Abstract
Purpose
To determine health related quality of life (QOL) during and after neoadjuvant chemoradiotherapy and surgery for patients with pancreatic adenocarcinoma.
Methods and materials
Participants of a prospective phase II multi-institutional trial treated with neoadjuvant chemoradiation followed by surgery, completed QOL questionnaires (EORTC-QLQC30, EORTC-PAN26 and FACT-Hep) at baseline, after 2 cycles of neoadjuvant therapy, after surgery, at 6 months from initiation of therapy and at 6-month intervals for 2 years. Mean scores were compared to baseline. Change >10% was considered a minimal clinically important difference.
Results
Of 71 participants in the trial, 55 were eligible for QOL analysis. Compliance ranged from 32-74%. EORTC-QLQ C30 global QOL did not significantly decline after neoadjuvant therapy, while, the FACT G, showed a statistically but not clinically significant decline (−8, P = 0.02). This was in parallel to deterioration in physical functioning (−14.1, P = 0.001), increase in diarrhea (+16.7, P = 0.044) and an improvement in pancreatic pain (−13, P = 0.01) as per EORTC-PAN26. Due to poor patient compliance in the non-surgical group, long-term analysis was performed only from surgically resected participants (n=36). Among those, global QOL returned to baseline levels after 6 months, remaining near baseline through the 24-month visit.
Conclusions
The study regimen consisting of 2 cycles of neoadjuvant therapy was completed without a clinically significant QOL deterioration. A transient increase in gastrointestinal symptoms and a decrease in physical functioning were seen after neoadjuvant chemoradiation. In those patients who underwent surgical resection, most domains returned back to baseline levels by 6 months.
BACKGROUND
Approximately 20-30% of patients who undergo pancreatectomy for pancreatic adenocarcinoma do not receive adjuvant therapy, often due to slow recovery following surgery. While adjuvant chemotherapy and/or radiotherapy has been shown to improve local recurrence and survival, it is associated with treatment related morbidity and mortality [1-4]. Neoadjuvant therapy increases the likelihood that patients will receive chemotherapy and radiation, without incurring delays resulting from postoperative recovery. Further, the potential for therapy related downstaging of the primary tumor might allow higher rates of margin-negative resection, a particularly important issue in borderline resectable cases [5]. Specific benefits for the incorporation of neoadjuvant radiation therapy include the ability to treat with smaller radiotherapy volumes thus limiting toxicity. By limiting toxicity, neoadjuvant radiation therapy can be delivered concurrently with full dose chemotherapy [6,7].
The impact of neoadjuvant chemoradiation on the quality of life (QOL) of patients with pancreatic cancer is not known, as prospective QOL data in this patient population is limited. Given the short life expectancy of these patients and the high potential for treatment related toxicity, it is important to understand how QOL can be affected during and following completion of neoadjuvant treatment. Preservation of function and well being in everyday life has become a key component of the health care goals for cancer patients [8,9]. This is especially important in the neoadjuvant setting where deterioration of health-related QOL and performance status may impact patients’ ability to tolerate a major pancreatectomy. The working hypothesis of this study was that global QOL did not decrease from baseline to re-evaluation after 2 cycles of neoadjuvant chemoradiation.
METHODS
Study design
This is a sub-study of a prospective multi-institutional phase II trial. [10] Patients with untreated pancreatic adenocarcinoma consented to receive neoadjuvant chemoradiation with full dose gemcitabine and oxaliplatin with accrual occurring between July 2007 and February 2010. Eligible participants were required to have pathologic confirmation of pancreatic adenocarcinoma and resectable or borderline resectable staging based on the National Comprehensive Cancer Network guidelines [11]. Eligibility criteria also included: a Zubrod performance status of ≤ 2 with no previous chemotherapy or radiotherapy and adequate bone marrow, liver and kidney organ function.[10,12] To be eligible for the QOL sub-study, participants were required to have adequate knowledge of English. Ethics approval was obtained from each institution participating in this trial.
Treatment plan
Gemcitabine 1000 mg/m2 was infused over 30 minutes on days 1, 8 and 15 of a treatment cycle. Oxaliplatin 85 mg/m2 was infused following gemcitabine over 90 minutes on days 1 and 15. Radiation therapy was given concurrently during cycle 1 of chemotherapy, five times a week with a total dose of 30 Gy in 15 fractions over 3 weeks. Three-dimensional planning was used, limiting the target volumes to gross disease with a 1-cm margin, allowing no elective lymph node irradiation. If participants were deemed resectable at re-staging, surgery was performed 2-4 weeks after the last chemotherapy dose. If the participant were considered not resectable, he or she would continue to cycle 3 and 4 of chemotherapy. If participants underwent resection, two additional cycles of adjuvant gemcitabine and oxaliplatin was recommended. Patients were followed for up to two years. After recurrence, patients were encouraged to complete quality of life questionnaires until the end of follow-up.
