS | 59.89 | 45.83 | A | |
| Nem_Aradu.A02_92631394_Rev_VIC | GAAGGTCGGAGTCAACGGATTCAATCCCCATATCTAGTGTCTTCTGT | AS | 60.94 | 42.31 | G | |
| Nem_Aradu.A04_109789467_Rev_ | CCAAAGCTCTTTTCCAGGTT | AF | 58.44 | 45 | (As) ≠ (BatSten = GregSten) ≠ (Ad = Ah = Am)* | |
| Nem_Aradu.A04_109789467_Fwd_FAM | GAAGGTGACCAAGTTCATGCTCAATAGAAACAGCAAAGCAATGG | AS | 60.98 | 39.13 | A | |
| Nem_Aradu.A04_109789467_Fwd_VIC | GAAGGTCGGAGTCAACGGATTCAATAGAAACAGCAAAGCAATGA | AS | 59.44 | 34.78 | G | |
| TOG906490_74_A04_106874754_Fwd_ | TTCATTCCATAAGCCCAACC | AF | 59.76 | 45 | (As = BatSten = GregSten) ≠ (Ad = Ah = Am)* | |
| TOG906490_74_A04_106874754_Rev_VIC | GAAGGTCGGAGTCAACGGATTAACTTTTCGAATCCTCATATTGCT | AS | 59.57 | 33.33 | A | |
| TOG906490_74_A04_106874754_Rev_FAM | GAAGGTGACCAAGTTCATGCTTTTTCGAATCCTCATATTGCG | AS | 60.05 | 38.1 | C | |
| TOG937303_589_A04_108564975_Rev_ | CCATCACAAAAGAACAAAACAAC | AF | 58.57 | 34.78 | ND | |
| TOG937303_589_A04_108564975_Fwd_FAM | GAAGGTGACCAAGTTCATGCTAATTACTCGTTGGAGTAGTTGATGG | AS | 59.85 | 40 | G | |
| TOG937303_589_A04_108564975_Fwd_VIC | GAAGGTCGGAGTCAACGGATTGAATTACTCGTTGGAGTAGTTGATGA | AS | 59.96 | 38.46 | A | |
| Nem_Aradu.A04_113373632_Rev_ | TCCTCATCATCATCTTTCTCCA | AF | 59.63 | 40.91 | ND | |
| Nem_Aradu.A04_113373632_Fwd_FAM | GAAGGTGACCAAGTTCATGCTAGGTTGGTCAAGGGTTTCAG | AS | 59.04 | 50 | A | |
| Nem_Aradu.A04_113373632_Fwd_VIC | GAAGGTCGGAGTCAACGGATTAGGTTGGTCAAGGGTTTCAA | AS | 59.42 | 45 | G | |
| TOG896942_133_A09_114770700_Fwd_ | AAAGAAAGGGCTCCCTAATTTC | AF | 59.16 | 40.91 | (As = BatSten = GregSten) ≠ (Ad = Ah = Am)* | |
| TOG896942_133_A09_114770700_Rev_FAM | GAAGGTGACCAAGTTCATGCTGGGCACAAAAATTCGCTACA | AS | 61 | 45 | T | |
| TOG896942_133_A09_114770700_Rev_VIC | GAAGGTCGGAGTCAACGGATTGGCACAAAAATTCGCTACG | AS | 59.32 | 47.37 | C | |
| Nem_Aradu.A04_114769893_Fwd_ | TCAAGTCGTGTGTTCTCTACACC | AF | 59.32 | 47.83 | (As = BatSten = GregSten = Ad = Ah = Am) | |
| Nem_Aradu.A04_114769893_Rev_FAM | GAAGGTGACCAAGTTCATGCTTCTTGTGACATGAGCTACAACTTCT | AS | 59.53 | 40 | C | |
| Nem_Aradu.A04_114769893_Rev_VIC | GAAGGTCGGAGTCAACGGATTTTGTGACATGAGCTACAACTTCG | AS | 60.35 | 43.48 | A | |
| Nem_Aradu.A04_115457181_Rev_ | TGTGGACAGATGGAAAACACA | AF | 59.99 | 42.86 | (As = GregSten) ≠ (BatSten = Ad = Ah = Am)* | |
| Nem_Aradu.A04_115457181_Fwd_VIC | GAAGGTCGGAGTCAACGGATTTTCGGCGTTGGACTGTG | AS | 60.4 | 58.82 | G | |
| Nem_Aradu.A04_115457181_Fwd_FAM | GAAGGTGACCAAGTTCATGCTCTTCGGCGTTGGACTGTA | AS | 58.35 | 55.56 | A | |
| Nem_Aradu.A04_117955004_Fwd_ | TCACGGTCCATGTATTCAGC | AF | 59.53 | 50 | (As = GregSten = BatSten = Am) ≠ (Ad = Ah)* | |
| Nem_Aradu.A04_117955004_Rev_VIC | GAAGGTCGGAGTCAACGGATTCGTTAGCAGTTGGACAAACAAAC | AS | 59.76 | 50 | G | |
| Nem_Aradu.A04_117955004_Rev_FAM | GAAGGTGACCAAGTTCATGCTCGTTAGCAGTTGGACAAACAAAT | AS | 60.95 | 43.48 | A | |
| Nem_Aradu.A04_121132127_Rev_ | AGATTTTCTGGGCCCATTTT | AF | 59.78 | 40 | (As = BatSten = GregSten) ≠ (Ad = Ah = Am)* | |
| Nem_Aradu.A04_121132127_Fwd_VIC | GAAGGTCGGAGTCAACGGATTGCCAAGCAAAGTAATGCCG | AS | 61.67 | 52.63 | G | |
| Nem_Aradu.A04_121132127_Fwd_FAM | GAAGGTGACCAAGTTCATGCTGCCAAGCAAAGTAATGCCA | AS | 59.82 | 47.37 | A | |
| Nem_Aradu.A04_121183243_Rev_ | AAGGTTGGGAATGTCAAGGA | AF | 59.38 | 45 | (As = BatSten = GregSten = Ad = Ah = Am) | |
| Nem_Aradu.A04_121183243_Fwd_FAM | GAAGGTGACCAAGTTCATGCTAACTGGTAGGTTTGGAAATAATCG | AS | 59.7 | 37.5 | C | |
| Nem_Aradu.A04_121183243_Fwd_VIC | GAAGGTCGGAGTCAACGGATTAAACTGGTAGGTTTGGAAATAATCC | AS | 59.91 | 36 | G | |
| Nem_Aradu.A09_112396428_Fwd_ | TATGATTGGCCCCCTAAATG | AF | 59.62 | 45 | (As = BatSten = GregSten) ≠ Ad ≠ (Ah = Am)* | |
| Nem_Aradu.A09_112396428_Rev_FAM | GAAGGTGACCAAGTTCATGCTAGCCCCCTCTTCTAAAACAAC | AS | 58.77 | 47.62 | A | |
| Nem_Aradu.A09_112396428_Rev_VIC | GAAGGTCGGAGTCAACGGATTCAGCCCCCTCTTCTAAAACAAT | AS | 60.81 | 45.45 | G | |
| Nem_Aradu.A09_112396635_Rev_ | CCTGGCTTCATGTTTGATGA | AF | 59.65 | 45 | Ad ≠ (As = BatSten = GregSten = Ah = Am) | |
| Nem_Aradu.A09_112396635_Fwd_VIC | GAAGGTCGGAGTCAACGGATTAATGTTACAAAAGGATCCCCAG | AS | 59.24 | 40.91 | G | |
| Nem_Aradu.A09_112396635_Fwd_FAM | GAAGGTGACCAAGTTCATGCTAATGTTACAAAAGGATCCCCAA | AS | 59.59 | 36.36 | A | |
| Nem_Aradu.A09_112399976_Rev_ | TGACGAGAAGGGGAAAGAAA | AF | 59.78 | 45 | (As = BatSten = GregSten = Ad = Ah = Am) | |
| Nem_Aradu.A09_112399976_Fwd_FAM | GAAGGTGACCAAGTTCATGCTAATCCTATTACTAAATCGCTGCTTTT | AS | 59.27 | 30.77 | C | |
| Nem_Aradu.A09_112399976_Fwd_VIC | GAAGGTCGGAGTCAACGGATTAATCCTATTACTAAATCGCTGCTTTC | AS | 59.65 | 34.62 | G | |
| Nem_Aradu.A09_112901114_Rev_ | CTCCCCAATTTCTCAGCAAG | AF | 59.81 | 50 | (As) ≠ (BatSten = GregSten) ≠ (Ad = Ah = Am)* | |
