Abstract
Background
Altered mental status is a significant predictor of mortality in inpatients. Several scales exist to characterize mental status, including the AVPU (Alert, responds to Voice, responds to Pain, Unresponsive) scale, which is used in many early warning scores in the general ward setting. The use of the Glasgow Coma Scale (GCS) and Richmond Agitation Sedation Scale (RASS) is not well established in this population.
Objective
To compare the accuracies of AVPU, GCS, and RASS for predicting inpatient mortality
Design
Retrospective cohort study
Setting
Single urban academic medical center
Participants
Adult inpatients on the general wards
Measurements
Nurses recorded GCS and RASS on consecutive adult hospitalizations. AVPU was extracted from the eye subscale of the GCS. We compared the accuracies of each scale for predicting in-hospital mortality within 24 hours of a mental status observation using area under the receiver operating characteristic curves (AUC).
Results
295,974 paired observations of GCS and RASS were obtained from 26,873 admissions; 417 (1.6%) resulted in in-hospital death. GCS and RASS more accurately predicted mortality than AVPU (AUC 0.80 and 0.82, respectively vs. 0.73; p<0.001 for both comparisons). Simultaneous use of GCS and RASS produced an AUC of 0.85 (95% CI: 0.82-0.87; p<0.001 when compared to all three scales).
Conclusions
In ward patients, both GCS and RASS were significantly more accurate predictors of mortality than AVPU. In addition, combining GCS and RASS was more accurate than any scale alone. Routine tracking of GCS and/or RASS on general wards may improve accuracy of detecting clinical deterioration.
Keywords: Mental Status, AVPU, Glasgow Coma Score, Richmond Agitation Sedation Scale, Mortality Prediction, In-Hospital Mortality
INTRODUCTION
Altered mental status (AMS), characterized by abnormal changes in a patient’s arousal and/or cognition, is a significant predictor of hospital mortality.1–3 Yet despite its prevalence3–5 and importance, up to three-quarters of AMS events go unrecognized by caregivers.6–8 Acute changes in mental status, often caused by delirium in the hospitalized patient,3 can present non-specifically making it difficult to detect and distinguish from other diagnoses such as depression or dementia.7, 9 Further complicating the recognition of AMS, numerous and imprecise qualitative descriptors such as “confused” and “alert and oriented” are used in clinical practice to describe the mental status of patients.10 Thus, more objective measures may result in improved detection of altered mental status and in earlier diagnostic and therapeutic interventions.
In critically ill patients, several scales have been widely adopted for quantifying mental status. The Richmond Agitation and Sedation Scale (RASS) was created to optimize sedation.11 The Glasgow Coma Scale (GCS) was developed for head trauma patients12 and is now a standardized assessment tool in intensive care units,13 the emergency department,14 and the pre-hospital setting.15 In addition, a simplified scale, AVPU (Alert, responsive to Verbal stimuli, responsive to Painful stimuli, and Unresponsive) was initially used in the primary survey of trauma patients16 but is now a common component of early warning scores and Rapid Response activation criteria, such as the Modified Early Warning Score.17–18 In fact, in a systematic review of 72 distinct early warning scores, 89% of the scores used AVPU as the measure of mentation.17 However, the utility of these three scales is not well established in the general ward setting. Our aim was therefore to compare the accuracies of AVPU, GCS, and RASS for predicting mortality in hospitalized general ward patients in order to provide insight into the accuracy of these different scores for clinical deterioration.
METHODS
Study Setting and Protocol
We conducted an observational cohort study of consecutive adult general ward admissions from July 2011 through January 2013 at a 500-bed, urban U.S. teaching hospital. During the study period, no early warning scoring systems were in place on the hospital wards. Rapid response teams (RRT) responding to altered mental status would do so without specific thresholds for activation. During this period, nurses on the general floors were expected to record each patient’s GCS and RASS score in the electronic health record (EPIC Systems Corporation; Verona, WI) as part of the routine patient assessment at least once every 12-hour shift. AVPU assessments were extracted from the eye component of the GCS. “A” was assigned to a GCS Eye score of 4 (opens eyes spontaneously), “V” to a score of 3 (opens eyes in response to voice), “P” to a score of 2 (opens eyes in response to painful stimuli), and “U” to a score of 1 (does not open eyes). To avoid comparison of mental status scores at different time points, only concurrent GCS and RASS scores, documented within 10 minutes of one another, were included in the analysis.
