Abstract
Background
Rising overdose fatalities among US veterans suggest veterans taking prescription opioids may be at risk for overdose. However, it is unclear whether veterans prescribed chronic opioids are aware of this risk.
Objectives
The objective of this study was to identify risk factors and determine awareness of risk for opioid overdose in veterans treated with opioids for chronic pain, using veterans treated with methadone or buprenorphine for opioid use disorder as a high-risk comparator group.
Methods
Ninety veterans on chronic opioid medication for either opioid use disorder or pain management completed a questionnaire assessing risk factors, knowledge, and self-estimate of risk for overdose.
Results
Nearly all veterans in both groups had multiple overdose risk factors although individuals in the pain management group had on average a significantly lower total number of risk factors than did individuals in the opioid use disorder group (5.9 v. 8.5, p<0.0001). On average, participants treated for pain management scored slightly but significantly lower on knowledge of opioid overdose risk factors (12.1 v. 13.5, p<0.01). About 70% of participants, regardless of group, believed their overdose risk was below that of the average American adult. There was no significant relationship between self-estimate of overdose risk and either number or knowledge of opioid overdose risk factors.
Discussion
Our results suggest that veterans in both groups underestimated their risk for opioid overdose. Expansion of overdose education to include individuals on chronic opioids for pain management and a shift in educational approaches to overdose prevention may be indicated.
Keywords: overdose, veterans, opioids, chronic pain, medication assisted treatment, risk assessment
Introduction
Overdose is now a public health crisis in the United States1. Deaths due to opioid overdose (OOD) have increased dramatically since the mid-1990’s1, 2 such that unintentional poisoning is now the leading cause of injury-related death among Americans age 25–643. Increased overdoses due to prescription opioid analgesics are responsible for most of this increase4. Opioid-using patients across a variety of health care settings, not just those identified as people who misuse substances, may be at risk for overdose.
It is unclear how frequently physicians warn patients about the risks of overdose when prescribing opioids5. Although researchers have identified factors associated with increased risk for OOD, patients taking prescribed opioids may or may not be aware of these specific risk factors. Even those who are aware of risk factors may not identify themselves as being at risk. Optimistic bias is present when an individual believes his or her personal risk for a particular outcome is lower than for others in a similar risk group6, 7. This bias has been documented in studies of medical risks ranging from osteoporosis8 to HIV9 to heart disease10. Among people who misuse substances, several studies have noted that individuals with multiple HIV transmission risk factors tended to perceive their personal risk of contracting HIV as low11–13. A recent qualitative study found that people who misuse opioids other than heroin often perceive these opioids as being safe from the risk of overdose, even though most of the interviewees had experienced one or more overdoses in the past14.
Studies of non-prescription use of opioids have identified risk factors associated with overdose. Some are not modifiable, such as a history of prior overdose15–17, a history of incarceration or arrest18–20, and male gender16, 17, 21. Others are modifiable, such as injection drug use17, 22, use of alcohol15, 20, 22, 23, use of benzodiazepines/sedatives22, 23, and use of cocaine15, 22. Additionally, the period immediately following release from incarceration has been identified as a high risk period for drug-related death, primarily due to overdose24. The risk of drug-related death is estimated to be 3 to 8 times as likely in the first 2 weeks of release as in the ensuing 10 weeks25.
While there are well established risk factors for overdose from the non-prescription use of opioids, the potential for overdose from prescribed opioids, especially for treatment of chronic pain, is less well understood. Thus far, opioid dose, opioid type, and co-prescription of benzodiazepines have been identified as risk factors for overdose among individuals receiving prescribed opioids. Increasing dose of prescribed chronic opioid therapy, expressed in morphine-equivalents (ME), is directly associated with increasing risk of overdose23, 26, 27. Methadone, when prescribed for pain, is disproportionately represented among overdose related deaths28, suggesting that individuals prescribed methadone as opposed to an alternative opioid for pain relief may also be at higher risk for fatal overdose. Finally, co-prescription of benzodiazepines and opioids is associated with a dose-dependent increased risk of overdose death29 in US veterans.
The rise in OOD in the population of veterans seeking treatment through the Veterans Health Administration (VA) closely mirrors that of the US population. A recent study also demonstrated a population-level association between trends in VA opioid prescribing by state and overdose deaths in veterans by state30. Risk factors for death from accidental overdose (including opioid overdose) among veterans include male sex, age between 30 and 59, and having a general psychiatric or substance use disorder31. Veterans may be at particular risk for OOD given that they have high rates of pain treated with prescription opioids32: between FY 2004 and FY 2008, about 32% of individuals treated nationwide through the VA received at least one prescription for opioids for pain27. Thus, veterans’ hospitals are an important setting for studying the risk of prescription OOD.
Individuals treated for opioid use disorder with methadone or buprenorphine are likely to have easily identifiable risk factors for OOD, whereas determining risk level for veterans on chronic opioids for pain is more challenging. OOD prevention education is usually provided prior to prescription of either methadone or buprenorphine for opioid use disorder; in fact, this is a federally mandated component of patient orientation in methadone clinics33. On the other hand, OOD education is not usually a focus for individuals on opioids for chronic pain, partly because it is not clear whether such education is needed. The purpose of this study was to identify the presence of risk factors and determine the risk awareness for OOD in veterans treated with opioids for chronic pain, using veterans treated with methadone or buprenorphine for opioid use disorder as a high-risk comparator group. Clarifying the risk factors and risk awareness for OOD in veterans treated with opioids for chronic pain could help determine whether and how OOD education should be administered to these veterans.