| Nem_Aradu.A09_112901114_Fwd_FAM | GAAGGTGACCAAGTTCATGCTAGGTGTTGACAGAATTACAACCG | AS | 60.32 | 43.48 | A | |
| Nem_Aradu.A09_112901114_Fwd_VIC | GAAGGTCGGAGTCAACGGATTGAGGTGTTGACAGAATTACAACCA | AS | 60.32 | 41.67 | G | |
| Nem_Aradu.A09_114001128_Rev_ | TTAAAGCCCCTGCTTTTTCA | AF | 59.83 | 40 | (As = BatSten = GregSten) ≠ (Ad = Ah = Am)* | |
| Nem_Aradu.A09_114001128_Fwd_FAM | GAAGGTGACCAAGTTCATGCTATGAGGGAACAACCAGCACTA | AS | 59.61 | 47.62 | C | |
| Nem_Aradu.A09_114001128_Fwd_VIC | GAAGGTCGGAGTCAACGGATTTGAGGGAACAACCAGCACTC | AS | 61.26 | 55 | A | |
| DS_c14276_456_A09_115161052_Rev_ | AGGAGTCATGGGATGGAATG | AF | 59.74 | 50 | (As = Ad = GregSten) ≠ (BatSten = Ah = Am)* | |
| DS_c14276_456_A09_115161052_Fwd_VIC | GAAGGTCGGAGTCAACGGATTTTTGGAAACATCAGCAAAGGA | AS | 60.6 | 38.1 | A | |
| DS_c14276_456_A09_115161052_Fwd_FAM | GAAGGTGACCAAGTTCATGCTTGGAAACATCAGCAAAGGC | AS | 59.79 | 47.37 | C | |
| TOG896078_413_A09_116503861_Rev_ | GTGGAAGAAATAGCAAAATGGA | AF | 58.25 | 36.36 | (As = BatSten = GregSten) ≠ (Ad = Ah = Am)* | |
| TOG896078_413_A09_116503861_Fwd_VIC | GAAGGTCGGAGTCAACGGATTAAGGAGTTATGGAGATGGTAAGTTTT | AS | 59.02 | 34.62 | T | |
| TOG896078_413_A09_116503861_Fwd_FAM | GAAGGTGACCAAGTTCATGCTAAGGAGTTATGGAGATGGTAAGTTTC | AS | 59.41 | 38.46 | C | |
| TOG903757_1119_A09_116533871_Rev_ | CCCAAGAAGCAGGGTACTTT | AF | 58.32 | 50 | (As = BatSten = GregSten = Ad = Ah = Am) | |
| TOG903757_1119_A09_116533871_Fwd_VIC | GAAGGTCGGAGTCAACGGATTACTTGATTTGATATGAGATTTCCTG | AS | 57.83 | 32 | G | |
| TOG903757_1119_A09_116533871_Fwd_FAM | GAAGGTGACCAAGTTCATGCTCACTTGATTTGATATGAGATTTCCTC | AS | 59.45 | 34.62 | C |
LG, Linkage group; TM, melting temperature; GC%, GC content; AF, Allele Flanking; As, Arachis stenosperma; BatSten, (Arachis batizocoi × A. stenosperma)4x, GregSten = (Arachis gregoryi × A. stenosperma)4x; Ad, Arachis duranensis; Ah, Arachis hypogaea; Am, Arachis monticola; AS, Allele specific; ND, Not defined/assay did not work.
Dye: Reference (A. duranensis) alleles are coupled with VIC and alternative (A. stenosperma) alleles are coupled with FAM.
Asterisk indicate assays that distinguish A. stenosperma-derived allotetraploids from A. hypogaea.
KASP assays were performed with the following genotypes: the diploids A. duranensis V14167 (A-reference genome), A. stenosperma V10309 (A-genome), the wild allotetraploid A. monticola accessions Pl219824 and Pl405933, the induced allotetraploids (A. batizocoi K9484 × A. stenosperma V10309)4x (here called BatSten) and (A. gregoryi V6389 × A. stenosperma V10309)4x (here called GregSten), and six A. hypogaea cultivars (Runner IAC-886, Tifrunner, Tifguard, GA-06G, NC3033, IAC69007). Reactions consisted of 2 μl of KASP 2X reaction mix, 0.055 μl of assay primer mix (12 mM of each allele-specific primer, and 30 mM of common primer), and 20 ng of genomic DNA, in a 4 µl volume. A C1000 Thermal Cycler (Bio-Rad) was used with the following cycling conditions: 94° for 15 min, nine cycles of 94° for 20 sec, touchdown starting at 65° for 60 sec (decreasing 0.8° per cycle), 29 cycles of 94° for 20 sec, and 57° for 60 sec. In order to improve the results, a second KASP program was run as following: nine cycles of 94° for 20 sec and 57° for 60 sec. Fluorescence was read by a LightCycler 480 Instrument II (Roche Life Science), and analyzed using the LightCycler 480 software (V.1.5.1). Three technical replicates were performed for each KASP assay.
In order to test correlation of KASP markers with nematode resistance, 20 of the most contrasting lines were selected to be assayed with the 15 KASP primers that successfully distinguished the synthetic allotetraploids from cultivated peanut.
Results
Nematode screening
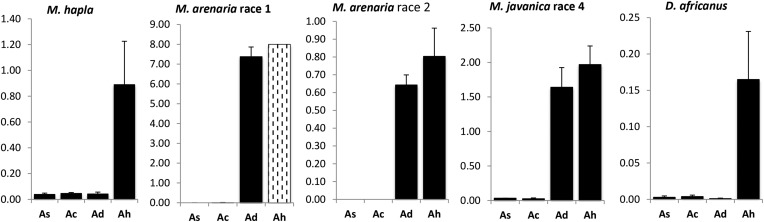
A. stenosperma and A. cardenasii were resistant to all nematode species tested, hosting no gall or egg production (Figure 1). A. duranensis was resistant to M. hapla and D. africanus, and comparable to cultivated peanut in susceptibility to M. javanica, and M. arenaria race 2 (Tukey HSD, P = 0.05). To M. arenaria race 1, A. duranensis was partially resistant (Tukey HSD, P = 0.05, Figure 1). Resistance was evaluated as reproductive factor (RF). The peanut cultivar Tatu, used as positive control, was susceptible to all nematode species tested, and low RF was observed in the bioassays against M. hapla and M. arenaria race 2 and D. africanus. This was because, in all these very susceptible plants, the root system was severely damaged and fragmented, not sustaining large quantities of nematodes. All A. hypogaea plants inoculated with M. arenaria race 1 died before the end of the experiment (Figure 1).
Figure 1.
