Abstract
The NADPH oxidases (NOX) represent a family of 7 related transmembrane enzymes that share a basic structural paradigm and the common ability to utilize NADPH to synthesize superoxide and other reactive oxygen species (ROS). NOX isoforms are distinguished from each other by their amino acid sequences, expression levels in different cell types, the mechanisms of enzyme activation and the type of ROS that are generated. NOX5 was the last NOX family member to be identified and in the past decade and a half we have gained significant insights into how NOX5 produces ROS, the cell types where it is expressed and the functional significance of NOX5 in health and disease. The objective of this review is to highlight accumulated and recent knowledge of the genetic and enzymatic regulation of NOX5 and the importance of NOX5 in human physiology and pathophysiology.
Keywords: NOX5, NADPH oxidase, reactive oxygen species, post-translational regulation, genetic regulation, significance, review
Introduction
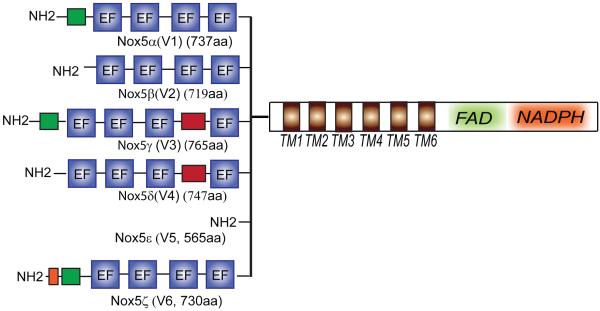
The first reported clones of NOX5 were identified in 2001 and the mRNA sequence encoded a 6 transmembrane spanning protein and a C-terminal NADPH binding dehydrogenase domain with homology to other NOX family members1, 2. Also revealed was a unique N-terminal extension that contained 4 calcium-binding EF hands which clearly distinguished NOX5 as the only strictly calcium-dependent NOX enzyme. When ectopically expressed in transfected cells, NOX5 produced low levels of superoxide that could be rapidly increased by the elevation of intracellular calcium. Also unique to NOX5 was the ability to generate ROS from a single gene product, absent the need for cytosolic or specific accessory proteins. NOX5 mRNA was found most strongly expressed in testis, spleen, and lymph nodes and also in vascular cells2. Since 2001, we have learned that there are multiple (6) splice variants of NOX5, that the enzymatic regulation is more complex than the simple binding of calcium to EF-hands, that NOX5 can bind to cytosolic proteins that regulate its stability and activity, that there are numerous SNP including some with high frequency and that NOX5 is expressed in a wide variety of cell types and that its activity and expression level are modified in various disease states. In the following sections we shall summarize these contributions to our knowledge of NOX5.
Enzymatic regulation of NOX5
Calcium-dependent regulation
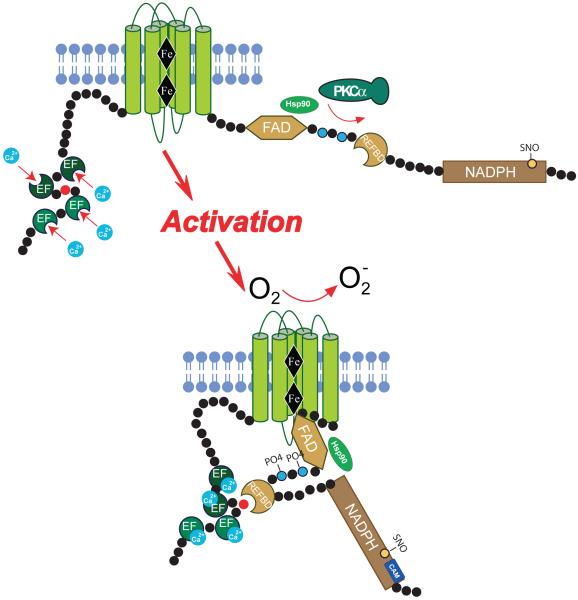
Based on homology with the other NOX isoforms, NOX5 is considered to have the same 6 transmembrane topology supporting 2 heme moieties (proposed to be mediated by H286, H300, H374 and H387 using the sequence for NOX5αV1 which is the longest functional isoform as shown in Figure 1) and a C-terminal FAD and NADPH binding dehydrogenase domain. Unique to NOX5 is a significant N-terminal extension2. Calcium is essential for the activity of NOX5 and ROS are not synthesized to any appreciable degree in a calcium-free buffer or from truncated NOX5 enzymes lacking the N-terminal region2-4. The N- terminus of NOX5 contains 2 pairs of 4 EF hands that are distinguished in their relative affinity for calcium, the proximal N-terminal pair having a lower affinity to calcium than the distal pair3, 5. The current paradigm for activation is that upon elevation of calcium, the occupation of the EF hands triggers a conformational change in the N-terminus that promotes interaction with a C-terminal region thus facilitating electron flow from NADPH to oxygen bound to the heme moieties for the eventual production of superoxide and attendant reactive oxygen species. The C-terminal region of NOX5 contains an auto-inhibitory domain that restricts enzyme activity, and has been designated the Regulatory EF-hand Binding Domain (REFBD, aa 656-679). The calcium-bound N-terminus of NOX5 binds to the REFBD and displaces it, removing the autoinhibition and facilitating enzyme activation3, 6 (Figure 2). However, there are a number of caveats to this relatively simple model of enzyme activation. Firstly, the amount of calcium required to fully activate NOX5 is quite high and secondly, the ability of NOX5 to produce ROS under conditions where resting levels of intracellular calcium do not change appreciably. These observations suggest that additional mechanisms may influence NOX5 activity.
Figure 1.
Schematic illustrating the differences in the NH2-terminal region of the 6 known NOX5 splice variants.
Figure 2.
Cartoon illustrating key regulatory post-translational modifications and the mechanism of activation of NOX5. Notes: illustration not drawn to scale and the REFBD is predicted to lie within multiple regions that mediate NADPH binding. Abbreviations: EF, calcium-binding EF hands, Fe, heme moieties, FAD, Flavin adenine dinucleotide, NADPH, nicotinamide adenine dinucleotide phosphate, PKC, protein kinase C, CAM, calmodulin, Hsp90, heat shock protein 90, REFBD, regulatory EF-hand Binding Domain PO4, phosphorylation, SNO, S-nitrosylation.
Phosphorylation
The amount of calcium required to half maximally activate NOX5 is approximately 1μM, which represents a relatively low affinity for calcium compared to other calcium regulated proteins2. Given the range of intracellular calcium concentrations that occur in most cells, the activation of NOX5 via this pathway alone is likely to be inefficient. This prompted the search for additional pathways of post translational regulation and in 2007, it was shown that PMA activates NOX5, in a unique manner that was independent from changes in intracellular calcium. Furthermore, PMA could synergize with low or submaximal concentrations of calcium-mobilizing agonists to elicit significantly greater levels of ROS production4, 7. The mechanism underlying this ability is the direct phosphorylation of NOX5 within a C-terminal region that contains a cluster of serine and threonine residues (T512 and S516, NOX5V1). Substitution of these residues to alanine, which cannot be phosphorylated, attenuates the ability of protein kinase C (PKC) to activate NOX5. In contrast, mutation of these residues to negatively charged amino acids, which mimic phosphorylation, resulted in an enzyme with enhanced ability to generate ROS 4. The PKC isoform mediating this response is most likely to be PKCα as genetic silencing of PKCα reduced the ability of PMA to activate NOX5 and a constitutively active form of PKCα robustly increased NOX5 activity8. Interestingly, silencing of PKCε also reduced PMA-stimulated ROS production from NOX5 but the expression of active variants of PKCε, strongly reduced NOX5 activity. The reason for this is not yet clear, but perhaps is indicative of differences in subcellular location, cross talk between PKC isoforms and substrate specificity. The ability to activate NOX5 was not observed with all PKC isoforms and the silencing of PKCδ actually increased PMA-stimulated NOX5 activity whereas silencing PKCθ was without effect8. PKC can also activate secondary kinases and it has been shown that the ability of PMA to stimulate NOX5 may, at least in part, involve the kinase ERK9. The ability of PMA to stimulate NOX5, is reduced in the presence of MEK1 inhibitors (PD98059 and U0126), by silencing MEK1 or expression of a dominant negative MEK1 and occurrs via the phosphorylation of S516 and to a lesser degree by T512. Unlike PKCα, the expression of a constitutively active form of MEK1 was not sufficient to increase NOX5 activity suggesting that the MEK/ERK1/2 pathway is necessary but not sufficient to regulate the PMA-dependent activation of NOX59. PKC-dependent pathways are not the only signaling pathways that can influence the phosphorylation and activity of NOX5. Indeed, the ability of calcium-mobilizing agonists to regulate NOX5 can be reduced by inhibitors of calcium-calmodulin-dependent kinases10, 11. CAMKII was shown to directly activate NOX5 via the phosphorylation of S493 (numbers corresponding to NOX5V1)10. The tyrosine kinase ABL1 has been shown to mediate activation of NOX5 in response to hydrogen peroxide, and while an active kinase is necessary, it is not yet known if this involves the direct tyrosine phosphorylation of NOX512. Based on prediction tools (GPS) there are likely numerous serine, threonine and tyrosine residues on NOX5 and numerous kinases that may influence the post-translational phosphorylation and activation of NOX5. Most of these await further identification. In summary, the ability of the N-terminal EF hand of NOX5 to interact with the C-terminal REFBD is regulated by the direct phosphorylation of the C-terminal region and it is likely that there are a multitude of kinases and sites of phosphorylation, beyond that described above, that can impact the activation of NOX5. The synergistic interaction between changes in phosphorylation and calcium-dependent occupation of the EF-hands provides for additional levels of control and diversifies the ability of NOX5 to produce an appropriate level of ROS in response to a stimulus (Summarized in Table 1 and Figure 2).
