Abstract
Serotonergic neurons have been implicated in a broad range of processes, but the principles underlying their effects remain a puzzle. Here, we ask how these neurons influence the tendency of larval zebrafish to swim in the light and avoid regions of darkness. Pharmacological inhibition of serotonin synthesis reduces dark avoidance, indicating an involvement of this neuromodulator. Calcium imaging of tph2-expressing cells demonstrates that a rostral subset of dorsal raphe serotonergic neurons fire continuously while the animal is in darkness, but are inhibited in the light. Optogenetic manipulation of tph2 neurons by channelrhodopsin or halorhodopsin expression modifies preference, confirming a role for these neurons. In particular, these results suggest that fish prefer swimming in conditions that elicits lower activity in tph2 serotonergic neurons in the rostral raphe.
Animals have an innate motivation to explore their surroundings, as this can help secure essential resources such as food. The pattern of exploration is shaped by characteristics of the environment, with one important feature being illumination. Animals as diverse as rodents1,2, adult fish3,4,5, fruit fly larvae6 and nematodes7 show a strong tendency to avoid regions that are brightly lit. Others, such as larval zebrafish8 and adult fruit flies9, prefer the light. How are these responses generated? One possibility is that light causes reflexive motor activity. In larval zebrafish, a decrease in illumination triggers turning10,11, while an increase in illumination causes forward swimming12,13. Such responses, together with other basic rules, can fully account for the ability of larval zebrafish to avoid regions of darkness12,13. However, light (or darkness for some animals) is not neutral but is innately aversive. This is suggested by observations that confining adult fish to a white compartment causes freezing14, and that rodents will learn an instrumental response to terminate exposure to light15. Moreover, anxiety, which increases the perception of threat16, affects preference in the light/dark assay. Drugs that reduce anxiety, such as diazepam, increase entry into regions that are normally avoided8,17,18, while anxiogenics increase avoidance. Thus, patterns of exploration in environments with uneven illumination reflect avoidance of an aversive stimulus.
The preference for light or darkness is further modified by factors such as time of day, level of arousal, age and even olfactory stimulation2,19,20,21,22, indicating that neural circuits driving the response are subject to considerable modulation23. One modulator that has been implicated is serotonin. Evidence for this is provided by observations that buspirone, a partial agonist of the 5HT1A receptor, decreases avoidance of the aversive zone in the light-dark assay8,22,24, as does serotonin depletion with para-chlorophenylalanine (pCPA)25. In vertebrates, serotonin is produced by discrete clusters of cells, most prominently in the midbrain raphe26. A long-standing theory is that serotonin is released from the raphe in response to aversive stimuli27,28. Because buspirone can inhibit raphe serotonergic neurons, where 5HT1A is a somatic autoreceptor29, the reduction of preference following buspirone treatment indicates that excitation of raphe serotonergic neurons drives avoidance. In the case of larval zebrafish, this means that darkness should cause an increase in serotonin release. However, increases in serotonin appears to be associated with increased forward swimming in the light, rather than turning away from the dark12. It has also been suggested that activation of the dorsal raphe encodes positive reward, rather than aversive stimuli, based on optogenetic stimulation experiments in mice30. Given these observations and the findings that pharmacological manipulations of serotonin signalling give conflicting results2,25 – e.g. an antagonist and agonist of 5HT1A both have the same effect in the light/dark assay25- the role of serotonergic neurons in controlling the preference for light versus darkness is unclear.
One approach to resolve how serotonergic neurons function in a particular behaviour is to record their activity while the animal is engaged in that behaviour, or, if this is not possible, then while the animal is exposed to sensory stimuli triggering that behaviour. In mammals, this has been done to a large extent with electrical recordings31, and responses to both aversive and rewarding stimuli were found32,33. However, in almost all cases, the identity of the recorded cells had not been independently characterized34. This raises the possibility of errors in interpretation, as the raphe contains a mixture of cell types35. Recently, by recording molecularly-defined cells in awake mice, the dorsal raphe nucleus was found to contain serotonergic neurons with diverse responses36. Phasic excitation was detected, in different cells, at the presentation of both appetitive and aversive cues. Tonic firing, which appears to reflect mood or brain state37, was also detected. Here, again, there was variability, with some neurons firing tonically when the animal has been repeatedly exposed to aversive stimuli while others fired tonically following repeated rewards. These observations emphasize the complexity of serotonergic neurons in vertebrates, even within a single nucleus.
Larval zebrafish have a serotonergic system that is simple and yet similar to other vertebrates in many respects38. A major advantage of the zebrafish larva as an experimental system is its amenability to optical recording of genetically defined neurons. Hence, the response of whole populations can be analysed. Here, by combining imaging of larval zebrafish with optogenetic manipulation, we provide evidence that dark-evoked excitation operates in conjunction with light-evoked inhibition of serotonergic neurons to influence the response to light and darkness.
Results
Pharmacological manipulation of serotonin synthesis affects preference
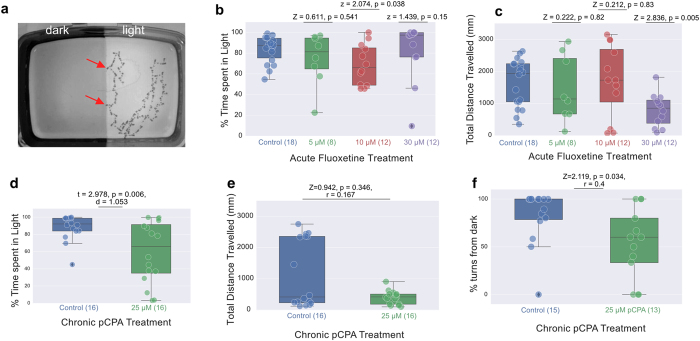
Larval zebrafish display a preference for swimming in light over darkness (Fig. 1a). An involvement of serotonin in larval dark avoidance has been previously suggested, based on the effects of buspirone8. As is the case in adults24, acute treatment with the selective serotonin reuptake inhibitor, fluoxetine, did not reduce preference in larvae (Kruskal-Wallis ANOVA test, p = 0.207, Fig. 1b) although the highest concentration (30 μM) significantly reduced the total distance traveled (Wilcoxon Rank Sum test, p = 0.005, Fig. 1c). Treatment with pCPA, which inhibits serotonin synthesis, increased the time spent in the dark compartment (independent t-test, p = 0.006, Fig. 1d); this is again similar to the effect seen in adult zebrafish25. Although there was no significant decrease in total distance traveled (Wilcoxon Rank Sum test, p = 0.346, Fig. 1e), a post-hoc visual analysis of swimming trajectory from video files indicated that pCPA-treated fish failed to turn away when they swim from the light towards darkness, in contrast to controls (Wilcoxon Rank Sum test, p = 0.034, Fig. 1f). Hence, a reduction of serotonin appears to reduce dark avoidance.