Reproductive factor of the nematodes Meloidogyne hapla, M. arenaria race 1, M. arenaria race 2, M. javanica race 4 and Ditylenchus africanus, observed in A. stenosperma (As), A. cardenasii (Ac), A. duranensis (Ad), and A. hypogaea cv. IAC Tatu (Ah). White bar with dashed vertical lines represents minimum expected RF of peanut plants that, due to heavy infestation, died before the end of the experiment.
Population phenotyping
The F6 RIL population used here was produced by single seed descent from a cross of A. duranensis × A. stenosperma. Individuals show varying degrees of fertility. Pollen viability of segregating individuals ranged from 80% to 99.3%, reflecting the genetic distance of the parents. The population showed large variability for all traits tested.
Nematode resistance:
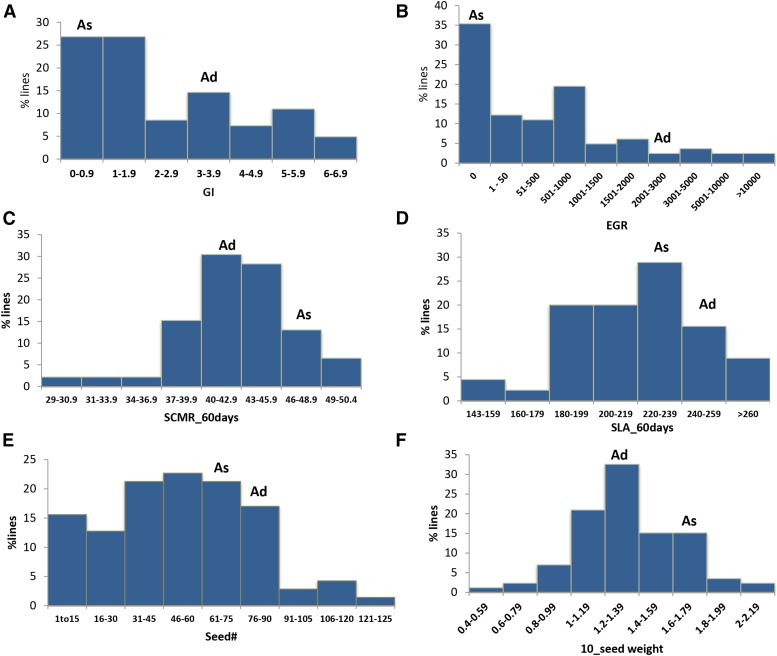
Evaluation was performed in the greenhouse for 2 years (2011 and 2013). Different severity levels were observed between experiments, with lower egg production levels in the second than in the first bioassay experiment. This difference might have been due to different environmental conditions, and also different nematode inoculum viability. All data were used for QTL identification but only the egg production data obtained from experiment I was used for statistical analyses, and evaluation of allele effects. Frequency distribution based on the pooled data for GI and EGR was strongly biased toward resistance. Some transgressive segregation was observed. For both GI datasets, most individuals had midparent values (54), only four were as, or more, resistant than A. stenosperma V10309 (0.3 ± 0.11) and 24 were more susceptible than A. duranensis K7988 (3.6 ± 0.86) (P < 0.05). For EGR, however, 31 individuals were more resistant than V10309 (10.16 ± 9.09), 42 had midparent values and only nine were more susceptible than K7988 (2188.89 ± 730.54) (P < 0.05). (Figure 2, A and B, and File S1). This showed that several lines that had high levels of root-galling supported only low levels of egg production. The susceptible peanut cultivar, Florunner had low infection rates. Wild species and segregating individuals showed smaller galls than Florunner. Pearson correlation between GI and EGR in the RIL population was significant [r2 (82) = 0.47, P = 0.01], but low enough to indicate that different genes might control the GI and nematode reproduction responses (Wang et al. 2012).
Figure 2.
Frequency distribution of resistance to Meloidogyne arenaria race 1 (A–B), drought-related traits (C–D), and yield traits (E–F) in recombinant inbred lines (F6) derived from a cross of A. duranensis K7988 (Ad) with A. stenosperma V10309 (As). The means of the parents are significantly different (P < 0.05).
Drought-related traits:
The two parents showed little variation for the drought-related traits analyzed, with values differing numerically, but not statistically significantly (Kruskal-Wallis, P < 0.05). A. stenosperma: TR/LA = 0.245 ± 0.036 g/cm; SCMR (60) = 46.03 ± 2.08; SLA (60) = 209.58 ± 5.70 g/cm. A. duranensis: TR/LA = 0.323 ± 0.041 g/cm; SCMR (60) = 42.8 ± 1.72; SLA (60) = 239.09 ± 13.58 g/cm. The population showed large transgressive segregation for SCMR and SLA (Figure 2, C and D).
Agronomic and domestication traits:
Phenotypic evaluations were performed at different generations (F5 and F6) and places. Values were normally distributed for most traits for most years. With the exception of NLB and RRA, the means of the parents for all traits evaluated were significantly different (P < 0.05). A. stenosperma produced fewer but heavier seeds than A. duranensis. Comparison of the means of the parents and the segregating genotypes reveals that, for all traits, there was transgressive segregation in the progenies (Figure 2, E and F). This is particularly interesting for seed characteristics; for instance, 11 individuals outperformed both parents in seed production and five in seed weight.
Construction of improved genetic map
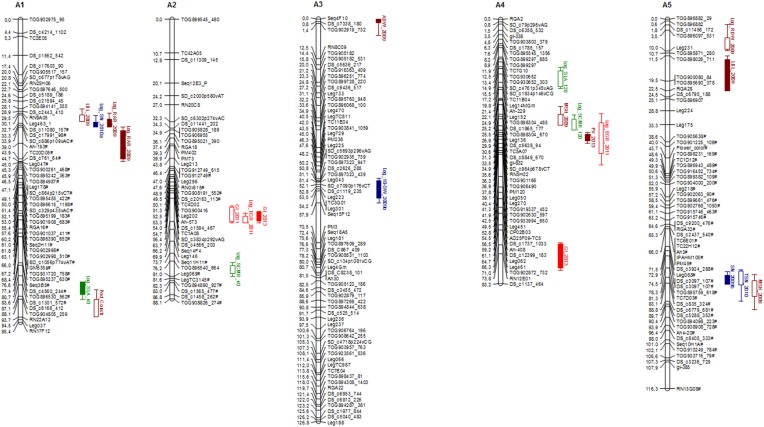
Initially, a total of 1404 polymorphic markers were used for linkage map construction using JoinMap. With LOD scores ranging from 7 to 20, 1108 marker loci were mapped into 10 LGs, with a total distance of 490.4 cM (data not shown). These markers included 528 SSR, 511 SNP, 56 anchor, and 13 RGAs (resistance gene analog) markers. This map showed many genomic regions saturated of cosegregating markers. After removal of all but one of each set of cosegregating markers, and including the 296 (1404–1108) markers that did not map with JoinMap, a framework map was then constructed using Mapmaker. Using a minimum LOD score of 9.0 and a maximum recombination fraction of 0.35, 502 markers mapped onto 10 LGs, spanning a total map distance of 1004.1 cM. These markers included 316 SNPs, 96 SSRs, 72 anchor, 17 RGAs, and one morphological (flower color) marker. LGs were numbered according to the F2 reference map (Moretzsohn et al. 2005). LGs ranged from 81.7 cM (with 48 markers) to 126.8 cM (68 markers), with an average distance of 2.0 cM between adjacent markers. A total of 269 (53.6%) out of the 502 mapped markers deviated from the expected 1:1 ratio at P < 0.05 level. Of these, 165 markers were skewed toward A. duranensis and 104 markers toward A. stenosperma. LGs 1, 4, 6, 7, 8, and the upper portion of LG 2 had an excess of A. stenosperma alleles, while LGs 3, 5, 9, 10, and the inner portion LG 2, an excess of A. duranensis alleles. All linkage groups have distorted markers, with LGs 3, 4, 5, and 9 being almost entirely composed of distorted markers. Distorted markers at P < 0.05 are indicated by # in Figure 3.