Quality of Life
We utilized the European Organization for Research and Treatment in Cancer Quality of Life Questionnaire version 3.0 (EORTC–QLQ C30) [13,14], the EORTC–PAN 26 module [15], and the Functional Assessment of Cancer Therapy Hepatobiliary & Pancreatic subscale (FACT-Hep) to measure changes in QOL [16-18]. Two scales were used to measure changes in global health related QOL; the FACT global health measure (G), which consists of four health related QOL dimensions: physical well being (consisting of seven items), social well being (seven items), emotional well being (six items) and functional well being (seven items) [17] and the EORTC-QLQ C30 global health / QOL domain (consisting of only two items). Changes in functioning and symptoms were measured using the EORTC-QLQ C30 and PAN 26. These do not generate a summary score; rather each scale is individually scored, ranging from 0 to 100, with functioning subscales defined such that higher scores represent better QOL and symptom subscales defined such that higher scores indicate more symptoms (worse QOL). The disease specific module - Hepatobiliary Cancer Subscale (FACT-Hep HCS) – (consisting of 18 items) was used as an independent assessment of concerns related to hepatobiliary cancers.
Questionnaires were administered at baseline and at re-evaluation following completion of cycle 2 of neoadjuvant therapy before surgery. Thereafter QOL data was solicited from all patients (resected and non-resected) at 6 months after the initiation of the treatment protocol and at 6-month intervals until 24 months.
Quality of life compliance
Compliance was calculated as the percentage of QOL-eligible patients alive at each study time-point. [19]
Data Analysis
Characterizing the change in global QOL from baseline to re-evaluation after 2 cycles of neoadjuvant therapy was the primary outcome. The QOL outcomes measured included the EORTC-QLQ C30 global health score and the physical and emotional functioning scales; the FACT G, the FACT-Hep HCS and the EORTC-PAN26. The minimal clinically important difference (MCID) in overall quality of life was defined as a 10% change, which corresponds to a 10-point difference in the FACT G or in the EORTC-QLQ-C30 global health scale. In order to detect the MCID with at most 5% type I error and 80% power, a sample size of at least 21 paired patient responses was required. [20,21] In order to estimate changes in the continuous QOL outcomes, linear, mixed-effects, repeated-measure models, were constructed with the within subject correlation expected to follow an autoregressive structure. Mean estimates and 95% confidence intervals were calculated for each study measurement point. P values at or below 0.05 were considered statistically significant.
RESULTS
Patient description
There were 71 participants enrolled onto the clinical trial. The QOL component of this study was started after 7 participants were already included into the trial. Of 64 potential participants eligible for QOL, 57 completed at least one QOL instrument and 55 were included in the final analysis (Figure 1). All further references below relate to these 55 participants.
Figure 1.
Diagram of participants and sample evolution: This is a flow chart of the evaluable patients grouped by analysis (No. 1 and 2) indicating the number of patients with analyzable QOL instruments at each subsequent step and time-point. “Other” refers to one patient removed from study due to non-compliance with treatment protocol.
There were 29 men and 26 women, median age 64 (range: 42–82) years. At diagnosis, 36/55 (65%) patients had borderline resectable disease and 19/55 (35%) had resectable disease. Most participants (36/55, 66%) underwent successful surgical resection. Following resection, 26/36, 72% participants received adjuvant chemotherapy.
The median overall survival was 18.2 months [95% confidence interval (CI): 13-26.9 months], 10.9 (95% CI: 6.1-12.6 months) for those who were not resected and 27.1 months (95% CI: 21.2-47.1 months) for resected participants. Median disease free survival was 11 months (95% CI: 4-21 months).
Table 1 shows the QOL compliance for the entire population. Participants who underwent curative surgical resection were more likely to have longitudinal QOL measurements after baseline and to comply with the 6-month and beyond QOL questionnaires. Of 19 participants who were not resected, QOL compliance at 6 months was 26% (5/19) and at 24 months, 15% (2//13). Due to this poor compliance, analysis was divided into two components for two different populations: those who completed 2 cycles of neoadjuvant therapy and those who underwent successful R0 or R1 resection. Data from non-resected participants contributed to the assessment of the neoadjuvant period but was excluded from analysis thereafter. (Figure 1)
Table 1.
Compliance of Quality of life questionnaires throughout the trial
| QOL questionnaires analyzed | Patients alive at each time-point | Compliance | |
|---|---|---|---|
| N | N | % | |
| Baseline | 53 | 55 | 96 |
| Post neoadjuvant therapy | 39 | 53 | 74 |
| Postsurgical visit pre-adjuvant | 23 | 51 | 45 |
| 6-month follow-up | 21 | 51 | 41 |
| 12-month follow-up | 19 | 40 | 48 |
| 18-month follow-up | 13 | 34 | 38 |
| 24-month follow-up | 10 | 33 | 32 |
Table 1. Compliance of quality of life (QOL) questionnaires was calculated as the percentage of the QOL-eligible patients alive at each study time-point.
Quality of life after two cycles of neoadjuvant therapy
There was a trend to reduced global QOL as measured by the EORTC-QLQ C30 global health domain between the baseline assessment and re-evaluation after 2 cycles of neoadjuvant therapy. The decrease in global QOL as measured by the FACT G (−8.4, P = 0.02) met statistical but not clinical significance criteria (Table 2). The EORTC physical functioning subscale −14.1, P = 0.0014) showed a definite change, reaching both the statistical and the clinical significant threshold.
Table 2.
