Abstract
In most forms of glomerular diseases, loss of size selectivity by the kidney filtration barrier is associated with changes in the morphology of podocytes. The kidney filtration barrier is comprised of the endothelial lining, the glomerular basement membrane, and the podocyte intercellular junction, or slit diaphragm. The cell adhesion proteins nephrin and neph1 localize to the slit diaphragm and transduce signals in a Src family kinase Fyn-mediated tyrosine phosphorylation-dependent manner. Studies in cell culture suggest nephrin phosphorylation-dependent signaling events are primarily involved in regulation of actin dynamics and lamellipodium formation. Nephrin phosphorylation is a proximal event that occurs both during development and following podocyte injury. We hypothesized that abrogation of nephrin phosphorylation following injury would prevent nephrin-dependent actin remodeling and foot process morphological changes. Utilizing a biased screening approach, we found nonreceptor Src homology 2 (sh2) domain-containing phosphatase Shp2 to be associated with phosphorylated nephrin. We observed an increase in nephrin tyrosine phosphorylation in the presence of Shp2 in cell culture studies. In the human glomerulopathies minimal-change nephrosis and membranous nephropathy, there is an increase in Shp2 phosphorylation, a marker of increased Shp2 activity. Mouse podocytes lacking Shp2 do not develop foot process spreading when subjected to podocyte injury in vivo using protamine sulfate or nephrotoxic serum (NTS). In the NTS model, we observed a lack of foot process spreading in mouse podocytes with Shp2 deleted and smaller amounts of proteinuria. Taken together, these results suggest that Shp2-dependent signaling events are necessary for changes in foot process structure and function following injury.
INTRODUCTION
Podocytes are highly differentiated epithelial cells with membrane extensions that arborize over the basement membrane in a highly polarized manner. The terminal branches of these actin-rich membrane extensions, called foot processes, interdigitate with each other, forming specialized intercellular junctions called slit diaphragms. Podocytes undergo flattening of the foot processes, or effacement, in most forms of glomerular diseases that present with protein leaks in the urine. Foot process effacement correlates with failure of the filtration barrier and development of proteinuria in both human diseases and animal models of podocyte dysfunction. The strong correlation between foot process morphological changes and failure of the filtration barrier suggests that prevention or reversal of effacement would be beneficial.
Nephrin is a transmembrane protein of the immunoglobulin superfamily that is located at the slit diaphragm (1). Nephrin's ability to regulate actin dynamics in a phosphorylation-dependent manner has been demonstrated by us and other investigators (2–6). A critical role for nephrin is suggested by the lack of normal foot process development in mice lacking nephrin or humans born with nephrin mutations (7, 8). In vitro studies have shown that engagement of the nephrin extracellular domain results in Src family kinase Fyn-dependent tyrosine phosphorylation of the nephrin cytoplasmic domain (6, 9, 10). Phosphorylated nephrin then recruits the Src homology 2 (sh2) domain-containing proteins Nck1/Nck2, the p85 subunit of phosphatidylinositol 3-kinase (PI3K), and Crk (3–6, 11) and other components of the actin polymerization complex (2, 3, 5, 12, 13). Mice with the Src family kinase Fyn deleted develop proteinuria and foot process defects that are evident at 7 weeks of age (10, 14). Mice with Fyn and Yes simultaneously deleted demonstrated a more severe phenotype than those with Fyn deletion alone (10).
Beyond development, the role of nephrin in podocyte homeostasis is not well understood. In vitro studies have demonstrated increased podocyte migration following activation of nephrin (5). Podocyte injury models using puromycin aminonucleoside and protamine sulfate show an increase in nephrin tyrosine phosphorylation (2, 3, 6), suggesting a related role of nephrin-mediated signaling in podocyte injury/repair. Careful evaluation of the state of nephrin phosphorylation in health and disease has been limited by the lack of availability of phosphospecific antibodies for nephrin. A major obstacle in investigating the relevance of nephrin phosphorylation following injury has been our lack of understanding of the molecular mechanisms that regulate nephrin phosphorylation itself.
Here, we present data showing that the nonreceptor tyrosine phosphatase Shp2 associates with nephrin in a phosphorylation-dependent manner. Shp2, encoded by the gene PTPN11, is highly conserved and is expressed ubiquitously. The structurally related phosphatase Shp1, encoded by the gene PTPN6, though primarily reported to be present in hematopoietic cells, was recently demonstrated to also be expressed in podocytes (15, 16). Shp2 has two sh2 domains, a catalytic domain, and a C terminus containing two regulatory tyrosine sites, Y542 and Y580 (17, 18). Shp2 is essential for development, as Shp2 mutation or deletion in mice results in embryonic lethality (19, 20). Shp2 plays a vital role in the regulation of Src kinase activity by regulating the protein complex associated with Src kinase activation (21–24). In most receptor tyrosine kinase signaling events, Shp2 is required for full activation of the signaling cascade (18, 25–27).
Given the role of Shp2 in the Src family kinase Fyn and activation and our finding that Shp2 interacts with nephrin, we hypothesized that Shp2 acts proximally to nephrin tyrosine phosphorylation and might be necessary for regulating nephrin-dependent actin dynamics. We observed an increase in nephrin tyrosine phosphorylation and Src kinase activation in the presence of Shp2 in a cell culture model of nephrin ligation. Concordantly, in mouse models of podocyte injury, nephrin tyrosine phosphorylation failed to increase in the absence of Shp2. Furthermore, deletion of Shp2 in mouse podocytes results in lack of foot process spreading in both protamine sulfate and nephrotoxic serum (NTS) models (28) of podocyte injury. These results suggest that Shp2-dependent signaling events are necessary for foot process structural changes following podocyte injury.
MATERIALS AND METHODS
Antibodies.
Purified rabbit polyclonal antibodies against nephrin (1) and phosphonephrin (p-nephrin) antibodies (which recognize phosphorylation on mouse nephrin Y1191 and Y1208 residues) (6) were previously described. Phospho-nephrin antibodies (generated against human nephrin Y1217 and Y1193 residues) were obtained from Epitomics (Burlingame, CA). Antibodies against Shp2, phospho-Shp2 (p-Shp2) (Y542 and Y580), Src, p-Src (Y527 and Y416), and Fyn were obtained from Cell Signaling Technologies (Danvers, MA). Anti-CD16 (clone 3G8) antibody was obtained from BD Bioscience; rhodamine- and DyLight-conjugated goat anti-mouse IgG were obtained from Pierce; glutathione S-transferase (GST)–horseradish peroxidase (HRP), β-actin, synaptopodin, phosphotyrosine, and Flag antibodies were obtained from Sigma-Aldrich. The 50A9 antibody was a gift from K. Tryggvason (65).
Plasmids.
A plasmid encoding wild-type human Shp2 was a kind gift from Benjamin Neel (University of Toronto, Toronto, Canada) (22). GST-tagged full-length Shp2 (GST-Shp2 FL) and GST-tagged Shp2 fragments containing the N-terminal sh2 domain, the C-terminal sh2 domain, and tandem sh2 (both N- and C-terminal sh2 domains) were subcloned with the full-length Shp2 construct as the template using standard PCR-based cloning techniques. The CD16-hemagglutinin (HA) construct was a gift from B. J. Mayer (University of Connecticut) (66). Constructs encoding fusion proteins consisting of the CD16 extracellular domain, CD7 transmembrane domain, and nephrin cytoplasmic domain and their mutants were generated using PCR-based techniques. Restriction digestion and DNA sequencing were used to confirm all construct sequences.
Immunoprecipitation and immunoblotting.
Proteins were extracted from plasma membranes in radioimmunoprecipitation assay (RIPA) buffer (phosphate-buffered saline [PBS] containing 0.1% SDS, 1% Nonidet P-40, 0.5% sodium deoxycholate, and 100 mM potassium iodide). HALT phosphatase and protease inhibitors were added to the RIPA buffer. Lysates were sonicated with 3 or 4 bursts of 5 to 10 s using Branson sonicators at the lowest setting. Endogenous immunoprecipitations were performed by extracting tissue in RIPA buffer containing 0.1% bovine serum albumin (BSA).
Cell culture.
Transient transfections were carried out in human podocyte cells (a gift from Moin Saleem, University of Bristol, United Kingdom) cultured in RPMI medium with Glutamax (Invitrogen) and supplemented with 10% fetal bovine serum (FBS) (Invitrogen Corp.) and 200 U/ml penicillin and streptomycin (Roche Applied Science), along with ITS (insulin, transferrin, and selenium) (Invitrogen Corp.). Transfections were performed using Lipofectamine 2000 (Invitrogen Corp.), Fugene HD (Roche), and electroporation with Amaxa Nucleofactor II (Amaxa Biosystem) According to the manufacturer's directions.
Nephrin phosphotyrosine mapping using synthetic oligopeptides.
Oligopeptides (11- to 18-mers) with and without phosphorylation on tyrosine residues were synthesized (Sigma Genosys; PEPScreen). The peptides were dissolved according to the manufacturer's recommendations. Solutions containing equimolar amounts of the peptides were made in 50 mM Tris buffer (pH 8.0). The peptides were blotted onto polyvinylidene difluoride (PVDF) membranes using a dot blot apparatus. The membranes were blocked with 5% milk solution in Tris-buffered saline with 0.1% Tween. Purified recombinant GST-Shp2 FL and GST-tagged fragments of Shp2 were used to overlay PVDF membranes blotted with the oligopeptides. The membranes were then probed using an anti-GST monoclonal antibody (Sigma, St. Louis, MO).
BiFC experiments.