Location and time stamped GCS and RASS scores, demographics, and in-hospital mortality data were obtained from the hospital’s Clinical Research Data Warehouse (CRDW), which is maintained by the Center for Research Informatics (CRI) at The University of Chicago. The study protocol and data collection mechanisms were approved by the University of Chicago Institutional Review Board (IRB #16995A).
Statistical Analysis
Baseline admission characteristics were described using proportions (%) and measures of central tendency (mean, standard deviations [SD]; median, inter-quartile ranges [IQR]). Patient severity of illness at first ward observation was calculated using the Modified Early Warning Score (MEWS).19 All mental status observations during a patient’s ward stay were included in the analysis. Odds ratios for 24-hour mortality following an abnormal mental status score were calculated using generalized estimating equations (GEE) with an exchangeable correlation structure to account for the correlation of scores within the same patient, as more than one abnormal mental status score may have been documented within the 24 hours preceding death. Spearman’s rank correlation coefficients (ρ) were used to estimate the correlation between AVPU, GCS, and RASS scores.
The predictive accuracies of AVPU, GCS, RASS, and the subscales of GCS were compared using area under the receiver operating characteristic curve (AUC), with mortality within 24 hours of a mental status observation as the primary outcome and the mental status score as the predictor variable. While AUCs are typically used as a measure of discriminative ability, this study used AUCs to summarize both sensitivity and specificity across a range of cutoffs, providing an overall measure of predictive accuracies across mental status scales. To estimate the AUCs, AVPU, GCS, and GCS subscales were entered into a logistic regression model as ordinal variables whereas RASS was entered as a nominal variable due to its positive and negative components, and predicted probabilities were calculated. In addition, a combined model was fit where GCS and RASS were classified as categorical independent variables. AUCs were then calculated by utilizing the predicted probabilities from each logistic regression model using the trapezoidal rule.20 A sensitivity analysis was performed to estimate the internal validity of the RASS model using ten-fold cross-validation.
Pre-defined subgroup analyses were performed that compared the accuracies of AVPU, GCS, and RASS for predicting 24-hour mortality in patients above and below the median age of the study population, and between patients who underwent surgery during their admission or not (surgical vs medical). All tests of significance used a two-sided p-value less than 0.05. All data analysis was performed using Stata version 13.0 (Statacorp, College Station, TX).
RESULTS
During the study period, 313,577 complete GCS and 305,177 RASS scores were recorded in the electronic health record by nursing staff. A total of 26,806 (17,603 GCS and 9,203 RASS) observations were excluded due to non-simultaneous measurement of the other score, resulting in 295,974 paired mental status observations. These observations were obtained from 26,873 admissions in 17,660 unique patients, with a median MEWS at ward admission of 1 (IQR 1-1). The mean patient age was 57 (SD 17) years and 23% were surgical patients (Table 1). Patients spent a median 63.9 (IQR 26.7-118.6) hours on the wards per admission and contributed a median of 3 (IQR 2-4) paired observations per day, with 91% of patients having at least two observations per day. A total of 417 (1.6%) general ward admissions resulted in death during the hospitalization, with 354 mental status observations occurring within 24 hours of a death. In addition, 26,618 (99.9%) admissions had at least one paired mental status observation within the last 24 hours of their ward stay.
Table 1.
Baseline characteristics of hospital admissions
| Total number of admissions, n | 26,873 |
| Total number of unique patients, n | 17,660 |
| Age, mean (SD), years | 57 (17) |
| Female sex, n (%) | 14,293 (53) |
| Race, n (%) | |
| White | 10,516 (39) |
| Black | 12,580 (47) |
| Other/Unknown | 3,777 (14) |
| Admission MEWS, median (IQR) | 1 (1–1) |
| Days on ward, median (IQR) | 5 (3–10) |
| Observations per person, per day, median (IQR) | 3 (2–4) |
| Underwent surgery during hospitalization, n (%) | 6,141 (23) |
| Deaths, n (%) | 417 (1.6) |
Characteristics are stratified at the hospital admission level.
Abbreviations: n, number of observations; SD, standard deviation; IQR, interquartile range.