Figure 3.
A genetic linkage map of the A-genome of Arachis obtained through the analysis of 90 F6 plants, generated from a cross between A. duranensis K7988 and A. stenosperma V10309. Numbers on the left of each group are Kosambi map distances (cM). QTL are indicated as colored bars running alongside linkage groups. Colors/textures are according to categories: red, nematode resistance; green, drought-related traits; blue, productivity; and brown, domestication and other agronomic traits. Distorted markers at P < 0.05 were identified by #.
QTL identification
The framework map, containing 502 markers, was used for QTL analysis. LOD significance threshold estimated for each trait ranged from 2.9 to 22.6, and only QTL with LOD values exceeding these values were included. At least one QTL was detected for 26 of the 29 traits analyzed, with a total of 52 QTL mapped by CIM. No significant QTL was identified for nematode eggs per root_2013 (EGR_2013), 10-seed weight_2009a (10-SW_2009a), and root length (RL). A summary of QTL is provided in Table 2 and described with more details in File S1.
Table 2. QTL identified for resistance to Meloidogyne arenaria race 1 (RKN), domestication, agronomic and drought-related traits on an A. duranensis x A. stenosperma F6 population.
| Trait Category | Trait Symbol | LGa | Positionb | Nearest Marker/Interval | LODc | Additive Effectd | R2 (%)e |
|---|---|---|---|---|---|---|---|
| RKN resistance | GI_2011 | 2 | 66.2 | seq14F4 | 6.1 | 0.957 | 16.5 |
| 9 | 40.3 | Leg199 | 6.8 | 0.868 | 16.7 | ||
| GI_2013 | 2 | 68.9 | seq14F4/Leg146 | 6.2 | 0.908 | 17.9 | |
| 4 | 74.8 | RN12E01 | 4.2 | −0.724 | 13.4 | ||
| 9 | 41.5 | Leg199/Leg1Gm | 3.4 | 0.519 | 8.7 | ||
| Log_EGR_2011 | 2 | 67.2 | seq14F4 / Leg146 | 15.0 | 1.053 | 43.7 | |
| 4 | 39.1 | Leg050 | 3.1 | 0.432 | 5.7 | ||
| 9 | 41.9 | Leg199/Leg1Gm | 6.0 | 0.577 | 11.9 | ||
| Drought-related | Log_SCMR_40 | 2 | 78.2 | TOG896540_664 / Leg069 | 4.9 | −0.332 | 17.0 |
| 6 | 88.2 | IPAHM-171a / DS_c9222_228 | 9.3 | 0.443 | 31.2 | ||
| 7 | 17.4 | DS_c4177_150 | 3.7 | 0.243 | 9.5 | ||
| 10 | 49.0 | SD_c1259p293vAC | 3.3 | 0.228 | 8.5 | ||
| Log_SCMR_60 | 10 | 73.5 | TOG902928_388 / TOG896385_429 | 3.1 | 0.288 | 11.1 | |
| Log_SCMR_120 | 4 | 33.5 | gi-832 | 3.5 | −0.326 | 10.7 | |
| 6 | 84.2 | RN0x06 | 4.4 | 0.275 | 13.3 | ||
| Log_SLA_40 | 1 | 83.6 | DS_c1301_572 | 3.4 | 0.380 | 9.9 | |
| 9 | 74.0 | SD_c3430p305vAG | 3.2 | 0.353 | 9.0 | ||
| Log_SLA_60 | 7 | 82.1 | DS_c7479_398 | 3.3 | −0.419 | 11.1 | |
| Log_SLA_120 | 4 | 19.2 | TC11B04 / Leg14MGm | 3.5 | 0.476 | 12.1 | |
| 9 | 3.9 | TOG895054_163 | 6.4 | −0.628 | 20.3 | ||
| Domestication/ | SN_2009a | 9 | 15.0 | TOG894454_341 | 3.7 | 18.499 | 13.6 |
| Agronomic traits | SN_2009b | 5 | 85.8 | DS_c835_324 / DS_c6779_681 | 5.9 | −65.428 | 26.0 |
| Log_SN_2010a | 1 | 33.3 | DS_c17991_98 | 4.8 | 0.395 | 15.7 | |
| 9 | 48.3 | TOG896362_355 / SD_c2057p484vGT | 3.4 | 0.338 | 11.4 | ||
| 10 | 42.9 | IPAHM-689 | 3.2 | −0.290 | 9.3 | ||
| Log_SN_2010b | 7 | 64.5 | TOG904989_542 | 4.1 | 0.639 | 18.6 | |
| 9 | 46.3 | TOG896362_355 / SD_c2057p484vGT | 3.4 | 0.529 | 12.9 | ||
| Log_10-SW_2009b | 3 | 52.8 | DS_c1119_235 | 3.4 | 0.238 | 12.1 | |
| Log_10-SW_2010a | 9 | 44.1 | Leg1Gm / TOG896362_355 | 4.2 | 0.207 | 15.2 | |
| 10 | 42.9 | IPAHM-689 | 3.9 | −0.170 | 12.3 | ||
| Log_10-SW_2010b | 7 | 64.9 | TOG904989_542 | 3.8 | 0.191 | 14.7 | |
| Pod_Constr | 1 | 92.1 | TOG904805_259 / RN22A12 | 3.9 | −5.421 | 14.2 | |
| PL | 6 | 115.0 | TOG895571 | 3.2 | −15.379 | 11.1 | |
| MSH | 4 | 30.6 | TC5A07 | 3.9 | 2.171 | 13.4 | |
| 5 | 89.7 | DS_c5288_353 | 4.0 | −2.357 | 10.2 | ||
| 10 | 65.8 | DS_c10522_129 | 3.3 | −1.803 | 8.3 | ||
| LBL | 1 | 31.1 | Leg463_1 / DS_c11080_157 | 9.9 | 27.289 | 32.0 | |
| 5 | 19.5 | TOG895690_378 | 3.7 | 14.566 | 8.9 | ||
| NLB | 8 | 95.3 | TC22C01 | 3.2 | 1.025 | 10.7 | |
| 10 | 40.4 | TC31C09 / IPAHM-689 | 5.1 | −1.371 | 19.1 | ||
| ADW | 3 | 0.0 | Seq4F10 | 3.3 | 3.645 | 9.3 | |
| 6 | 56.4 | Leg346 / TC7C06 | 5.3 | 3.602 | 15.6 | ||
| 9 | 63.5 | TOG896078_413 | 3.8 | −3.012 | 10.5 | ||
| Log_RDW | 5 | 6.5 | TOG896097_531 / Leg231 | 3.5 | 0.235 | 14.3 | |
| 6 | 63.0 | TOG896979_290 | 4.0 | 0.238 | 16.4 | ||
| Log_RRA | 1 | 33.3 | DS_c17991_98 | 3.5 | 0.132 | 11.2 | |
| 1 | 39.0 | Ah-193 | 4.5 | 0.146 | 13.9 | ||
| 10 | 57.1 | TC7H11 | 3.4 | 0.160 | 11.0 | ||
| TB | 6 | 56.4 | Leg346 / TC7C06 | 6.7 | 5.124 | 21.2 | |
| 9 | 63.5 | TOG896078_413 | 3.1 | −3.460 | 9.5 | ||
| PV | 4 | 36.9 | TOG906490 | 3.3 | 1.793 | 10.7 |
LOD, logarithm of the odds; GI, gall index; EGR eggs/g of root; SCMR, SPAD chlorophyll meter reading; SN, seed number; SW, seed weight; Pod_Constr, pod constriction; PL, peg length; MSH, main stem height; LBL, lateral branch length; NLB, number of lateral branches; LBL, lateral branch length; ADW, aerial dry weight; RDW, root dry weight; RRA weight ratio root/aerial part; TB, total biomass; PV, pollen viability.