Table 1.
Kinases regulating the phosphorylation and activity of NOX5. AA numbers for NOX5V1
| Kinase | Site(s) of phosphorylation |
NOX5 Activity | Reference |
|---|---|---|---|
| PKCα | S508, T512, S516 | Increased | 8 |
| PKCε | ND* | Decreased | 8 |
| CAM Kinase II | S493 | Increased | 10 |
| ERK | S516 | Increased | 9 |
| Abl | ? | Increased | 12 |
ND: Not Determined
Protein:protein interactions
NOX5 was initially reported as a protein that could function independently and without the need for cytoplasmic subunits. This remains true today, but a number of proteins have since been identified that bind to NOX5 and can modify its calcium-dependent activity or expression (Summarized in Table 2). The first described cytosolic protein that can influence NOX5 activity was calmodulin13. The C-terminus of NOX5 contains a calmodulin binding site that is situated close to the C- terminal NADPH binding site (aa689-707). Although equivalent CAM binding sites are well conserved on other NOX enzymes, the binding of calcium-bound calmodulin to NOX5, but not to other NOX isoforms, was shown to increase its sensitivity to calcium and provides another avenue for calcium-sensitization in addition to phosphorylation. Given the location of the calmodulin binding motif it can be anticipated that calmodulin binding promotes electron transfer, as with other NADPH-dependent proteins such as eNOS, but the precise mechanism by which this occurs in NOX5 is not yet known.
Table 2.
NOX5 protein binding partners and effect on NOX5 activity.
| Protein | Binding Site on NOX5 |
NOX5 Activity | Reference |
|---|---|---|---|
| Calmodulin | C-terminal aa689-707 |
Increased | 13 |
| c-Abl | ND | Increased | 12 |
| PKCα | ND | Increased | 8 |
| Cav-1 | ND | Decreased | 17 |
| Hsp90 | C-terminal | Increased | 24, 28 |
| Hsp70 | C-terminal | Decreased | 25 |
| CHIP | ND | Decreased | 25 |
ND: Not Determined
Caveolin-1 (Cav-1) is an integral membrane protein and the major structural protein of the flask shaped plasma membrane organelle, caveolae. Cav-1 has a complex biology and in addition to its architectural role in caveolae, it binds to and regulates the activity and subcellular location of numerous signaling molecules. Dysregulation of Cav-1 has been documented in a number of disease states including atherosclerosis, inflammation, fibrosis and pulmonary hypertension14. Previous studies have shown that Cav-1 is necessary to support stimulus-dependent coupling of NOX2, presumably via improved coordination of protein:protein interactions at the plasma membrane15, 16. However, a direct interaction been Cav-1 and NOX isoforms was not appreciated until recently17. Cav-1 binds directly to NOX5 (and NOX2) and suppresses the activity of NOX1-3 and 5. Inhibition is mediated by the scaffolding domain of Cav-1 and can be mimicked by peptides linked to a cell penetrating sequence. While seemingly paradoxical, the relationship between enzyme location and allosteric inhibition by caveolin-1 has been shown before and is best characterized by the mechanisms regulating eNOS activity. Numerous groups have shown that eNOS must reside at plasma membrane caveolae for maximal activity and at rest is inhibited by binding to caveolin-1. The influx of calcium displaces caveolin-1 from eNOS and activates the enzyme14. Similarly, calcium-calmodulin can effectively displace Cav-1 from NOX217. Whether this relationship exists for NOX5 remains to be determined. In an additional mechanism of action, Cav-1 can also regulate the expression levels of NOX2 and NOX4, but not NOX517 and this occurs secondary to the established actions of Cav-1 to repress pro-inflammatory signaling. Collectively, these data present a paradigm where Cav-1 is necessary for optimal activation of NOX enzymes, by supporting the existence of caveolae to enable efficient signaling, but also a repressor of ROS production through direct enzyme binding and inhibition of pro-inflammatory signaling. Accordingly, in disease states where there is a loss of Cav-1 expression, this may result in increased expression and activity of NOX enzymes and greater production of ROS as has been shown17-20. Adding to the complexity, NOX-derived ROS may also impact cellular signaling via the increased phosphorylation of Cav-121.
In addition to the above listed proteins, a number of kinases have now been shown to bind to NOX5 including ABL1 and PKCα which associate in a stimulus dependent manner that is dependent on an active kinase8, 12. A prior study has shown that NOX5 can bind p22phox, but others have shown that it does not22. The C-terminal domain of NOX5 contains regions that mediate the binding of NOX5 to itself and the formation of a tetrameric complex23. The function of the NOX5 tetramer is not yet established, but it may regulate NOX5 activity and or affect stabilization of the protein.
Molecular Chaperones
In addition to the above listed proteins, NOX5 (and also NOX1-3) bind to the chaperones, Hsp90 and Hsp70. Inhibitors of Hsp90 acutely reduce the ability of NOX5 to produce superoxide and chronic inhibition stimulates the degradation of NOX5 protein expression. These data suggest that NOX5 is a client protein or substrate of Hsp90 and this is supported by co-immunoprecipitation and bioluminescence resonance energy transfer studies that show the direct binding of NOX5 to Hsp9024. Inhibitors of Hsp90 that target the N-terminal domain ATP binding pocket promote the dissociation of Hsp90 and increase the association of Hsp70 to NOX5 and also recruit CHIP (Carboxyl-terminus of Hsp70 Interacting Protein) an Hsp70 regulated E3 ubiquitin ligase25. This pathway promotes the ubiquitination of NOX5 and its subsequent proteolysis. The mechanisms by which Hsp90 influences the activity of NOX5 remain incompletely defined. Based on other Hsp90 client proteins, the possible mechanisms by which Hsp90 regulates NOX5 are manifold and include the maintenance of tertiary structure, insertion of heme, subcellular localization or the regulation of kinase activity. Current evidence suggests that acute inhibition of Hsp90 selectively inhibits the production of superoxide but does not impact hydrogen peroxide production. How this occurs is not yet known, but studies of NOX4 and DUOX enzymes reveal that the extracellular loops of these enzymes can rapidly convert superoxide to hydrogen peroxide resulting in enzymes that emit primarily hydrogen peroxide 26, 27. Thus Hsp90 may have a structural role in coordinating the stability or position of the extracellular loop regions of NOX5. A major obstacle to this mechanism is the intracellular location of Hsp90 and how it would impact the extracellular loops of NOX5 or the intraorganelle loops of NOX5 that is localized to the endoplasmic reticulum 4, 22. The ratio of Hsp90 to Hsp70 bound to NOX5 can alter the balance of enzyme stability and ROS production, with greater binding of Hsp70 promoting reduced ROS production and enzyme instability. This may have implications in the beneficial mechanism of drugs such as BGP-15 and GGA that can increase Hsp70 to promote the degradation of NOX5 (and NOX1-3) and reduce ROS production. Alternatively, diseases such as inflammation, cancer and atherosclerosis have been shown to have increased Hsp90 or reduced Hsp70 levels and this may be a mechanism that contributes to the increased production of ROS. This was recently shown in an experimental model of atherosclerosis where inhibition of Hsp90 not only reduced the size and complexity of vascular lesions but reduced the levels of ROS and the expression of NOX1 and 2 28and also in a model of type II diabetes in which Hsp90 inhibitors potently reduced ROS production and NOX expression in isolated leukocytes, lung tissue, human blood vessels and aorta from db/db mice25.