Material and Methods
Both outpatient clinics participating in this study are located in a VA hospital in a mid-sized mid-Western city. The Opioid Treatment Clinic (OTC) provides group and individual counseling as well as methadone or buprenorphine treatment for approximately 310 veterans with opioid use disorder. The Pain Management Clinic (PMC) provides comprehensive pain management, including counseling, physical therapy, acupuncture, chiropractic treatment, and pharmacologic treatment with opioid and other analgesic prescriptions, to about 150 veterans with chronic pain conditions. To be included in this study, veterans had to be receiving opioid medication prescribed by a VA medical provider for a minimum of 3 consecutive months and had to be receiving treatment from either the OTC or PMC, but not both, at the time of study entry. The study was approved by the requisite institutional review board and VHA research and development committees.
Veterans in both clinics were recruited from July 2013 to January 2014 through fliers posted in approved locations at each clinic. Fliers requested that interested patients who had been taking opioid medication for at least 3 months call a designated study number to participate in a survey about veterans’ perceptions of risk for overdose on opioid medications and interest in overdose treatment. Additionally, veterans in the OTC received a flier at the dosing window of the clinic, while veterans in the PMC received a flier from the clinic nurse at the beginning of their appointments. One hundred thirteen veterans were screened for participation and of these 106 met inclusion criteria. Veterans meeting inclusion criteria were scheduled for a face-to-face interview. Ninety veterans completed the study interview; 16 did not attend their interview sessions. Approximately 20% of the total estimated patient population of the two clinics participated in the survey.
All veterans completed the written informed consent process prior to beginning the 45 minute, verbally administered study interview. Prior to the study interview, research staff read the following definition of overdose to each participant: “When we use the term overdose, we mean when a toxic amount of drug or drugs overwhelms the body such that a person is no longer able to respond to others or breathe adequately. An overdose can occur with prescription or recreational drugs, or a combination of both, and can be deliberate or accidental.” This definition, along with a list of common opioid and sedative medications, was provided in written form for the participant to refer to throughout the interview. During the first part of the interview, with permission from the participant, research staff recorded all active prescriptions in the VA electronic medical record for opioids and sedatives (benzodiazepines, barbiturates, and muscle relaxants), including the type of medication, the dose and frequency of the medication, and how long the medication had been prescribed. Veterans received a small monetary reimbursement for their participation.
The interview included questions about demographics and medical/psychiatric conditions. It also contained multiple items about personal OOD risk factors. The AUDIT-C was used to assess alcohol use34 and the DAST-10 was used to assess drug use35. History of overdose, arrests, and intravenous drug use were assessed through additional interview questions. Opioid and sedative use were assessed through both the interview and a review of the participant’s medical record; because participants were taking a variety of opioid medications, we converted their total daily opioid dose to the equianalgesic morphine equivalent (ME) dose, where 1 ME = 1 mg oral morphine per day36. Participants were asked to assess their risk of OOD in two ways: 1) participants were asked, “Compared to the average American adult, what do you think is your risk of overdosing on opioids in the next year?” (higher versus the same or lower, referred to as “comparative risk self-estimate” in this paper); 2) participants were asked to estimate the percent chance that they would overdose on opioids in the next year using a visual analog scale (referred to as “absolute risk self-estimate” in this paper). Participant knowledge of OOD risk factors was assessed with an 18 question true/false test. Participants were instructed to consider whether each item was a risk factor for OOD in general, not whether it was a risk factor for them personally. Some items on the true/false test were modified from Domain A of the Opioid Overdose Knowledge Scale 37, a previously validated measure. However, we did not use the full OOKS for several reasons: it focused exclusively on heroin users, making some items (i.e., increase in heroin purity, switching from smoking to injecting heroin) inappropriate for participants who did not use heroin; it included multiple questions about symptoms and treatment of OOD which were not relevant to our study; and all answers in Domain A of OOKS were “true,” which we thought might lead our participants to lose focus after answering multiple items. Some false risk factors (such as, “Not getting enough sleep”) were therefore included to maintain participant engagement during the test. The risk factor knowledge test used in this study was piloted in a sample of 250 individuals in community treatment for substance use; validation of the test is ongoing.
All data analyses were completed using SAS, Version 9.3 (SAS Institute, Inc., Cary, North Carolina). Statistical tests were conducted at a 5% Type I error rate with Bonferroni correction for multiple comparisons. Baseline demographics and medication use were compared between the two clinics using Wilcoxon rank-sum, chi-square, or Fisher’s exact tests as appropriate. Percent of participants with each identified OOD risk factor and percent of participants correctly identifying each risk factor on the knowledge test was compared across clinics using Fisher’s exact tests. Mean number of participant risk factors and total score on the knowledge test were compared by clinic and by comparative risk self-estimate using the Wilcoxon rank-sum test. Pearson correlation coefficients were determined to compare number of risk factors with knowledge of risk factors, absolute risk self-estimate with knowledge of risk factors, and absolute risk self-estimate with number of risk factors.