Quality of life during after two cycles of neoadjuvant therapy compared to baseline.
| QOL Endpoint | [N] Mean (95% CI) | Post –Pre Neoadjuvant Therapy (N=36) | ||
|---|---|---|---|---|
| Instrument / Scale | Baseline | End of 2nd cycle | Estimated Mean difference (95°% CI) | P-value |
| EORTC – C30 | ||||
| Global Health | [53] 65.2 (59.4 – 71.0) | [39] 57.5 (48.7 – 66.2) | −7.8 (−17.0 – 1.5) | 0.0987 |
| Physical Functioning | [53] 87.9 (84.2 – 91.6) | [39] 74.7 (66.5 – 83.0) | −14.1* (−21.3 – −6.8) | 0.0014 |
| Emotional Functioning | [53] 68.9 (62.2 – 75.7) | [39] 64.9 (56.8 – 72.9) | −0.2 (−8.2 – 7.7 | 0.9999 |
| FACT | ||||
| G | [53] 80.8 (77.0 – 84.6) | [39] 71.6 (66.1 – 77.1) | −8.4 (−13.3 – −3.5) | 0.0201 |
| HCS | [50] 55.3 (52.2 – 58.3) | [39] 51.1 (47.7 – 54.6) | −4.0 (−7.4 – −0.6) | 0.0895 |
| PAN-26 | ||||
| Pancreatic Pain | [53] 33.8 (28.1 – 39.5) | [39] 23.3 (17.2 – 29.4) | −12.7* (4.5 – 21.0) | 0.0107 |
Table 1. EORTC-QLQ C30, EORTC-PAN 26, FACT-Hep G and HCS presented as medians and means with range scores at baseline and at re-evaluation following two cycles of neoadjuvant therapy. Highlighted numbers represent statistically significant differences with p value <0.05. Asterisk (*) represents a minimal clinically important difference. A high score on a functional scale represents a high/healthy level of functioning, but a high score for a symptom scale represents a high level of symptomatology / problems.
The hepatobiliary cancer subscale of the FACT-Hep remained similar throughout neoadjuvant therapy and the pancreatic pain subscale (EORTC PAN-26 −12.7, P = 0.01) significantly decreased, suggesting less reported pain after adjuvant therapy than before (Table 2).