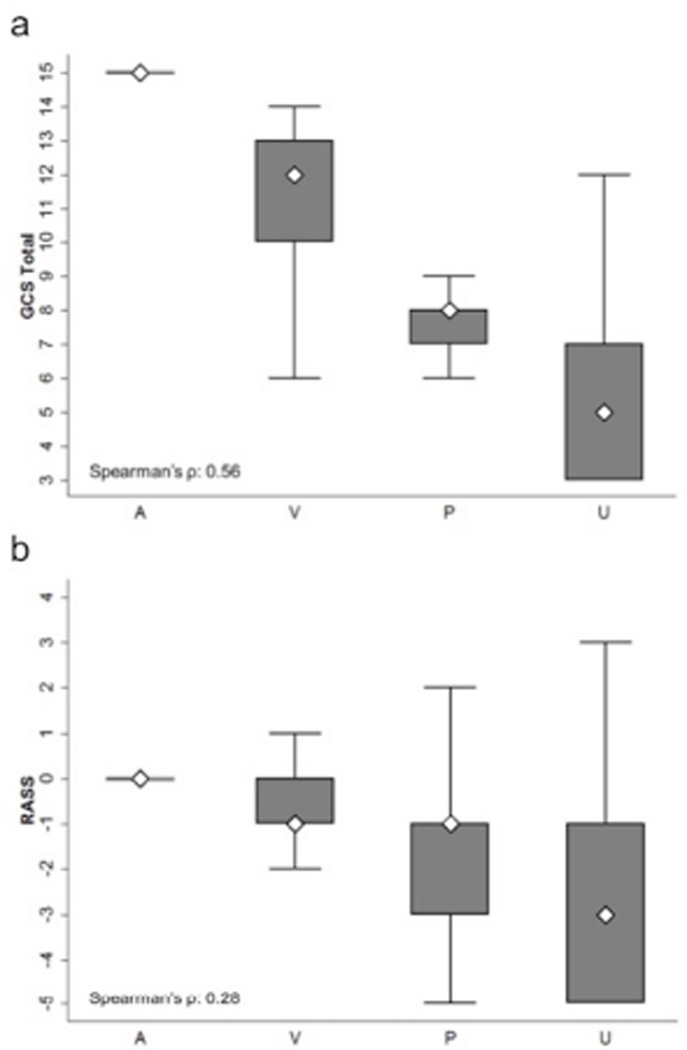
AVPU was moderately correlated with GCS (Spearman’s ρ=0.56; Figure 1a) and weakly correlated with RASS (Spearman’s ρ=0.28; Figure 1b). GCS scores were also weakly correlated to RASS (Spearman’s ρ=0.13, p<0.001). Notably, AVPU mapped to distinct levels of GCS, with “Alert” associated with a median GCS total score of 15, “Voice” a score of 12, “Pain” a score of 8, and “Unresponsive” a score of 5. Abnormal mental status scores on any scale were associated with significantly higher odds of death within 24 hours than normal mental status scores (Table 2). This association was consistent within the three subscales of GCS and for scores in both the sedation (< 0) and agitation (> 0) ranges of RASS.
Figure 1.
Score correlations between (1a) AVPU and GCS Total, and between (1b) AVPU and RASS*
*Boxes indicate interquartile range (25th to 75th percentiles), whiskers indicate 5th to 95th percentiles and diamonds indicate median. Each correlation significant at P<0.001. Abbreviations: AVPU, Alert-Voice-Pain-Unresponsive; GCS, Glascow Coma Scale; RASS, Richmond Agitation Sedation Scale.
Table 2.
Odds of mortality within 24 hours of an abnormal mental status score
| Mental Status Score | Observations, n (%) | Odds Ratio for mortality (95%CI) |
|---|---|---|
| GCS Eye (AVPU) | ||
| 4 (Alert) | 289,857 (98) | Reference |
| <4 (Not Alert) | 6,117 (2) | 33.8 (23.9–47.9) |
| GCS Verbal | ||
| 5 | 277,862 (94) | Reference |
| 4 | 11,258 (4) | 4.7 (2.8–7.9) |
| <4 | 6,854 (2) | 52.7 (38.0–73.2) |
| GCS Motor | ||
| 6 | 287,441 (97) | Reference |
| <6 | 8,533 (3) | 41.8 (30.7–56.9) |
| GCS Total | ||
| 15 | 276,042 (93) | Reference |
| 13,14 | 12,437 (4) | 5.2 (3.3–8.3) |
| <13 | 7,495 (3) | 55.5 (40.0–77.1) |
| RASS | ||
| >0 | 6,867 (2) | 8.5 (5.6–13.0) |
| 0 | 275,708 (93) | Reference |
| <0 | 13,339 (5) | 25.8 (19.2–34.6) |
Odds ratios, with 95% confidence intervals (CI) comparing the probability of mortality within 24 hours of an abnormal mental status score to the probability of mortality within 24 hours of a normal mental status score (“Reference”). All calculations control for clustering of observations within the same admission. All odds ratios were significant at P<0.001.