The bimolecular fluorescence complementation (BiFC) assay utilizes the ability of two nonfluorescent fragments of a fluorescent protein to associate and form a fluorescent complex. We were able to study the interaction between nephrin and Shp2 by using this principle. T. K. Kerppola (University of Michigan) (32, 33) provided constructs that were used to generate reagents for these experiments. Briefly, two fragments corresponding to residues 1 to 154 (YN155) and 155 to 238 (YC155) were attached to the C terminus of a CD16/CD7 nephrin chimera and Shp2. An HA tag was substituted for the nephrin cytoplasmic domain for control experiments. Cells were transfected with the appropriate constructs and clustered using anti-CD16, followed by rhodamine-labeled mouse IgG, as described previously (2–6). We also generated individual and compound tyrosine mutations to identify the specific nephrin cytoplasmic domain tyrosine residues that interact with Shp2. Human podocyte cells were transfected with CD16/CD7 chimeric constructs. Thirty hours following transfection, RPMI medium was removed and replaced with fresh medium containing 1 μg/ml CD16 antibody (clone 3G8; Beckman Coulter). The cells were maintained on ice for 1 h. At this point, the cells were washed twice with PBS, 1 μg/ml rhodamine-conjugated or unlabeled anti-mouse IgG (Pierce Biotechnology) was added to the medium, and incubation was continued at 37°C for 30 min. Cells for immunofluorescence assays were washed 3 times with PBS and fixed with cytoskeleton buffer. The composition of the cytoskeleton buffer stock was 10 mM 2-CN-morpholinoethanesulfonic acid, 138 mM KCl, 3 mM MgCl2, 2 mM EGTA, and sucrose to a final concentration of 0.32 M. On the day of use, 20% paraformaldehyde was added to the cytoskeleton buffer stock to achieve a final concentration of 4%. Coverslips were mounted on glass slides using ProLong Gold antifade reagent (Life Technologies, NY). Samples were analyzed by fluorescence confocal microscopy with an Olympus FV-500 microscope using a 100× oil immersion objective lens and Fluoview software (version TIEMPO 4.3; Olympus). Images were processed using Adobe Photoshop software. All images were acquired at 1,024- by 1,024-pixel resolution.
Generation of mice with podocyte-specific Shp2 deleted.
Shp2-floxed mice (a kind gift from Benjamin Neel, University of Toronto, Toronto, Canada) were described previously and maintained on a mixed C57BL/6 and 129sv background (34). Shp2fl/fl mice were crossed with Cre mice, where Cre recombinase is driven by the podocyte-specific podocin promoter NPHS2, as described previously (31). For experiments, Shp2fl/fl podocin Cre+/− (homozygous for the floxed Shp2 allele and heterozygous for podocin-Cre) mice were used. For controls, littermates homozygous for the floxed Shp2 allele but lacking podocin-Cre were used.
Mouse kidney perfusion.
Perfusion of mouse kidneys with protamine sulfate was carried out as described previously (2, 6, 35). Briefly, mice were anesthetized with a combination of ketamine and xylazine; the core temperature of the mice was monitored with a rectal probe, and the animals were maintained at 37°C throughout the procedure using a heating pad apparatus. Kidneys were perfused with solutions maintained at 37°C through the abdominal aorta at a pressure of 120 mm Hg and an infusion rate of 12 ml/min. Perfusion was carried out with Hanks balanced salt solution (HBSS) for 2 min, followed by perfusion with protamine sulfate (2 mg/ml in HBSS; Sigma) for 15 min. This was followed with wash out using HBSS for 5 min. For effacement recovery, following perfusion with protamine sulfate, mice were perfused with heparin sulfate (1 mg/ml in HBSS; Sigma) for 15 min followed by wash out using HBSS. Glomeruli were isolated using graded sieving as described previously (2, 3, 6). For phosphatase inhibitor studies, mice were perfused with HBSS containing 100 mM NSC878777 (Tocris Bioscience). The University Committee on the Use and Care of Animals Institutional Review Board at the University of Michigan Medical School approved all animal experiments. All work was conducted in accordance with the principles and procedures outlined in the National Institutes of Health Guidelines for the Care and Use of Experimental Animals.
Nephrotoxic nephritis model.
The nephrotoxic nephritis experiments were performed as described previously (11, 37, 38). In brief, 8-week-old Shp2fl/fl,Cre+ mice and control Shp2fl/fl,Cre− littermates were injected retro-orbitally with sheep anti-rat glomerular lysate (NTS) or sheep IgG (control) at a concentration of 1.5 mg per mouse (approximately 25-g body weight). Urine was collected at 24 and 48 h postinjection. For transmission (TEM) and scanning (SEM) electron microscope analysis, mice were perfusion fixed for 24 or 48 h, as described previously (2).
Isolation of glomeruli using magnetic beads.
To isolate glomeruli, mice were perfused through the heart with magnetic 4.5-μm-diameter Dynabeads (Life Technologies) at 8 × 107 dilution in PBS. The kidneys were removed, minced into 1-mm cubes, and digested with collagenase (1 mg/ml collagenase A in 100 U/ml DNase I in HBSS) at 37°F for 30 min with gentle agitation. The collagenase-digested tissue was gently pressed through a 100-μm sieve using a flattened pestle. The filtered cells were passed through a new strainer and collected. The cell suspension was centrifuged at 200 × g for 5 min. The supernatant was discarded, and the cell pellet was resuspended in HBSS. The Dynabead-containing glomeruli were isolated using a magnet and washed at least three times with HBSS. The tissue was kept over ice throughout the procedure except for the initial incubation with collagenase. The protocol has been described in detail by Takemoto et al. (39). This method provides a highly pure glomerular preparation (close to 98% purity).
Flotation gradient preparation.
Glomeruli isolated from mouse kidneys were homogenized at 4°C with a Dounce homogenizer in a buffer containing 250 mM sodium chloride, 5 mM EDTA, 1 mM sodium fluoride, 1 mM sodium orthovanadate, 1 mM pyrophosphate, and a protease inhibitor mixture tablet (Roche). To the homogenate, Triton X-100 was added to a final concentration of 1% and mixed well. The mixture was maintained on ice for 15 min prior to mixing with 60% Optiprep (Sigma) to obtain a final density of 40%. Five milliliters of this mixture was added to precooled centrifuge tubes and then layered with 3 ml of 30% Optiprep, followed by 4 ml of 5% Optiprep. The Optiprep dilutions were obtained by diluting 60% Optiprep with Tricine buffer containing 20 mM Tricine, 0.25 mM sucrose, and 1 mM EDTA. The gradient was centrifuged at 169,044 × g in a Beckman SW1Ti rotor for 3 h at 4°C. The Triton X-100-insoluble membrane fraction (detergent-resistant membrane fractions [DRMs]) was observed at the interface between 5% and 30% Optiprep densities. Top-to-bottom 1-ml fractions were collected and analyzed as indicated.
EM and slit diaphragm frequency analysis.
Preparation of mouse kidneys for SEM and TEM were performed by standard methods. For SEM, 20 glomeruli of each sample were analyzed with an Amray 1910 field emission scanning electron microscope at the University of Michigan Microscope and Image Analysis core facility. For TEM, samples were examined by the Philips CM-100 transmission electron microscope at the University of Michigan Microscope and Image Analysis core facility. Slit diaphragm frequency using TEM was assessed by counting the foot process junctions per micrometer of basement membrane. At least 10 samples were analyzed under each experimental condition.
Study approval.
All animal studies were approved by the University Committee on the Use and Care of Animals Institutional Review Board at the University of Michigan School of Medicine. Deidentified human kidney biopsy samples were obtained for immunostaining from the Department of Pathology at the University of Michigan. Control kidney tissue was obtained from the unaffected area of tumor nephrectomy samples.
ImageJ quantitation statistical analysis.
Data are presented as means and standard errors of the mean throughout the text unless otherwise specified. The numbers of experiments performed are mentioned in the figure legends. All experiments were performed at least 3 times. ImageJ software was used to quantify the density of protein bands on Western blots. Statistical comparisons were performed using two-tailed t tests where applicable. A P value of ≤0.05 was considered to represent a statistically significant difference.
RESULTS
The sh2 domain-containing protein Shp2 interacts with nephrin in a tyrosine phosphorylation-dependent manner.
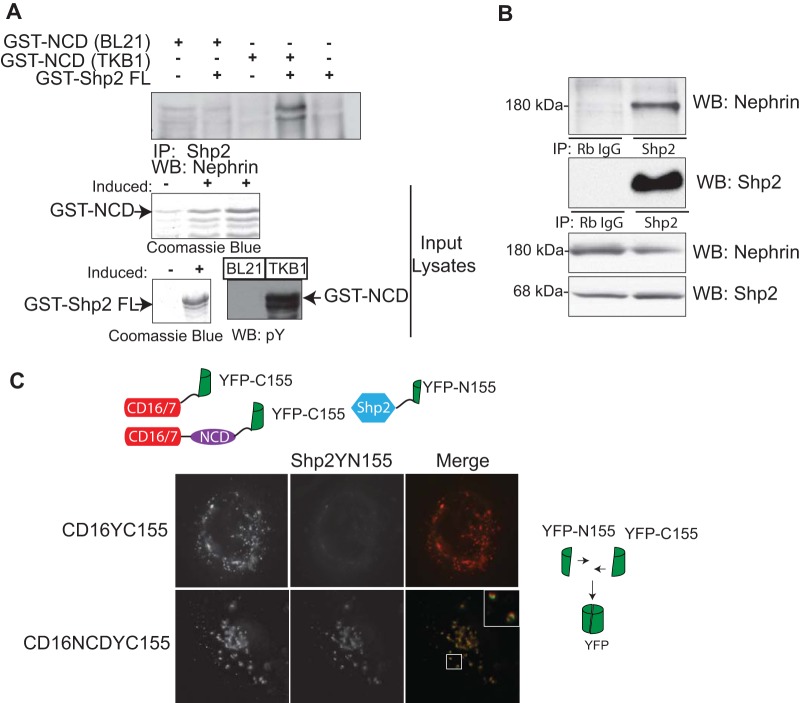
To identify proteins that interact with nephrin in a tyrosine phosphorylation-dependent manner, we generated a library of His-tagged sh2 domains of sh2 domain-containing proteins. Purified recombinant His-tagged proteins were blotted onto a PVDF membrane and incubated with phosphorylated (expressed in Escherichia coli TKB1) or unphosphorylated GST-tagged recombinant nephrin cytoplasmic domain. Using this biased approach, we found that Shp2 interacts with phosphorylated nephrin (data not shown). We further confirmed the interaction between nephrin and Shp2 using a full-length GST-Shp2 fusion protein (Fig. 1A). Similar to our findings in the initial screen, we observed interaction of full-length Shp2 only with tyrosine-phosphorylated nephrin. Using coimmunoprecipitation, we were also able to confirm the interaction of endogenous nephrin and Shp2 in glomerular lysates (Fig. 1B).