Subcellular localization
The subcellular location of a protein can have a crucial impact on its activity29. The intracellular location of NOX5 is thought to not only regulate the ability to generate ROS but to differentially influence the function of molecules in close proximity that are modified by superoxide or secondary ROS. In transfected cells and also in cultured human endothelial cells and prostate cancer cells, NOX5 is present primarily on intracellular membranes with a distribution that is consistent with the endoplasmic reticulum (ER) 4, 7, 22, 30, 31. Expression of WT or GFP fusion proteins have yielded consistent results and agree with studies showing that other NOX isoforms are also found in the ER 32-35. In addition to the ER, NOX5 has been detected at the plasma membrane7, 30. Traffic from intracellular membranes to the plasma membrane can be mediated by polybasic domains within the N-terminus of NOX5 (PBR-N) that bind to phosphatidylinositol 4,5-bisphosphate, a phospholipid enriched at the plasma membrane. The PBR-N is highly conserved in most orthologs of NOX5 and may account for the variable expression of NOX5 at the plasma membrane in a number of cell types. The C-terminus of NOX5 contains another conserved polybasic domain that has been called the PBR-C. Mutation of this region reduces NOX5 enzyme activity but does not affect its intracellular location. The functional relevance of intra versus extracellular superoxide derived from NOX5 remains to be established.
S-nitrosylation and SUMOylation
A reciprocal relationship exists between nitric oxide (NO) and superoxide. Increased superoxide has long been established to react avidly with and inactivate NO signaling36. More recently, NO has been shown to reduce superoxide production from NOX isoforms37including NOX5 with a rank order potency of NOX1 > NOX3 > NOX5 > NOX2, whereas NOX4 was refractory to the effects of NO38. The mechanisms underlying NOX enzyme inhibition are a direct modification to the enzyme that was not tyrosine nitration, glutathiolation, or phosphorylation. Instead, the S-nitrosylation of NOX5 was confirmed using the biotin-switch assay. Mass spectrometry identified 4 major sites of S-nitrosylation, C107, C246, C519 and C694. Of these, C694 was the most relevant to changes in function and a conservative mutation to serine, reduced NOX5 activity, S-nitrosylation and the ability of NO to suppress superoxide production. The ability of NO to suppress NOX5-derived superoxide was observed with both NO donors and endogenously produced NO in endothelial cells and LPS-challenged smooth muscle and was reversed with the denitrosylases thioredoxin 1 and GSNO reductase38. These results suggest that NO signaling may provide tonic inhibition of NOX isoforms and that loss of NO may reciprocally increase ROS production.
Protein SUMOylation (SUMO, small ubiquitin-related modifier) provides protection from cellular stress, including excessive levels of oxidative stress39. Increased SUMOylation was found to significantly reduce NOX5 (and other NOX isoform) activity in a variety of cell types, but the mechanisms by which SUMOylation decreased ROS production remain unresolved. Direct enzyme activity is decreased in isolated assays with excess calcium and NADPH but the level of enzyme expression and phosphorylation remain unchanged. There is no evidence supporting the post-translational attachment of SUMO (small ubiquitin-related modifier) to NOX540and the ability of SUMO to reduce the activity of all of the NOX enzymes suggesting a more general mechanism.
Genetic regulation of NOX5
In humans, the gene for NOX5 is located on chromosome 15 and from this gene, six reported isoforms can be expressed (shown in Fig. 1). The different isoforms originate from distinct promoter sites and translational start sites as well as the alternate splicing of exon 6. The proteins they encode are unique at the N-terminus and share a conserved C-terminal region. The modifications of the N-terminus have varying effects on the function of each isoform (Table 3). The alpha (V1) and beta (V2) isoforms exhibit approximately equal activity and produce equivalent amounts superoxide and hydrogen peroxide when expressed ectopically in transfected cells, irrespective of the type of stimulation. The NOX5 V1 contains an N-terminal extension and it remains to be determined whether this addition confers any unique properties in the regulation of NOX5 activity. In contrast, the γ (V3), δ (V4) and ε (V5) isoforms are catalytically inactive in transfected cells and do not produce appreciable superoxide or hydrogen peroxide at a variety of expression levels41. The NOX5 V3 and V4 isoforms contain an insertion between the 3rd and 4th EF hand that may either alter calcium-binding to NOX5 or the ability of the N-terminus to trigger ROS production. The first pair of EF hands has a lower affinity for calcium and disruption by mutagenesis does not significantly impact NOX5 function 3suggesting that they are less crucial for NOX5 function. NOX5ε (V5) lacks both pairs of N-terminal EF hands and the absence of enzyme activity in this isoform suggest that they are essential for displacing autoinhibitory domains and/or triggering electron flow. The activity of the NOX5ζ (V6) has not yet been studied, but based on homology to the other isoforms; it is likely to be active. The relative expression levels of the 6 NOX5 isoforms varies with cell type and NOX5 V3-5 can function as dominant negatives when co-expressed with the active isoforms, NOX5 V1 and V2 as they have intact C-terminal regions, critical for oligomerization, and insertions that appear to disrupt enzyme activity in the N-terminal region. 23, 41.
Table 3.
Splice isoforms of NOX5 and respective enzyme activity.
NOX5 Polymorphisms
In comparison to the other NOX family members, the gene for NOX5 is relatively large (~126kb). Within this region there are numerous intragenic, intronic and exonic single nucleotide polymorphisms that have the potential to regulate the expression level of NOX5 and also to impact its enzymatic function. The coding sequence for the NOX5 protein has greater than 108 reported nonsense, missense and synonymous SNPs (NCBI) with frequencies varying from MAF (Minor Allele Frequency) 0.0005 to 0.31. Missense SNPs which alter the amino acid composition are present in regions of NOX5 that may impact enzyme function including EF hands, transmembrane regions and the NADPH binding C-terminus. One SNP that is validated (rs34406284) and two non-validated SNPs (rs369517329, rs370082662) represent missense mutations that encode stop codons. Individuals with genes harboring these SNPs would express truncated NOX5 enzymes that are predicted to be inactive. The enzymatic consequences of a selection of the more frequent SNPs that reside in critical areas of the enzyme, have recently been assessed43. Of the 15 missense mutations encoded by SNPs that were evaluated, 7 resulted in proteins that were expressed well but had little or no enzyme activity (M95K, S254R, T271M, R437Q, R548H, G560R, V707A, NOX5V1). Some of these SNPs had relatively high frequencies such as the R548H (10.62%). Notably there were no gain of function mutants. The demographics of SNPs in the NOX5 gene are varied and an interesting finding is that the SNP encoding R548H has a relatively high frequency among Asians and Africans versus Europeans with South Americans being intermediate (0.115 African Americans, 0.208 Kenyans, 0.119 Nigerians and 0 for Western and Northern Europeans, 0 from Great Brittain, Italy and Spain and 0.086 for Mexicans, 0.046 for Puerto Ricans, 0.067 Columbians). The SNP encoding W272Ter was predominantly found in Africans (0.074 in African Americans, 0.0625 in Kenyans and 0.108 in Nigerians) versus 0 in other populations. The loss of calcium-dependent activity in the M95K (M77K in NOX5V2) has also revealed important information about the mechanisms by which NOX5 is activated. The M95 lies between the 2 pairs of EF hands and close to a stretch of hydrophobic amino acids. The SNP encoding a substitution of the hydrophobic methionine to a charged lysine (M95K, rs112069106) resulted in an enzyme with greatly impaired calcium-dependent activity. In contrast, a more conservative substitution of M95 with the hydrophobic valine (rs34097994), resulted in a less significant decrease in activity. Methionine residues in calmodulin have been shown to mediate the interaction with substrates. It is possible that M95, as the only methionine residue in this region, is critical for interaction with the C-terminal REFBD and enzyme activation (Figure 2). While SNPs encoding missense or nonsense mutations within the exons of NOX5 have the potential to directly alter enzyme function, they may represent only part of the impact of genetic differences. SNPs in intragenic regions may influence promoter activity and in introns, RNA splicing. Similarly, synonymous mutations within exons may disrupt duons and also alter gene expression. The above findings bolster the significance of a question that has been raised previously, “;are there NOX5 knockout humans”. Given the frequency of SNPs encoding NOX5 enzymes with no or close to zero enzyme activity (0.23% to 10.62%) the likelihood of humans inheriting 2 non-functioning alleles must be significant and in some populations, notably Africans, more likely. NOX5 may be dispensable and some species such as mice and rats seem to do fine without a gene for NOX5. On the other hand, the most frequent exonic missense SNP in NOX5 (31%) encodes a well-tolerated mutation (L352F) that has minimal effect on enzyme activity. Regardless, the identification of humans devoid of a functioning NOX5 would be an important step forward in our understanding of the physiological and pathophysiological significance of this isoform as detailed below.