Abbreviations: AVPU, Alert-Voice-Pain-Unresponsive; GCS, Glascow Coma Scale; RASS, Richmond Agitation Sedation Scale; n, number of observations.
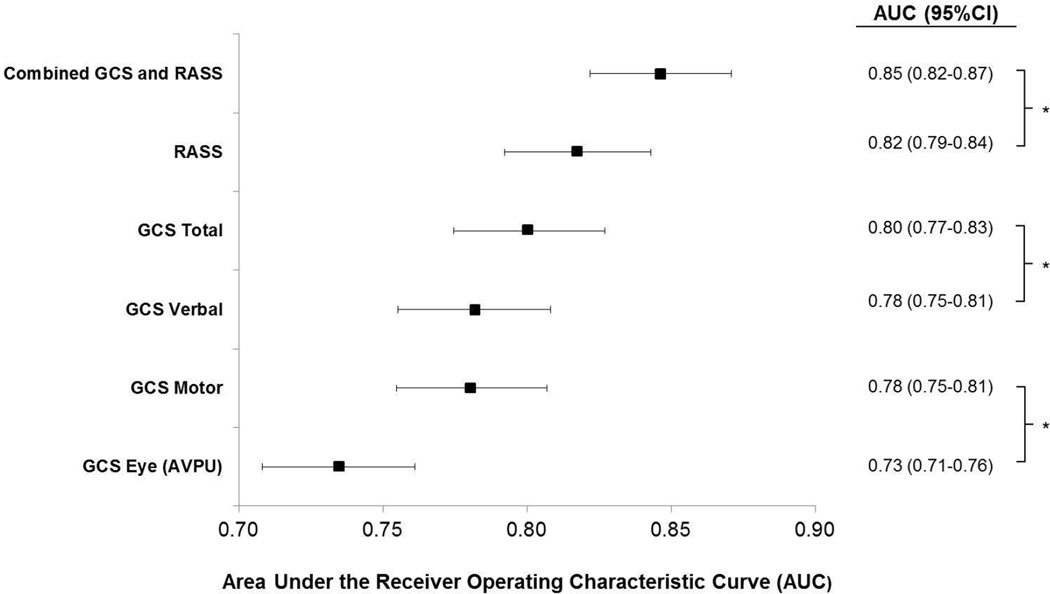
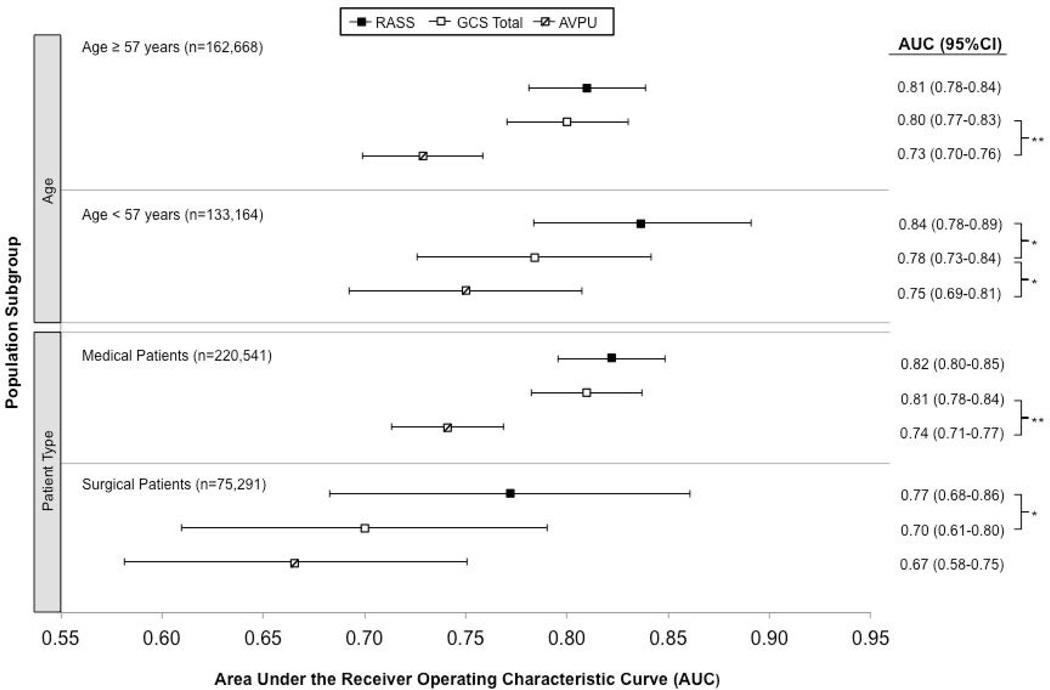
AVPU was the least accurate predictor of mortality (AUC 0.73 [95% CI 0.71-0.76]) while simultaneous use of GCS and RASS was the most accurate predictor (AUC 0.85 [0.82-0.87], Figure 2). The accuracies of GCS and RASS were not significantly different from one another in the total study population (AUC 0.80 [0.77-0.83] and 0.82 [0.79-0.84], respectively; p=0.13). Ten-fold cross-validation to estimate the internal validity of the RASS model resulted in a lower AUC (0.78 [0.75-0.81]) for RASS as a predictor of 24-hour mortality. Subgroup analysis indicated that RASS was more accurate than GCS in younger patients (<57 years old) and in surgical patients (Figure 3).