Linkage group.
Map position in Kosambi cM.
Maximum LOD score.
Positive values indicate that higher-value alleles come from A. duranensis K7988, and negative values indicate that higher-value alleles come from A. stenosperma V10309.
Proportion of the total phenotypic variance explained by the QTL.
Nematode resistance:
Three major QTL for both the root-galling (GI) and egg production (EGR) components of nematode resistance evaluated were consistently identified; these mapped in LGs 02, 04 and 09. On LG02, the closest marker was seq14F4 (Ferguson et al. 2004), and the QTL mapped in the same marker interval (66.2–68.9 cM), with LOD scores between 6.1 and 15.0, for GI_2011 and 2013, and for EGR_2011. These QTL explained between 16.5 and 43.7% of the total phenotypic variance. For the QTL on LG09, the closest marker was Leg199 (Bertioli et al. 2009), in map interval 40.3–41.9 cM, with LOD scores between 3.4 and 6.8, and explaining 8.7–16.7% of phenotypic variance. The third QTL was identified on LG04 for EGR_2011, close to marker Leg050 (Bertioli et al. 2009), on map position 39.1 cM, with LOD 3.1,and explaining 5.7% of the phenotypic variance. For all these QTL, resistance was derived from A. stenosperma (Table 2 and Figure 3). An additional QTL was identified on LG04 for GI_2013, close to marker RN12E01 (Moretzsohn et al. 2005), on map position 74.8 cM, with maximum LOD of 4.2, explaining 13.4% of the phenotypic variance. This was the only QTL that conferred resistance derived from A. duranensis (Table 2 and Figure 3).
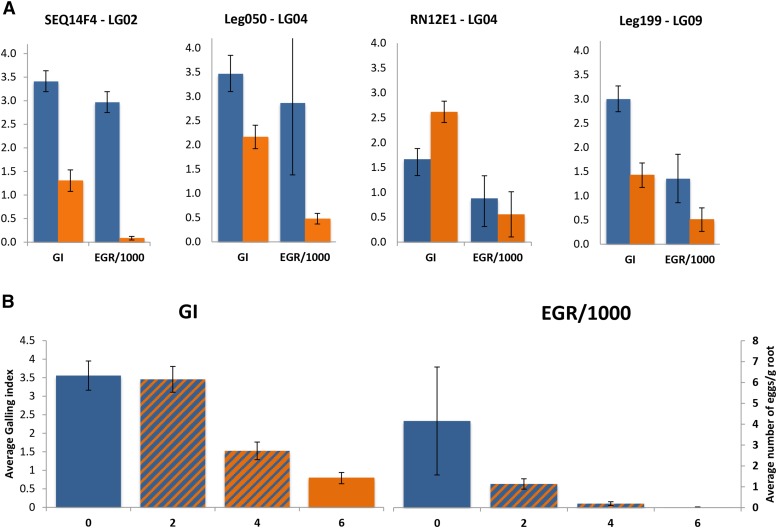
With the analyses of the phenotypic effects of nearest markers linked to QTL contributing to nematode resistance, we found that the presence of the A. stenosperma allele of locus Seq14F4 (LG02) contributed to a reduction of 61.8% of GI, and 92.6% of EGR (Figure 4A). On average, individuals carrying the A. stenosperma allele of locus Leg050 (LG04) had a reduction of 37.7% on GI, and 83.3% on EGR. For the locus Leg199 (LG09), the reductions were 52.5% for GI, and 62.6% for EGR.
Figure 4.
(A) Effect of QTL-linked markers for galling index (GI) and nematode egg production (EGR/1000) on mean phenotypic value (± SE). The markers are distributed on three different linkage groups: Seq14F4 - LG02, Leg050 and RN12E1 – LG04 and Leg199 - LG09. (B) Effect of combination of QTL based on genotypic classes carrying zero to six A. stenosperma favorable alleles Seq14F4, Leg050 and Leg199.
Drought-related traits:
SLA and SCMR were evaluated at different times of plant development and were treated separately. Five QTL were identified for SLA and seven for SCMR, in seven different LGs. No clear clustering of QTL was observed. The strongest QTL, explaining 31.2% of the phenotypic variation for SCMR_40, was located on LG06, linked to markers IPAHM-171a / DS_c9222_228.
Agronomic/domestication traits:
Agronomic and domestication traits were evaluated in different years. A total of 31 QTL was identified. As expected for these polygenic traits, several QTL explaining a small percentage of phenotypic variance were found. Alleles from both parents contributed to an increase of seed number and weight, as well as for MSH, NLB, ADW, and TB. For two domestication traits, pod constriction (PC), and peg length (PL), alleles derived from A. duranensis reduced the lengths. For each of these traits, only one QTL was identified. For the other traits (LBL, RDW, RRA, and PV), A. stenosperma alleles increased the phenotypic values. A few QTL were consistent between years and some were found in similar positions in different populations. QTL for seed weight (LG07), seed number (LG05), and main stem height (LG04), coincided with the linkage groups with QTL found by (Fonceka et al. 2012). Similarly, seed weight (LG07), pod constriction (LG01), and main stem height (LG04 and LG05), were found in similar positions as on the B-population A. ipaënsis × A. magna (Leal-Bertioli et al. 2015b). One QTL for seed number colocalized with a strong QTL for nematode resistance on LG09 (Figure 3).
KASP primer design and validation on tetraploid backgrounds
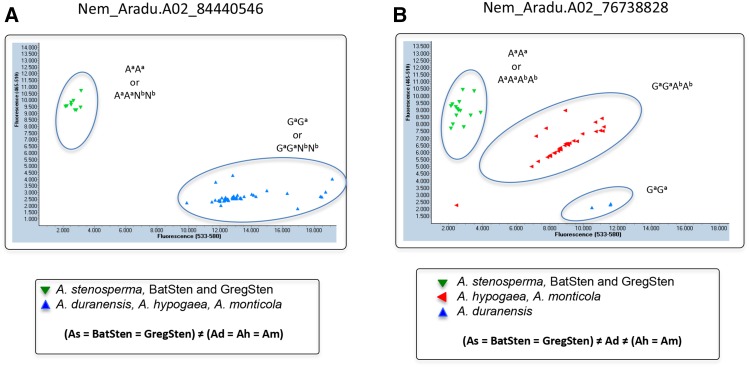
Twenty-five KASP assays were designed for the three genomic regions of A. stenosperma-derived QTL for nematode resistance, in LG02, 04 and 09. Sixteen successfully distinguished A. stenosperma (As) and its derived synthetic allotetraploids from A. duranensis (Ad) and the A-subgenome component of all A. hypogaea (Ah) tested; seven did not distinguish A. stenosperma from A. duranensis and A. hypogaea, and only two assays failed (Table 1). One assay curiously did not distinguish A. stenosperma from the A component of A. hypogaea, but did from the As-derived induced allotetraploids. Different useful cluster configurations were observed, and are listed in Table 1: (1) in seven assays, A. stenosperma clusters with the induced allotetraploids, BatSten and GregSten, and A. duranensis clusters with the peanut cultivars and A. monticola [(As = BatSten = GregSten) ≠ (Ad = Ah = Am)] (Figure 5A); (2) In three assays, A. duranensis is distinguished from all other genotypes, A. stenosperma clusters with the induced allotetraploids, BatSten and GregSten, and A. monticola clusters with all peanut cultivars [(As = BatSten = GregSten) ≠ Ad ≠ (Ah = Am)] (Figure 5B); (3) in two assays, A. stenosperma is distinguished from all genotypes, and the induced allotetraploids form a cluster, the A. duranensis, A. hypogaea, and A. monticola form a third cluster [(As) ≠ (BatSten = GregSten) ≠ (Ad = Ah = Am)]. Three other clustering configurations can also be useful for distinguishing both induced allotetraploids from peanut: [(As = Ah = Am) ≠ Ad ≠ (BatSten = GregSten)], [(As = GregSten = BatSten = Am) ≠ (Ad = Ah)], and two were useful for distinguishing GregSten: [(As = GregSten) ≠ (BatSten = Ad = Ah =Am)] and (As = Ad = GregSten) ≠ (BatSten = Ah = Am). All useful assays are marked with an asterisk on Table 1. Ten out of the 15 successful KASP assays showed significant Pearson correlation (P = 0.05) with nematode resistance (Table 3).