Results
Ninety individuals completed the survey, 52 from the OTC and 38 from the PMC. Table 1 displays characteristics of respondents. The median VA-prescribed ME dose for PMC participants was 35 ME (interquartile range 70). The median total ME dose, based on self-reported use of all prescribed and illicit opioids, for PMC participants (excluding 2 individuals who used heroin, for which ME could not be calculated) was 56 ME (interquartile range 70). The median VA-prescribed ME dose for OTC participants was 430 ME (interquartile range 230); 77% of OTC participants were prescribed methadone and the remainder were taking buprenorphine. It was not possible to calculate the total ME dose (all prescribed plus all illicit) for OTC participants due to the inability to estimate ME for heroin. Seventy-nine percent of OTC and 58% of PMC participants (p<0.05) reported that they had “never” taken an extra dose of their VA-prescribed opioid medication, while 11% of OTC and 13% of PMC participants (NS) reported taking an extra dose at least once per week. Twenty-five percent of OTC and 29% of PMC (NS) participants reported opioid-related aberrant behaviors (defined as taking more than the prescribed amount of their VA-prescribed opioids, using opioids obtained from non-VA prescribers, or using illicit opioids) at least once in the last 3 months. Seventeen percent of OTC and 58% of PMC participants (p<0.001) were prescribed sedative medications (including benzodiazepines, other hypnotics such as zolpidem, and muscle relaxants such as carisoprodol); 6% OTC and 29% PMC (p<0.01) participants were prescribed benzodiazepines specifically. Six percent of OTC and 5% of PMC participants (NS) reported sedative-related aberrant behaviors (defined as taking more than the prescribed amount of their VA-prescribed sedatives, using sedatives obtained from non-VA prescribers, or using illicit sedatives) at least once in the last 3 months.
Table 1.
Characteristics of participating veterans in the PMC and OTC
| Pain Management Clinic n=38 Mean (standard deviation) OR Percent (n) | Opioid Treatment Clinic n=52 Mean (standard deviation) OR Percent (n) | |
|---|---|---|
| Age | 55.2 (10.9) | 46.2 (12.7) |
| Male | 94.7% (36) | 92.3% (48) |
| Race | ||
| Caucasian | 68.4% (26) | 86.5% (45) |
| African American | 18.5% (7) | 3.9% (2) |
| Other | 13.2% (5) | 9.6% (5) |
| Hispanic ethnicity | 2.6% (1) | 7.7% (4) |
| High school graduate or GED | 89.5% (34) | 98.1% (51) |
| Married | 55.3% (21)A | 19.2% (10)A |
| Housing | ||
| Lives alone | 26.3% (10)A | 32.7% (17)A |
| Lives with family | 68.4% (26)A | 32.7% (17)A |
| Lives with non-family | 5.3% (2)A | 34.6% (18)A |
| Lives with another opioid user | 21.1% (8) | 35.3% (18) |
| Homeless | 0.0% (0) | 11.5% (6) |
| OIF/OEF status* | 15.8% (6) | 26.9% (14) |
| Combat experience | 50.0% (19) | 38.5% (20) |
| Service connected disability | 50.0% (19) | 48.1% (225) |
| Medical conditions | ||
| COPD or asthma | 23.7% (9) | 17.3% (9) |
| Liver disease | 23.7% (9) | 40.4% (21) |
| Kidney disease | 10.5% (4) | 3.9% (2) |
| Sleep apnea | 31.6% (12) | 25.0% (13) |
| Heart Disease | 29.0% (11) | 13.5% (7) |
| Psychiatric conditions | ||
| Depression | 47.4%(18) | 80.8% (42) |
| Severe mental illness** | 15.8% (6) | 17.3% (9) |
| PTSD | 36.8% (14) | 57.7% (30) |
| PHQ-2 | 1.7 (2.0) | 2.4 (2.1) |
| Substance use | ||
| Daily Tobacco user | 50.0% (19) | 73.1% (38) |
| Used IVD in life | 18.4% (7)C | 71.2% (37)C |
| AUDIT-C | 1.8 (2.6) | 1.3 (2.2) |
| DAST-10 | 2.6 (3.3)B | 8.6 (1.2)B |
Served in Operation Iraqi Freedom/Operation Enduring Freedom
Bipolar disorder, schizophrenia, or schizoaffective disorder
Group differences significant at p<0.05 with Bonferroni correction for multiple comparisons
Group differences significant at p<0.01 with Bonferroni correction for multiple comparisons
Group differences significant at p<0.001 with Bonferroni correction for multiple comparisons
Table 2 displays all potential OOD risk factors assessed for participants. The average number of risk factors was significantly higher for participants in the OTC compared to participants in the PMC (8.5 v. 5.9, p<0.0001). There was no significant correlation between participants’ number of risk factors and their knowledge of OOD risk factors (Pearson’s rho=0.18, p=0.09). OTC participants scored slightly but significantly higher on average than PMC participants on a test of their knowledge of OOD risk factors (13.5 v. 12.1 out of a possible 18, p<0.01). Table 3 provides detailed information on the results for each question. Of note, over 90% of both groups recognized that taking higher than usual amounts of opioids, mixing alcohol or other drugs with opioids, using opioids from more than one doctor, and injecting opioids were risk factors for OOD. On the other hand, less than 70% of either group recognized liver/kidney disease and sleep apnea as risk factors. Several items illustrated differences in knowledge between the two groups. A higher percentage of OTC participants than PMC participants recognized that using opioids alone, using soon after release from incarceration, using after a week or more of no use, and having breathing problems were risk factors for OOD. However, after controlling for multiple comparisons, the only difference between groups that remained significant was “taking opioids after a week or more of not using any” (p<0.001). Some misconceptions were also present. More than half of both groups thought that not getting enough sleep was a risk factor for OOD. Around 40% of OTC participants incorrectly thought that being overweight was also a risk factor, whereas less than 20% of PMC participants made this error.