FIG 1.
(A) Shp2 binds to nephrin in a tyrosine phosphorylation-dependent manner. Purified recombinant full-length-GST-tagged Shp2 was incubated with tyrosine-phosphorylated GST-nephrin recombinant protein expressed in E. coli TKB1 or GST-nephrin expressed in E. coli BL21. Immunoprecipitation (IP) and immunoblotting (Western blotting [WB]) were performed with the indicated antibodies. Only tyrosine-phosphorylated nephrin associated with Shp2. Tyrosine phosphorylation of nephrin expressed in E. coli TKB1 was confirmed using phosphotyrosine antibody. Recombinant protein expression was confirmed using Coomassie blue. (B) Shp2 interacts with nephrin in glomerular lysates. A coimmunoprecipitation experiment using glomerular lysate showed interaction between endogenous nephrin and Shp2 (Rb IgG, rabbit IgG). (C) In vitro BiFC showing interaction of nephrin with Shp2. A construct encoding a chimeric protein with a CD16 extracellular domain, CD7 transmembrane domain, and nephrin cytoplasmic domain fused with a C-terminal fragment of YFP (YFP-C155) was generated. Full-length Shp2 was fused with an N-terminal fragment of YFP (YFP-N155). Following transfection in human podocytes, CD16 chimeras were clustered by the addition of anti-CD16 antibody (primary), followed by anti-mouse IgG (secondary) conjugated with rhodamine. Close approximation of the two YFP fragments resulted in reconstitution of the fluorescent protein and emission of green fluorescence. A CD16 construct fused with YFP-C155 lacking the nephrin cytoplasmic domain served as a control. Original magnification, ×630.
Nephrin and Shp2 demonstrate BiFC.
To better define the nephrin-Shp2 interaction, we used a novel method of BiFC (32, 40). Both CD16-nephrin and Shp2 were fused to complementary fragments of a fluorescent reporter protein (yellow fluorescent protein [YFP]) (Fig. 1C). We employed the previously described CD16 clustering model in which CD16 is fused to the cytoplasmic domain of nephrin (NCD) and clustering is induced with anti-CD16 (2–6), as it provides a robust model for nephrin phosphorylation-dependent signaling. We hypothesized that only a close physical interaction of nephrin with Shp2 would bring the two YFP fragments in proximity to reconstitute the native three-dimensional structure of YFP and emit a fluorescent signal. We observed robust fluorescence when CD16NCD-YC155 (YFP-C155, the YFP C-terminal 155 residues) was clustered in the presence of Shp2YN155 (YFP-N155, the YFP N-terminal 155 residues). In contrast, this fluorescent signal was lacking in the control (Fig. 1C), which contains the YFP-C155 fragment only at its C terminus and lacks the nephrin cytoplasmic domain. Mouse monoclonal CD16 antibody, followed by Texas Red-labeled anti-mouse IgG, was used to cluster and visualize CD16 chimeras at the cell membrane.
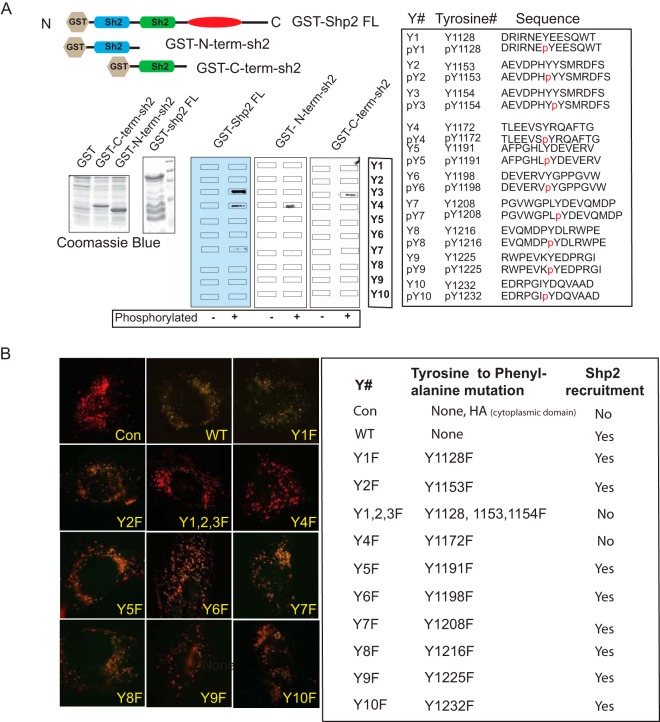
Nephrin cytoplasmic domain tyrosine residues Y1154 and Y1172 are important for the nephrin-Shp2 interaction.
A peptide array screening method was used to identify tyrosine residues in nephrin that are required for the interaction of nephrin and Shp2. Synthetic 11- to 15-mer tyrosine-phosphorylated oligopeptides that overlapped each of the 10-tyrosine residues present in the mouse nephrin cytoplasmic domain were synthesized (Sigma-Aldrich). As controls, corresponding oligopeptides without tyrosine phosphorylation were used. Peptide arrays immobilized on PVDF membranes were probed with full-length GST alone (GST), GST-Shp2 FL, and GST-tagged N-terminal sh2 domain and C-terminal sh2 domain (Fig. 2A). By this approach, the Shp2 N-terminal sh2 domain and C-terminal sh2 domain bound to a phosphorylated oligopeptide spanning nephrin Y1172 and Y1154 residues, respectively. Full-length GST-Shp2 bound to both nephrin Y1154 and Y1172. GST alone did not bind to the peptides and served as a control for the GST (data not shown). We further confirmed these findings using BiFC. Human podocytes expressing CD16-nephrin constructs with single or compound Y-to-F mutations in the cytoplasmic domains fused with the C-terminal YFP fragment (YC155) and Shp2YN155 (the N-terminal YFP fragment) were clustered as described above. Similar to our peptide array findings, nephrin Y1172F (Y4F) mutation resulted in abrogation of the nephrin-Shp2 interaction (Fig. 2B). Despite numerous attempts, we observed low expression of the CD16-nephrin Y1154F (Y3F) construct. To circumvent this, we used a triple Y-to-F mutation, Y1128F, Y1153F, and Y1154F (Y1,2,3F), along with single Y1128F (Y1) and Y1153F (Y2) mutations. We observed abrogation of the nephrin-Shp2 interaction when the triple mutant was expressed. The single mutation involving nephrin tyrosine residues Y1128 and Y1153 had a robust nephrin-Shp2 interaction, suggesting the necessity for the Y1154 residue in nephrin-Shp2 interaction using BiFC. Tyrosine-to-phenylalanine mutations of other nephrin cytoplasmic domain tyrosine residues did not affect the nephrin-Shp2 interaction.
FIG 2.
(A) Mapping of nephrin tyrosine residues that interact with Shp2 using a synthetic-peptide array. Synthetic 11- to 15-mer tyrosine-phosphorylated (p) and unphosphorylated oligopeptides that overlapped each of the 10 tyrosine residues of the nephrin cytoplasmic domain were blotted onto a PVDF membrane. The membrane was then incubated with purified recombinant GST-tagged full-length Shp2, the Shp2 N-terminal sh2 domain, and the Shp2 C-terminal sh2 domain. Recombinant protein expression was confirmed using Coomassie blue. Immunoblotting with anti-GST antibody showed that the Shp2 N-terminal and C-terminal sh2 domains associated with nephrin Y4 and Y3, respectively. The full-length Shp2 containing both sh2 domains associated with both nephrin Y3 and Y4. (B) Mapping of nephrin tyrosine residues that interact with Shp2 using BiFC. CD16/CD7 chimeras with nephrin cytoplasmic domains containing tyrosine-to-phenylalanine mutations fused with C-terminal fragments of YFP (YFP-C155) were generated. CD16 clustering experiments were performed on cells expressing a CD16 chimeric construct and constructs encoding full-length Shp2 fused with N-terminal YFP (YFP-N155). Reconstitution of YFP was not observed in cells expressing Y4F and Y1,2,3F. The table on the right shows the various mutations and tyrosine residue positions in mouse nephrin. Con, control that has an HA tag; WT, wild type. Original magnification, ×630.
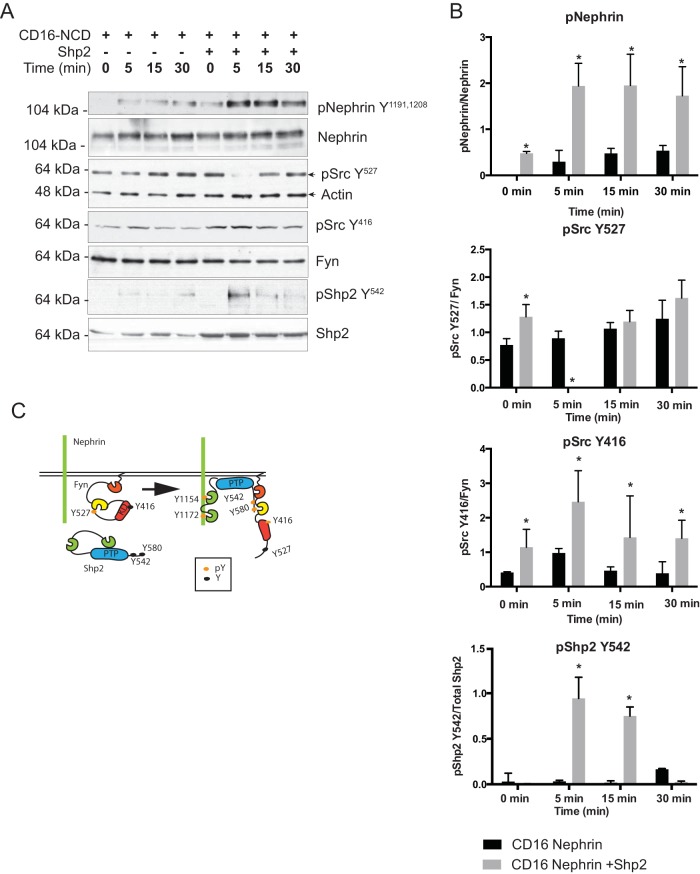
There is an increase in nephrin phosphorylation in the presence of Shp2.