Functional Significance of NOX5
A substantial body of literature exists that outlines the contributions of other NOX enzymes to both physiological and pathophysiological processes42. By comparison, NOX5 remains enigmatic. An appreciation of similarly important roles for NOX5 has been delayed due to the later discovery of this isoform, the lack of specific tools and inhibitors and its absence from the genomes of mice and rats. Nonetheless, there is a growing focus on the importance of NOX5 as discussed below and summarized in table 4.
Table 4.
Cell type and tissue specific expression, regulation and functional significance of Nox5 variants under physiology/pathophysiology conditions.
| System | Cell type/tissue |
Main variants |
Stimulus | Regulation | Function | Disease | Referenc es |
|---|---|---|---|---|---|---|---|
| Cardio- vascular system |
blood vessels |
V1-V5 | NF-kB, AP-1, and STAT1/STA T3 |
Atherosclerosis, myocardial infarction, hypertension |
41, 45, 48, 50 |
||
| endothelial cells |
V1, V4, V5 |
Thrombin / ionomycin |
inactivate NO, alter signaling through kinases, promotes apoptosis, stimulates proliferation, migration and angiogenesis |
Endothelial dysfunction, atherosclerosis, hypertension |
22,42, 41, 48, 53, 52 |
||
| smooth muscle cell |
V1-V4 | angiotensin II, endothelin- 1, PDGF, TNFα and IFNγ |
stimulating Erk phosphorylation, stimulates proliferation and migration, upregulation of calcium-activated potassium channels |
Atherosclerosis, cardiovascular disease |
22, 39, 42, 47,48, 53 |
||
| myocytes | Myocardial infarction | 50 | |||||
| Urinary system |
podocytes | V2 | angiotensin II, TGF-β |
filtration barrier function and systolic BP |
diabetic nephropathy | 11 | |
| Skin | keratinocyte s |
Calcium, SPC |
Atopic dermatitis | 55, 56 | |||
| Immune System |
Spleen, lymph nodes, Bone marrow cells |
2 | |||||
| Reproductiv e System |
Spermatocyt es, prostate, uterus, ovaries and placenta |
Calcium | sperm motility, muscular contractions and egg laying, |
Reproductive System disease |
1, 2, 60, 59, 60 |
||
| Multiple system cancer cells |
prostate, pancreatic, endometrial, liver, thyroid, breast, melanoma, hairy cell leukemia6666 6666 and esophageal |
V6 | Acid, Calcium | calcium- sensitive transcription factor, CREB and the PAF- stimulation of STAT5 |
increased proliferation, reduced apoptosis and DNA damage (induction of PGE2, DNMT1, NFkB and ROS induced DNA damage) |
Prostate cancer (81% positive for Nox5) >ovarian (70%) >melanoma(64%) >breast (61%) = glioblastoma (61%) ≥colon (60%) >lung (56%) >lymphoma (44%) |
31, 44, 59, 63, 64, 66, 67, 68, 69, 70 |
Cell Specific Expression of NOX5
As mentioned above, identification of the promoter regions of NOX5, which are, in part, responsible for regulating expression levels in various cell types and tissues, remains incompletely understood. Recent cloning of a putative promoter region of NOX5 revealed binding sites for NF-kB, AP-1, and STAT1/STAT3 44 but this region is not likely to encompass all of the regulatory elements or explain cell specific expression in certain cell types. The highest levels of NOX5 expression are observed in the spleen and testis2 and lower levels are seen in the vasculature, the gastrointestinal tract, fetal organs and various cancers 1, 2, 45. Increased NOX5 expression can be triggered by a variety of mechanisms including acid- treatment of esophageal adenocarcinoma cells, thrombin-treatment of endothelial cells 22, 46and treatment of smooth muscle cells with angiotensin II, endothelin-147, PDGF48, TNFα41and IFNγ44. Epigenetics may have a more profound role in the regulation of NOX5 expression in different cells types. The expression of NOX5 has been shown to be negatively influenced by DNA methylation49which may account for cell-specific regulation and changes in expression in disease states. The relatively ratio of different splice variants of NOX5 appears to vary with NOX5α(V1) the predominant form in blood vessels41, NOX5βand δ(V2 and V3) in endothelial cells22, NOX5α-γ(V1-V4) in smooth muscle cells 22, NOX5β(V2) in podocytes11and NOX5ε(V6) in Barrett's esophageal adenocarcinoma cells46.
Cardiovascular System
In 2001, NOX5 expression was first reported in cultured human smooth muscle cells and to a lesser extent, in endothelial cells 2. More recently, NOX5 has been detected in intact human and primate blood vessels22, 41, 50-52 and is expressed primarily in the intima and media. Increased NOX5 expression has been observed in human atherosclerosis 50, human myocardial infarction52 and human hypertension 47 but not in less advanced vascular lesions in primates51. The primary isoforms expressed in human blood vessels are the functional isoforms, NOX5α(V1) and NOX5β(V2) 41 whereas endothelial cells in culture express V1, V4 and V5 and vascular smooth muscle cells express NOX5 V1-V4 22. In vivo, the significance of endogenously expressed NOX5 to the development of cardiovascular disease in humans is not yet known. In isolated vascular cells, however, there is more evidence to support a functional role.
Endothelium
In human endothelial cells, 3 isoforms of NOX5α-ε (V1-V5) can be detected which generate ROS in response to thrombin and ionomycin22. It is not yet clear how the V3-V5 isoforms of NOX5, which are inactive in transfected cells, produce ROS in vascular cells. This suggests that there may be alternative mechanisms that are absent in transfected cells or reflect the difficulties detecting the low levels of ROS produced in vascular cells. In endothelial cells, NOX5 can function to inactivate nitric oxide signaling and endothelium-derived relaxation53, alter signaling through multiple kinase pathways (increased phosphorylation of Erk, Jnk, P38 and JAK2), promotes apoptosis at high concentrations 41, stimulate proliferation 22, migration 54 and angiogenesis54. The primary splice variant of NOX5 in blood vessels is NOX5α with β expressed to a lesser degree. Other splice variants of NOX5 were not detected41. The discrepancy between the expression levels of the different NOX5 splice variants in intact blood vessels versus that in cultured cells is not yet clear and may relate to the specific blood vessels studied or the isoform specific probes.