Table 2.
Risk factors for opioid overdose identified in participating veterans in the PMC and OTC
| Percent with this risk factor for opioid overdose | ||
|---|---|---|
| Risk factor for opioid overdose | Pain Management Clinic n=38 Percent (n) | Opioid Treatment Clinic n=52 Percent (n) |
| Male | 94.7% (36) | 92.3% (48) |
| Caucasian | 68.4% (26) | 86.5% (45) |
| Mental illness | 57.9% (22) | 86.5% (45) |
| Arrested more than 3 times in life | 47.4% (18) | 67.3% (35) |
| Divorced | 34.2% (13) | 53.9% (28) |
| Daily prescribed opioid dose >50 ME but ≤ 100 ME | 31.6% (12)B | 1.9% (1)B |
| Sleep apnea | 31.6% (12) | 25.0% (13) |
| Using benzodiazepines | 29.0% (11) | 11.5% (6) |
| DAST positive score | 23.7% (9)C | 98.1% (51)C |
| AUDIT-C positive score | 23.7% (9) | 23.1% (12) |
| Liver disease | 23.7% (9) | 40.4% (21) |
| COPD or asthma | 23.7% (9) | 17.3% (9) |
| Daily prescribed opioid dose >100 ME | 21.1% (8)C | 98.1% (51)C |
| Past overdose | 21.1% (8) | 53.9% (27) |
| Past intravenous drug use | 18.4% (7)C | 71.2% (37)C |
| Never married | 10.5% (4) | 19.2% (10) |
| Kidney disease | 10.5% (4) | 3.9% (2) |
| Did not finish high school | 10.5% (4) | 1.9% (1) |
| Taking opioids prescribed by more than one doctor | 7.9% (3) | 1.9% (1) |
| Average number of potential risk factors | 5.9 (STD=2.6, median=6) | 8.5 (STD= 1.7, median=9) |
Group differences significant at p<0.05 with Bonferroni correction for multiple comparisons
Group differences significant at p<0.01 with Bonferroni correction for multiple comparisons
Group differences significant at p<0.001 with Bonferroni correction for multiple comparisons
Table 3.
Performance of veterans in the PMC and OTC on a knowledge-based test of opioid overdose risk factors. Test was true-false; correct answer in parenthesis.
| Percent identifying item as a risk factor for opioid overdose | ||
|---|---|---|
| Is this a risk factor for opioid overdose? | Pain Manageme nt Clinic n=38 Percent (n) | Opioid Treatme nt Clinic n=52 Percent (n) |
| Taking opioids from more than one doctor (T) | 100.0% (38) | 94.2% (49) |
| Taking larger than usual doses of opioids (T) | 97.4% (37) | 98.1% (51) |
| Injecting opioids (T) | 97.4% (37) | 92.3% (48) |
| Taking opioids and recreational drugs at the same time (T) | 94.7% (36) | 98.1% (51) |
| Drinking alcohol while taking opioids (T) | 94.7% (36) | 96.2% (50) |
| Taking opioids and sedatives at the same time (T) | 89.5% (34) | 98.1% (51) |
| Taking opioids again soon after release from jail or prison (T) | 68.4% (26) | 90.4% (47) |
| Not getting enough sleep (F) | 60.5% (23) | 65.4% (34) |
| Taking opioids with no one else around (T) | 52.6% (20) | 78.9% (41) |
| Taking opioids after a week or more of not using any (T) | 50.0% (19)C | 88.5% (46)C |
| Having liver or kidney disease (T) | 50.0% (19) | 69.2% (36) |
| Having breathing problems like asthma, emphysema, or COPD (T) | 44.7% (17) | 73.1% (38) |
| Having sleep apnea (T) | 34.2% (13) | 53.9% (27) |
| Being a tobacco smoker (F) | 29.0% (11) | 17.3% (9) |
| Not drinking enough water (F) | 21.1% (8) | 17.3% (9) |
| Being overweight (F) | 15.8% (6) | 42.3% (22) |
| Being female (F) | 15.8% (6) | 7.7% (4) |
| Having acid reflux (GERD) (F) | 13.2% (5) | 7.7% (4) |
Group differences significant at p<0.05 with Bonferroni correction for multiple comparisons
Group differences significant at p<0.01 with Bonferroni correction for multiple comparisons
Group differences significant at p<0.001 with Bonferroni correction for multiple comparisons
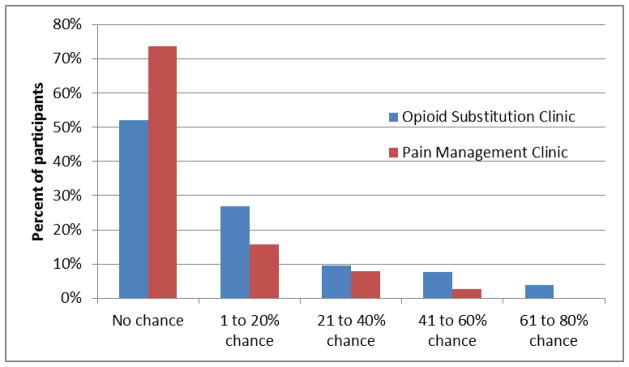
When asked to compare their risk for overdosing on opioids in the next year to the average American adult (comparative risk self-estimate), 70% of all participants estimated their risk as lower. There was no significant difference in comparative risk self-estimate when compared by clinic (65% in OTC v. 76% in PMC). Comparative risk self-estimate was not reflective of actual risk factors or knowledge of risk factors. Individuals who estimated themselves as having a lower than average risk had a mean of 7.2 risk factors while those who estimated themselves as having the same or higher risk had a mean of 7.8 risk factors (NS). The mean score on the test of knowledge of OOD risk factors was 12.8 for participants estimating their risk as lower versus 13.2 for participants estimating their risk as the same or higher (NS). Figure 1 displays the results of participants’ estimates of their risk for OOD in the next year on a visual analog scale (absolute risk self-estimate). Fifty-two percent (n=27) of OTC participants and 74% (n=28) of PMC participants estimated their risk as 0. The average absolute risk self-estimate was 13.5 for OTC participants and 7.0 for PMC participants (p=0.05). There was a correlation between number of risk factors and absolute risk self-estimate on the visual analog scale (Pearson’s rho=0.32, p<0.002). There was no correlation between absolute risk self-estimate and score on the test of knowledge of OOD risk factors (Pearson’s rho=0.13, p =0.22).