We used the CD16 clustering model to assess the state of nephrin phosphorylation in the presence of Shp2. We observed a robust increase in the intensity of nephrin phosphorylation on Y1191 and Y1208 residues when Shp2 was transfected (Fig. 3A) compared to cells that expressed CD16-nephrin alone. Nephrin phosphorylation was apparent within 5 min following clustering and was higher at all time points when Shp2 was present. Review of the literature revealed a prominent role of Shp2 in activation of Src family kinase activity (22, 24, 29). Src family kinases (Fyn, Yes, Lyn, etc.) and Shp2 have similar mechanisms of activation following a proximal signaling event. In its inactive state, phosphorylation on Fyn's C-terminal Y527 results in a closed configuration, which hides the kinase domain (Fig. 3C) (24). Dephosphorylation of Y527 results in unfolding of the tertiary structure of Fyn, leading to autophosphorylation of Y416 in its kinase domain and activation of Fyn. Similarly, phosphorylation of Shp2 Y542 interacts intramolecularly with the N-terminal sh2 domain to relieve basal inhibition of the phosphatase, whereas phosphorylation at Y580 stimulates the PTPase activity by interaction with the C-terminal sh2 domain (18, 29). In the CD16 model system, following clustering, we observed a simultaneous decrease in Src Y527 phosphorylation and an increase in Src Y416 autophosphorylation (Fig. 3A). Src Y416 and Y527 antibodies cross-react with other members of the Src family kinases, including Fyn and Yes, at equivalent sites. Changes in Src Y416 and Y527 phosphorylation represent changes in phosphorylation at Y416 and Y527 of multiple members of Src family kinases, including Fyn. The Src kinase Fyn has been shown to be primarily responsible for nephrin tyrosine phosphorylation in both in vitro and in vivo studies (10). Since there is no specific phosphoantibody that reacts with Fyn Y416 and Y527, the Src Y416 and Src Y527 antibody is used to assess the changes in Fyn phosphorylation at those specific tyrosine residues. Following clustering, we also observed an increase in Shp2 Y542 phosphorylation concurrent with nephrin phosphorylation. Quantification using ImageJ densitometry revealed 2- to 3-fold-higher levels of nephrin phosphorylation at 15 min in the presence of Shp2 (Fig. 3B). These observations suggest that in the presence of Shp2, nephrin phosphorylation is both enhanced and sustained longer.
FIG 3.
(A) There is enhanced nephrin tyrosine phosphorylation in the presence of Shp2. Cells expressing CD16/CD7/NCD and the indicated plasmids were clustered as described previously. The cells were lysed in RIPA buffer and sonicated at different time points. The lysates were resolved using SDS-PAGE, and the blots were probed with the indicated antibodies. There was enhanced nephrin phosphorylation in the presence of Shp2. At the same time, there was an increase in p-Src (Y416) and p-Shp2 Y542 phosphorylation. (B) Quantification of nephrin phosphorylation following CD16-nephrin clustering in the presence and absence of Shp2. In the presence of Shp2, there was a 3-fold increase in nephrin phosphorylation at the 15- and 30-min time points. The data are mean values and standard errors of the mean. *, P < 0.001 using a two-tailed t test. (C) Schematic of Src kinase regulation by Shp2. All Src kinases, including Fyn, have two regulatory tyrosines, Y416 and Y527. In their basal inactive state, Src kinases are phosphorylated on Y527, resulting in a closed configuration, which hides the kinase domain. Dephosphorylation of Y527 results in unfolding of the tertiary structure of Src kinase, autophosphorylation of Y416 in the kinase domain, and activation of the kinase enzymatic activity. We incorporated the nephrin tyrosine residues (Y1154 and Y1172) identified by us as essential for nephrin-Shp2 interaction. Furthermore, phosphorylation of two tyrosine residues on the C terminus of Shp2, Y542 and Y580, resulted in similar unfolding of the tertiary structure of Shp2, thus making its phosphatase domain available for enzymatic activity.
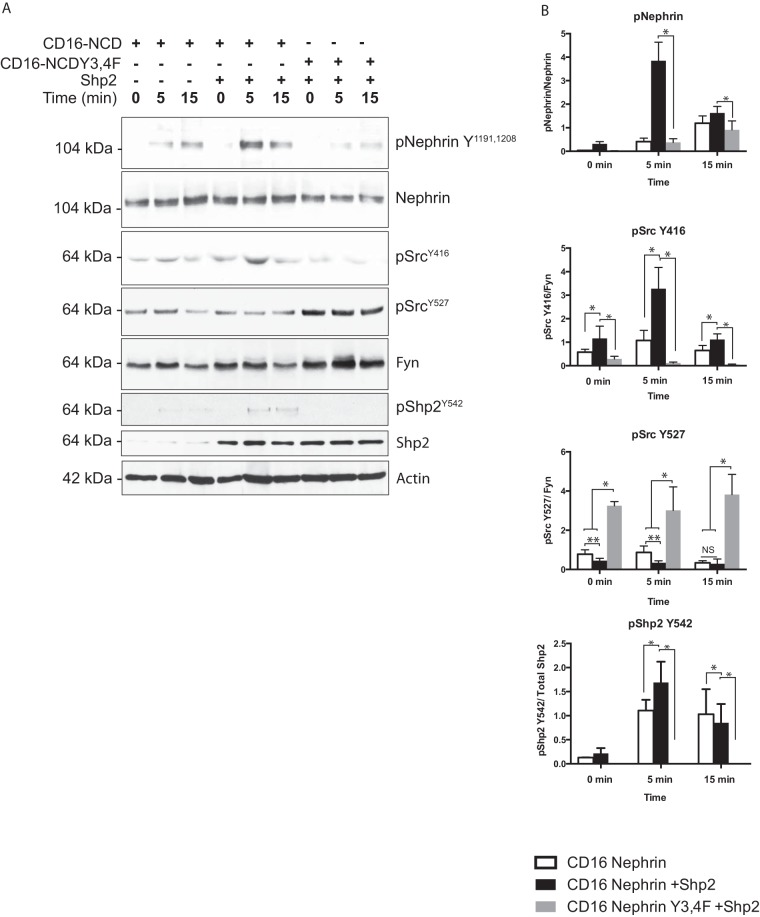
Shp2-dependent nephrin phosphrylation is attenuated when nephrin Y3 and Y4 are mutated.
To examine the change in nephrin phosphorylation when the nephrin-Shp2 interaction is perturbed, we transfected CD16-nephrin carrying tyrosine-to-phenylalanine mutations on nephrin Y1154 (Y3) and Y1172 (Y4), along with Shp2. The Y3F single mutant is not expressed well following transfection; to circumvent this, we used the Y3,4F compound mutation. There was a decrease in nephrin phosphorylation when CD16-nephrin Y3,4F mutants were clustered in the presence of Shp2 (Fig. 4A). The increase in Shp2 Y542 and Src Y416 phosphorylation seen following CD16-nephrin clustering was also abrogated when nephrin Y3 and Y4 residues were mutated. Quantification of nephrin, Src, and Shp2 phosphorylation was done by densitometry using ImageJ software (Fig. 4B). In the presence of Shp2, nephrin Y1191 and Y1208 phosphorylation was significantly higher at each time point than that of controls (without Shp2) (P < 0.001). Attenuation of nephrin phosphorylation when nephrin was mutated at Y3 and Y4 was also statistically significant at each time point compared to clustering of CD16-nephrin in the presence or absence of Shp2 (P < 0.001). Shp2 Y542 phosphorylation was higher at 5- and 15-min time points when Shp2 was transfected. Similarly, Src Y416 phosphorylation was also attenuated when the CD16-nephrin Y3 and Y4 mutant was clustered in the presence of Shp2. These data suggest that nephrin tyrosine residues Y3 and Y4, identified previously as the Shp2 binding sites, are necessary for Shp2-dependent enhancement of nephrin phosphorylation.
FIG 4.
Nephrin phosphorylation is attenuated when nephrin Y1154 (Y3) and Y1172 (Y4) residues are mutated. (A) Nephrin phosphorylation following CD16-nephrin clustering was markedly attenuated when a CD16− nephrin construct carrying the Y3,4F mutation was transfected along with Shp2. Similarly, there was attenuation of Shp2 Y542 and Src Y416 phosphorylation following clustering of CD16-nephrin chimeras carrying the Y3,4F (double) mutations. (B) Quantification of bands using densitometry showed a statistically significant increase in nephrin phosphorylation in the presence of Shp2 compared to the control at each time point (*, P < 0.001; **, P < 0.01; NS, not significant using the ANOVA test). The decrease in nephrin phosphorylation following clustering of mutant CD16-nephrin (Y3,4F) in the presence of Shp2 was also statistically significant compared to clustering of wild-type CD16-nephrin in the presence of Shp2. The data are mean values and standard errors of the mean.