Figure 2.
Predictive accuracies of mental status scales (and GCS subscales) for mortality within 24-hours of a mental status observation
*P<0.001; Area under the receiver operating characteristic curve (AUC) with whiskers indicating 95% confidence intervals (95% CI) for predicting mortality occurring within 24 hours of a mental status observation. AUCs are shown for each mental status scale, for the combination of GCS and RASS, and for the three subscales of GCS.
Abbreviations: AUC, Area under the receiver operating characteristic curve; AVPU, Alert-Voice-Pain-Unresponsive; GCS, Glascow Coma Scale; RASS, Richmond Agitation Sedation Scale.
Figure 3.
Predictive accuracies of AVPU, GCS, and RASS for mortality within 24 hours of a mental status observation: Subgroup analysis based on age and surgical status
*P<0.05, **P<0.001; Area under the receiver operating characteristic curve (AUC) with whiskers indicating 95% confidence intervals (95% CI) for predicting mortality occurring within 24 hours of a mental status observation, analyzed at the observation level and stratified by patient age (below or greater than or equal to the median age of 57 years) and surgical status (patient with surgery during hospitalization or medical patient only).
Abbreviations: AUC, Area under the receiver operating characteristic curve; AVPU, Alert-Voice-Pain-Unresponsive; GCS, Glascow Coma Scale; RASS, Richmond Agitation Sedation Scale.
Removal of the 255 admissions missing a paired mental status observation within the last 24 hours of their ward stay resulted in no change in the AUC values. A sensitivity analysis for prediction of a combined secondary outcome of 24-hour ICU transfer or cardiac arrest yielded lower AUCs for each mental status scale, with no change in the association between scales.
DISCUSSION
To our knowledge, this study is the first to compare the accuracies of AVPU, GCS, and RASS for predicting mortality in the general ward setting. Similar to McNarry and Goldhill, we demonstrated that AVPU scores mapped to distinct levels of GCS. While our study reports the same median GCS scores of 15 and 8 for AVPU levels of “Alert” and “Pain” respectively, we indicate slightly lower corresponding median GCS scores for AVPU scores of “Voice” (12 vs.13) and “Unresponsive” (5 vs. 6) than their previous work.21 We found that AVPU was the least accurate predictor of mortality within 24 hours of an observation, and the combination of GCS and RASS was the most accurate. RASS was at least as accurate of a predictor for 24-hour mortality in comparison to GCS total in the overall study population. However, the RASS score was the most accurate individual score in surgical and younger patients. These findings suggest that changing from the commonly used AVPU scale to the RASS and/or GCS would improve the prognostic ability of mental status assessments on the general wards.
Buist and colleagues have previously demonstrated altered mental status to be one of the strongest predictors of death on the wards. In that study, a GCS score of 3 and a decrease in GCS score by more than two points were independently associated with mortality (odds ratio 6.1 [95% CI: 3.1 – 11.8] and 5.5 [95% CI: 2.6 – 11.9], respectively).22 We have also previously shown that after adjusting for vital signs, being unresponsive to pain was associated with a 4.5-fold increase in the odds of death within 24 hours,23 while Subbe and colleagues showed a relative risk ratio of 5.2 [95% CI 1.5-18.1]) for the combined endpoint of cardiac arrest, death at 60 days, or admission to the intensive care/ high dependency unit.19 In the current study, the magnitude of these associations was even stronger, with a GCS score <13 correlating with a 55-fold increase in the odds of death, compared to a normal GCS, and not being alert being associated with a 33.8-fold increase in the odds of death. This difference in magnitude is likely a product of the univariate nature of the current analysis, compared to both the Buist and Churpek et al studies, which adjusted for vital signs, thereby lessening the impact of any single predictor. Since this study was designed to compare mental status variables to one another for future model inclusion and all the analyses were paired, confounding by additional predictors of death was not a concern.