Figure 5.
Screenshots of two examples of Arachis A-genome SNP genotyping using KASP assays. Both assays (Nem_Aradu.A02_84440546 and Nem_Aradu.A02_76738828) show differentiationcbetween A. duranensis V14167 and the A-genome of A. hypogaea from the wild species A. stenosperma V10309, and the induced allotetraploids BatSten and GregSten. In (A), two clusters are present: one with A. duranensis, A. monticola and all A. hypogaea cultivars, and another with A. stenosperma and induced allotetraploids [noted in Table 1 as (As = BatSten = GregSten) ≠ (Ad = Ah = Am)]. In (B), three clusters are present, with an intermediate cluster of A. hypogaea and A. monticola [noted in Table 1 as (As = BatSten = GregSten) ≠ Ad ≠ (Ah =Am)]. Diploid and tetraploid genotypes are indicated near each cluster. A and G refer to the DNA bases; the subscript letters refer to the A and B subcomponent genomes of peanut. N is used when B subgenome bases are not detected.
Table 3. Pearson correlations between KASP assays and components of nematode resistance on segregating lines of the RIL F6 population A. duranensis × A. stenosperma. Significant correlations at 0.05 level ≥ I0.344I (values with asterisks).
| KASP Assay | LG | Pseudomolecule Position | Trait | |||
|---|---|---|---|---|---|---|
| GI_2011 | EGR_2011 | Log_EGR_2013 | GI_2013 | |||
| Nem_Aradu.A02_76738828 | 2 | 76738828 | −0.362* | 0.086 | −0.358* | −0.497* |
| Nem_Aradu.A02_84440546 | 2 | 84440546 | −0.493* | 0.084 | −0.464* | −0.523* |
| Nem_Aradu.A02_84440594 | 2 | 84440594 | −0.555* | −0.325 | −0.599* | −0.596* |
| DS_c1614_886_A02_88903581 | 2 | 88903581 | −0.693* | −0.505* | −0.750* | −0.722* |
| TOG894171_695_A02_92486807 | 2 | 92486807 | −0.600* | −0.021 | −0.553* | −0.600* |
| TOG906490_74_A04_106874754 | 4 | 106874754 | −0.647* | −0.518* | −0.698* | −0.339 |
| Nem_Aradu.A04_109789467 | 4 | 109789467 | −0.542* | −0.385* | −0.562* | −0.358* |
| Nem_Aradu.A04_121132127 | 4 | 121132127 | −0.221 | −0.261 | −0.291 | −0.172 |
| Nem_Aradu.A09_112396428 | 9 | 112396428 | −0.353* | −0.319 | −0.312 | −0.125 |
| Nem_Aradu.A09_112901114 | 9 | 112901114 | −0.396* | −0.293 | −0.335 | −0.142 |
| Nem_Aradu.A09_114001128 | 9 | 114001128 | −0.400* | −0.207 | −0.243 | −0.374* |
| TOG896942_133_A09 | 9 | 114770700 | −0.197 | −0.226 | −0.149 | 0.074 |
| DS_c14276_456_A09_115161052 | 9 | 115161052 | −0.227 | −0.263 | −0.229 | −0.138 |
| TOG896078_413_A09_116503861 | 9 | 116503861 | −0.130 | −0.235 | −0.156 | 0.011 |
| TOG903757_1119_A09_116533871 | 9 | 116533871 | −0.130 | −0.235 | −0.156 | 0.011 |
Discussion
Currently, the only source of resistance to M. arenaria used in commercial peanut cultivars comes from A. cardenasii. This wild A-genome species harbors a number of loci that reduce RNK infestation (Burow et al. 2014) but, to our knowledge, only one, localized on a large chromosomal segment mapping to LG09, has been introgressed into commercial peanut cultivars (Nagy et al. 2010). Molecular markers for this chromosomal segment are used in marker-assisted breeding to expedite its incorporation into new cultivars (Chu et al. 2011). However, the use of a single source of resistance is clearly vulnerable to being overcome through virulence selection, and there is interest in identifying new sources.
The multiple disease resistances and close relationship of A. stenosperma to the A-subgenome of cultivated peanut have stimulated interest in its use in breeding programs. It is now being used in programs in the USA, Brazil, India and Senegal. The A. stenosperma accession studied here, V10309, was shown previously to be resistant to M. arenaria. The expression of genes involved in the hypersensitive response and production of secondary metabolites related to pathogen defense is triggered shortly following nematode challenge (Proite et al. 2007; Guimarães et al. 2010; Morgante et al. 2013). Microscopically, at least two mechanisms of resistance are apparent: prepenetration (physical or chemical root barriers), and a postpenetration classical hypersensitive response (Proite et al. 2008). Here we extend the known resistances of this A. stenosperma accession to M. hapla, M. javanica race 4 and D. africanus (Figure 1), and genetically map the resistance for M. arenaria. For mapping, we worked in the genetically simplified context of a diploid population. The maternal parent of this population was the most probable A-genome ancestral species of cultivated peanut A. duranensis (accession K7988), and the paternal A. stenosperma. While the close relationships of these species to the A-subgenome of A. hypogaea ensure a good chance that QTL will be applicable for crop breeding, the diploid genetics reduces allelic interactions and avoids complexities of tetrasomic recombination (Leal-Bertioli et al. 2015a).
Although the main focus of this work was the identification of QTL for nematode resistance, this population was also evaluated for several other traits. The parents of the mapping population had similar values for drought-related traits (SCMR and SLA); nevertheless, transgressive segregation was observed, and QTL and marker associations were identified. Also, although A. stenosperma and A. duranensis are both wild species, they differ somewhat in phenotypes that are strongly selected during domestication: A. duranensis has shorter pegs and pod constrictions. Transgressive segregation was also observed for these traits, with 31 lines having shorter pegs and pod constrictions than both parents. QTL were identified for these and other plant architectural traits. Many of these traits are complex and quantitative, and will depend on environment and genetic ploidy (Leal-Bertioli et al. 2012). Nevertheless their identification enriches the information content of this A-genome map, and they can be easily cross-referenced to the genome sequence of A. duranensis.
Four QTL that contribute to RKN resistance were identified, on LG02, 04 and 09. For three of them (closest linked markers Seq14F4, Leg050 and Leg199), the presence of the A. stenosperma alleles greatly reduces both root-galling (GI) and egg production (EGR/1000) (Figure 4, A and B). For the other QTL, with closest linked marker RN12E01, the effect was opposite: A. stenosperma alleles increased root-galling. It is worth noting that, for the diploid population, the susceptible parent (A. duranensis K7988) is much more resistant than A. hypogaea. Therefore, the effects of the resistances conferred by wild species alleles in the context of the highly susceptible cultivated peanut genetic background are likely to be larger than the effects measured here. The genome location of all these QTL is different to the A. cardenasii chromosomal segment currently used in commercial cultivars. Therefore, in principle, multiple sources of resistance, derived from different QTL (Figure 4B) could be harbored in peanut cultivars for improved, and potentially more durable, resistance.