As discussed in our previous paper, baseline global quality of life was associated with improved survival in the multivariate analysis (P < 0.05).[10]
Quality of life following successful resection
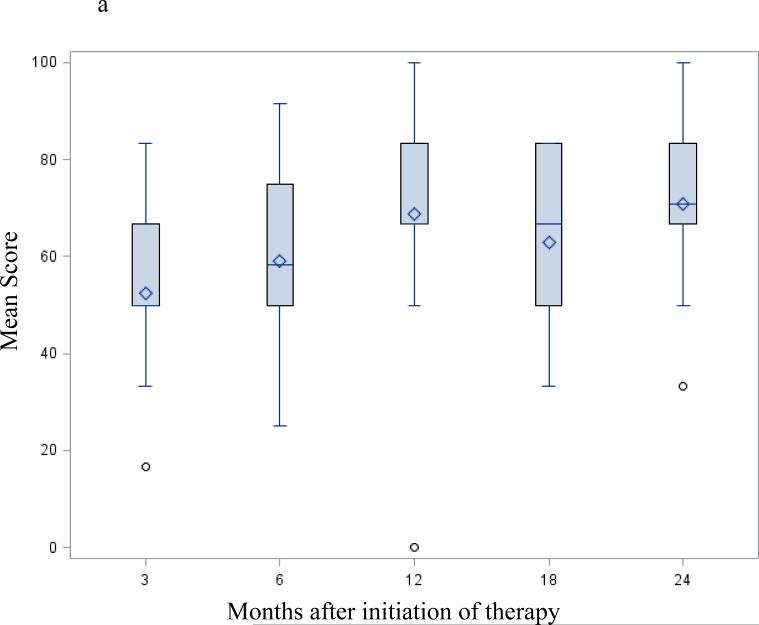
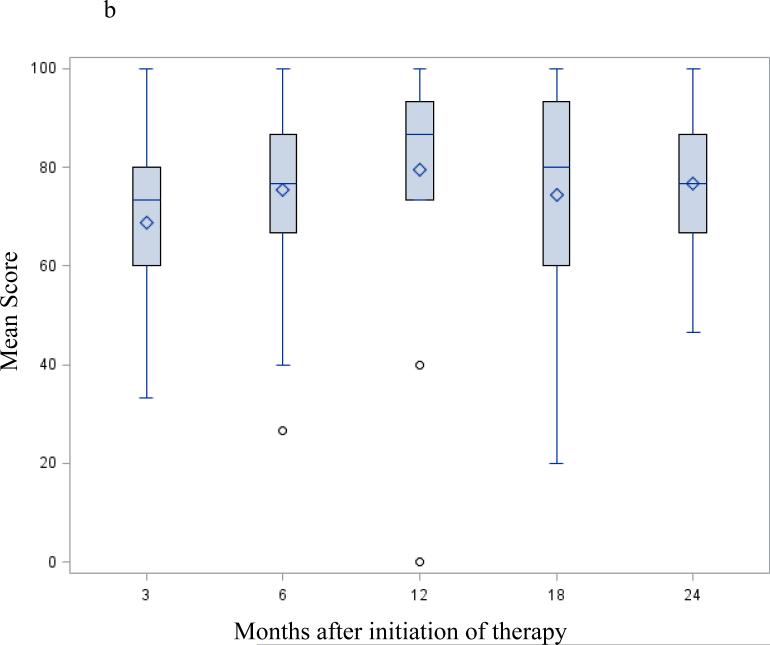
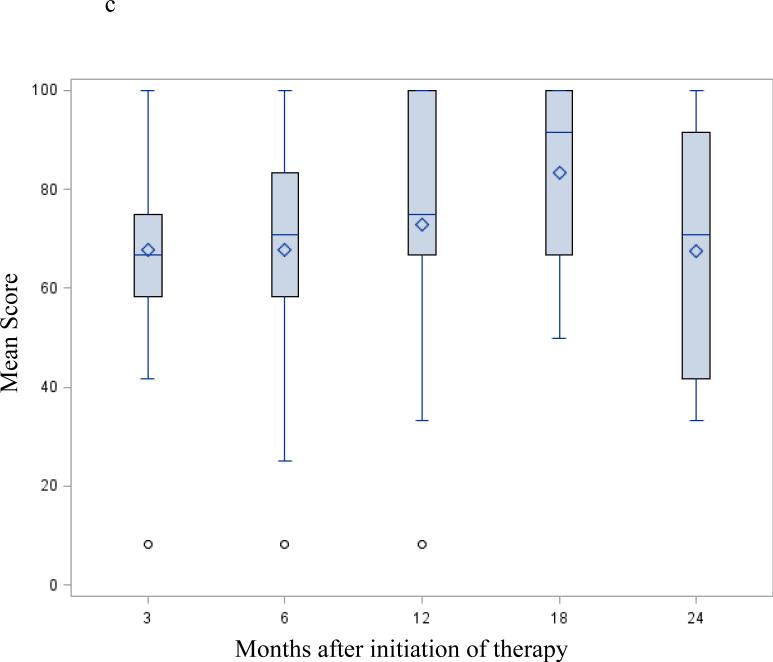
Following successful resection, global QOL as measured by the EORTC-QLQ C30 global health domain (Figure 2a) and the FACT G (Table 3) showed a trend to continuous improvement. At 12 months it remained unchanged up to the 24-month visit. Changes in the physical and emotional functioning subscales from the EORTC-QLQ C30 (Figure 2b-c) followed a similar pattern – started low at the 3 month - postoperative preadjuvant visit then progressively increasing up to the 24-month visit.
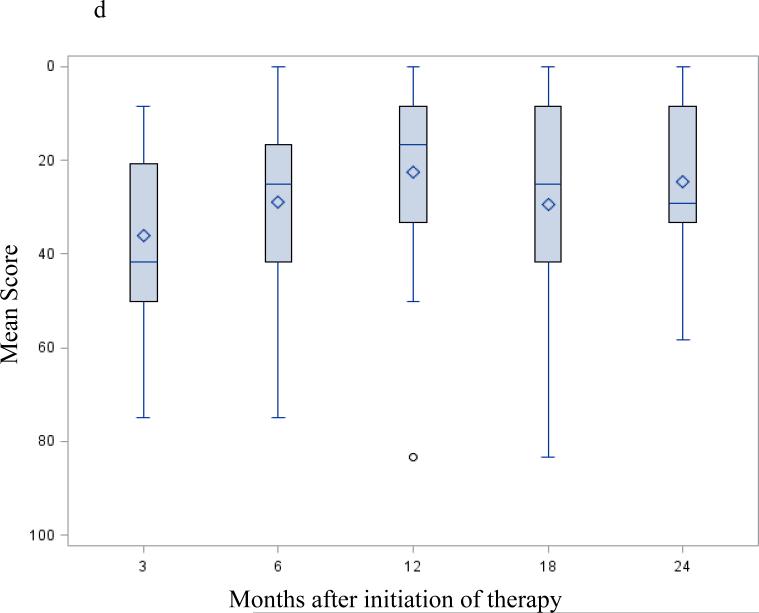
Figure 2.
EORTC-QLQ C30 / PAN 26 mean score change after successful surgery; selected functioning and symptoms subscale: Mean scores following successful resection – months from initiation of protocol therapy. Three months from initiation of therapy correspond to the post-operative / pre-adjuvant visit. Fig. 2a EORTC-QLQ C30 global health QOL; Fig. 2b EORTC-QLQ C30 physical functioning subscale; Fig. 2c EORTC-QLQ C30 emotional functioning subscale; Fig. 2d EORTC PAN26 pancreatic pain subscale. A high score on a functional scale (a-b-c) represents a high/healthy level of functioning, but a high score for a symptom scale (d) represents a high level of symptomatology / problems. None of these changes were statistically significant.
Table 3.