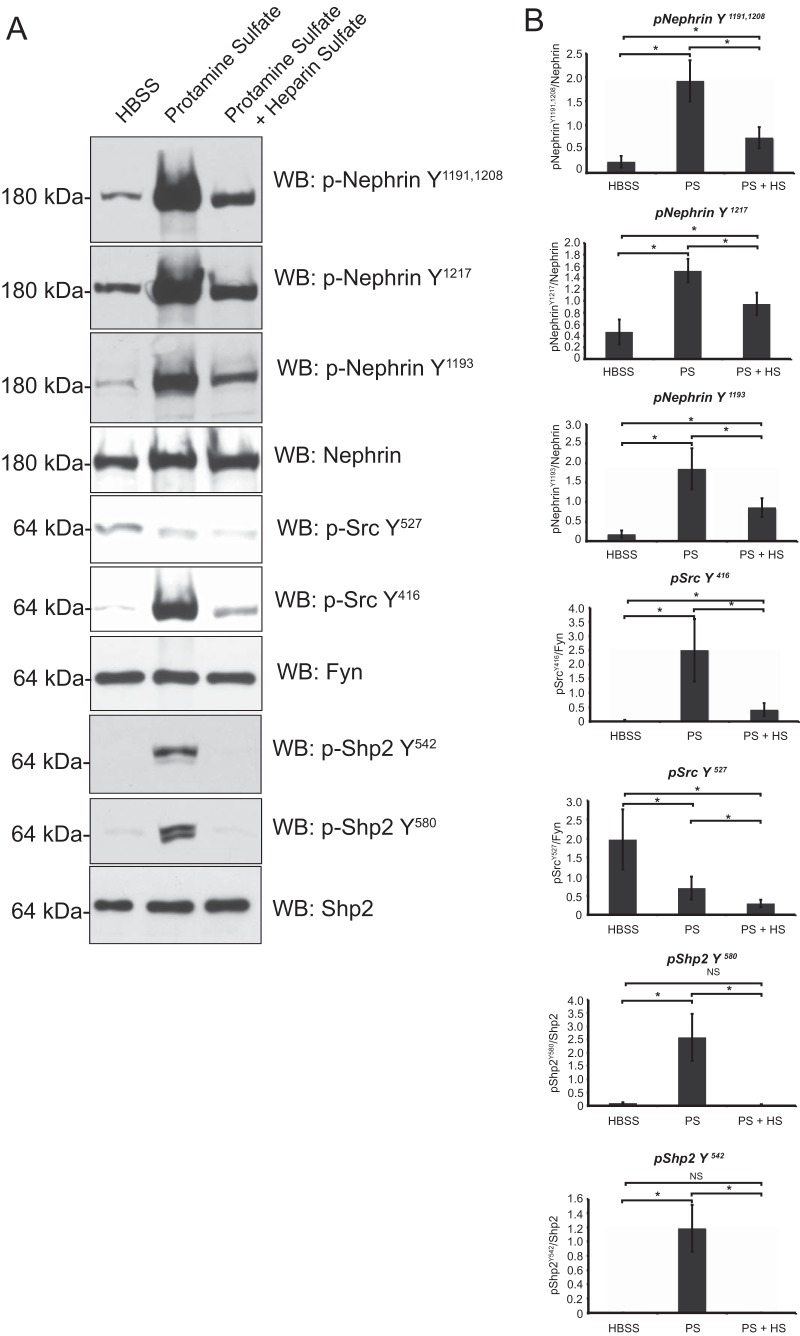
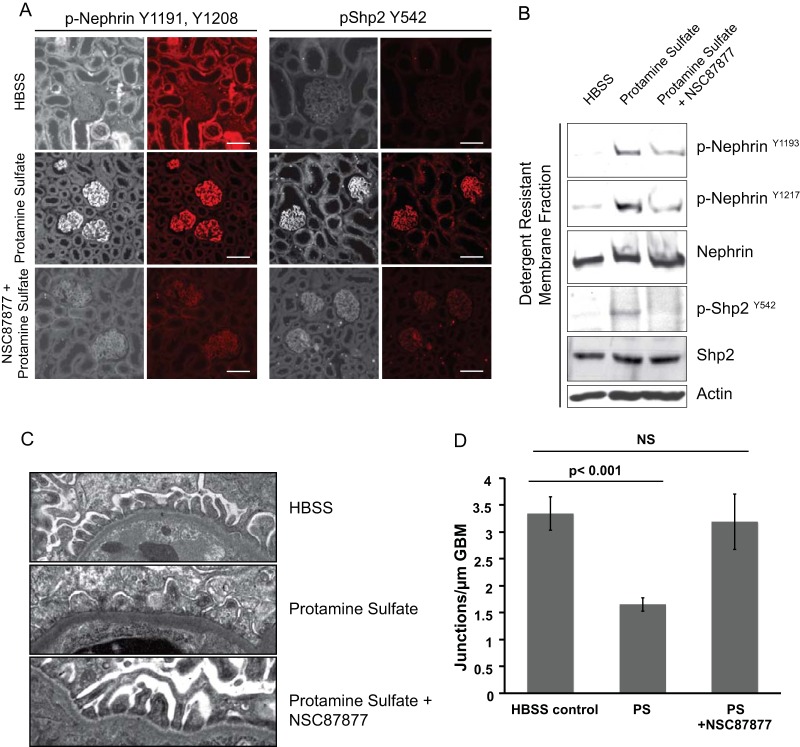
Phosphorylation events take place in detergent-resistant membrane fractions of glomeruli isolated from mouse kidneys perfused with protamine sulfate in vivo.
Among the various models of podocyte injury, protamine sulfate provides a rapid and reversible model of injury with changes in the podocyte cytoskeleton. We expected changes in nephrin, Src kinase, and Shp2 phosphorylation following protamine sulfate injury. We examined glomerular detergent-resistant membrane fractions for these changes, as they provide enrichment of these components (Fig. 5A). We observed an increase in nephrin tyrosine phosphorylation using antibodies against the mouse nephrin Y1191 and Y1208 residues, as well as the commercially available antibodies against the human nephrin Y1193 and Y1217 residues. The latter antibodies are numbered based on the human nephrin amino acid sequence and correspond to the Y1191 and Y1208 residues, respectively, in mouse nephrin. We observed an increase in Src Y416 and Shp2 Y542 and Y580 phosphorylation. There was a decrease in Src Y527 phosphorylation that indicated increased Src kinase activity (Fig. 5A). Quantification of phosphorylation using ImageJ software from 4 different experiments was performed and is shown in Fig. 5B. Following infusion of heparin sulfate (an antidote for protamine sulfate), we observed reversal toward the baseline of tyrosine phosphorylation on nephrin, Src Y416, and Shp2 tyrosine residues.
FIG 5.
Phosphoproteome analysis of isolated glomerular detergent-resistant membrane fractions following protamine sulfate injury. (A) Glomeruli were isolated from mouse kidneys perfused with HBSS, protamine sulfate, and protamine sulfate followed by heparin sulfate. The glomerular lysates were fractionated using the flotation gradient method to obtain the DRMs. Following resolution of lysates using SDS-PAGE, the membranes were blotted with the indicated antibodies. The blots are representative of 3 independent experiments. (B) Quantification of nephrin, Shp2, and Src phosphorylation using densitometry. Glomeruli from mouse kidneys perfused with HBSS, protamine sulfate (PS), and protamine sulfate followed by heparin sulfate (PS + HS) were isolated and fractioned using a flotation gradient. Detergent-resistant membrane fractions were resolved using SDS-PAGE and blotted for the indicated antibodies (Fig. 4). ImageJ software was used to quantify the bands from three different experiments. There was an increase in nephrin tyrosine phosphorylation using three different antibodies against residues necessary for nephrin-Nck interaction. There was an increase in Shp2 activation, suggested by an increase in tyrosine phosphorylation of Shp2 Y542 and Y580 following protamine sulfate perfusion. There was an increase in Src tyrosine phosphorylation on the Y416 residue and a decrease in tyrosine phosphorylation of the Src Y527 residue following protamine sulfate perfusion. Heparin sulfate reversed the phosphorylation on nephrin, Shp2, and Src Y416. Src Y527 dephosphorylation persisted following heparin sulfate perfusion. The data are mean values ± standard errors of the mean. *, P < 0.001; NS, not significant using a two-tailed t test.
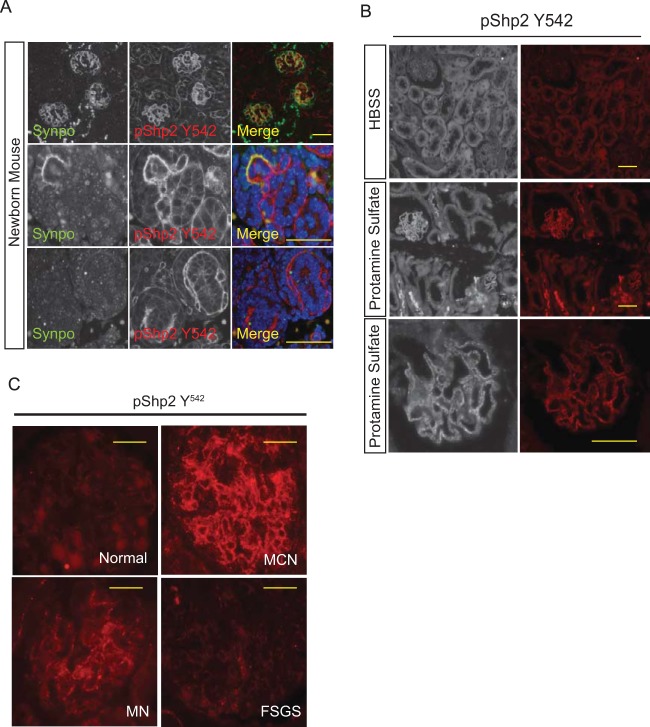
There is increased Shp2 activity during podocyte development and following injury.