One of the potential strengths of RASS over GCS and AVPU is its ability to measure agitation levels, in addition to depressed mentation, a feature which has been shown to be present in up to 60% of delirium episodes.24 This may also explain why RASS was the most accurate predictor of mortality in our subset of younger patients and surgical patients, since hyperactive delirium is more common in younger and healthier patients, which surgical patients tend to be as compared to medical patients.25–26 In this study, we found negative RASS scores portending a worse prognosis than positive ones, which supports previous findings that hypoactive delirium had a higher association with mortality than hyperactive delirium at 6 months (hazard ratio 1.90 vs 1.37) and at 1 year (hazard ratio 1.60 vs 1.30) in elderly patients at post-acute care facilities in two separate studies.27–28 However, a study of patients undergoing surgery for hip fracture found that patients with hyperactive delirium were more likely to die or be placed in a nursing home at one month follow-up when compared to patients with purely hypoactive delirium (79% vs 32%, p=0.003).29
We found the assessment of RASS and GCS by ward nurses to be highly feasible. During the study period, nurses assessed mental status with the GCS and RASS scales at least once per 12-hour shift in 91% of patients. GCS has been shown to be reliably and accurately recorded by experienced nurses (reliability coefficient = 0.944 with 96.4% agreement with expert ratings).30 RASS can take less than 30 seconds to administer, and in previous studies of the ICU setting has been shown to have over 94% nurse compliance for administration,31 and good inter-rater reliability (weighted kappa 0.66 and 0.89, respectively).31–32 Further, in a prior survey of 55 critical care nurses, 82% agreed that RASS was easy to score and clinically relevant.31
This study has several limitations. First, it was conducted in a single academic institution which may limit generalizability to other hospitals. Second, baseline cognition and comorbidities were not available in the dataset so were unable to conduct additional subgroup analyses by these categories. However, we used age and hospital admission type as proxies. Third, the AVPU scores in this study were extracted from the eye subset of the GCS scale, as AVPU was not directly assessed on our wards during the study period. Clinical assessment of mental status on the AVPU scale notes the presence of any active patient response (eg. eye-opening, grunting, moaning, movement) to increasingly noxious stimuli. As such, our adaptation of AVPU using only eye-opening criteria may underestimate the true number of patients correctly classified as alert, or responding to vocal/painful stimuli. However, a sensitivity analysis comparing directly assessed AVPU during a three-year period prior to the study implementation at our institution and AVPU derived from the GCS Eye subscale for the study period indicated no difference in predictive value for 24-hour mortality. Fourth, we did not perform trend analyses for change from baseline mental status or evolution of AMS, which may more accurately predict 24-hour mortality than discrete mental status observations. Finally, the three scales we compared differ in length, which may bias the AUC against AVPU, a four point scale with a trapezoidal ROC curve compared to the smoother curve generated by the 15-point GCS scale, for example. However, the lack of discrimination of the AVPU is the likely source of its lesser accuracy.
CONCLUSION
In the general ward setting, routine collection of GCS and RASS is feasible and both are significantly more accurate for predicting mortality than the more commonly used AVPU scale. In addition, the combination of GCS and RASS has greater accuracy than any of the three individual scales. RASS may be particularly beneficial in the assessment of younger and/or surgical patients. Routine documentation and tracking of GCS and/or RASS by nurses may improve the detection of clinical deterioration in general ward patients. In addition, future early warning scores may benefit from the inclusion of GCS and/or RASS en lieu of AVPU.
Acknowledgments
Source of Funding: Drs. Churpek and Edelson have a patent pending (ARCD. P0535US.P2) for risk stratification algorithms for hospitalized patients. Dr. Churpek is supported by a career development award from the National Heart, Lung, and Blood Institute (K08 HL121080). Dr. Edelson has received research support from the National Heart, Lung, and Blood Institute (K23 HL097157), Philips (Andover, MA), the American Heart Association (Dallas, TX), Laerdal Medical (Stavanger, Norway), and Early Sense (Tel Aviv, Israel). She has ownership interest in Quant HC (Chicago, IL), which is developing products for risk stratification of hospitalized patients.
Footnotes
Conflicts of Interest: All other authors report no conflicts of interest.