Quality of life for the FACT G and FACT-Hep HCS for the post-surgery period for those undergoing successful resection
| Adjuvant Chemotherapy Period Estimated Mean (95% CI) | Long-term Follow-up Period Estimated Mean (95% CI) | ||||
|---|---|---|---|---|---|
| Instrument/Scale | Pre-adjuvant | Post-adjuvant | 12 months | 18 months | 24 months |
| FACT | |||||
| G | 72.1 (66-78) | 76.7 (72-81) | 82.6 (77-89) | 84.2 (78-91) | 81.6 (72-91) |
| HCS | 48.7 (46-62) | 51.9 (49-55) | 56.2 (53-59) | 57.8 (54-61) | 56.5 (52-61) |
Table 2. FACT G and FACT-Hep HCS presented as mean QOL and 95% confidence interval (CI) scores over time. None of these differences were statistically significant (P <0.05).
Changes in the FACT-Hep HCS were not significantly different however there was a trend towards improved quality levels by 12 months, which remained similarly unchanged up to the 24-month visit (Table 3). Other symptoms that increased significantly following neoadjuvant full dose chemotherapy with conformal RT (both clinically and statistically) were diarrhea (+14, P < 0.001), limb weakness (+ 24, P = 0.001) and fatigue (+19, P = 0.003), reaching a peak level at 6 months and returning back to baseline at 12 months and beyond.
DISCUSSION
There is limited literature evaluating the impact of neoadjuvant therapy on QOL in pancreatic cancer [16]. This prospective analysis examined the QOL outcomes of patients with pancreatic adenocarcinoma who received neoadjuvant full dose gemcitabine and oxaliplatin with concomitant conformal radiation, followed by surgery and two postoperative cycles of chemotherapy [10]. Our results indicate that while this neoadjuvant treatment protocol is associated with an initial decrease in global health-related QOL, it was generally well tolerated and patients were able to undergo resection with an acceptable level of QOL. In patients who went on to curative surgical resection, the decrease in global QOL observed after 2 cycles of neoadjuvant therapy gradually returned back to baseline levels within 6 months of initiation of treatment and was preserved for up to 24 months.
We suspect that one of the main determinants of changes in global QOL (EORTC-QLQ C30) in those who underwent 2 cycles of neoadjuvant therapy was physical functioning. This subscale of the EORTC-QLQ C30 demonstrated a statistical and clinically significant decrease.
The summary measures of the FACT G did not show a clinically significant change. On the other hand, specific item subscales from the EORTC-PAN 26 module, such as diarrhea, fatigue and limb weakness did demonstrate a clinically and statistically significant, while transient worsening. [21] Our findings are consistent with the results published by Short et al, where the impact of 6-month adjuvant chemoradiotherapy did not decrease QOL compared to baseline using the EORTC-QLQ-C30 and PAN26. [22]
The instrument of choice for determining QOL changes in the neoadjuvant setting has not yet been clearly determined [23,24]. The scales utilized in this study complement each other and give robust information on symptoms and how they affect patients’ QOL. The FACT-Hep with its many component questions and subscales, is theoretically more sensitive in detecting QOL changes in patients with hepatobiliary diseases while the EORTC-QLQ C30 – global QOL scale (with only two items) provides a multidimensional QOL assessment that may be easier for clinicians to use in directing therapy based on a single score. This is the most probable reason for its widespread use in most large clinical trials. [13,16]
Compliance was one of the major limitations of our study; it proved adequate in the re-evaluation visit after cycle 2 of neoadjuvant therapy, but it decreased thereafter. This was primarily due to the fact that patients who were not resected for medical reasons or for cancer progression did not complete QOL questionnaires – presumably as a result of poor state of health and emotional despair and/or discharge to community hospital or palliative care for follow-up at the time of recurrence. This limitation shared by most longitudinal QOL studies in patients with terminal cancer such as pancreatic cancer. This compares to other major pancreatic cancer protocols [25-28], including the study by Conroy et al where compliance for questionnaire completion over time in surviving participants varied between 40-95%. [25,29] In our study, long-term outcomes on QOL are representative mostly of those participants without disease progression after neoadjuvant therapy who underwent curative surgery. QOL outcomes on patients deemed unresectable after neoadjuvant therapy or who were not resected at the time of operation (i.e. occult metastases) are unknown; therefore, comparisons between patients who underwent surgery and those who were not resected were not possible to make.