Smooth muscle
NOX5 expression has been detected in vascular smooth muscle cells from human coronary arteries, aorta and blood vessels of the spleen and lung 2, 41, 47, 48, 50and in cultured cells, the splice variants NOX5V1-V4 have been detected. Increased NOX5 expression in vascular smooth muscle cells alters intracellular signaling (predominantly stimulating Erk phosphorylation), stimulates smooth muscle proliferation 41, 48, migration 55 and the upregulation of calcium-activated potassium channels 55.
Myocardium
There is little information on the role of NOX5 in the heart. A recent study has shown that NOX5 is expressed in both blood vessels and myocytes of the heart and that expression is increased post myocardial infarction52. The functional significance of NOX5 in myocytes is not yet established.
Kidney
In diabetic humans there is a significant upregulation of NOX5 expression in the kidney. The majority of staining is localized to a specific cell type, the podocytes as determined by co-staining with the podocyte specific marker, nephrin. Podocytes are responsible for regulating the process of filtration via interaction and interdigitation with capillaries in the glomerulus. Challenging human podocytes with angiotensin II resulted in a robust upregulation of NOX5 expression whereas high glucose had no direct effect. The primary NOX5 isoform in podocytes was NOX5β and the other isoforms were not detected. To assess the functional significance of NOX5 in the kidneys in vivo, podocyte specific NOX5β transgenic animals were created in mice which naturally lack the gene for NOX5. These animals had no change in podocyte number but increased activation of cytoskeletal proteins, early onset albuminuria, podocyte foot process effacement, increased blood pressure and enhanced sensitivity to glomerlular damage in diabetes. These elegant studies advance a pathologic role of NOX5 in the diabetic kidney11. Given the critical role of angiotensin II in the induction of NOX5 expression in diabetes, it is likely that NOX5 also contributes to renal damage in other diseases with high angiotensin II such as hypertension. NOX5 expression has also been detected in human renal proximal tubules and is significantly increased in cells from individuals with hypertension56.
Skin
The skin is the largest organ in humans and surprisingly little is known about the expression of NOX5 in the various cells that comprise the multiple layers of skin. Studies have reported that NOX5 is well expressed in keratinocytes, contributing to calcium-dependent ROS production and that expression can be upregulated by proinflammatory agents such as sphingosylphosphorylcholine, SPC 57, 58.
The Immune System
NOX5 was first characterized as a gene that is highly expressed in the spleen and lymph nodes but not in circulating lymphocytes2. Bone marrow cells have been shown to express NOX5, but transcripts are not detected in differentiated immune cells. The role of NOX5 in immune cells remains poorly defined.
The Reproductive System
NOX5 mRNA is highly abundant in the testes and in particular is found in pachytene spermatocytes and to a lesser extent in round spermatids. High levels of expression are seen in the lumen of the seminiferous tubules, associated with maturing spermatids and in ejaculated spermatozoa NOX5 was present in the flagella region and the acrosome12, 59. ROS production in sperm is calcium-dependent and sensitive to NOX inhibitors and it is proposed that NOX5-derived ROS contributes to sperm motility 60. NOX5 expression is also observed in the prostate and expression levels can be significantly higher in prostate cancer cells61. In females, NOX5 is expressed in the uterus, ovaries and placenta1, 2. As with male reproductive function, the lack of direct evidence, specifically genetic strategies to selectively inhibit NOX5, places limits on our understanding of the precise contribution of NOX5 to the function of the female reproductive system. This is more clear in drosophila where an ortholog of NOX5 (dNOX) has been shown to be important for muscular contractions and egg laying62. Mechanistically, the elevation of intracellular calcium triggers the activation of dNOX which generates ROS and triggers additional calcium entry. This positive feedback loop facilitates a greater muscular contraction. The loss of dNOX reduces intracellular calcium levels, weakens ovarian contraction and renders the insects sterile. The mechanism by which dNOX promotes calcium influx is not yet known. In humans, the importance of NOX5 to reproduction is not yet known. Greater information on the existence of individuals and families with inactivating SNPs in NOX5 (as discussed above) may help to resolve this issue.
Cancer
Increased ROS have been proposed as both causative molecules in the development of cancer and accessory molecules that contribute to an enhanced rate of proliferation. Some cancerous cells produce large amounts of ROS and overexpress NOX enzymes and under express antioxidant defense enzymes45, 63. However, the lack of efficacy of antioxidants in vivo to prevent or treat cancer (and cardiovascular disease) has limited the broad acceptance of the importance of ROS in cancer. Many reasons have been proposed to rationalize the failure of antioxidants including potency, timing, the lack of specificity and a decrease in endogenous defenses against oxidants. In vitro, where the conditions can be more tightly controlled, increased expression of NOX can, on its own, promote the transformation and uncontrolled growth of cells64. Increased expression of NOX5 has been documented in a number of cancers or cancer cell lines including prostate 31, pancreatic 65, endometrial, liver, thyroid, breast, melanoma61, hairy cell leukemia66and esophageal cancer46. NOX5 protein and mRNA have been detected in human prostate cancer and the prostate cancer cell lines LNCaP, PC-3 and DU 14531, 61. In DU145 cells, ROS production is calcium-dependent and antisense knockdown of NOX5, but not p22phox or NOX2, inhibits both ROS production and cellular proliferation. Similarly in PC-3 cells, silencing of NOX5 expression decreased proliferation and increased apoptosis and these effects were mediated by increased signaling through multiple kinases 67. Barrett’s esophagus (BE) is a metaplasia of the lower esophagus due to chronic acid exposure. BE has a strong connection with esophageal adenocarcinoma (EA) which is a cancer with a poor prognosis. A number of studies have implicated NOX5 as a pathogenic mechanism linking excess acid to the increased production of ROS, increased proliferation, reduced apoptosis and DNA damage 46, 68, 69. Acid increases the expression of NOX5 in esophageal adenocarcinoma cells via the calcium-sensitive transcription factor, CREB46 and also via the PAF-stimulation of STAT570. Mechanisms by which NOX5 contributes to adenocarcinoma behavior include the induction of PGE2, DNMT1, NFkB71and ROS induced DNA damage. An interesting observation is that these effects are mediated by the NOX5ε(V5) isoform that lacks EF hands. Other studies have shown that this isoform is inactive 2, 41. How calcium regulates the activity of NOX5V5 in the human Barrett's Adenocarcinoma cell line (FLO) remains an unanswered question. NOX5 mRNA is also highly expressed in hairy cell leukemia (HCL)66, breast cancer (ZR-75), melanoma (SK-MEL 5) with low levels reported in colorectal, hematopoietic, lung and brain cancer cell lines 61. Protein expression of NOX5 is increased in cancers with varying frequencies prostate (81% positive for NOX5) >ovarian (70%) >melanoma(64%) >breast (61%) = glioblastoma (61%)>colon (60%) >lung (56%) >lymphoma (44%)72.
Summary
NOX5 was first identified 15 years ago and since that time we have accumulated an impressive amount of knowledge about its molecular regulation. However, the importance of NOX5 to human physiology and pathophysiology remains a large and importunate question. Given the lack of animal models that are not already “NOX5 knockouts” we are prevented from adopting time honored approaches to modeling disease in rodents using loss of function genetic strategies. The study by Holterman et al11, have taken the opposite strategy of expressing NOX5 in a specific cell type in a transgenic mouse. In this instance the mouse, which naturally lacks NOX5, provides an advantage in gain of function strategies to overexpress NOX5 in specific cell types. Interestingly, this NOX5-transgenic mouse recapitulates many aspects of renal disease that are seen in humans. We now know, based on SNPs, that there are likely to be (or should be) humans that have zero to very low NOX5 function. The impact of the loss of NOX5 on reproduction, cancer and cardiovascular disease would answer many of the above questions about the significance of NOX5 to human physiology and pathophysiology. However, it remains possible that NOX5 may be essential and that NOX5 knockout humans may not exist. A further limitation of studies addressing a functional role of NOX5 is the lack of a specific inhibitor. If NOX5 is important in the development of disease, as the studies in transgenic mice seem to suggest, a selective NOX5 inhibitor may have important clinical efficacy in a number of diseases ranging from atherosclerosis, diabetes, hypertension and cancer.