Figure 1.
Participant estimate of risk for overdose on opioids in the next year, using visual analog scale graded from 0–100%
Discussion
In our study sample, we found that every participant had at least 2 risk factors for OOD and the majority had at least 6. Although patients in the OTC did have more risk factors on average, the mean number of risk factors for PMC participants was also high. With the exception of risk factors directly related to substance use disorders (for example, DAST score or prior use of intravenous drugs), there were few other differences between risk factors for each group. Many of the study veterans being treated with opioids for chronic pain had OOD risk profiles similar to those being treated with methadone or buprenorphine for opioid use disorder. Veterans being treated for chronic pain also scored lower on knowledge of OOD risk factors than did those being treated for opioid use disorder, suggesting individuals being treated for chronic pain might be less aware of their risk.
Veterans in both clinics did fairly well overall on the test of overdose knowledge, consistent with prior studies done among non-veterans38, 39. While it is encouraging that over 90% of veterans recognized a range of important OOD risk factors, there were certain risk factors neither group was apt to recognize. Many veterans in both groups did not identify liver disease or sleep apnea as potential risks for overdose in persons using opioids, even though these conditions are particularly prevalent in the veteran population. PMC participants were significantly less likely than OTC participants to recognize that using opioids after a week or more of not using was a risk factor for overdose. This risk is often emphasized in OOD education programs for people who inject drugs, where brief periods of enforced abstinence (due to detox, jail, or drug availability) are common. However, individuals in pain management programs can also experience brief periods of abstinence if they overuse their prescriptions and then run out of medication prior to scheduled refill. Increased education about OOD risks for individuals who are not identified as having substance use disorders may be needed, especially for those on high dose opioids.
Our study also revealed that about a quarter of the individuals in both the OTC and PMC samples were misusing their medications at least occasionally. Among PMC participants, the median ME of total opioids used was over 160% of the median ME dose prescribed through the VA, suggesting that some individuals were using substantially more opioids (either prescribed by non-VA physicians, obtained from family or acquaintances, or purchased illicitly) than prescribed by their VA providers. In two large studies26, 27, one of which focused on veterans, opioid therapy doses of just 50–100mg ME per day were associated with significantly increased risk for unintentional overdose: the median total dose of PMC participants was 56 ME. Almost 30% of individuals in the PMC group were also prescribed benzodiazepines, further increasing their risk for overdose.
Although all of the veterans surveyed, regardless of treatment group, had risk factors for OOD, 70% of participants estimated their risk as lower than the average American. There was no correlation between a participant’s total number of risk factors and his/her own estimate of risk as compared to the general population. We interpret this as an example of the optimistic bias that has already been demonstrated in multiple studies of health risk perception8–10. Participants were somewhat better at recognizing their risk when asked to estimate the chance that they would overdose within the next year rather than when asked to make a comparison with the “average American.” Although participants remained over-optimistic, with more than half estimating their chance as 0, the correlation between number of risk factors and estimate of absolute risk suggests that individuals had some awareness of the effects of personal risk factors on chance of OOD.