Additionally, our study was not powered to analyze the association of individual symptoms with global QOL. For example, the increase in fatigue and limb weakness at re-evaluation after cycle 2 of chemotherapy with subsequent improvement at 12 months implies a causal relationship to the parallel change in physical functioning (EORTC-QLQ C30) and well being (FACT-Hep). Such analysis would be helpful in determining what symptoms contribute most to changes in global QOL and perhaps explain the differences obtained in different instruments. Lastly, our study lacks a control group without neoadjuvant chemoradiation. A previously reported randomized controlled trial comparing adjuvant chemoradiation therapy with observation in pancreatic cancer, demonstrated a better global QOL in the treatment arm with a mean postoperative global QOL score of 73 compared to 82 in our study. These data suggest that the detriment observed in QOL after the administration of neoadjuvant chemoradiation is similar to that observed in the adjuvant setting based on historical controls. [30] Historical comparisons with other neoadjuvant protocols cannot be performed; as to our knowledge, there are no other previous studies in this field. A larger randomized controlled trial evaluating the effect of neoadjuvant chemoradiation therapy on long-term outcomes and QOL is needed. The experience and knowledge gained on studies like ours have led to the design of larger trials that would eventually answer these questions. A large intergroup study (Alliance A021101) is underway evaluating the feasibility of performing such a trial, specifically assessing the accrual rate and treatment-related toxicity and treatment delay during preoperative therapy. [5]
The good tolerability of this regimen is in part due to the low dose of radiation used and the small field size (clinical target volume= gross tumor volume plus 1 cm). It would be difficult to compare to the more commonly used 50 Gy – 5-FU combination, however; this study provides an important foundation of prospective patient self-reported QOL data that can be used to compare to future regimens combining higher dose radiation therapy – Intensity-Modulated Radiation Therapy (IMRT) / Stereotactic Body Radiation Therapy (SBRT) - with newer chemotherapy regimens such as FOLFIRINOX or gemcitabine / nab-paclitaxel; as such, our study will be one of the first well-designed phase II clinical trials to evaluate the QOL of patients undergoing neoadjuvant chemoradiation therapy for pancreatic cancer and will serve as reference for future neoadjuvant therapy trials.
Strengths of this study include the prospective nature of the phase II multi-center trial and the use of two different scores to determine global QOL, taken from two well-validated QOL instruments, as well as the use of three QOL instruments to evaluate the symptomatology of patients throughout the study period.
In summary, this neoadjuvant regimen can be delivered with good tolerability, albeit with a statistically significant transient decline in global QOL at re-evaluation after 2 cycles of neoadjuvant therapy. This decrease in QOL returns back to baseline levels among those who underwent successful surgery at >6 months following initiation of treatment. This is consistent with other pancreatic cancer studies that show a decline in QOL shortly after neoadjuvant treatment with a subsequent improvement or stabilization of QOL following therapy [22,25,29]. The results of this study provide unique data on QOL in the neoadjuvant setting that can be compared to other neoadjuvant regimens for patients with resectable or borderline resectable pancreatic cancer and can be used as a reference for future studies. Clinicians administering this neoadjuvant therapy regimen will find the results of this study useful for the clinical management of their patients and to determine the effect of therapy on patients’ QOL. Measurement of health-related QOL in patients with disease progression after neoadjuvant therapy and those who do not undergo surgical resection should be studied further in future trials.
Summary.
The effects of neoadjuvant chemoradiation on the quality of life of patients with pancreatic cancer are not well understood. This phase II trial of neoadjuvant chemoradiation with gemcitabine and oxaliplatin shows a trend towards decreased quality of life after 2 cycles of neoadjuvant therapy with a return to baseline in those who undergo subsequent curative resection. In summary, this neoadjuvant regimen can be delivered with good tolerability, without affecting the possibility of surgical resection.
Acknowledgements
This research is supported in part by the National Institutes of Health through the University of Michigan Cancer Center Support Grant (5 p30 CA46592) by use of the Cancer Center Clinical Trials Office and Biostatistics Core and in part by Sanofi.
Dr Zalupski received research support from Sanofi for his reported clinical trial. Dr Bekaii-Saab receives support from Sanofi as a consultant. Dr Wei has received honoraria from Sanofi.
This research is supported in part by Sanofi-Aventis
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: Other Conflicts of Interest: None
Presented in part at the American Society of Clinical Oncology Gastrointestinal Cancers Symposium; San Francisco, CA; 2013 and at the Society of Surgical Oncology 66th Annual Cancer Symposium; Washington DC; 2013.