REFERENCES
- 1.Ely EW, Shintani A, Truman B, et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA. 2004;291(14):1753–1762. doi: 10.1001/jama.291.14.1753. [DOI] [PubMed] [Google Scholar]
- 2.Pompei P, Foreman M, Rudberg MA, Inouye SK, Braund V, Cassel CK. Delirium in hospitalized older persons: outcomes and predictors. J Am Geriatr Soc. 1994;42(8):809–815. doi: 10.1111/j.1532-5415.1994.tb06551.x. [DOI] [PubMed] [Google Scholar]
- 3.Siddiqi N, House AO, Holmes JD. Occurrence and outcome of delirium in medical in-patients: a systematic literature review. Age Ageing. 2006;35(4):350–364. doi: 10.1093/ageing/afl005. [DOI] [PubMed] [Google Scholar]
- 4.Levkoff SE, Evans DA, Liptzin B, et al. Delirium. The occurrence and persistence of symptoms among elderly hospitalized patients. Arch Intern Med. 1992;152(2):334–340. doi: 10.1001/archinte.152.2.334. [DOI] [PubMed] [Google Scholar]
- 5.Dyer CB, Ashton CM, Teasdale TA. Postoperative delirium. A review of 80 primary data-collection studies. Arch Intern Med. 1995;155(5):461–465. doi: 10.1001/archinte.155.5.461. [DOI] [PubMed] [Google Scholar]
- 6.Inouye SK, Foreman MD, Mion LC, Katz KH, Cooney LM Jr. Nurses' recognition of delirium and its symptoms: comparison of nurse and researcher ratings. Arch Intern Med. 2001;161(20):2467–2473. doi: 10.1001/archinte.161.20.2467. [DOI] [PubMed] [Google Scholar]
- 7.Armstrong SC, Cozza KL, Watanabe KS. The misdiagnosis of delirium. Psychosomatics. 1997;38(5):433–439. doi: 10.1016/S0033-3182(97)71420-8. [DOI] [PubMed] [Google Scholar]
- 8.Ely EW, Stephens RK, Jackson JC, et al. Current opinions regarding the importance, diagnosis, and management of delirium in the intensive care unit: a survey of 912 healthcare professionals. Crit Care Med. 2004;32(1):106–112. doi: 10.1097/01.CCM.0000098033.94737.84. [DOI] [PubMed] [Google Scholar]
- 9.Farrell KR, Ganzini L. Misdiagnosing delirium as depression in medically ill elderly patients. Arch Intern Med. 1995;155(22):2459–2464. [PubMed] [Google Scholar]
- 10.Simpson CJ. Doctors and nurses use of the word confused. Br J Psychiatry. 1984;145:441–443. doi: 10.1192/bjp.145.4.441. [DOI] [PubMed] [Google Scholar]
- 11.Sessler CN, Gosnell MS, Grap MJ, et al. The Richmond Agitation-Sedation Scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med. 2002;166(10):1338–1344. doi: 10.1164/rccm.2107138. [DOI] [PubMed] [Google Scholar]
- 12.Teasdale G, Jennett B. Assessment and prognosis of coma after head injury. Acta Neurochir (Wien) 1976;34(1-4):45–55. doi: 10.1007/BF01405862. [DOI] [PubMed] [Google Scholar]
- 13.Bastos PG, Sun X, Wagner DP, Wu AW, Knaus WA. Glasgow Coma Scale score in the evaluation of outcome in the intensive care unit: findings from the Acute Physiology and Chronic Health Evaluation III study. Crit Care Med. 1993;21(10):1459–1465. doi: 10.1097/00003246-199310000-00012. [DOI] [PubMed] [Google Scholar]
- 14.Holdgate A, Ching N, Angonese L. Variability in agreement between physicians and nurses when measuring the Glasgow Coma Scale in the emergency department limits its clinical usefulness. Emerg Med Australas. 2006;18(4):379–384. doi: 10.1111/j.1742-6723.2006.00867.x. [DOI] [PubMed] [Google Scholar]
- 15.Menegazzi JJ, Davis EA, Sucov AN, Paris PM. Reliability of the Glasgow Coma Scale when used by emergency physicians and paramedics. J Trauma. 1993;34(1):46–48. doi: 10.1097/00005373-199301000-00008. [DOI] [PubMed] [Google Scholar]
- 16.Alexander RH, Proctor HJ American College of Surgeons. Advanced trauma life support program for physicians: ATLS. 5th. Chicago, IL: American College of Surgeons; 1993. Committee on Trauma. [Google Scholar]