The difference between the two OOD risk estimates (one using comparative risk and one using absolute risk) might be explained by the “representative heuristic,” one of the driving components of optimistic bias. The representative heuristic describes the tendency for individuals who are asked to judge something relative to a prototype (“the average American adult”) to imagine instead a prototype that is stereotypical of the risk category (“someone who might overdose on opioids”)40. When an individual compares herself to this “risky” prototype, she then judges herself at lower risk than the stereotyped image. The representative heuristic may have been particularly likely in veterans in the OTC sample, who may associate with individuals at much higher risk for overdose than would be representative of the US population as a whole. When participants were asked to estimate their own absolute risk, without making a comparison to another group, the representative heuristic component of optimistic bias was no longer present, resulting in a more accurate self-assessment. Other aspects of optimistic bias, such as the tendency to focus on personal factors that decrease risk7 and to overestimate personal control6, remained in play, however; thus, even when asked about absolute risk, participants underestimated their risk.
Our findings suggest that OOD education efforts may be more salient if emphasis is placed on absolute risk rather than relative risk, for example, by identifying personal risk factors and discussing how these risk factors increase a particular individual’s risk of OOD. In a pain clinic setting, this might include highlighting personal medical and psychiatric problems that increase risk for OOD independent of a history of substance abuse. Because individuals tend to judge the risk of others as higher than their own risk40, focusing on the benefits of OOD education for at-risk family and friends may also be more effective than focusing only on the individual receiving the education. Finally, eliciting and reflecting on personal experiences of OOD, if any, may help individuals better recognize their risk for OOD, making them more likely to be invested in education and prevention programs.
Our study has several limitations and should be regarded as a starting point for more definitive studies of OOD risk factors and risk awareness. First, we recruited a relatively small convenience sample of veterans from two VA clinics at a single hospital. Our study sample cannot be assumed to be representative of these clinics, the larger VA population, or the U.S. population as a whole. Particularly in the PMC sample, individuals who had prior experience with OOD or for whom OOD was of personal relevance may have been more interested in and willing to participate in this survey, resulting in an over-estimate of the risk factors for the total PMC population receiving opioid treatment for chronic pain. Second, our study did not include a truly exhaustive assessment of OOD risk factors. Certain risk factors, such as alcohol use, were not assessed as effectively as they could have been (i.e., the AUDIT-C uses a time frame of the past year, which may not accurately reflect current use). Use of cocaine was not assessed at all due to concerns that this might affect responses focused specifically on opioid overdose, nor did we include any assessment or measure of pain in our survey. Additionally, it is difficult to assess the relative importance of various reported risk factors in contributing to overall risk of overdose. Therefore, the assessment of risk in this study is necessarily incomplete. Third, although the concept of equianalgesic doses of opioids allows comparison of opioids of varying potencies41, 42, these calculations are over-simplifications which do not take into account the many factors that contribute to the potency of a given analgesic in a particular individual42. Conversion calculations often vary from publication to publication43 and are particularly problematic for methadone and fentanyl44, 45. There are limited data published on equianalgesic calculations for sublingual, rather than parenteral or transdermal, buprenorphine. Nonetheless, we believe the utility of comparing opioids of differing potencies outweighs the known problems of this method.
Despite these study limitations, we can draw tentative conclusions. Veterans receiving chronic prescription opioids, such as those in treatment for opioid use disorder or on opioids for chronic pain, may be at risk for OOD and not fully aware of this risk, making it critical for providers to raise awareness of OOD in these settings. Our study suggests that at least some veterans on chronic opioids for pain have similar risk factors for OOD as patients receiving opioid agonist treatment for addiction. These veterans are much more likely to be missed in education and prevention campaigns focused on OOD. It is critical that this group not be excluded from education efforts. We believe that all patients on chronic opioids above 50mg ME, including those without substance use diagnoses, should be considered for prevention programs focusing on OOD education and naloxone distribution.
Acknowledgments
This report is based upon work supported by the Department of Veterans Affairs and the Research in Addiction Medicine Scholars (RAMS) Program, National Institute on Drug Abuse Award Number R25DA033211. Dr. Stein is a recipient of a NIDA Mid-Career Investigator Award (K24 DA00512).
Footnotes
Disclaimer
The contents do not represent the views of the Department of Veterans Affairs or the United States Government.
Contributors
All authors have reviewed and approved the manuscript. All authors contributed substantially to its content.
Conflict of Interest
The authors have no conflict of interest to declare.
References
- 1.Centers for Disease Control and Prevention. Vital signs: overdoses of prescription opioid pain relievers-United States, 1999–2008. Morbidity & Mortality Weekly Report. 2011;60:1487–1492. [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. Opioid overdoses in the United States. Journal of Pain & Palliative Care Pharmacotherapy. 2012;26:44–47. doi: 10.3109/15360288.2011.653875. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. National Center for Injury Prevention and Control. [Accessed Oct 20 2014];Data and Statistics Web-based Injury Statistics Query and Reporting System. 2005 http://www.cdc.gov/injury/wisqars/LeadingCauses.html.