Figure 1. Effect of pharmacological manipulation on light preference.
(a) An example of larval zebrafish behaviour when given a choice between light and dark zones in a tank. The image shows the trace of 1 fish, over a period of 66.7 seconds. Traces were obtained by minimum projection of a t-stack consisting of recordings made at 12 frames/second under infrared incident illumination. The fish remained within the light zone. When it approached the border (arrows), it turned back into the light. (b, c) The effect of three different concentrations of fluoxetine on light preference, as shown by percentage of time spent in the light (b) and distance travelled (c). The highest concentration used caused a reduction in locomotion, but did not reduce preference for the light. (d,e) pCPA caused a reduction in percentage of time spent in the light (d) and in distance travelled (e). Fish that approached the dark turned away less frequently following exposure to pCPA (f). Fish that never approached the boundary between light and dark were excluded from turn frequency analysis. In panels b-f, box plots are drawn such that the box extends between the lower (25%) and upper (75%) quartiles of the data with a line at the median. The whiskers represent the distribution of the data. Each circle represents data from individual fish and circle with black diamonds show the outliers. For distributions of data not satisfying the normality test, p-value was obtained using the Wilcoxon Rank Sum Test (Z is the test statistic, r is the non parametric effect size, panels b,c,e,f) else an independent T-test (t is the t-statistic, d is Cohen’s d, panel d) was used. Statistical comparisons were always made between control fish and drug treated fish. Number of fish in each condition is provided within brackets on the x-axis.
A subpopulation of tph2 neurons in the dorsal raphe are inhibited by light and excited by darkness
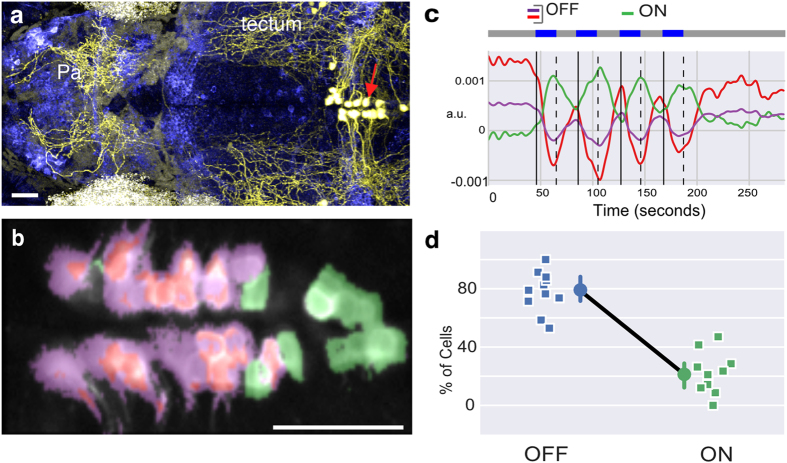
To directly assess whether serotonergic neurons in the dorsal raphe nucleus are affected by light and darkness, two-photon imaging was performed on transgenic zebrafish larvae in which the tryptophan hydroxylase 2 (tph2) promoter39 (Fig. 2a) was used to drive the genetically encoded calcium indicator GCaMP6s40. In the first experiment, fish were exposed to alternating periods of light and darkness (n = 11 fish, 244 cells). The majority of neurons (189 cells) displayed a drop in fluorescence during light ON (Fig. 2b–d), relative to the period of light OFF; these are termed the OFF neurons. The decrease in fluorescence persisted throughout the period of light, and was seen in all fish. A minority of cells (55 cells) displayed the opposite change, and these were located at the caudal end of the nucleus; these are the ON neurons. Tph2 is also expressed in the pretectum41,42, and these neurons showed both ON and OFF responses (Figure S1). Hence, either light or darkness can excite tph2 serotonergic neurons.
Figure 2. Response of dorsal raphe serotonergic neurons to light and darkness.
(a) Serotonergic neurons (red arrow) in the dorsal raphe of a 5 day-old zebrafish, as labelled by Kaede (coloured yellow) in a Tg(tph2:GAL4, UAS:Kaede) line. Axons extend broadly in the brain, which is pan-neurally labelled (blue). (b) Activity in the raphe of a 9 day-old Tg(tph2:GCaMP6s) fish, in response to four pulses of light. Pixels have the same color scheme as the activity profiles shown in panel c (see Methods). (c) Time series of three clusters obtained by running k-means clustering following principal component analysis (PCA) with two components on the data in panel (b). Blue light was delivered during the periods indicated by the blue bars. (d) Average percentage of tph2 neurons in the raphe excited (ON neurons) or inhibited (OFF neurons) by light (n = 11 fish; squares are individual fish, circles indicate mean and error bar indicates 95% c.i. around the mean). Anterior is to the left in panels (a,b) Pa: pallium; scale bar = 25 μm.