Nephrin is tyrosine phosphorylated during development and following injury (3, 6, 30). Since Shp2 is proximal to Src kinase activation, we hypothesized that Shp2 should also be tyrosine phosphorylated during podocyte development and following injury. We examined newborn mouse kidney sections for evidence of concurrent Shp2 activation, given that glomerular maturation continues after birth in mice. We observed increased Shp2 Y542 phosphorylation in kidney sections from newborn mice, suggesting increased Shp2 activity (Fig. 6A). The staining pattern of p-Shp2 overlaps with synaptopodin in developing and young glomeruli, though it appears that Shp2 precedes the appearance of synaptopodin (Fig. 6A, bottom) during development. A similar increase in Shp2 Y542 phosphorylation was observed in mouse kidney sections following perfusion with protamine sulfate (Fig. 6B).
FIG 6.
(A) Shp2 is tyrosine phosphorylated in developing glomeruli. Newborn mouse kidney sections were prepared as described in Materials and Methods. The sections were analyzed by indirect immunofluorescence microscopy following immunostaining with p-Shp2 (Y542) antibody. Synaptopodin antibody was used to identify podocytes in the section. (Top row) Lower-magnification images of kidney cortex. (Bottom rows) Higher-magnification images of developing glomeruli. Scale bars, 20 μm. (B) Shp2 is tyrosine phosphorylated following perfusion with protamine sulfate. Adult mouse kidneys were perfused with HBSS and protamine sulfate prior to perfusion fixation with paraformaldehyde. Kidney sections were analyzed by indirect fluorescence microscopy. Magnification, ×200 (top 2 rows) and ×400 (bottom row). Scale bars, 20 μm. (C) Enhanced Shp2 phosphorylation in human glomerular diseases. Representative images show p-Shp2 Y542 antibody staining of paraffin-embedded kidney sections from healthy subjects and from patients with minimal-change nephrosis (MCN), membranous nephropathy (MN), and focal segmental glomerulosclerosis (FSGS). Kidney biopsy sections from 5 or more patients in each disease category were immunostained. Scale bars, 20 μm.
Increased Shp2 activity in human glomerular disease.
Podocyte injury in human glomerular diseases should have a similar increase in nephrin phosphorylation, as well as an increase in Shp2 activity. Available phosphonephrin antibodies do not work well with human kidney sections. As a surrogate for nephrin phosphorylation, we tested for changes in Shp2 Y542 phosphorylation in kidney biopsy sections from patients with various glomerular diseases. Phospho-Shp2 antibody was characterized to confirm its specificity (see Fig. S1 in the supplemental material). We observed an increase in Shp2 Y542 phosphorylation in both minimal-change nephrosis and membranous nephropathy (Fig. 6C). Interestingly, increase in Shp2 Y542 phosphorylation was not observed in focal segmental glomerulosclerosis. These results suggest that Shp2 plays a role in signaling events that occur following podocyte injury in a subset of human glomerular diseases.
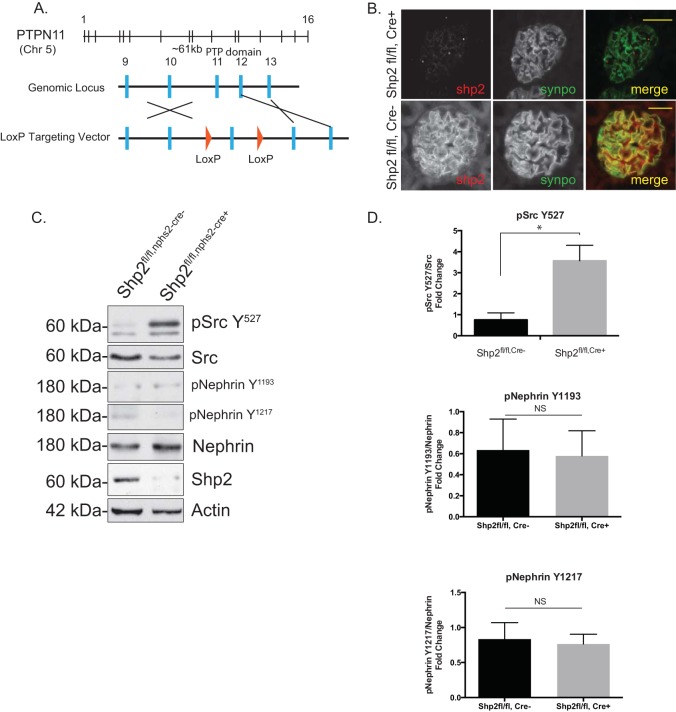
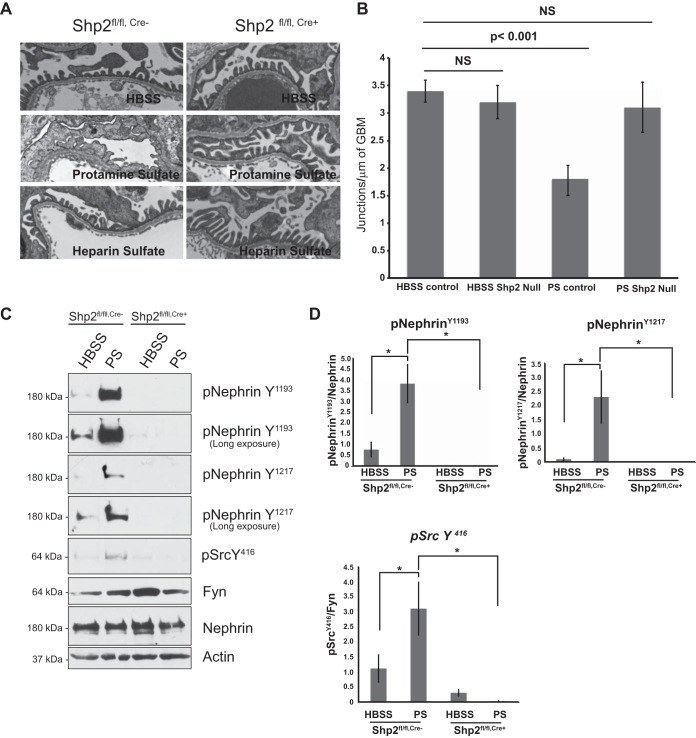
Mice with a podocyte-specific deletion of Shp2 are resistant to protamine sulfate injury.
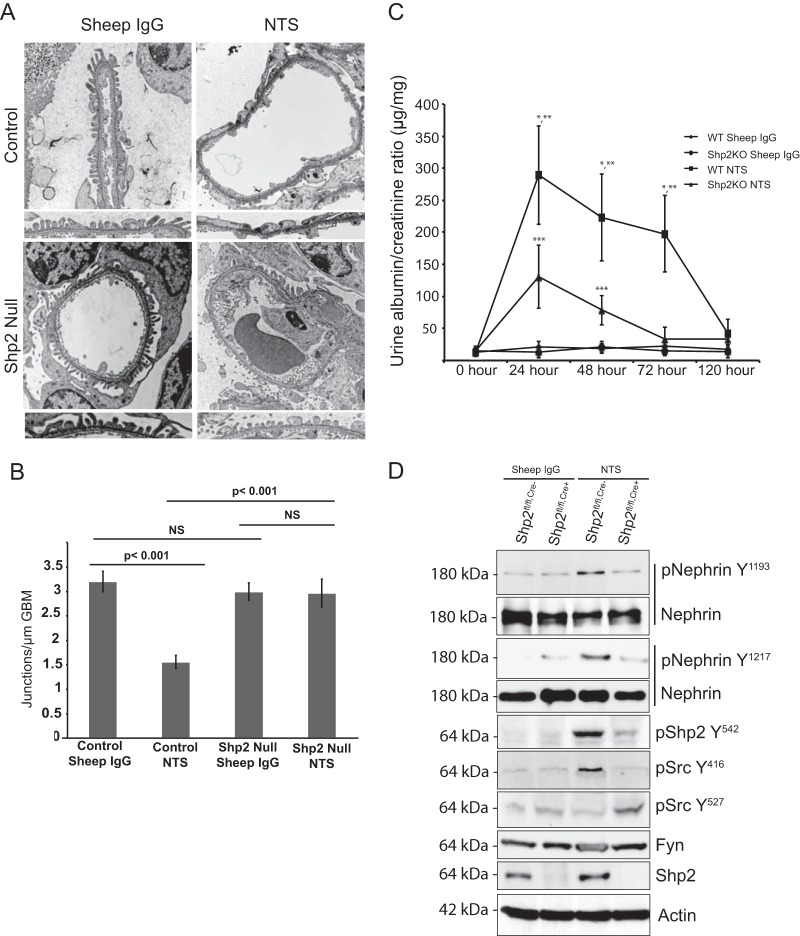
To test the role of Shp2 in vivo, Shp2 was deleted specifically in murine podocytes by breeding Shp2 floxed mice (with the LoxP sequence flanking exon 11, as generated by Neel et al. [34]) with podocin-Cre mice (31) (Fig. 7A). The mice were healthy at birth, and there was no evidence of proteinuria even at 20 months of age. Deletion of Shp2 was confirmed by immunostaining on paraffin-embedded kidney sections from 1-month-old Shp2fl/fl,Cre+ and Shp2fl/fl,Cre− mice (Fig. 7B). High levels of Src Y527 phosphorylation in glomerular lysates from Shp2 knockout (KO) mice indicate decreased Src kinase activity in the absence of Shp2 in vivo (Fig. 7C and D). Nephrin phosphorylation on Y1193 and Y1217 residues is low at baseline and does not change significantly in the absence of Shp2. Lack of a developmental phenotype, though surprising, has been observed in other podocyte-specific-deletion mouse models (2, 11). We speculated that functional loss of Shp2 might become evident when challenging mice using the protamine sulfate model of podocyte injury. Three-month-old Shp2fl/fl,Cre+ mice and their littermate controls were perfused via the renal artery with protamine sulfate or control HBSS buffer solution. We observed protection of foot processes from spreading in the mice with Shp2 deleted following protamine sulfate perfusion (Fig. 8A). This observation was confirmed by evaluation of the podocyte intercellular junction frequency by TEM, where control mice had a significantly reduced junction frequency after injury compared to mice with Shp2 deleted, which remained similar to HBSS-perfused control mice (Fig. 8B). There was a decrease in nephrin phosphorylation in the Shp2 knockout mouse glomeruli when probed with antibody against the Y1193 and Y1217 nephrin residues (Fig. 8C). Quantification of blots using ImageJ software is shown in Fig. 8D. The observation that there were higher levels of Src Y527 phosphorylation in the absence of Shp2 in vivo, along with decreased levels of Fyn-dependent nephrin Y1192 and Y1217 phosphorylation following injury, suggests that Shp2 is necessary for Fyn activation within the nephrin signaling complex.