- 17.Smith GB, Prytherch DR, Schmidt PE, Featherstone PI. Review and performance evaluation of aggregate weighted 'track and trigger' systems. Resuscitation. 2008;77(2):170–179. doi: 10.1016/j.resuscitation.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 18.Smith GB, Prytherch DR, Schmidt PE, Featherstone PI, Higgins B. A review, and performance evaluation, of single-parameter"track and trigger" systems. Resuscitation. 2008;79(1):11–21. doi: 10.1016/j.resuscitation.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 19.Subbe CP, Kruger M, Rutherford P, Gemmel L. Validation of a modified Early Warning Score in medical admissions. QJM. 2001;94(10):521–526. doi: 10.1093/qjmed/94.10.521. [DOI] [PubMed] [Google Scholar]
- 20.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44(3):837–845. [PubMed] [Google Scholar]
- 21.McNarry AF, Goldhill DR. Simple Bedside Assessment of level of consciousness: comparison of two simple assessment scales with the Glascow Coma Scale. Anaesthesia. 2004;59(1):34–37. doi: 10.1111/j.1365-2044.2004.03526.x. [DOI] [PubMed] [Google Scholar]
- 22.Buist M, Bernard S, Nguyen TV, Moore G, Anderson J. Association between clinically abnormal observations and subsequent in-hospital mortality: a prospective study. Resuscitation. 2004;62(2):137–141. doi: 10.1016/j.resuscitation.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 23.Churpek MM, Yuen TC, Edelson DP. Predicting clinical deterioration in the hospital: the impact of outcome selection. Resuscitation. 2013;84(5):564–568. doi: 10.1016/j.resuscitation.2012.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peterson JF, Pun BT, Dittus RS, et al. Delirium and its motoric subtypes: a study of 614 critically ill patients. J Am Geriatr Soc. 2006;54(3):479–484. doi: 10.1111/j.1532-5415.2005.00621.x. [DOI] [PubMed] [Google Scholar]
- 25.Angles EM, Robinson TN, Biffl WL, et al. Risk factors for delirium after major trauma. Am J Surg. 2008;196(6):864–869. doi: 10.1016/j.amjsurg.2008.07.037. [DOI] [PubMed] [Google Scholar]
- 26.Meagher DJ, O'Hanlon D, O'Mahony E, Casey PR, Trzepacz PT. Relationship between symptoms and motoric subtype of delirium. J Neuropsychiatry Clin Neurosci. 2000;12(1):51–56. doi: 10.1176/jnp.12.1.51. [DOI] [PubMed] [Google Scholar]
- 27.Yang FM, Marcantonio ER, Inouye SK, et al. Phenomenological subtypes of delirium in older persons: patterns, prevalence, and prognosis. Psychosomatics. 2009;50(3):248–254. doi: 10.1176/appi.psy.50.3.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kiely DK, Jones RN, Bergmann MA, Marcantonio ER. Association between psychomotor activity delirium subtypes and mortality among newly admitted post-acute facility patients. J Gerontol A Biol Sci Med Sci. 2007;62(2):174–179. doi: 10.1093/gerona/62.2.174. [DOI] [PubMed] [Google Scholar]
- 29.Marcantonio E, Ta T, Duthie E, Resnick NM. Delirium severity and psychomotor types: their relationship with outcomes after hip fracture repair. J Am Geriatr Soc. 2002;50(5):850–857. doi: 10.1046/j.1532-5415.2002.50210.x. [DOI] [PubMed] [Google Scholar]
- 30.Rowley G, Fielding K. Fielding, Reliability and accuracy of the Glasgow Coma Scale with experienced and inexperienced users. Lancet. 1991;337(8740):535–538. doi: 10.1016/0140-6736(91)91309-i. [DOI] [PubMed] [Google Scholar]
- 31.Pun BT, Gordon SM, Peterson JF, et al. Large-scale implementation of sedation and delirium monitoring in the intensive care unit: a report from two medical centers. Crit Care Med. 2005;33(6):1199–1205. doi: 10.1097/01.ccm.0000166867.78320.ac. [DOI] [PubMed] [Google Scholar]
- 32.Vasilevskis EE, Morandi A, Boehm L, et al. Delirium and sedation recognition using validated instruments: reliability of bedside intensive care unit nursing assessments from 2007 to 2010. J Am Geriatr Soc. 2011;59(Suppl 2):S249–S255. doi: 10.1111/j.1532-5415.2011.03673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]