To deploy these resistance QTL for crop improvement, we have previously developed A. stenosperma-derived artificially induced allotetraploids that are sexually compatible with cultivated peanut (Leal-Bertioli et al. 2015c). In this study we developed new KASP markers around the QTL of interest using the genome sequence of A. duranensis (http://www.peanutbase.org); confirmed the marker associations with nematode resistance and tested them in tetraploid genotypes. A. stenosperma-derived induced allotetraploids (BatSten and GregSten) were distinguished from all the peanut cultivars, including Tifguard, which harbors A. cardenasii-derived RKN resistance. In ongoing work these markers will be used to facilitate the selection of backcrossed progeny that harbor the A. stenosperma QTL of interest, and the testing of their function in a tetraploid genetic background.
Supplementary Material
Acknowledgments
The authors would like to acknowledge the technical assistance of Leandro Mesquita, Leonardo Fonseca, and Igor Bacon (CENARGEN, Brazil), Silvio Santos (UCB, Brazil), Eric Antepenko, Jenny Leverett (UGA, United States) and Teresa Mullens (UCR, United States). We thank Dr. Ignácio Godoy (IAC, Brazil) for providing DNA of peanut line 69007. This work was supported by the Generation Challenge Program Tropical Legumes 1; National Council for Scientific and Technological Development of Brazil (CNPq); MARS Inc., The Peanut Foundation, and host institutions.
Footnotes
Supporting information is available online at www.g3journal.org/lookup/suppl/doi:10.1534/g3.115.023044/-/DC1
Communicating editor: E. Akhunov
Literature Cited
- Bertioli D., Moretzsohn M., Madsen L., Sandal N., Leal-Bertioli S., et al. , 2009. An analysis of synteny of Arachis with Lotus and Medicago sheds new light on the structure, stability and evolution of legume genomes. BMC Genomics 10(1): 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertioli, D.J., P. Ozias-Akins, Y. Chu, K.M. Dantas, S.P. Santos et al., 2014 The use of SNP markers for linkage mapping in diploid and tetraploid peanuts. G3 (Bethesda) 4(1):89–96. [DOI] [PMC free article] [PubMed]
- Bridge J., Page S. L. J., 1980. Estimation of root-knot nematode infestation levels on roots using a rating chart. Trop. Pest Manage. 26: 296–298. [Google Scholar]
- Burow M., Simpson C., Starr J., Paterson A., 2014. Transmission genetics of chromatin from a synthetic amphidiploid to cultivated peanut (Arachis hypogaea L.). broadening the gene pool of a monophyletic polyploid species. Genetics 159(2): 823–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caillaud M. C., Dubreuil G., Quentin M., Perfus-Barbeoch L., Lecomte P., et al. , 2008. Root-knot nematodes manipulate plant cell functions during a compatible interaction. J. Plant Physiol. 165(1): 104–113. [DOI] [PubMed] [Google Scholar]
- Carneiro R. M. D. G., Carneiro R. G., Neves D. I., Almeida M. R. A., 2003. A new race of Meloidogyne javanica on Arachis pintoi in the state of Parana. Nematologia Brasileira 27(2): 219–221. [Google Scholar]
- Chu Y., Wu C., Holbrook C. C., Tillman B., Person G., et al. , 2011. Marker-assisted selection to pyramid nematode resistance and the high oleic trait in peanut. Plant Genome 4(2): 110–117. [Google Scholar]
- Churchill G. A., Doerge R. W., 1994. Empirical threshold values for quantitative trait mapping. Genetics 138: 963–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Waele D., Jones B. L., Bolton C., Van den Berg E., 1989. Ditylenchus destructor in hulls and seeds of peanut. J. Nematol. 21: 10–15. [PMC free article] [PubMed] [Google Scholar]
- Doerge R. W., Churchill G. A., 1996. Permutation tests for multiple loci affecting a quantitative character. Genetics 142: 285–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong W., Holbrook C. C., Timper P., Brenneman T. B., Mullinix B. G., 2007. Comparison of methods for assessing resistance to Meloidogyne arenaria in peanut. J. Nematol. 39(2): 169–175. [PMC free article] [PubMed] [Google Scholar]
- Eisenback J. D., Bernard E. C., Starr J. L., Lee T. A., Tomaszewski E. K., 2003. Meloidogyne haplanaria n. sp. (Nematoda: Meloidogynidae), a root-knot nematode parasitizing peanut in Texas. J. Nematol. 35: 395–403. [PMC free article] [PubMed] [Google Scholar]
- Ferguson M. E., Burow M. D., Schulze S. R., Bramel P. J., Paterson A. H., et al. , 2004. Microsatellite identification and characterization in peanut (A. hypogaea L.). Theor. Appl. Genet. 108: 1064–1070. [DOI] [PubMed] [Google Scholar]
- Fonceka D., Tossim H.-A., Rivallan R., Vignes H., Faye I., et al. , 2012. Fostered and left behind alleles in peanut: interspecific QTL mapping reveals footprints of domestication and useful natural variation for breeding. BMC Plant Biol. 12(1): 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia G. M., Stalker H. T., Kochert G., 1995. Introgression analysis of an interspecific hybrid population in peanuts (Arachis hypogaea L.) using RFLP and RAPD markers. Genome 38(1): 166–176. [DOI] [PubMed] [Google Scholar]
- Guimarães P., Brasileiro A., Proite K., de Araújo A., Leal-Bertioli S., et al. , 2010. A study of gene expression in the nematode resistant wild peanut relative, Arachis stenosperma, in response to challenge with Meloidogyne arenaria. Trop. Plant Biol. 3(4): 183–192. [Google Scholar]
- Guimarães P., Brasileiro A., Morgante C., Martins A., Pappas G., et al. , 2012. Global transcriptome analysis of two wild relatives of peanut under drought and fungi infection. BMC Genomics 13(1): 387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holbrook C. C., Noe J. P., 1990. Resistance to Meloidogyne arenaria in Arachis spp. and the implications on development of resistant peanut cultivars. Peanut Science 17: 35–38. [Google Scholar]
- Holbrook C. C., Stalker H. T., 2003. Peanut breeding and genetic resources. Plant Breed. Rev. 22: 297–355. [Google Scholar]
- Holbrook C. C., Timper P., Culbreath A. K., Kvien C. K., 2008. Registration of ‘Tifguard’ peanut. Journal of Plant Registrations 2: 2. [Google Scholar]
- Hussey R. S., Barker K. R., 1973. A comparison of methods of collecting inocula of Meloidogyne spp., including a new technique. Plant Disease Reporter 57: 1025–1028. [Google Scholar]
- Lander E., Green P., Abrahamson J., Barlow A., Daly M., et al. , 1987. MAPMAKER: an interactive computer package for constructing primary genetic maps of experimental and natural populations. Genomics 1: 174–181. [DOI] [PubMed] [Google Scholar]
- Langmead B., Salzberg S. L., 2012. Fast gapped-read alignment with Bowtie 2. Nat. Methods 9(4): 357–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leal-Bertioli S. C. M., Jose A. C. V. F., Alves-Freitas D. M. T., Moretzsohn M. C., Guimarães P. M., et al. , 2009. Identification of candidate genome regions controlling disease resistance in Arachis. BMC Plant Biol. 9(1): 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leal-Bertioli S. C. M., Farias M. P., Silva P. T., Guimarães P. M., Brasileiro A. C. M., et al. , 2010. Ultrastructure of the initial interaction of Puccinia arachidis and Cercosporidium personatum with leaves of Arachis hypogaea and Arachis stenosperma. J. Phytopathol. 158(11–12): 792–796. [Google Scholar]
- Leal-Bertioli S. C. M., Bertioli D. J., Guimarães P. M., Pereira T. D., Galhardo I., et al. , 2012. The effect of tetraploidization of wild Arachis on leaf morphology and other drought-related traits. Environ. Exp. Bot. 84: 17–24. [Google Scholar]
- Leal-Bertioli S., Shirasawa K., Abernathy B., Moretzsohn M., Chavarro C., et al. , 2015a Tetrasomic recombination is surprisingly frequent in allotetraploid Arachis. Genetics 199(4): 1093–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leal-Bertioli, S.C., U. Cavalcante, E.G. Gouveia, C. Ballen-Taborda, K. Shirasawa et al., 2015b Identification of QTLs for rust resistance in the peanut wild species Arachis magna and the development of KASP markers for marker assisted selection. G3 (Bethesda) 5(7):1403–1413. [DOI] [PMC free article] [PubMed]
- Leal-Bertioli S. C. M., Santos S. P., Dantas K. M., Inglis P. W., Nielen S., et al. , 2015c Arachis batizocoi: a study of its relationship to cultivated peanut (A. hypogaea) and its potential for introgression of wild genes into the peanut crop using induced allotetraploids. Ann. Bot. (Lond.) 115(2): 237–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Handsaker B., Wysoker A., Fennell T., Ruan J., et al. , 2009. The Sequence Alignment/Map format and SAMtools. Bioinformatics 25(16): 2078–2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lincoln, S., M.J. Daly and E. S. Lander, 1992 Mapping genes controlling quantitative traits with MAPMAKER/QTL. Technical Report, Whitehead Institute, Cambridge, MA.