FIG 7.
Podocyte-specific deletion of Shp2. (A) Schematic representation of the floxed Shp2 allele showing two LoxP sites (arrowheads) flanking exon 11 of Shp2. (B) Paraffin-embedded mouse kidney sections from Shp2fl/fl (control) and Shp2fl/fl,Cre+ (Shp2-null) mice were double stained for Shp2 (red) and synaptopodin (synpo) (green) as a podocyte marker. Scale bars, 20 μm. (C) Src activation is decreased in the absence of Shp2. Detergent-resistant membrane fractions from the glomerular lysates of Shp2fl/fl (control) and Shp2fl/fl,Cre+ (Shp2-null) mouse kidneys were probed with Src Y527 and phosphonephrin antibody (Y1193 and Y1217). There was enhanced phosphorylation at the Src Y527 residue in the absence of Shp2. (D) Quantification of Src Y527 and nephrin phosphorylation. The data are mean values and standard errors of the mean. *, P < 0.001; NS, not significant using a two-tailed t test (10 or more animals were analyzed in each group).
FIG 8.
Podocytes from mice with Shp2 deleted are protected from protamine sulfate injury. (A) Foot process spreading is not seen in Shp2fl/fl,Cre+ (Shp2-null) mice following protamine sulfate perfusion. Shown are TEM images of mouse kidneys perfused with HBSS (control), protamine sulfate, or protamine sulfate followed by heparin sulfate. The results are representative of 5 mice per group. Original magnification, ×5,200. (B) Numbers of junctions per micrometer of GBM, as seen by TEM. The data are means ± standard errors of the mean. (C) Glomeruli from Shp2fl/fl,Cre− (control) and Shp2fl/fl,Cre+ (Shp2-null) mouse kidneys perfused with HBSS and protamine sulfate were isolated using Dynabeads. Detergent-resistant membrane fractions were prepared, resolved using SDS-PAGE, and blotted with the indicated antibodies. There was a decrease in nephrin phosphorylation in Shp2fl/fl,Cre+ (Shp2-null) kidneys following perfusion with protamine sulfate. The blots are representative of 3 independent experiments. (D) Quantification of nephrin and Src phosphorylation in Shp2fl/fl,Cre+ and Shp2fl/fl,Cre− mouse glomerular lysates following perfusion with HBSS and protamine sulfate. Detergent-resistant membrane fractions from mouse glomerular lysates were resolved using SDS-PAGE and blotted with the indicated antibodies. ImageJ software was used to quantify the bands from three different experiments using 4 or 5 animals in each group. The increase in nephrin Y1193 and Y1217 and Src Y416 observed following protamine sulfate perfusion in Shp2fl/fl,Cre− mouse kidney glomerular lysates was abrogated in the Shp2fl/fl,Cre+ mouse. The data are means ± standard errors of the mean. *, P < 0.001.
Inhibition of Shp2 activity using a small-molecule inhibitor is protective in protamine sulfate injury.
In order to test available small-molecule inhibitors of Shp2 as a potential therapeutic modality, we perfused 2-month-old mice with NSC87877, a specific inhibitor of Shp2 phosphatase activity (41–43). The mice were perfused with NSC87877 30 min prior to perfusion of protamine sulfate. Site-directed mutagenesis and molecular modeling suggested that NSC87877 binds to the catalytic cleft of Shp2 (43). There was a reduction in both nephrin and Shp2 phosphorylation in mice that were perfused with NSC87877 (Fig. 9A) prior to protamine sulfate using indirect immunofluorescence imaging. Detergent-resistant membrane fractions from isolated glomeruli from mice perfused with NSC878777 had a similar decrease in nephrin and Shp2 phosphorylation (Fig. 9B). Importantly, TEM analysis of kidney sections showed lack of foot process spreading in animals that were perfused with NSC87877 prior to protamine sulfate compared to those that received protamine sulfate only (Fig. 9C and D). These observations suggest a potential benefit of inhibiting Shp2 activity in podocytes to prevent foot process effacement and proteinuria.
FIG 9.
The Shp2 inhibitor NSC87877 protects podocytes from protamine sulfate injury. (A) Indirect immunofluorescence images of paraffin-embedded mouse kidney sections following perfusion with HBSS (control), protamine sulfate, and NSC87877 prior to protamine sulfate. The sections were stained with p-nephrin Y1191 and Y1208 and p-Shp2 Y542 antibodies. Scale bars, 20 μm. (B) Immunoblots of glomerular lysates from mouse kidneys showing a decrease in nephrin tyrosine phosphorylation when probed with p-nephrin Y1193 and Y1217 antibodies in mice perfused with the Shp2 inhibitor NSC87877 prior to protamine sulfate. There was a decrease in p-Shp2 Y542 levels, as well. (C) TEM images of wild-type mice perfused with HBSS (control), protamine sulfate, or NSC87877 prior to protamine sulfate. The results are representative of at least 3 mice per group. Original magnification, ×5,200. (D) Numbers of junctions per micrometer of GBM as seen by TEM. The data are means ± standard errors of the mean.
Foot process spreading and increase in nephrin phosphorylation were not seen in mice with podocyte-specific Shp2 deleted following injury by nephrotoxic serum.
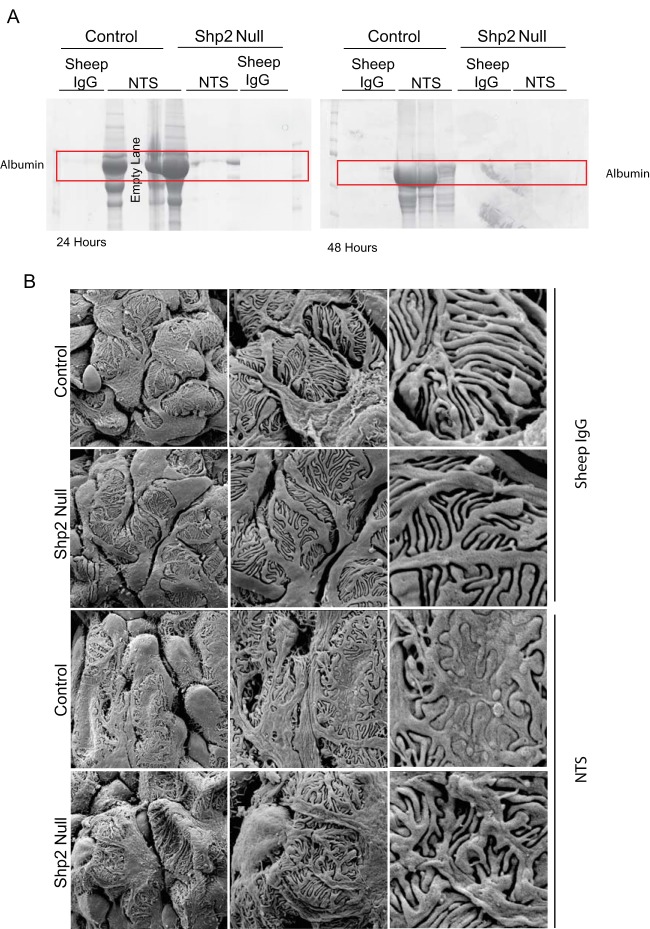
We examined an alternate podocyte injury model using the previously described nephrotoxic nephritis model (28, 37). Unlike the protamine sulfate model, the NTS model allows us to examine both the functional and the morphological significance of Shp2 deletion in mouse podocytes. Mice were injected with sheep anti-rat glomerular lysate antiserum (NTS) intraorbitally, or sheep IgG was used as a control. Following NTS injection, there was an increase in albuminuria within 24 h in wild-type mice (Fig. 10A). Though Shp2fl/fl,Cre+ mice had a slight increase in albuminuria at the 24-h time point, it resolved completely by 72 h, when it was similar to that of the control in magnitude (Fig. 11C). SEM (Fig. 10B) and TEM (Fig. 11A) 48 h following NTS injection showed a lack of foot process effacement in Shp2fl/fl,Cre+ mice compared to Shp2fl/fl,Cre− mice (control). Morphometric analysis of the junction frequency per micrometer of glomerular basement membrane surface (GBM) showed preservation of foot process morphology in Shp2fl/fl,Cre+ mice (Fig. 11B). Similar to the protamine sulfate model, glomerular lysates showed an increase in nephrin Y1193 and Y1217 phosphorylation following NTS injection in the Shp2fl/fl,Cre− wild-type animals. The increase in nephrin phosphorylation was abrogated in lysates from Shp2fl/fl,Cre+ mouse glomeruli (Fig. 11D). At the same time, there was a rise in Shp2 Y542 and Src Y416 phosphorylation and a decrease in Src Y527 phosphorylation in control animals, suggesting activation of both Shp2 and Src kinase in this model. Quantification of the bands using densitometry is shown in Fig. S2 in the supplemental material. These observations are consistent with our findings in the protamine sulfate model of podocye injury and, additionally, provide functional data in regard to proteinuria, which is not feasible in the protamine sulfate model.
FIG 10.
Podocyte-specific Shp2 deletion attenuates proteinuria induced by NTS. (A) Urine was collected from mice 24 and 48 h after injection of NTS or control sheep IgG. Ten microliters of urine was loaded in each lane. (Quantification of albumin and creatinine in urine is shown in Fig. S1 in the supplemental material.) (B) SEM of Shp2fl/fl and Shp2fl/fl, podocin CreTg/+ mouse glomeruli 48 h after injection of NTS or control sheep IgG. Original magnifications, ×3,000 (column 1), ×7,000 (column 2), and ×20,000 (column 3). The data are representative of 6 (A) and 3 (B) mice per group.