Shock-induced excitation in the raphe is inhibited by light
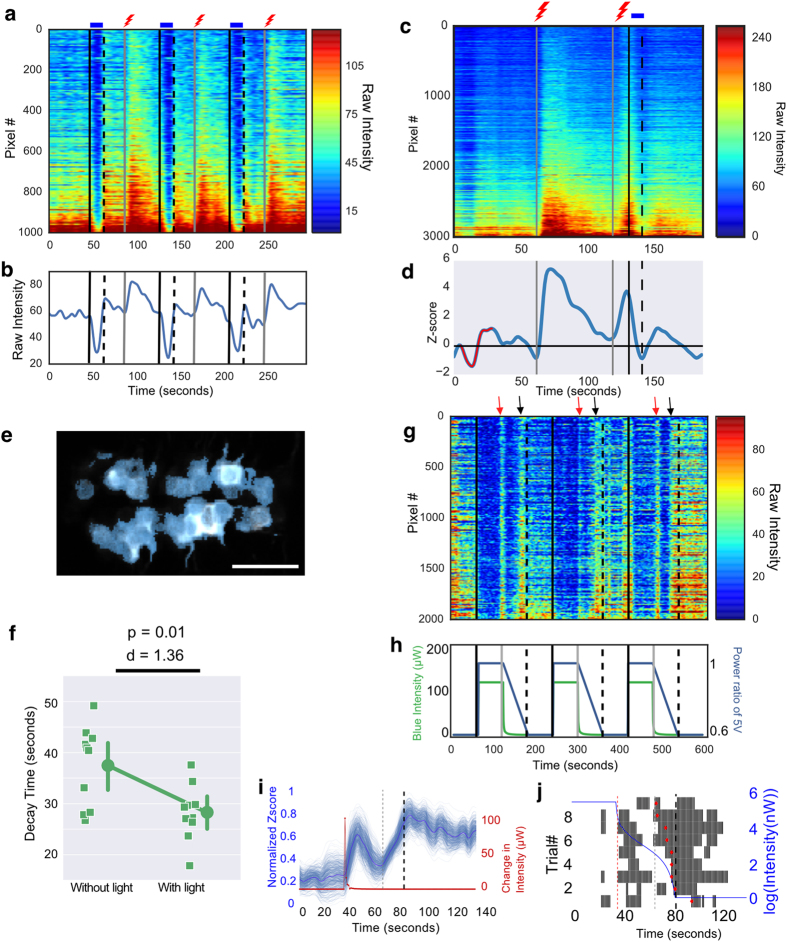
We tested how raphe neurons that are inhibited by light and excited by darkness (the OFF neurons) respond to a different stimulus, to determine whether excitation is specific to darkness. To do this, fish were exposed to a transient electric shock as well as alternating periods of light and dark. OFF neurons were excited by the shock (Fig. 3a,b; n = 13 fish; 308 cells), with shock-induced excitation lasting beyond the duration of the shock. A similar excitation was also seen in the caudal raphe, indicating that the excitation here may not be tied strictly to reward value, because both shock (an aversive stimulus) and light (which is not aversive) triggered excitation.
Figure 3. Response of dorsal raphe serotonergic neurons to shock and light.
(a,b) Pixel-wise (a) and averaged (b) response in one fish. Light caused a decrease in activity, whereas darkness and shock caused an increase. (c–f) Effect of a 10-second light pulse on shock-evoked activity. Two 1-second pulses of shock were given 60 seconds apart and light was delivered 10 seconds after the second shock. (c) The response in one fish. (d) A single activity related cluster obtained from k-means clustering of the normalized data. (e) Cells in the raphe corresponding to the cluster shown in (d). (f) Time for shock-evoked fluorescence to drop to the minimum value, with or without delivery of light, in 10 fish. Circles represent the mean. Error bars are 95% c.i. (g–j) Response to gradually decreasing light. (g) Neurons are inhibited in the light, and excited at the start of the decrease (red arrows), and close to darkness (black arrows). (h) Voltage applied to the LED (blue trace) and measured intensity (green trace). (i) Data compiled from three fish, each exposed to three trials (total of 9 trials). The red trace shows difference in intensity between two consecutive time points. The bold blue trace is the mean activity across 9 trials. The thinner lines represent resamples of the data obtained by 500 bootstrap iterations. There is a transient excitation following the large change in illumination. (j) Raster plots showing spike times obtained by temporal deconvolution of the mean calcium traces in each trial. The trials are sorted in ascending order of the half maxima. Black lines correspond to individual spikes of action potentials. The red diamonds indicate the half maxima of the second peak response to decreasing illumination. In 8/9 trials, the second response starts before the LED intensity turns 0. Red dashed line: time point with the large change in illumination. Grey dashed line: earliest half maxima. Bold black dashed line: time-point when the LED intensity was 0. p-value in (f) is based on Paired T-test. d is Cohen’s d. Scale bar = 25 μm.
The sustained excitation in dark-excited raphe neurons appeared to be inhibited by the delivery of blue light. This was confirmed by comparing the time for GCaMP6s fluorescence to reach a minimum after the shock, with or without the presence of light (Fig. 3c–f; n = 10 fish, 253 cells; Cohen’s d = 1.36, Paired T-test p = 0.01). Hence, an increase in irradiance can reduce tonic activity of serotonergic neurons following an aversive stimulus of a different modality. This indicates that the effect of light on raphe neurons is not simply a loss of dark-induced excitation, but is an active inhibition.
We next imaged fish that were exposed to a gradual decrease in illumination, by progressively reducing the voltage supply to the LEDs. This was primarily an attempt to mimic illumination changes that would accompany movement of the fish towards darkness in a light/dark assay. Two discrete excitation responses were detected, one correlating with the start of decrease in intensity, and the second towards the end (Fig. 3g; n = 3 fish, 66 cells). The presence of two responses may be caused by the fact that decrease in light intensity was unexpectedly non-linear in our experiment (Fig. 3h). This pattern of change indicates that a sharp change in luminance (decrease of 107 μW) as well as the absence of light below a threshold (average luminance of 0.107 ± 0.11 μW), which are both aversive stimuli43, can cause excitation in a population of dorsal raphe serotonergic neurons (Fig. 3i,j). Excitation to the sharp drop in luminance was transient (average duration of response: 10.22 ± 4.68 s, n = 3 fish), in contrast to the sustained excitation seen with shock (average duration of response: 30.09 ± 0.75 s, n = 10 fish).