- Linsley E. G., Cazier M. A., 1963. Further observations on bees which take pollen from plants of the genus Solanum. Pan-Pac. Entomol. 39: 1–18. [Google Scholar]
- Michelotto M. D., Barioni W., Jr, Resende M. D. V., Godoy I. J., Leonardecz E., et al. , 2015. Identification of fungus resistant wild accessions and interspecific hybrids of the genus Arachis. PLoS One . 10.1371/journal.pone.0128811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moretzsohn M., Leoi L., Proite K., Guimarães P., Leal-Bertioli S., et al. , 2005. A microsatellite-based, gene-rich linkage map for the AA genome of Arachis (Fabaceae). Theor. Appl. Genet. 111(6): 1060–1071. [DOI] [PubMed] [Google Scholar]
- Morgante C. V., Brasileiro A. C. M., Roberts P. A., Guimarães L. A., Araújo A. C. G., et al. , 2013. A survey of genes involved in Arachis stenosperma resistance to Meloidogyne arenaria race 1. Funct. Plant Biol. 40(12): 1298–1309. [DOI] [PubMed] [Google Scholar]
- Nagy E., Chu Y., Guo Y., Khanal S., Tang S., et al. , 2010. Recombination is suppressed in an alien introgression in peanut harboring Rma, a dominant root-knot nematode resistance gene. Mol. Breed. 26(2): 357–370. [Google Scholar]
- Nagy E. D., Guo Y., Tang S., Bowers J. E., Okashah R. A., et al. , 2012. A high-density genetic map of Arachis duranensis, a diploid ancestor of cultivated peanut. BMC Genomics 13(1): 469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson S. C., Simpson C. E., Starr J. L., 1989. Resistance to Meloidogyne arenaria in Arachis spp. germplasm. J. Nematol. 21: 654–660. [PMC free article] [PubMed] [Google Scholar]
- Norden A. J., Lipscomb R. W., Carver W. A., 1969. Registration of ‘Florunner’ peanuts. Crop Sci. 9: 850. [Google Scholar]
- Oliphant A., Barker D. L., Stuelpnagel J. R., Chee M. S., 2002. BeadArray technology: Enabling an accurate, cost-effective approach to high-throughput genotyping. Biotechniques 32: S56. [PubMed] [Google Scholar]
- Oostenbrink M., 1966. Major characteristics of the relation between nematodes and plants. Mededelingen Landbouwhogeschool Wageningen 66: 1–46. [Google Scholar]
- Proite K., Leal-Bertioli S., Bertioli D., Moretzsohn M., da Silva F., et al. , 2007. ESTs from a wild Arachis species for gene discovery and marker development. BMC Plant Biol. 7(1): 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proite K., Carneiro R., Falcão R., Gomes A., Leal-Bertioli S., et al. , 2008. Post-infection development and histopathology of Meloidogyne arenaria race 1 on Arachis spp. Plant Pathol. 57(5): 974–980. [Google Scholar]
- Shirasawa K., Bertioli D. J., Varshney R. K., Moretzsohn M. C., Leal-Bertioli S. C. M., et al. , 2013. Integrated consensus map of cultivated peanut and wild relatives reveals structures of the A and B genomes of Arachis and divergence of the legume genomes. DNA Res. 20(2): 173–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson C. E., Starr J. L., 2001. Registration of ‘COAN’ peanut. Crop Sci. 41: 918. [Google Scholar]
- Simpson C. E., Nelson S. C., Starr L. J., Woodard K. E., Smith O. D., 1993. Registration of TxAG-6 and TxAG-7 peanut germplasm lines. Crop Sci. 33(6): 1418. [Google Scholar]
- Simpson C. E., Starr J. L., Church G. T., Burrow M. D., Paterson A. H., 2003. Registration of NemaTAM peanut. Crop Sci. 43: 1561. [Google Scholar]
- Simpson C. E., Starr J. L., Baring M. R., Burow M. D., Cason J. M., et al. , 2013. Registration of ‘Webb’ peanut. Journal of Plant Registrations 7(3): 265–268. [Google Scholar]
- Stalker H. T., Moss J. P., 1987. Speciation, cytogenetics and utilization of Arachis species. Adv. Agron. 41: 1–39. [Google Scholar]
- Stalker H. T., Beute M. K., Shew B. B., Barker K. R., 2002. Registration of two root-knot nematode-resistant peanut germplasm lines. Crop Sci. 42(1): 312–313. [DOI] [PubMed] [Google Scholar]
- Van Ooijen, J., 2006 JoinMap 4. Software for the calculation of genetic linkage maps in experimental populations. Wageningen: Kyazma, B.V.
- Voorrips R. E., 2002. MapChart: software for the graphical presentation of linkage maps and QTLs. J. Hered. 93(1): 77–78. [DOI] [PubMed] [Google Scholar]
- Wang C., Ulloa M., Mullens T. R., Yu J. Z., Roberts P. A., 2012. QTL analysis for transgressive resistance to root-knot nematode in interspecific cotton (Gossypium spp.) progeny derived from susceptible parents. PLoS One 7(4): e34874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, S., C. J. Basten, and Z. B. Zeng, 2006 Windows QTL Cartographer 2.5. Department of Statistics, North Carolina State University, Raleigh, NC. [Google Scholar]
- Wendt C. D., Swart A., Vrain T. C., Webster J. M., 1995. Ditylenchus africanus sp. N. from South Africa; a morphological and molecular characterization. Fundam. Appl. Nematol. 18: 241–250. [Google Scholar]
- Zeng Z. B., 1993. Theoretical basis for separation of multiple linked gene effects in mapping quantitative trait loci. Proc. Natl. Acad. Sci. USA 90(23): 10972–10976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Z. B., 1994. Precision mapping of quantitative trait loci. Genetics 136(4): 1457–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.