REFERENCES
- 1.Cheng G, Cao Z, Xu X, van Meir EG, Lambeth JD. Homologs of gp91phox: cloning and tissue expression of Nox3, Nox4, and Nox5. Gene. 2001;269:131–40. doi: 10.1016/s0378-1119(01)00449-8. [DOI] [PubMed] [Google Scholar]
- 2.Banfi B, Molnar G, Maturana A, Steger K, Hegedus B, Demaurex N, Krause KH. A Ca(2+)-activated NADPH oxidase in testis, spleen, and lymph nodes. J Biol Chem. 2001;276:37594–601. doi: 10.1074/jbc.M103034200. [DOI] [PubMed] [Google Scholar]
- 3.Banfi B, Tirone F, Durussel I, Knisz J, Moskwa P, Molnar GZ, Krause KH, Cox JA. Mechanism of Ca2+ activation of the NADPH oxidase 5 (NOX5) J Biol Chem. 2004;279:18583–91. doi: 10.1074/jbc.M310268200. [DOI] [PubMed] [Google Scholar]
- 4.Jagnandan D, Church JE, Banfi B, Stuehr DJ, Marrero MB, Fulton DJ. Novel mechanism of activation of NADPH oxidase 5. calcium sensitization via phosphorylation. J Biol Chem. 2007;282:6494–507. doi: 10.1074/jbc.M608966200. [DOI] [PubMed] [Google Scholar]
- 5.Wei CC, Reynolds N, Palka C, Wetherell K, Boyle T, Yang YP, Wang ZQ, Stuehr DJ. Characterization of the 1st and 2nd EF-hands of NADPH oxidase 5 by fluorescence, isothermal titration calorimetry, and circular dichroism. Chem Cent J. 2012;6:29. doi: 10.1186/1752-153X-6-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tirone F, Radu L, Craescu CT, Cox JA. Identification of the binding site for the regulatory calcium-binding domain in the catalytic domain of NOX5. Biochemistry. 2010;49:761–71. doi: 10.1021/bi901846y. [DOI] [PubMed] [Google Scholar]
- 7.Serrander L, Jaquet V, Bedard K, Plastre O, Hartley O, Arnaudeau S, Demaurex N, Schlegel W, Krause KH. NOX5 is expressed at the plasma membrane and generates superoxide in response to protein kinase C activation. Biochimie. 2007;89:1159–67. doi: 10.1016/j.biochi.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 8.Chen F, Yu Y, Haigh S, Johnson J, Lucas R, Stepp DW, Fulton DJ. Regulation of NADPH oxidase 5 by protein kinase C isoforms. PLoS One. 2014;9:e88405. doi: 10.1371/journal.pone.0088405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pandey D, Fulton DJ. Molecular regulation of NADPH oxidase 5 via the MAPK pathway. Am J Physiol Heart Circ Physiol. 2011;300:H1336–44. doi: 10.1152/ajpheart.01163.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pandey D, Gratton JP, Rafikov R, Black SM, Fulton DJ. Calcium/calmodulin-dependent kinase II mediates the phosphorylation and activation of NADPH oxidase 5. Mol Pharmacol. 2011;80:407–15. doi: 10.1124/mol.110.070193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holterman CE, Thibodeau JF, Towaij C, Gutsol A, Montezano AC, Parks RJ, Cooper ME, Touyz RM, Kennedy CR. Nephropathy and elevated BP in mice with podocyte-specific NADPH oxidase 5 expression. J Am Soc Nephrol. 2014;25:784–97. doi: 10.1681/ASN.2013040371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.El Jamali A, Valente AJ, Lechleiter JD, Gamez MJ, Pearson DW, Nauseef WM, Clark RA. Novel redox-dependent regulation of NOX5 by the tyrosine kinase c-Abl. Free Radic Biol Med. 2008;44:868–81. doi: 10.1016/j.freeradbiomed.2007.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tirone F, Cox JA. NADPH oxidase 5 (NOX5) interacts with and is regulated by calmodulin. FEBS Lett. 2007;581:1202–8. doi: 10.1016/j.febslet.2007.02.047. [DOI] [PubMed] [Google Scholar]
- 14.Fridolfsson HN, Roth DM, Insel PA, Patel HH. Regulation of intracellular signaling and function by caveolin. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2014 doi: 10.1096/fj.14-252320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu G, Ye RD, Dinauer MC, Malik AB, Minshall RD. Neutrophil caveolin-1 expression contributes to mechanism of lung inflammation and injury. American journal of physiology Lung cellular and molecular physiology. 2008;294:L178–86. doi: 10.1152/ajplung.00263.2007. [DOI] [PubMed] [Google Scholar]
- 16.Lobysheva I, Rath G, Sekkali B, Bouzin C, Feron O, Gallez B, Dessy C, Balligand JL. Moderate caveolin-1 downregulation prevents NADPH oxidase-dependent endothelial nitric oxide synthase uncoupling by angiotensin II in endothelial cells. Arterioscler Thromb Vasc Biol. 2011;31:2098–105. doi: 10.1161/ATVBAHA.111.230623. [DOI] [PubMed] [Google Scholar]
- 17.Chen F, Barman S, Yu Y, Haigh S, Wang Y, Dou H, Bagi Z, Han W, Su Y, Fulton DJ. Caveolin-1 is a negative regulator of NADPH oxidase-derived reactive oxygen species. Free Radic Biol Med. 2014;73:201–13. doi: 10.1016/j.freeradbiomed.2014.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pavlides S, Tsirigos A, Vera I, Flomenberg N, Frank PG, Casimiro MC, Wang C, Fortina P, Addya S, Pestell RG, Martinez-Outschoorn UE, Sotgia F, Lisanti MP. Loss of stromal caveolin-1 leads to oxidative stress, mimics hypoxia and drives inflammation in the tumor microenvironment, conferring the “reverse Warburg effect”: a transcriptional informatics analysis with validation. Cell cycle. 2010;9:2201–19. doi: 10.4161/cc.9.11.11848. [DOI] [PubMed] [Google Scholar]
- 19.Karuppiah K, Druhan LJ, Chen CA, Smith T, Zweier JL, Sessa WC, Cardounel AJ. Suppression of eNOS-derived superoxide by caveolin-1: a biopterin-dependent mechanism. Am J Physiol Heart Circ Physiol. 2011;301:H903–11. doi: 10.1152/ajpheart.00936.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao YY, Zhao YD, Mirza MK, Huang JH, Potula HH, Vogel SM, Brovkovych V, Yuan JX, Wharton J, Malik AB. Persistent eNOS activation secondary to caveolin-1 deficiency induces pulmonary hypertension in mice and humans through PKG nitration. The Journal of clinical investigation. 2009;119:2009–18. doi: 10.1172/JCI33338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Basset O, Deffert C, Foti M, Bedard K, Jaquet V, Ogier-Denis E, Krause KH. NADPH oxidase 1 deficiency alters caveolin phosphorylation and angiotensin II-receptor localization in vascular smooth muscle. Antioxid Redox Signal. 2009;11:2371–84. doi: 10.1089/ars.2009.2584. [DOI] [PubMed] [Google Scholar]
- 22.BelAiba RS, Djordjevic T, Petry A, Diemer K, Bonello S, Banfi B, Hess J, Pogrebniak A, Bickel C, Gorlach A. NOX5 variants are functionally active in endothelial cells. Free Radic Biol Med. 2007;42:446–59. doi: 10.1016/j.freeradbiomed.2006.10.054. [DOI] [PubMed] [Google Scholar]