Optogenetic manipulation of tph2 serotonergic neurons alters preference
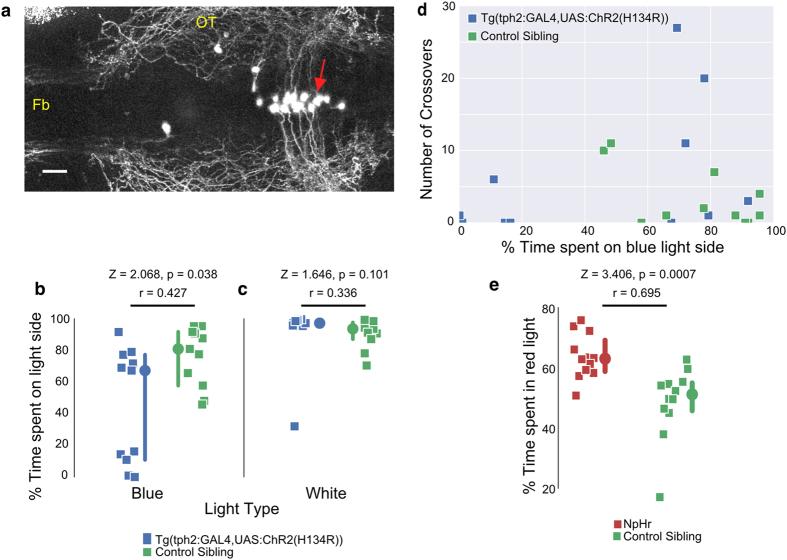
If avoidance of the dark is caused by an increase in the activity of raphe serotonergic neurons in darkness, then triggering activity in the light should induce avoidance of light. To test this, we expressed a variant of channelrhodopsin, ChR2 (H134R)44, using the tph2 driver. The presence of an eYFP tag enabled identification of fish and neurons carrying the transgene (Fig. 4a). Fish were placed in a chamber with a choice between blue light (20 W/mm2) and darkness. While some fish expressing ChR2 (H134R)-eYFP retained a preference for the blue light, a significant number displayed a preference for the dark (Fig. 4b). After the blue light/dark assay, all fish were immediately transferred to a normal white light/dark assay as a control and all fish stayed in the light (Fig. 4c). An analysis of the number of crossovers in the blue light experiment (Fig. 4d) indicates that there are three classes of behaviour – fish that stay in the dark, those that stay in the light and those that cross several times. Siblings not expressing ChR2 (H134R)-eYFP, in contrast, remained in the light and rarely crossed over.
Figure 4. Effect of optogenetic manipulation of tph2 neurons on preference.
(a) Dorsal view of a Tg(tph2:GAL4, UAS:ChR2(H124R)-eYFP) larva, with anterior to the left. Channelrhodopsin is expressed in the dorsal raphe (red arrow). The image is a maximal projection of optical sections covering 122 μm. (b,c) The percentage of time spent in the light side of the tank, with either blue (b) or white (c) light. All control sibling fish preferred the side with blue or white light. However, fish expressing ChR2 (H134R)-eYFP under the tph2 driver had a significant preference for the dark side when blue light was used. (d) The number of crossovers between the blue light and the dark side as a function of their percentage of time spent on the blue light side. (e) Preference of eNpHR3.0-expressing fish and their siblings for red over green (n = 12 each). There is a significant change towards red in fish expressing halorhodopsin (p = 0.0007). Fb: forebrain; OT: optic tectum; scale bar = 25 μm. Gamma = 0.65 was applied to panel (a). In panels c and e, each square represents one fish (n = 11 fish in each group). Circles are the median and error bar indicates 95% c.i. around the median. p-value is obtained from Wilcoxon Rank-sum test. Z is the test statistic; r is the non-parametric effect size.
The bimodal response seen with ChR2, i.e. the preference for either light or darkness, could be due to several reasons (see Discussion). One possibility is that excitation of a subset of serotonergic neurons is rewarding, while excitation of another subset signals punishment, with random expression driving greater activation in one of the subsets. In this scenario, inhibition of the first subset would be non-rewarding, while inhibition of the second is rewarding. Hence, optogenetic inhibition of tph2 neurons in a two-choice assay would be predicted to also give a bimodal distribution. To test this, we placed fish expressing halorhodopsin (the eNpHR3.0 variant45) in tph2 neurons, in a chamber where they could swim either in green or red light, which have differential effects on halorhodopsin. Control fish, which were siblings without halorhodopsin expression, spent equal time in both regions of the tank, whereas the eNpHR3.0 expressing fish showed a shift towards the red light (Fig. 4e). This uniform shift is inconsistent with a dual role for inhibition in preference.
Discussion
We have investigated the mechanism underlying the preference of larval zebrafish for light over darkness. Pharmacological manipulation with pCPA indicates that serotonin has a role in causing dark avoidance, consistent with the published effect of buspirone8. Activity imaging demonstrates that darkness excites a majority of tph2 serotonergic neurons within the raphe, a structure that has been implicated in response to aversive stimuli27, further supporting the possibility that release of serotonin is involved in dark avoidance. Moreover, expression of ChR2 under the tph2 driver, which would enable blue light to depolarize serotonergic neurons, yielded fish with reduced preference for blue light. Together, these results indicate that excitation of tph2 serotonergic neurons influences avoidance of darkness, potentially by increasing anxiety. However, not all tph2 serotonergic neurons are excited by darkness. Some of these neurons, which are located more caudally in the raphe, are inhibited by darkness and excited by light. The dorsal raphe of zebrafish, like those of other vertebrates, consists of two subnuclei - B6 and B7 - that are organized rostro-caudally and have distinct targets, inputs and co-transmitters41. The responses to light and darkness may thus be segregated by subnuclei, indicating that serotonergic neurons have more than one role in the preference for light.
Some evidence for this is provided by the bimodal response seen in fish expressing ChR2 in tph2 neurons: while many avoid the light, a number retain dark avoidance. This is unlikely to be due simply to lack of ChR2, as expression in the raphe was confirmed in all fish. The bimodal response may reflect the fact that additional factors, such as internal state, act via other pathways to over-ride the effects of serotonin in some fish. Alternatively, where the fish swims may depend on the relative level of serotonergic neuron activation under different stimuli, with there being a winner-take-all outcome. For example, in some fish, stronger depolarization of dark-excited neurons by blue light relative to darkness, due to higher levels of ChR2 expression, may cause avoidance of the light; in others, stronger activation of these neurons by darkness may lead to avoidance of the dark.
Another possibility is that excitation in some raphe neurons is rewarding30,46,47. It might be argued that a stochastically higher level of expression in one subset of neurons biases preference. However, photoinhibition by expression of eNpHR3.0 with the same tph2 driver did not lead to a bimodal outcome in a green/red choice assay. Thus, the assumption that some raphe neurons encode for reward and others encode for punishment was not supported by our data because that assumption will predict a bimodal result in both photostimulation (ChR2) and photoinhibition (eNpHR3.0) experiments.