- 4.Paulozzi LJ, Budnitz DS, Xi Y. Increasing deaths from opioid analgesics in the United States. Pharmacoepidemiology & Drug Safety. 2006;15:618–627. doi: 10.1002/pds.1276. [DOI] [PubMed] [Google Scholar]
- 5.Beauchamp G, Winstanley E, Ryan S, Lyons M. Moving beyond misuse and diversion: the urgent need to consider the role of iatrogenic addiction in the current opioid epidemic. American Journal of Public Health. 2014;104:2023–2029. doi: 10.2105/AJPH.2014.302147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shepperd JA, Klein WM, Waters EA, Weinstein ND. Taking stock of unrealistic optimism. Perspectives on Psychological Science. 2013;8:395–411. doi: 10.1177/1745691613485247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weinstein ND. Unrealistic optimism about future life events. Journal of Personality and Social Psychology. 1980;39:806–820. [Google Scholar]
- 8.Giangregorio L, Dolovich L, Cranney A, et al. Osteoporosis risk perceptions among patients who have sustained a fragility fracture. Patient Education & Counseling. 2009;74:213–220. doi: 10.1016/j.pec.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown EJ. College students’ AIDS risk perception. Journal of Psychosocial Nursing & Mental Health Services. 1998;36:25–30. doi: 10.3928/0279-3695-19980901-16. [DOI] [PubMed] [Google Scholar]
- 10.Green J, Grant M, Hill KL, Brizzolara J, Belmont B. Heart disease risk perception in college men and women. Journal of American College Health. 2003;51:207–211. doi: 10.1080/07448480309596352. [DOI] [PubMed] [Google Scholar]
- 11.Singer M, Dai H, Weeks MR, Malave D. AIDS risk perception among women drug users in Hartford, CT. Women & Health. 1998;27:67–85. doi: 10.1300/J013v27n01_05. [DOI] [PubMed] [Google Scholar]
- 12.Crisp BR, Barber JG, Ross MW, Wodak A, Gold J, Miller ME. Injecting drug users and HIV/AIDS: risk behaviours and risk perception. Drug & Alcohol Dependence. 1993;33:73–80. doi: 10.1016/0376-8716(93)90035-o. [DOI] [PubMed] [Google Scholar]
- 13.Johnston CL, Marshall BDL, Qi J, et al. HIV knowledge and perceptions of risk in a young, urban, drug-using population. Public Health. 2011;125:791–794. doi: 10.1016/j.puhe.2011.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frank D, Mateu-Gelabert P, Guarino H, et al. High risk and little knowledge: overdose experiences and knowledge among young adult nonmedical prescription opioid users. International Journal of Drug Policy. 2015;26:84–91. doi: 10.1016/j.drugpo.2014.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coffin PO, Tracy M, Bucciarelli A, Ompad D, Vlahov D, Galea S. Identifying injection drug users at risk of nonfatal overdose. Academic Emergency Medicine. 2007;14:616–623. doi: 10.1197/j.aem.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 16.Stoove MA, Dietze PM, Jolley D. Overdose deaths following previous non-fatal heroin overdose: record linkage of ambulance attendance and death registry data. Drug & Alcohol Review. 2009;28:347–352. doi: 10.1111/j.1465-3362.2009.00057.x. [DOI] [PubMed] [Google Scholar]
- 17.Britton PC, Wines JD, Jr, Conner KR. Non-fatal overdose in the 12 months following treatment for substance use disorders. Drug and Alcohol Dependence. 2010;107:51–55. doi: 10.1016/j.drugalcdep.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jenkins LM, Banta-Green CJ, Maynard C, et al. Risk factors for nonfatal overdose at Seattle-area syringe exchanges. Journal of Urban Health. 2011;88:118–128. doi: 10.1007/s11524-010-9525-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dietze P, Jolley D, Fry CL, Bammer G, Moore D. When is a little knowledge dangerous? Circumstances of recent heroin overdose and links to knowledge of overdose risk factors. Drug and Alcohol Dependence. 2006;84:223–230. doi: 10.1016/j.drugalcdep.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 20.Seal KH, Kral AH, Gee L, et al. Predictors and prevention of nonfatal overdose among street-recruited injection heroin users in the San Francisco Bay Area, 1998–1999. American Journal of Public Health. 2001;91:1842–1846. doi: 10.2105/ajph.91.11.1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hall AJ, Logan JE, Toblin RL, et al. Patterns of abuse among unintentional pharmaceutical overdose fatalities. JAMA. 2008;300:2613–2620. doi: 10.1001/jama.2008.802. [DOI] [PubMed] [Google Scholar]
- 22.Brugal MT, Barrio G, De LFL, Regidor E, Royuela L, Suelves JM. Factors associated with non-fatal heroin overdose: assessing the effect of frequency and route of heroin administration. Addiction. 2002;97:319–327. doi: 10.1046/j.1360-0443.2002.00058.x. [DOI] [PubMed] [Google Scholar]
- 23.Dietze P, Jolley D, Fry C, Bammer G. Transient changes in behaviour lead to heroin overdose: results from a case-crossover study of non-fatal overdose. Addiction. 2005;100:636–642. doi: 10.1111/j.1360-0443.2005.01051.x. [DOI] [PubMed] [Google Scholar]