- 23.Kawahara T, Jackson HM, Smith SM, Simpson PD, Lambeth JD. Nox5 forms a functional oligomer mediated by self-association of its dehydrogenase domain. Biochemistry. 2011;50:2013–25. doi: 10.1021/bi1020088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen F, Pandey D, Chadli A, Catravas JD, Chen T, Fulton DJ. Hsp90 regulates NADPH oxidase activity and is necessary for superoxide but not hydrogen peroxide production. Antioxid Redox Signal. 2011;14:2107–19. doi: 10.1089/ars.2010.3669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen F, Yu Y, Qian J, Wang Y, Cheng B, Dimitropoulou C, Patel V, Chadli A, Rudic RD, Stepp DW, Catravas JD, Fulton DJ. Opposing actions of heat shock protein 90 and 70 regulate nicotinamide adenine dinucleotide phosphate oxidase stability and reactive oxygen species production. Arterioscler Thromb Vasc Biol. 2012;32:2989–99. doi: 10.1161/ATVBAHA.112.300361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takac I, Schroder K, Zhang L, Lardy B, Anilkumar N, Lambeth JD, Shah AM, Morel F, Brandes RP. The E-loop is involved in hydrogen peroxide formation by the NADPH oxidase Nox4. J Biol Chem. 2011;286:13304–13. doi: 10.1074/jbc.M110.192138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ueyama T, Sakuma M, Ninoyu Y, Hamada T, Dupuy C, Geiszt M, Leto TL, Saito N. The Extracellular A-loop of Dual Oxidases Affects the Specificity of Reactive Oxygen Species Release. J Biol Chem. 2015 doi: 10.1074/jbc.M114.592717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Madrigal-Matute J, Fernandez-Garcia CE, Gomez-Guerrero C, Lopez-Franco O, Munoz-Garcia B, Egido J, Blanco-Colio LM, Martin-Ventura JL. HSP90 inhibition by 17-DMAG attenuates oxidative stress in experimental atherosclerosis. Cardiovascular research. 2012;95:116–23. doi: 10.1093/cvr/cvs158. [DOI] [PubMed] [Google Scholar]
- 29.Jagnandan D, Sessa WC, Fulton D. Intracellular location regulates calcium-calmodulin-dependent activation of organelle-restricted eNOS. Am J Physiol Cell Physiol. 2005;289:C1024–33. doi: 10.1152/ajpcell.00162.2005. [DOI] [PubMed] [Google Scholar]
- 30.Kawahara T, Lambeth JD. Phosphatidylinositol (4,5)-bisphosphate modulates Nox5 localization via an N-terminal polybasic region. Mol Biol Cell. 2008;19:4020–31. doi: 10.1091/mbc.E07-12-1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brar SS, Corbin Z, Kennedy TP, Hemendinger R, Thornton L, Bommarius B, Arnold RS, Whorton AR, Sturrock AB, Huecksteadt TP, Quinn MT, Krenitsky K, Ardie KG, Lambeth JD, Hoidal JR. NOX5 NAD(P)H oxidase regulates growth and apoptosis in DU 145 prostate cancer cells. Am J Physiol Cell Physiol. 2003;285:C353–69. doi: 10.1152/ajpcell.00525.2002. [DOI] [PubMed] [Google Scholar]
- 32.Ambasta RK, Kumar P, Griendling KK, Schmidt H, Busse R, Brandes RP. Direct interaction of the novel nox proteins with p22phox is required for the formation of a functionally active NADPH oxidase. Journal of Biological Chemistry. 2004;279:45935–45941. doi: 10.1074/jbc.M406486200. [DOI] [PubMed] [Google Scholar]
- 33.Bayraktutan U, Blayney L, Shah AM. Molecular characterization and localization of the NAD(P)H oxidase components gp91-phox and p22-phox in endothelial cells. Arterioscler Thromb Vasc Biol. 2000;20:1903–11. doi: 10.1161/01.atv.20.8.1903. [DOI] [PubMed] [Google Scholar]
- 34.Van Buul JD, Fernandez-Borja M, Anthony EC, Hordijk PL. Expression and localization of NOX2 and NOX4 in primary human endothelial cells. Antioxid Redox Signal. 2005;7:308–17. doi: 10.1089/ars.2005.7.308. [DOI] [PubMed] [Google Scholar]
- 35.Chen K, Kirber MT, Xiao H, Yang Y, Keaney JF., Jr Regulation of ROS signal transduction by NADPH oxidase 4 localization. J Cell Biol. 2008;181:1129–39. doi: 10.1083/jcb.200709049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gryglewski RJ, Palmer RM, Moncada S. Superoxide anion is involved in the breakdown of endothelium-derived vascular relaxing factor. Nature. 1986;320:454–6. doi: 10.1038/320454a0. [DOI] [PubMed] [Google Scholar]
- 37.Selemidis S, Dusting GJ, Peshavariya H, Kemp-Harper BK, Drummond GR. Nitric oxide suppresses NADPH oxidase-dependent superoxide production by S-nitrosylation in human endothelial cells. Cardiovascular research. 2007;75:349–58. doi: 10.1016/j.cardiores.2007.03.030. [DOI] [PubMed] [Google Scholar]
- 38.Qian J, Chen F, Kovalenkov Y, Pandey D, Moseley MA, Foster MW, Black SM, Venema RC, Stepp DW, Fulton DJ. Nitric oxide reduces NADPH oxidase 5 (Nox5) activity by reversible S-nitrosylation. Free Radic Biol Med. 2012;52:1806–19. doi: 10.1016/j.freeradbiomed.2012.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bossis G, Melchior F. SUMO: regulating the regulator. Cell Div. 2006;1:13. doi: 10.1186/1747-1028-1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pandey D, Chen F, Patel A, Wang CY, Dimitropoulou C, Patel VS, Rudic RD, Stepp DW, Fulton DJ. SUMO1 negatively regulates reactive oxygen species production from NADPH oxidases. Arterioscler Thromb Vasc Biol. 2011;31:1634–42. doi: 10.1161/ATVBAHA.111.226621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pandey D, Patel A, Patel V, Chen F, Qian J, Wang Y, Barman SA, Venema RC, Stepp DW, Rudic RD, Fulton DJ. Expression and functional significance of NADPH oxidase 5 (Nox5) and its splice variants in human blood vessels. Am J Physiol Heart Circ Physiol. 2012;302:H1919–28. doi: 10.1152/ajpheart.00910.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 43.Wang Y, Chen F, Le B, Stepp DW, Fulton DJ. Impact of Nox5 polymorphisms on basal and stimulus-dependent ROS generation. PLoS One. 2014;9:e100102. doi: 10.1371/journal.pone.0100102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Manea A, Manea SA, Florea IC, Luca CM, Raicu M. Positive regulation of NADPH oxidase 5 by proinflammatory-related mechanisms in human aortic smooth muscle cells. Free Radic Biol Med. 2012;52:1497–507. doi: 10.1016/j.freeradbiomed.2012.02.018. [DOI] [PubMed] [Google Scholar]
- 45.Lambeth JD. Nox enzymes, ROS, and chronic disease: an example of antagonistic pleiotropy. Free Radic Biol Med. 2007;43:332–47. doi: 10.1016/j.freeradbiomed.2007.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fu X, Beer DG, Behar J, Wands J, Lambeth D, Cao W. cAMP-response element-binding protein mediates acid-induced NADPH oxidase NOX5-S expression in Barrett esophageal adenocarcinoma cells. J Biol Chem. 2006;281:20368–82. doi: 10.1074/jbc.M603353200. [DOI] [PubMed] [Google Scholar]
- 47.Montezano AC, Burger D, Paravicini TM, Chignalia AZ, Yusuf H, Almasri M, He Y, Callera GE, He G, Krause KH, Lambeth D, Quinn MT, Touyz RM. Nicotinamide adenine dinucleotide phosphate reduced oxidase 5 (Nox5) regulation by angiotensin II and endothelin-1 is mediated via calcium/calmodulin-dependent, rac-1-independent pathways in human endothelial cells. Circ Res. 2010;106:1363–73. doi: 10.1161/CIRCRESAHA.109.216036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jay DB, Papaharalambus CA, Seidel-Rogol B, Dikalova AE, Lassegue B, Griendling KK. Nox5 mediates PDGF-induced proliferation in human aortic smooth muscle cells. Free Radic Biol Med. 2008;45:329–35. doi: 10.1016/j.freeradbiomed.2008.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhu C, Yu ZB, Chen XH, Ji CB, Qian LM, Han SP. DNA hypermethylation of the NOX5 gene in fetal ventricular septal defect. Exp Ther Med. 2011;2:1011–1015. doi: 10.3892/etm.2011.