Excitation of dorsal raphe neurons has been linked to arousal39. We suggest that the excitation seen in the caudal raphe, which occurs in the light, may be involved in this phenomenon. Darkness does not appear to trigger arousal, as arousal is associated with prolonged excitation of the raphe39, and sudden darkness only causes transient excitation (Fig. 3g–j). Moreover, extended darkness, in contrast to light, does not cause increased locomotion11, which is one feature of arousal. It is known that the level of aversion to darkness is dependent on the contrast between the light and dark zone8. Activity in dorsal raphe increases perception of a light/dark boundary during arousal39. Hence, the ChR2-expressing fish may have a heightened avoidance of the dark because of increased visual sensitivity. This, coupled with the aversive effect of excitation of the anterior raphe by blue light, could generate the bimodal response.
We have found that light inhibits the excitation of raphe serotonergic neurons triggered by a shock. This suggests that light can actively inhibit activity induced by an aversive stimulus, and may thus have some rewarding properties. This would be consistent with previous experiments in rodents demonstrating the rewarding effects of intra-cranial self-administration of the GABAA agonist muscimol48 or of microinjection of the 5HT-1A agonist 8-OH-DPAT49 directly into the dorsal raphe nucleus. A rewarding effect of light in the light/dark preference assay may not have been detected previously12, as reward value in this case appears to be in the form of relief from aversion; light would not trigger approach in the absence of an aversive stimulus. The inhibitory effect of light may also explain null effect of acute fluoxetine treatment, which blocks serotonin reuptake after it is released at the synapse. If the neurons controlling aversion are already inhibited by light, this compound cannot further increase the level of serotonin.
In conclusion, the data here indicate that both excitation and inhibition of tph2 serotonergic neurons, most likely in the raphe, influence the preference of larval zebrafish for light over darkness. Potential targets of these neurons are regions of the forebrain that appear to be involved in this behaviour22. These data also support the picture that raphe serotonergic neurons are multifunctional34,36. We propose that the zebrafish can provide one experimental model to further investigate the principles of serotonin effects on vertebrate brain circuits, as it is a relatively simple system that enables a combination of behaviour and activity imaging of defined cells across the entire brain. To probe the role of neurons with distinct response characteristics, however, it will be necessary to use finer-grain drivers. While serotonergic neuron-specific drivers, such as the tph2:GAL4 driver used here, are better than wholesale activation of a particular region or pharmacological manipulation of whole tissues, the complexity of response suggests that manipulation needs to be carried out at the resolution of subnuclei or response types. Intersectional strategies may be useful in this respect, and will help clarify the effects of different levels of activity in different functional groups. This will enable the formulation and testing of theories that more fully reflect the complexity of serotonin function in the vertebrate brain.
Methods
Zebrafish lines
Experiments were carried out in accordance with protocols approved by the Institutional Animal Care and Use Committee of Biopolis. Zebrafish (Danio rerio) lines used for this study were: Tg (UAS:GCaMP6s)sq202, Tg (tph2:GAL4, UAS:Kaede)y22839, Tg (UAS:ChR2 (H134R)-eYFP)s1990t44, Tg (UAS:eNpHR3.0-mCherry)s1987t44 and AB wildtype.
GCaMP6s40 (Addgene plasmid 40753) was cloned downstream of UAS in a Tol2 vector, using the Gateway system, to create Tg(UAS:GCaMP6s)sq202.
Imaging was carried out on larvae in a nacre−/− background50. Animals were housed in a facility with lights on between 8 am and 10 pm. Larvae were grown in batches of 25–30, in 500 ml water from age 4 dpf. Animals were sorted on the basis of fluorescence for growing up, and selected at random for imaging.
Drug treatments
Fluoxetine
Fluoxetine hydrochloride (Sigma-Aldrich F132) was prepared as a 5 mM stock in E3 fish water (5 mM NaCl, 0.17 mM KCl, 0.33 mM CaCl2, 0.33 mM MgSO4). Acute treatment was carried out using 3 concentrations (5, 10 and 30 μM), based on previous reports using bath application in larval zebrafish12,51. At 14 dpf, larval zebrafish were put in the test tank for 10 minutes for habituation. The drug was then pipetted into the tank for another 10 minutes of acclimatization. After that, the fish were subject to the light/dark assay for another 10 minutes to observe acute drug effects. Controls were siblings from the same batch of experimental fish, which were treated in the same timeline except that the drug treatment was omitted.
pCPA
The tryptophan hydroxylase inhibitor pCPA (4-Chloro-DL-phenylalanine methyl ester hydrochloride; Sigma-Aldrich C3635) was dissolved in E3 fish water to make a 10 mM stock solution. Chronic treatment began by exposing 13 dpf larval zebrafish, in a group of 8–12 fish per dish, to 25 μM pCPA52. The light/dark test began 24 hours later, first with 10 minutes of acclimatisation in the test tanks. Fish were maintained in E3 with 25 μM pCPA throughout the experiment. Controls, which were siblings of the drug treated fish, were exposed to E3 fish water for 24 hours and tested in E3.
Calcium imaging
Zebrafish larvae (aged 5–11 dpf) were anaesthetized in mivacurium and embedded in low-melting temperature agarose (1.2–2.0% in E3) in a glass-bottom dish (Mat Tek). They were imaged on a Nikon two-photon microscope (A1RMP), attached to a fixed stage upright microscope, using a 25x water immersion objective (NA = 1.1). The femtosecond laser (Coherent Vision II) was tuned to 920 nm for GCaMP6s imaging. Images were collected in galvano-scanning mode with a 525/50 nm bandpass emission filter at 1 Hz. Fish were imaged during the day only, between 10 am and 7 pm. The facility lights were on from 8 am to 10 pm.
Light pulses were generated by a TMS Lite LED box with the peak wavelength at 470 nm. This was powered by a 24V adapter and controlled by TTL signals synchronized with image capture using a National Instruments DAQ board, all controlled by Nikon Elements software. A low-pass filter (450 nm “Tokyo blue 071”, Lee Filter) was used to reduce bleed-through of the light to the detector. Light intensity at the sample was 20 mW/mm2. To create blue light with gradually decreasing intensity, a DS1804 digital potentiometer was used to control a pair of 5 mm blue LEDs powered and controlled by an Arduino board. Light intensity was measured using the PM100A meter and S120VC sensor (Thorlabs).