References
- 1.Oettle H, Post S, Neuhaus P, Gellert K, Langrehr J, Ridwelski K, Schramm H, Fahlke J, Zuelke C, Burkart C, Gutberlet K, Kettner E, Schmalenberg H, Weigang-Koehler K, Bechstein WO, Niedergethmann M, Schmidt-Wolf I, Roll L, Doerken B, Riess H. Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: A randomized controlled trial. JAMA : the journal of the American Medical Association. 2007;297:267–277. doi: 10.1001/jama.297.3.267. [DOI] [PubMed] [Google Scholar]
- 2.Regine WF, Winter KA, Abrams RA, Safran H, Hoffman JP, Konski A, Benson AB, Macdonald JS, Kudrimoti MR, Fromm ML, Haddock MG, Schaefer P, Willett CG, Rich TA. Fluorouracil vs gemcitabine chemotherapy before and after fluorouracil-based chemoradiation following resection of pancreatic adenocarcinoma: A randomized controlled trial. JAMA : the journal of the American Medical Association. 2008;299:1019–1026. doi: 10.1001/jama.299.9.1019. [DOI] [PubMed] [Google Scholar]
- 3.Herman JM, Swartz MJ, Hsu CC, Winter J, Pawlik TM, Sugar E, Robinson R, Laheru DA, Jaffee E, Hruban RH, Campbell KA, Wolfgang CL, Asrari F, Donehower R, Hidalgo M, Diaz LA, Jr., Yeo C, Cameron JL, Schulick RD, Abrams R. Analysis of fluorouracil-based adjuvant chemotherapy and radiation after pancreaticoduodenectomy for ductal adenocarcinoma of the pancreas: Results of a large, prospectively collected database at the johns hopkins hospital. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008;26:3503–3510. doi: 10.1200/JCO.2007.15.8469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jemal A, Tiwari RC, Murray T, Ghafoor A, Samuels A, Ward E, Feuer EJ, Thun MJ. Cancer statistics, 2004. CA: a cancer journal for clinicians. 2004;54:8–29. doi: 10.3322/canjclin.54.1.8. [DOI] [PubMed] [Google Scholar]
- 5.Katz MH, Marsh R, Herman JM, Shi Q, Collison E, Venook AP, Kindler HL, Alberts SR, Philip P, Lowy AM, Pisters PW, Posner MC, Berlin JD, Ahmad SA. Borderline resectable pancreatic cancer: Need for standardization and methods for optimal clinical trial design. Annals of surgical oncology. 2013;20:2787–2795. doi: 10.1245/s10434-013-2886-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Katz MH, Pisters PW, Evans DB, Sun CC, Lee JE, Fleming JB, Vauthey JN, Abdalla EK, Crane CH, Wolff RA, Varadhachary GR, Hwang RF. Borderline resectable pancreatic cancer: The importance of this emerging stage of disease. Journal of the American College of Surgeons. 2008;206:833–846. doi: 10.1016/j.jamcollsurg.2007.12.020. discussion 846-838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Talamonti MS, Small W, Jr., Mulcahy MF, Wayne JD, Attaluri V, Colletti LM, Zalupski MM, Hoffman JP, Freedman GM, Kinsella TJ, Philip PA, McGinn CJ. A multi-institutional phase ii trial of preoperative full-dose gemcitabine and concurrent radiation for patients with potentially resectable pancreatic carcinoma. Annals of surgical oncology. 2006;13:150–158. doi: 10.1245/ASO.2006.03.039. [DOI] [PubMed] [Google Scholar]
- 8.Haddock MG, Swaminathan R, Foster NR, Hauge MD, Martenson JA, Camoriano JK, Stella PJ, Tenglin RC, Schaefer PL, Moore DF, Jr., Alberts SR. Gemcitabine, cisplatin, and radiotherapy for patients with locally advanced pancreatic adenocarcinoma: Results of the north central cancer treatment group phase ii study n9942. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2007;25:2567–2572. doi: 10.1200/JCO.2006.10.2111. [DOI] [PubMed] [Google Scholar]
- 9.Bernhard J, Dietrich D, Scheithauer W, Gerber D, Bodoky G, Ruhstaller T, Glimelius B, Bajetta E, Schuller J, Saletti P, Bauer J, Figer A, Pestalozzi BC, Kohne CH, Mingrone W, Stemmer SM, Tamas K, Kornek GV, Koeberle D, Herrmann R. Clinical benefit and quality of life in patients with advanced pancreatic cancer receiving gemcitabine plus capecitabine versus gemcitabine alone: A randomized multicenter phase iii clinical trial--sakk 44/00-cecog/pan.1.3.001. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008;26:3695–3701. doi: 10.1200/JCO.2007.15.6240. [DOI] [PubMed] [Google Scholar]
- 10.Kim EJ, Ben-Josef E, Herman JM, et al. A multi-institutional phase 2 study of neoadjuvant gemcitabine and oxaliplatin with radiation therapy in patients with pancreatic cancer. Cancer. 2013;119:2692–2700. doi: 10.1002/cncr.28117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Network NCC. Nccn practice guidelines for pancreatic cancer, version 2.2012. Book Nccn practice guidelines for pancreatic cancer, version 2.2012. 2012. Editor, editor^editors.
- 12.Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, Carbone PP. Toxicity and response criteria of the eastern cooperative oncology group. American journal of clinical oncology. 1982;5:649–655. [PubMed] [Google Scholar]
- 13.Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, Filiberti A, Flechtner H, Fleishman SB, de Haes JC, et al. The european organization for research and treatment of cancer qlq-c30: A quality-of-life instrument for use in international clinical trials in oncology. Journal of the National Cancer Institute. 1993;85:365–376. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 14.Groenvold M, Klee MC, Sprangers MA, Aaronson NK. Validation of the eortc qlqc30 quality of life questionnaire through combined qualitative and quantitative assessment of patient-observer agreement. Journal of clinical epidemiology. 1997;50:441–450. doi: 10.1016/s0895-4356(96)00428-3. [DOI] [PubMed] [Google Scholar]