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Guzik TJ, Chen W, Gongora MC, Guzik B, Lob HE, Mangalat D, Hoch N, Dikalov S, Rudzinski P, Kapelak B, Sadowski J, Harrison DG. Calcium-dependent NOX5 nicotinamide adenine dinucleotide phosphate oxidase contributes to vascular oxidative stress in human coronary artery disease. J Am Coll Cardiol. 2008;52:1803–9. doi: 10.1016/j.jacc.2008.07.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stanic B, Pandey D, Fulton DJ, Miller FJ., Jr. Increased epidermal growth factor-like ligands are associated with elevated vascular nicotinamide adenine dinucleotide phosphate oxidase in a primate model of atherosclerosis. Arterioscler Thromb Vasc Biol. 2012;32:2452–60. doi: 10.1161/ATVBAHA.112.256107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hahn NE, Meischl C, Kawahara T, Musters RJ, Verhoef VM, van der Velden J, Vonk AB, Paulus WJ, van Rossum AC, Niessen HW, Krijnen PA. NOX5 expression is increased in intramyocardial blood vessels and cardiomyocytes after acute myocardial infarction in humans. Am J Pathol. 2012;180:2222–9. doi: 10.1016/j.ajpath.2012.02.018. [DOI] [PubMed] [Google Scholar]
- 53.Zhang Q, Malik P, Pandey D, Gupta S, Jagnandan D, Belin de Chantemele E, Banfi B, Marrero MB, Rudic RD, Stepp DW, Fulton DJ. Paradoxical activation of endothelial nitric oxide synthase by NADPH oxidase. Arterioscler Thromb Vasc Biol. 2008;28:1627–33. doi: 10.1161/ATVBAHA.108.168278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pi X, Xie L, Portbury AL, Kumar S, Lockyer P, Li X, Patterson C. NADPH oxidase-generated reactive oxygen species are required for stromal cell-derived factor-1alpha-stimulated angiogenesis. Arterioscler Thromb Vasc Biol. 2014;34:2023–32. doi: 10.1161/ATVBAHA.114.303733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gole HK, Tharp DL, Bowles DK. Upregulation of intermediate-conductance Ca2+-activated K+ channels (KCNN4) in porcine coronary smooth muscle requires NADPH oxidase 5 (NOX5) PLoS One. 2014;9:e105337. doi: 10.1371/journal.pone.0105337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yu P, Han W, Villar VA, Yang Y, Lu Q, Lee H, Li F, Quinn MT, Gildea JJ, Felder RA, Jose PA. Unique role of NADPH oxidase 5 in oxidative stress in human renal proximal tubule cells. Redox Biol. 2014;2:570–9. doi: 10.1016/j.redox.2014.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Choi H, Kim S, Kim HJ, Kim KM, Lee CH, Shin JH, Noh M. Sphingosylphosphorylcholine down-regulates filaggrin gene transcription through NOX5-based NADPH oxidase and cyclooxygenase-2 in human keratinocytes. Biochemical pharmacology. 2010;80:95–103. doi: 10.1016/j.bcp.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 58.Benedyk M, Sopalla C, Nacken W, Bode G, Melkonyan H, Banfi B, Kerkhoff C. HaCaT keratinocytes overexpressing the S100 proteins S100A8 and S100A9 show increased NADPH oxidase and NF-kappaB activities. The Journal of investigative dermatology. 2007;127:2001–11. doi: 10.1038/sj.jid.5700820. [DOI] [PubMed] [Google Scholar]
- 59.Sabeur K, Ball BA. Characterization of NADPH oxidase 5 in equine testis and spermatozoa. Reproduction. 2007;134:263–70. doi: 10.1530/REP-06-0120. [DOI] [PubMed] [Google Scholar]
- 60.Musset B, Clark RA, DeCoursey TE, Petheo GL, Geiszt M, Chen Y, Cornell JE, Eddy CA, Brzyski RG, El Jamali A. NOX5 in human spermatozoa: expression, function, and regulation. J Biol Chem. 2012;287:9376–88. doi: 10.1074/jbc.M111.314955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Juhasz A, Ge Y, Markel S, Chiu A, Matsumoto L, van Balgooy J, Roy K, Doroshow JH. Expression of NADPH oxidase homologues and accessory genes in human cancer cell lines, tumours and adjacent normal tissues. Free radical research. 2009;43:523–32. doi: 10.1080/10715760902918683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ritsick DR, Edens WA, McCoy JW, Lambeth JD. The use of model systems to study biological functions of Nox/Duox enzymes. Biochem Soc Symp. 2004:85–96. doi: 10.1042/bss0710085. [DOI] [PubMed] [Google Scholar]
- 63.Szatrowski TP, Nathan CF. Production of large amounts of hydrogen peroxide by human tumor cells. Cancer Res. 1991;51:794–8. [PubMed] [Google Scholar]
- 64.Suh YA, Arnold RS, Lassegue B, Shi J, Xu X, Sorescu D, Chung AB, Griendling KK, Lambeth JD. Cell transformation by the superoxide-generating oxidase Mox1. Nature. 1999;401:79–82. doi: 10.1038/43459. [DOI] [PubMed] [Google Scholar]
- 65.Mochizuki T, Furuta S, Mitsushita J, Shang WH, Ito M, Yokoo Y, Yamaura M, Ishizone S, Nakayama J, Konagai A, Hirose K, Kiyosawa K, Kamata T. Inhibition of NADPH oxidase 4 activates apoptosis via the AKT/apoptosis signal-regulating kinase 1 pathway in pancreatic cancer PANC-1 cells. Oncogene. 2006;25:3699–707. doi: 10.1038/sj.onc.1209406. [DOI] [PubMed] [Google Scholar]
- 66.Kamiguti AS, Serrander L, Lin K, Harris RJ, Cawley JC, Allsup DJ, Slupsky JR, Krause KH, Zuzel M. Expression and activity of NOX5 in the circulating malignant B cells of hairy cell leukemia. J Immunol. 2005;175:8424–30. doi: 10.4049/jimmunol.175.12.8424. [DOI] [PubMed] [Google Scholar]
- 67.Holl M, Koziel R, Schafer G, Pircher H, Pauck A, Hermann M, Klocker H, Jansen-Durr P, Sampson N. ROS signaling by NADPH oxidase 5 modulates the proliferation and survival of prostate carcinoma cells. Molecular carcinogenesis. 2015 doi: 10.1002/mc.22255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhou X, Li D, Resnick MB, Wands J, Cao W. NADPH oxidase NOX5-S and nuclear factor kappaB1 mediate acid-induced microsomal prostaglandin E synthase-1 expression in Barrett’s esophageal adenocarcinoma cells. Mol Pharmacol. 2013;83:978–90. doi: 10.1124/mol.112.083287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li D, Cao W. Role of intracellular calcium and NADPH oxidase NOX5-S in acid-induced DNA damage in Barrett’s cells and Barrett’s esophageal adenocarcinoma cells. Am J Physiol Gastrointest Liver Physiol. 2014;306:G863–72. doi: 10.1152/ajpgi.00321.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Si J, Behar J, Wands J, Beer DG, Lambeth D, Chin YE, Cao W. STAT5 mediates PAF-induced NADPH oxidase NOX5-S expression in Barrett’s esophageal adenocarcinoma cells. Am J Physiol Gastrointest Liver Physiol. 2008;294:G174–83. doi: 10.1152/ajpgi.00291.2007. [DOI] [PubMed] [Google Scholar]
- 71.Hong J, Li D, Wands J, Souza R, Cao W. Role of NADPH oxidase NOX5-S, NF-kappaB, and DNMT1 in acid-induced p16 hypermethylation in Barrett’s cells. Am J Physiol Cell Physiol. 2013;305:C1069–79. doi: 10.1152/ajpcell.00080.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Antony S, Wu Y, Hewitt SM, Anver MR, Butcher D, Jiang G, Meitzler JL, Liu H, Juhasz A, Lu J, Roy KK, Doroshow JH. Characterization of NADPH oxidase 5 expression in human tumors and tumor cell lines with a novel mouse monoclonal antibody. Free Radic Biol Med. 2013;65:497–508. doi: 10.1016/j.freeradbiomed.2013.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]