The electric field stimulus was created by placing electric wires below the water surface. The wires were 4 cm apart oriented parallel to the anterior-posterior axis of the fish and connected to a Grass SD9 Stimulator providing 50 V, thus creating a 12.5 V/cm electric field. The electric field was applied for 1 second at 50 Hz.
Data analysis
Image Processing
Raw images from each z-plane were first sent through an x-y registration protocol to correct for horizontal/vertical shift after the first image by using normxcorr2 in Matlab (Mathworks). Where necessary, a median filter was applied to reduce noise and data was normalised using 25 seconds before light onset as a baseline (red trace shown in Fig. 3d). Imaging data was analysed using custom scripts in Python and plots were created using matplotlib and seaborn.
Data Analysis
Raw data in Fig. 3a,c,g were plotted by extracting pixels with high standard deviations corresponding to the raphe (around 1000–3000 pixels) and sorting them by their average response. To provide a spatial representation of activity, the Thunder algorithm53 was used. For Fig. 2b,c, PCA was performed to reduce noise and obtain a low dimensional spatial representation of pixels with similar temporal profiles. PCA components representing light evoked activity patterns that captured over 70% of the variance were used to reconstruct the data to a reduced dimension. K-means clustering was performed following PCA to identify signals with similar response profiles. For Fig. 3d and Figure S1 which had reduced noise, K-means was directly performed on the normalised data. K-means clustering partitions the n pixels into k clusters where the n pixels belong to a cluster with the closest mean. Each pixel in the raw data could then be grouped into the k different clusters it belongs to and differently coloured. This is shown in Figs 2b and 3e. With this, individual cells can be seen clearer and can be manually segmented/counted for response properties if necessary (for example in Fig. 2d).
Decay time in Fig. 3f was calculated by measuring the time taken for shock evoked activity to reach a global minimum over 50 seconds after shock delivery.
Analysing response to intensity change
To understand effects of intensity changes, fish were kept in 60 seconds of darkness before switching on the LED which remained at high intensity for 60 seconds after which the LED sharply decreased by 107 μW at 61 seconds and then gradually decreased to total darkness (0 W power) over the next 60 seconds. This is plotted in Fig. 3h. Three such trials were done on three different fish. The nine trials were then aligned to 20 second after light onset and 50 second after the LED’s power went to zero. To find if there is a threshold of light intensity below which the raphe neurons are activated in the intensity gradient experiment, mean calcium traces from each trial were converted to firing rates by temporal deconvolution54. Since calcium signals do not have a sharp rise and decay time, the estimated firing rates provided an easier way to calculate threshold. The firing rates were converted to spikes if they were 2 standard deviations above the mean and spike times were plotted as a raster plot in Fig. 3j. The peak firing rate after the LED went to 0W was calculated and the half maxima was defined as the time point that is 50% of the peak response.
Statistics
Prior to performing statistical comparisons, the Shapiro-Wilk test was used to confirm the null hypothesis that the data follows a normal distribution. If the null hypothesis was rejected, a non parametric Wilcoxon Rank Sum test was used and the median ±95% confidence interval around the median of the distributions were reported. If the distribution were normal, a Paired or independent student t-test was used and the mean ±95% confidence interval around the mean were reported. Effect sizes were reported when the sample sizes were comparable. When data was normally distributed, Cohen’s d was used. For non-normal distributions, a non parametric effect size was calculated as r = z/√N where z is the test statistic obtained from Wilcoxon Rank sum test and N is the total number of samples. For drug experiments when multiple concentrations were used (i.e. fluoxetine treatment), a one-way ANOVA on ranks (Kurskal-Wallis rank test) was used to determine treatment effect.
Optogenetic manipulation
Previously validated lines44 carrying the H134R variant55 or eNpHR3.0 variant45 fused to eYFP and mCherry respectively were used. Fish were sorted with a fluorescence stereomicroscope prior to behavioural testing. To activate ChR2 (H134R)-eYFP, a LED blue light box was used with intensity measured at 20 mW/mm2. The light was filtered by a low-pass (450 nm) filter (Tokyo Blue 071 from LEE Filter). For the light choice experiment, green (525 nm) and red (660 nm) light boxes (TMS Lite) were used, with intensity at 50 mW/mm2. Expression of ChR2 (H134R) and eNpHR3.0 was confirmed by imaging for the YFP or mCherry tag after behavioural testing using two-photon microscopy. All fish had expression in the dorsal raphe; expression in other regions, such as the pretectum, was not reproducible and did not correlate with behaviour.
Light/dark assay
The assay was conducted in transparent plastic tanks (dimensions: 43 mm width × 60 mm length × 30 mm height) filled with 50 mL of embryo water (840 mS) in a dark procedure room. To create the dark component, half of tank was covered by black 3M Vinyl tape on the exterior surface. Four such tanks were monitored simultaneously by a Logitech webcam (Model# Pro 9000) from above in a resolution of 800 × 600 pixels. To prevent social influence among fish, a black cardboard was placed between the tanks. To prevent influence from the experimenter, a cardboard box was used to isolate the entire set up such that the fish could not see the experimenter or the computer screen. To create the light compartment, white light was provided from below the tank by an Apple iPad Air in the strongest brightness level.
Real-time tracking of the fish location was achieved by custom-written Python codes using the opencv library. The recording lasted 10,000 frames with a speed of 16 fps, which equals to about 10.5 minutes. After the experiment ended, the x-y coordinates of each fish in each frame were exported to a Microsoft Excel file for further processing. When fish entered the dark side of the tank, the tracking algorithm would lose the fish. In such frames, 0-0 (x-y coordinates) was written to the computer memory. In the Excel sheet, a custom-written macro calculated the number of frames with 0-0 and the number of frames with non-zero x-y coordinates. Both numbers are divided by 10,000, thus representing the percentage of time the fish spent in the dark side and the light side.