- 24.Binswanger IA, Stern MF, Deyo RA, et al. Release from prison--a high risk of death for former inmates. N Engl J Med. 2007;356:157–165. doi: 10.1056/NEJMsa064115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Merrall ELC, Kariminia A, Binswanger IA, et al. Meta-analysis of drug-related deaths soon after release from prison. Addiction. 2010;105:1545–1554. doi: 10.1111/j.1360-0443.2010.02990.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dunn KM, Saunders KW, Rutter CM, et al. Opioid prescriptions for chronic pain and overdose: a cohort study. Annals of Internal Medicine. 2010;152:85–92. doi: 10.1059/0003-4819-152-2-201001190-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bohnert A, Valenstein M, Bair MJ, et al. Association between opioid prescribing patterns and opioid overdose-related deaths. JAMA. 2011;305:1315–1321. doi: 10.1001/jama.2011.370. [DOI] [PubMed] [Google Scholar]
- 28.Centers for Disease Control and Prevention. Vital signs: risk for overdose from methadone used for pain relief - United States, 1999–2010. Morbidity & Mortality Weekly Report. 2012;61:493–497. [PubMed] [Google Scholar]
- 29.Park TW, Saitz R, Ganoczy D, Ilgen MA, Bohnert A. Benzodiazepine prescribing patterns and deaths from drug overdose among US veterans receiving opioid analgesics: case-cohort study. British Medical Journal. 2015;350:h2698. doi: 10.1136/bmj.h2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bohnert A, Ilgen MA, Trafton JA, et al. Trends and regional variation in opioid overdose mortality among Veterans Health Administration patients, fiscal year 2001 to 2009. Clin J Pain. 2014;30:605–612. doi: 10.1097/AJP.0000000000000011. [DOI] [PubMed] [Google Scholar]
- 31.Bohnert A, Ilgen MA, Ignacio RV, McCarthy JF, Valenstein M, Blow FC. Risk of death from accidental overdose associated with psychiatric and substance use disorders. American Journal of Psychiatry. 2012;169:64–70. doi: 10.1176/appi.ajp.2011.10101476. [DOI] [PubMed] [Google Scholar]
- 32.Bennett AS, Elliott L, Golub A. Veterans’ health and opioid safety - contexts, risks, and outreach implications. Federal Practitioner. 2015;32:4–7. [PMC free article] [PubMed] [Google Scholar]
- 33.Substance Abuse and Mental Health Services Administration. Federal Guidelines for Opioid Treatment Programs. Rockville, MD: 2015. HHS Publication No. (SMA) PEP 15-FEDGUIDEOTP. [Google Scholar]
- 34.Bush K, Kivlahan D, McDonell M, Fihn S, Bradley K. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Archives of Internal Medicine. 1998;158:1789–1795. doi: 10.1001/archinte.158.16.1789. [DOI] [PubMed] [Google Scholar]
- 35.Yudko E, Lozhkina O, Fouts A. A comprehensive review of the psychometric properties of the Drug Abuse Screening Test. Journal of Substance Abuse Treatment. 2007;32:189–198. doi: 10.1016/j.jsat.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 36.Svendsen K, Borchgrevink P, Fredheim O, Hamunen K, Mellbye A, Dale O. Choosing the unit of measurement counts: the use of oral morphine equivalents in studies of opioid consumption is a useful addition to defined daily doses. Palliative Medicine. 2011;25:725–732. doi: 10.1177/0269216311398300. [DOI] [PubMed] [Google Scholar]
- 37.Williams A, Strang J, Marsden J. Development of Opioid Overdose Knowledge (OOKS) and Attitudes (OOAS) Scales for take-home naloxone training evaluation. Drug & Alcohol Dependence. 2013;132:383–386. doi: 10.1016/j.drugalcdep.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 38.Strang J, Manning V, Mayet S, et al. Overdose training and take-home naloxone for opiate users: prospective cohort study of impact on knowledge and attitudes and subsequent management of overdoses. Addiction. 2008;103:1648–1657. doi: 10.1111/j.1360-0443.2008.02314.x. [DOI] [PubMed] [Google Scholar]
- 39.Tobin KE, Sherman SG, Beilenson P, Welsh C, Latkin CA. Evaluation of the Staying Alive programme: Training injection drug users to properly administer naloxone and save lives. International Journal of Drug Policy. 2009;20:131–136. doi: 10.1016/j.drugpo.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 40.Shepperd JA, Carroll P, Grace J, Terry M. Exploring the causes of comparative optimism. Psychological Belgica. 2002;42:65–98. [Google Scholar]
- 41.Berdine HJ, Nesbit SA. Equianalgesic dosing of opioids. Journal of Pain & Palliative Care Pharmacotherapy. 2006;20:79–84. [PubMed] [Google Scholar]
- 42.Knotkova H, Fine PG, Portenoy RK. Opioid rotation: the science and the limitations of the equianalgesic dose table. Journal of Pain and Symptom Management. 2009;38:426–439. doi: 10.1016/j.jpainsymman.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 43.Shaheen PE, Walsh D, Lasheen W, Davis MP, Lagman RL. Opioid equianalgesic tables: are they all equally dangerous? Journal of Pain and Symptom Management. 2009;38:409–417. doi: 10.1016/j.jpainsymman.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 44.O’Bryant CL, Linnebur SA, Yamashita TE, Kutner JS. Inconsistencies in opioid equianalgesic ratios: clinical and research implications. Journal of Pain & Palliative Care Pharmacotherapy. 2008;22:282–290. doi: 10.1080/15360280802537241. [DOI] [PubMed] [Google Scholar]
- 45.Pereira J, Lawlor P, Vigano A, Dorgan M, Bruera E. Equianalgesic dose ratios for opioids a critical review and proposals for long-term dosing. Journal of Pain and Symptom Management. 2001;22:672–687. doi: 10.1016/s0885-3924(01)00294-9. [DOI] [PubMed] [Google Scholar]