- 15.Fitzsimmons D, Johnson CD, George S, Payne S, Sandberg AA, Bassi C, Beger HG, Birk D, Buchler MW, Dervenis C, Fernandez Cruz L, Friess H, Grahm AL, Jeekel J, Laugier R, Meyer D, Singer MW, Tihanyi T. Development of a disease specific quality of life (qol) questionnaire module to supplement the eortc core cancer qol questionnaire, the qlq-c30 in patients with pancreatic cancer. Eortc study group on quality of life. Eur J Cancer. 1999;35:939–941. doi: 10.1016/s0959-8049(99)00047-7. [DOI] [PubMed] [Google Scholar]
- 16.Heffernan N, Cella D, Webster K, Odom L, Martone M, Passik S, Bookbinder M, Fong Y, Jarnagin W, Blumgart L. Measuring health-related quality of life in patients with hepatobiliary cancers: The functional assessment of cancer therapy-hepatobiliary questionnaire. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2002;20:2229–2239. doi: 10.1200/JCO.2002.07.093. [DOI] [PubMed] [Google Scholar]
- 17.Cella DF, Tulsky DS, Gray G, Sarafian B, Linn E, Bonomi A, Silberman M, Yellen SB, Winicour P, Brannon J, et al. The functional assessment of cancer therapy scale: Development and validation of the general measure. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1993;11:570–579. doi: 10.1200/JCO.1993.11.3.570. [DOI] [PubMed] [Google Scholar]
- 18.Velanovich V. Using quality-of-life instruments to assess surgical outcomes. Surgery. 1999;126:1–4. doi: 10.1067/msy.1999.97994. [DOI] [PubMed] [Google Scholar]
- 19.Osoba D, Zee B. Completion rates in health-related quality-of-life assessment: Approach of the national cancer institute of canada clinical trials group. Statistics in medicine. 1998;17:603–612. doi: 10.1002/(sici)1097-0258(19980315/15)17:5/7<603::aid-sim807>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 20.Ringash J, O'Sullivan B, Bezjak A, Redelmeier DA. Interpreting clinically significant changes in patient-reported outcomes. Cancer. 2007;110:196–202. doi: 10.1002/cncr.22799. [DOI] [PubMed] [Google Scholar]
- 21.Osoba D, Rodrigues G, Myles J, Zee B, Pater J. Interpreting the significance of changes in health-related quality-of-life scores. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1998;16:139–144. doi: 10.1200/JCO.1998.16.1.139. [DOI] [PubMed] [Google Scholar]
- 22.Short M, Goldstein D, Halkett G, Reece W, Borg M, Zissiadis Y, Kneebone A, Spry N. Impact of gemcitabine chemotherapy and 3-dimensional conformal radiation therapy/5-fluorouracil on quality of life of patients managed for pancreatic cancer. International journal of radiation oncology, biology, physics. 2013;85:157–162. doi: 10.1016/j.ijrobp.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 23.Raut CP, Evans DB, Crane CH, Pisters PW, Wolff RA. Neoadjuvant therapy for resectable pancreatic cancer. Surgical oncology clinics of North America. 2004;13:639–661, ix. doi: 10.1016/j.soc.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 24.Heinrich S, Pestalozzi BC, Schafer M, Weber A, Bauerfeind P, Knuth A, Clavien PA. Prospective phase ii trial of neoadjuvant chemotherapy with gemcitabine and cisplatin for resectable adenocarcinoma of the pancreatic head. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008;26:2526–2531. doi: 10.1200/JCO.2007.15.5556. [DOI] [PubMed] [Google Scholar]
- 25.Conroy T, Desseigne F, Ychou M, Bouche O, Guimbaud R, Becouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, de la Fouchardiere C, Bennouna J, Bachet JB, Khemissa-Akouz F, Pere-Verge D, Delbaldo C, Assenat E, Chauffert B, Michel P, Montoto-Grillot C, Ducreux M, Groupe Tumeurs Digestives of U, Intergroup P Folfirinox versus gemcitabine for metastatic pancreatic cancer. The New England journal of medicine. 2011;364:1817–1825. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 26.Glimelius B, Hoffman K, Sjoden PO, Jacobsson G, Sellstrom H, Enander LK, Linne T, Svensson C. Chemotherapy improves survival and quality of life in advanced pancreatic and biliary cancer. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 1996;7:593–600. doi: 10.1093/oxfordjournals.annonc.a010676. [DOI] [PubMed] [Google Scholar]
- 27.Sadura A, Pater J, Osoba D, Levine M, Palmer M, Bennett K. Quality-of-life assessment: Patient compliance with questionnaire completion. Journal of the National Cancer Institute. 1992;84:1023–1026. doi: 10.1093/jnci/84.13.1023. [DOI] [PubMed] [Google Scholar]
- 28.Young T, Maher J. Collecting quality of life data in eortc clinical trials--what happens in practice? Psycho-oncology. 1999;8:260–263. doi: 10.1002/(SICI)1099-1611(199905/06)8:3<260::AID-PON383>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 29.Gourgou-Bourgade S, Bascoul-Mollevi C, Desseigne F, Ychou M, Bouche O, Guimbaud R, Becouarn Y, Adenis A, Raoul JL, Boige V, Berille J, Conroy T. Impact of folfirinox compared with gemcitabine on quality of life in patients with metastatic pancreatic cancer: Results from the prodige 4/accord 11 randomized trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2013;31:23–29. doi: 10.1200/JCO.2012.44.4869. [DOI] [PubMed] [Google Scholar]
- 30.Morak MJ, Pek CJ, Kompanje EJ, Hop WC, Kazemier G, van Eijck CH. Quality of life after adjuvant intra-arterial chemotherapy and radiotherapy versus surgery alone in resectable pancreatic and periampullary cancer: A prospective randomized controlled study. Cancer. 2010;116:830–836. doi: 10.1002/cncr.24809. [DOI] [PubMed] [Google Scholar]