The number of crossovers was calculated as the total number of frames where the fish swam from the light side to the dark side and stayed for at least 1 second. Frequency of turning away from the dark side was calculated by first determining the number of approaches to the light/dark boundary. This was defined as movement to within two body lengths (about 10 mm) of the boundary, in a trajectory that was close to perpendicular to the boundary. The fish was considered to turn if it changed direction by 90 degrees or more, while within the two body-length area in the light area or while within one body length into the dark side. The total number of turns divided by the total number of approaches is represented as percentage of turns from dark in Fig. 1f. A few fish were discarded from this analysis because they never moved to the boundary area during the experiment.
These automated (except the analysis of turn away from the dark side) analyses were applied equally to all fish. No animals were excluded. The sample size required was based on pilot studies using 40 wildtype fish from 4 different batches to establish the robustness of the assay, and a pilot study with ChR2 expressing fish. 3 independent batches of ChR2 fish were tested. The light choice assay was carried out on 2 independent batches of eNpHR3.0 fish, and sample size was based on a pilot study with a separate batch.
Additional Information
How to cite this article: Cheng, R.-K. et al. Activation and inhibition of tph2 serotonergic neurons operate in tandem to influence larval zebrafish preference for light over darkness. Sci. Rep. 6, 20788; doi: 10.1038/srep20788 (2016).
Supplementary Material
Acknowledgments
We thank Caroline Kibat for generation of the Tg(tph2:GCaMP6s) line and Michelle Kee for the image of wild type larvae in the light/dark assay (Figure 1a). We also thank Claire Wyart for providing transgenic lines for optogenetic manipulation, and Ajay Mathuru and Roger Herikstad for discussion.
Footnotes
Author Contributions R.K.C. and S.J. designed experiments. R.K.C. performed two-photon imaging, and designed and performed the light/dark assay. S.K. carried out data analysis. S.J. wrote the manuscript. All authors reviewed the manuscript.
References
- Campbell B. A. & Messing R. B. Aversion thresholds and aversion difference limens for white light in albino and hooded rats. Journal of experimental psychology 82, 353–359 (1969). [Google Scholar]
- Bourin M. & Hascöet M. The mouse light/dark box test. Eur J Pharmacol 463, 55–65 (2003). [DOI] [PubMed] [Google Scholar]
- Thorndike E. A note on the psychology of fishes. American Naturalist 33, 923–925 (1899). [Google Scholar]
- Maximino C. et al. A comparative analysis of the preference for dark environments in five teleosts. International Journal of Comparative Psychology 20, 351–367 (2007). [Google Scholar]
- Serra E. L., Medalha C. C. & Mattioli R. Natural preference of zebrafish (Danio rerio) for a dark environment. Braz. J. Med. Biol. Res. 32, 1551–1553 (1999). [DOI] [PubMed] [Google Scholar]
- Xiang Y. et al. Light-avoidance-mediating photoreceptors tile the Drosophila larval body wall. Nature 468, 921–926 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward A., Liu J., Feng Z. & Xu X. Z. S. Light-sensitive neurons and channels mediate phototaxis in C. elegans. Nat Neurosci 11, 916–922 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steenbergen P. J., Richardson M. K. & Champagne D. L. Patterns of avoidance behaviours in the light/dark preference test in young juvenile zebrafish: a pharmacological study. Behav Brain Res 222, 15–25 (2011). [DOI] [PubMed] [Google Scholar]
- Gong Z. et al. Two pairs of neurons in the central brain control Drosophila innate light preference. Science 330, 499–502 (2010). [DOI] [PubMed] [Google Scholar]
- Huang K.-H., Ahrens M. B., Dunn T. W. & Engert F. Spinal projection neurons control turning behaviors in zebrafish. Curr Biol 23, 1566–1573 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess H. A. & Granato M. Modulation of locomotor activity in larval zebrafish during light adaptation. J Exp Biol 210, 2526–2539 (2007). [DOI] [PubMed] [Google Scholar]
- Burgess H. A., Schoch H. & Granato M. Distinct retinal pathways drive spatial orientation behaviors in zebrafish navigation. Curr Biol 20, 381–386 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X. & Engert F. Navigational strategies underlying phototaxis in larval zebrafish. Front Syst Neurosci 8, 10.3389/fnsys.2014.00039 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaser R. E., Chadwick L. & McGinnis G. C. Behavioral measures of anxiety in zebrafish (Danio rerio). Behav Brain Res 208, 56–62 (2010). [DOI] [PubMed] [Google Scholar]
- Keller F. S. Light-aversion in the white rat. The Psychological Record 4, 235–250 (1941). [Google Scholar]
- Bishop S. J. Neurocognitive mechanisms of anxiety: an integrative account. Trends Cogn Sci (Regul Ed) 11, 307–316 (2007). [DOI] [PubMed] [Google Scholar]
- Crawley J. & Goodwin F. K. Preliminary report of a simple animal behavior model for the anxiolytic effects of benzodiazepines. Pharmacol Biochem Behav 13, 167–170 (1980). [DOI] [PubMed] [Google Scholar]
- Gebauer D. L. et al. Effects of anxiolytics in zebrafish: similarities and differences between benzodiazepines, buspirone and ethanol. Pharmacol Biochem Behav 99, 480–486 (2011). [DOI] [PubMed] [Google Scholar]
- Maximino C., de Brito T. M., Dias C. A. G. de M., Gouveia A. & Morato S. Scototaxis as anxiety-like behavior in fish. Nature protocols 5, 209–216 (2010). [DOI] [PubMed] [Google Scholar]
- Weil Z. M., Zhang Q., Hornung A., Blizard D. & Pfaff D. W. Impact of generalized brain arousal on sexual behavior. Proceedings of the National Academy of Sciences 107, 2265–2270 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson J. F., Whitlock K. E. & Partridge J. C. Zebrafish Preference for Light or Dark Is Dependent on Ambient Light Levels and Olfactory Stimulation. Zebrafish 8, 17–22 (2011). [DOI] [PubMed] [Google Scholar]
- Lau B. Y. B., Mathur P., Gould G. G. & Guo S. Identification of a brain center whose activity discriminates a choice behavior in zebrafish. Proc Natl Acad Sci USA 108, 2581–2586 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marder E. Neuromodulation of neuronal circuits: back to the future. Neuron 76, 1–11 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maximino C., da Silva A. W. B., Gouveia A. Jr. & Herculano A. M. Pharmacological analysis of zebrafish (Danio rerio) scototaxis. Prog Neuropsychopharmacol Biol Psychiatry 35, 624–631 (2011). [DOI] [PubMed] [Google Scholar]
- Maximino C. et al. Role of serotonin in zebrafish (Danio rerio) anxiety: relationship with serotonin levels and effect of buspirone, WAY 100635, SB 224289, fluoxetine and para-chlorophenylalanine (pCPA) in two behavioral models. Neuropharmacology 71, 83–97 (2013). [DOI] [PubMed] [Google Scholar]
- Jacobs B. L. & Azmitia E. C. Structure and function of the brain serotonin system. Physiol. Rev. 72, 165–229 (1992). [DOI] [PubMed] [Google Scholar]
- Deakin J. & Graeff F. 5-HT and mechanisms of defence. J Psychopharmacol 5, 305–315 (1991). [DOI] [PubMed] [Google Scholar]
- Dayan P. & Huys Q. J. M. Serotonin in affective control. Annual Review of Neuroscience 32, 95–126 (2009). [DOI] [PubMed] [Google Scholar]
- Norton W. H. J., Folchert A. & Bally-Cuif L. Comparative analysis of serotonin receptor (HTR1A/HTR1B families) and transporter (slc6a4a/b) gene expression in the zebrafish brain. J Comp Neurol 511, 521–542 (2008). [DOI] [PubMed] [Google Scholar]
- Liu Z. et al. Dorsal raphe neurons signal reward through 5-HT and glutamate. Neuron 81, 1360–1374 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranade S. P. & Mainen Z. F. Transient Firing of Dorsal Raphe Neurons Encodes Diverse and Specific Sensory, Motor, and Reward Events. J Neurophysiol 102, 3026–3037 (2009). [DOI] [PubMed] [Google Scholar]
- Nakamura K., Matsumoto M. & Hikosaka O. Reward-dependent modulation of neuronal activity in the primate dorsal raphe nucleus. J Neurosci 28, 5331–5343 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromberg-Martin E. S., Hikosaka O. & Nakamura K. Coding of task reward value in the dorsal raphe nucleus. J Neurosci 30, 6262–6272 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayan P. & Huys Q. Serotonin’s many meanings elude simple theories. Elife 4, 10.7554/eLife.07390 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissbourd B. et al. Presynaptic partners of dorsal raphe serotonergic and GABAergic neurons. Neuron 83, 645–662 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. Y., Amoroso M. W. & Uchida N. Serotonergic neurons signal reward and punishment on multiple timescales. Elife 4, 10.7554/eLife.06346 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi K., Nakao K. & Nakamura K. Appetitive and aversive information coding in the primate dorsal raphé nucleus. J Neurosci 35, 6195–6208 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillesaar C. The serotonergic system in fish. J Chem Neuroanat 41, 294–308 (2011). [DOI] [PubMed] [Google Scholar]
- Yokogawa T., Hannan M. C. & Burgess H. A. The dorsal raphe modulates sensory responsiveness during arousal in zebrafish. J Neurosci 32, 15205–15215 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T.-W. et al. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature 499, 295–300 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaspar P. & Lillesaar C. Probing the diversity of serotonin neurons. Philos Trans R Soc Lond, B, Biol Sci 367, 2382–2394 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillesaar C., Tannhäuser B., Stigloher C., Kremmer E. & Bally-Cuif L. The serotonergic phenotype is acquired by converging genetic mechanisms within the zebrafish central nervous system. Dev Dyn 236, 1072–1084 (2007). [DOI] [PubMed] [Google Scholar]
- Schnörr S. J., Steenbergen P. J., Richardson M. K. & Champagne D. L. Measuring thigmotaxis in larval zebrafish. Behav Brain Res 228, 367–374 (2012). [DOI] [PubMed] [Google Scholar]
- Arrenberg A. B., Del Bene F. & Baier H. Optical control of zebrafish behavior with halorhodopsin. Proceedings of the National Academy of Sciences 106, 17968–17973 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gradinaru V. et al. Molecular and Cellular Approaches for Diversifying and Extending Optogenetics. Cell 141, 154–165 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J., Jia C., Feng Q., Bao J. & Luo M. Prospective coding of dorsal raphe reward signals by the orbitofrontal cortex. J Neurosci 35, 2717–2730 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki K. W. et al. Optogenetic Activation of Dorsal Raphe Serotonin Neurons Enhances Patience for Future Rewards. Current Biology 24, 2033–2040 (2014). [DOI] [PubMed] [Google Scholar]
- Liu Z.-H. & Ikemoto S. The midbrain raphe nuclei mediate primary reinforcement via GABA(A) receptors. Eur J Neurosci 25, 735–743 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher P. J., Ming Z. H. & Higgins G. A. Conditioned place preference induced by microinjection of 8-OH-DPAT into the dorsal or median raphe nucleus. Psychopharmacology (Berl.) 113, 31–36 (1993). [DOI] [PubMed] [Google Scholar]
- Lister J. A., Robertson C. P., Lepage T., Johnson S. L. & Raible D. W. nacre encodes a zebrafish microphthalmia-related protein that regulates neural-crest-derived pigment cell fate. Development 126, 3757–3767 (1999). [DOI] [PubMed] [Google Scholar]
- Airhart M. J. et al. Movement disorders and neurochemical changes in zebrafish larvae after bath exposure to fluoxetine (PROZAC). Neurotoxicology and teratology 29, 652–664 (2007). [DOI] [PubMed] [Google Scholar]
- Airhart M. J. et al. Adverse effects of serotonin depletion in developing zebrafish. Neurotoxicology and teratology 34, 152–160 (2012). [DOI] [PubMed] [Google Scholar]
- Freeman J. et al. Mapping brain activity at scale with cluster computing. Nat Methods 11, 941–950 (2014). [DOI] [PubMed] [Google Scholar]
- Vogelstein J. T. et al. Spike inference from calcium imaging using sequential Monte Carlo methods. Biophys J 97, 636–655 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel G. et al. Light activation of channelrhodopsin-2 in excitable cells of Caenorhabditis elegans triggers rapid behavioral responses. Curr Biol 15, 2279–2284 (2005). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.