FIG 11.
(A) TEM of Shp2fl/fl and Shp2fl/fl, podocin CreTg/+ mouse glomeruli at 48 h after injection of NTS or control sheep IgG. Original magnification, ×4,800. (B) Numbers of junctions per micrometer of glomerular basement membrane, as seen by TEM 48 h after injection (means ± standard errors of the mean). (C) Quantification of proteinuria in NTS model of podocyte injury. Albumin and creatinine were quantified in urine samples obtained from Shp2fl/fl,Cre− and Shp2fl/fl,Cre+ mice following injection of sheep IgG (control) and NTS at various time points. There was a sharp rise in urinary albumin leakage following NTS injection in the Shp2fl/fl,Cre− mice that resolved over the next 5 to 7 days. There was a milder increase in albuminuria in the Shp2fl/fl,Cre+ mice following NTS injection that resolved completely by day 3. There was no increase in urine albumin leakage in the mice injected with sheep IgG that served as a control. The data are representative of 4 experiments with 3 mice per group. There was a statistically significant difference (*, P < 0.001) in albuminuria between the NTS-injected Shp2 WT and Shp2 KO animals at the 24-h, 48-h, and 72-h time points. There was a different statistically significant albuminuria between NTS- and sheep IgG-injected WT Shp2 mice at the 24-h, 48-h, and 72-h time points (**, P < 0.001). At 24 h and 48 h, NTS- and sheep IgG-injected Shp2 KO mice had statistically different albuminurias (***, P < 0.001). The data are means ± standard errors of the mean. (D) Glomeruli from Shp2fl/fl,Cre− and Shp2fl/fl,Cre+ mouse kidneys perfused with sheep IgG (control) and NTS were isolated using Dynabeads. Glomerular lysates were prepared, resolved using SDS-PAGE, and blotted with the indicated antibodies. Increase in nephrin phosphorylation (Y1193 and Y1217) was abrogated in mice with Shp2 deleted. The two blots shown for total nephrin represent separate experiments in which nephrin phosphorylation was assessed. There was a decrease in Src Y416 phosphorylation and enhanced Y527 phosphorylation in glomerular lysates from Shp2fl/fl,Cre+ mice following NTS perfusion compared to control and NTS-perfused Shp2fl/fl,Cre− mice. Shp2 Y542 phosphorylation was also decreased in Shp2fl/fl,Cre+ glomerular lysates following NTS perfusion compared to control and NTS-perfused Shp2fl/fl,Cre− mice. The data are representative of 3 mice per group. (Quantification of bands using densitometry from three separate experiments is shown in Fig. S2 in the supplemental material.)
DISCUSSION
Podocyte foot process spreading is a marker for human glomerular diseases that present with protein leakage. To develop treatment modalities that can prevent proteinuria, a good understanding of the signaling mechanisms that are involved in foot process spreading is of paramount importance. In vitro studies have shown that nephrin tyrosine phosphorylation transduces a signal that results in recruitment of a protein complex that is able to polymerize actin (2, 4, 6). Deletion of both nephrin and the adaptor protein Nck in podocytes results in a similar phenotype where foot processes fail to develop normally (4, 8). Nck plays a role in assembling a protein complex that is necessary for actin polymerization (44). Deletion of the Src kinase Fyn also results in foot process structural abnormality, which was noted to be independent of immune-mediated mechanisms (9). Both biochemical and genetic studies have established that Fyn binds to and tyrosine phosphorylates nephrin. Deletion of Fyn resulted in marked attenuation of nephrin tyrosine phosphorylation in detergent-resistant membrane fractions of glomerular lysates (10). Studies regarding nephrin tyrosine phosphorylation during mouse glomerular development and following injury remain controversial. The studies have shown both increases and decreases in nephrin phosphorylation following injury in both animal models and human glomerular diseases (6, 16, 35, 36, 45, 46). There are several possibilities that could explain the difference in observations made by various investigators in this regard. It is possible that less nephrin is detectable by immunochemistry because most of it becomes insoluble after phosphorylation and is trapped in the pellet along with actin. Alternatively, there could be differences in the affinities of various phosphonephrin antibodies due to unavailability of the epitope when tyrosine-phosphorylated nephrin is associated with the recruited complex along with actin. We have consistently observed an increase in nephrin tyrosine phosphorylation following injury when using the detergent-resistant membrane fractions as a result of enrichment of nephrin in the insoluble membrane fraction following phosphorylation. Nevertheless, the issue of nephrin phosphorylation remains controversial and requires further investigation.
In this study, we have focused on the interaction between nephrin and the receptor tyrosine phosphatase Shp2 and Shp2's effect on nephrin tyrosine phosphorylation. We have also examined the consequence of deletion of Shp2 in mouse podocytes. Kinases and phosphatases regulate all aspects of cellular function by switching on or switching off numerous signaling cascades. Cellular signaling events are tightly regulated by feedback loops, with simultaneous activation of both positive and negative regulatory signaling cascades (47). Shp2 has been shown to play a regulatory role in the feedback loop that activates Src kinases (21–24). A recent study showed a role for Shp2 in activating the Src family kinase Fyn in response to α6β4 integrin engagement (24). Specifically, Shp2 binds to a phosphorylated tyrosine residue on α6β4 integrin via its N-terminal Sh2 domain, resulting in activation of catalytic activity. Fyn is then recruited through an interaction with the phospho-Y580 residue in the C terminus of Shp2. Furthermore, Fyn phosphorylated Shp2 on Y542, resulting in a positive-feedback loop that contributed to sustained Shp2 and Fyn activity (24). In Ras-ERK signaling, Shp2 sequesters C-terminal Src kinase (Csk), a negative regulator of c-Src, thus positively regulating Src kinase activity.
Studies have revealed that both Shp1 and Shp2 are essentially inactive under basal conditions resulting from autoinhibition of the catalytic domain by the N-terminal sh2 domain. The catalytic activity is increased on binding of the sh2 domain to phosphotyrosine peptides. Singly phosphorylated peptides increase Shp2 activity 10-fold (48, 49), whereas engagement of both sh2 domains increases the activity by 100-fold (50, 51). Furthermore, there is evidence to suggest that binding of both sh2 domains of Shp2 to the receptor protein results in higher phosphatase activity and a stronger, more intense signal (50, 52, 53). Binding of Shp2 to nephrin phosphotyrosines Y1154 and Y1172 suggests a similar function of Shp2 in amplification of nephrin phosphorylation-dependent signaling cascades. In our study, mouse podocytes with Shp2 deleted did not develop foot process developmental abnormalities. We believe this is most likely due to the delayed deletion of Shp2 after podocyte and glomerular maturation has advanced to the capillary loop stage, at which time the Cre recombinase driven by the podocin promoter is first expressed and induces DNA recombination (31). Ultimately, the loss of targeted protein, in this case Shp2, likely depends on the half-life or stability of the mRNA and protein being deleted (54, 55). Completion of the initial developmental events while Shp2 is still available might explain the normal podocyte development. A recent publication showed the absence of an immediate phenotype following inducible RNA interference-mediated nephrin knockdown in a mature adult mouse (45). In the same study, the nephrin knockdown mice were susceptible to developing glomerulosclerosis following doxorubicin injury, suggesting a prominent role of nephrin during development and following injury (45). Consistent with these observations, podocytes with Shp2 deleted do not undergo foot process spreading in either the protamine sulfate or NTS model of podocyte injury. At the same time, there was no increase in nephrin phosphorylation following injury when Shp2 was deleted or its activity was chemically inhibited. Our study did not identify the kinase responsible for Shp2 activation within the nephrin-associated protein complex, especially since nephrin does not have its own kinase activity, nor did it identify the kinase responsible for phosphorylation of the nephrin Y1154/Y1172 residues, which are necessary for the nephrin-Shp2 interaction. It is plausible that basal Src kinase activity may be responsible for phosphorylation of the nephrin Y1154/Y1172 residues. Phosphorylation on nephrin Y1154 was shown to occur in vivo by γ-32P metabolic labeling (6). At the same time, in an in vitro tryptic peptide mapping method, the Src kinase Fyn was able to phosphorylate the nephrin Y1154 residue (6).
Following injury, podocytes retain their structure when focal adhesion regulatory proteins, such as focal adhesion kinase (FAK), Crk, and uPAR, are deleted in the mouse (11, 56, 57). Within this context, it is noteworthy that cells with Shp2 deleted display an increased number of focal adhesions reminiscent of focal adhesion kinase-deficient cells (58). Src kinases have also been shown to positively regulate FAK activity and FA turnover (59–62). A recent study found protection of podocytes from injury following inhibition of protein tyrosine phosphate 1B (PTP1B), as overexpression of PTP1B resulted in increased FAK phosphorylation and activity of Src kinases. Beyond Shp2's impact on nephrin phosphorylation, as suggested by our data, an increase in Shp2 activity has been shown to play an important role in activation of focal adhesion and integrin complex (24, 26). Our observation that mouse podocytes with Shp2 deleted are resistant to injury is consistent with these previous observations. We observed an increase in glomerular Shp2 activity in kidney sections obtained from patients with minimal-change nephrosis and membranous nephropathy but not in kidney sections from patients with focal segmental glomerulosclerosis. Interestingly, George et al. also reported absence of FAK and Cas phosphorylation in glomeruli in biopsy samples from patients with focal segmental glomerulosclerosis (11). A recent publication demonstrated an increase in Shp1 expression in podocytes in response to hyperglycemia, resulting in decreased nephrin tyrosine phosphorylation at residue Y1217 (16). Whether Shp1 affects Src kinase activity similarly to Shp2 has not been shown. Reiser et al. reported an increase in Shp2 and a decrease in Shp1 expression levels following puromycin injury (63). The specific roles of Shp tyrosine phosphatases in different disease models requires further investigation. The differences in Shp2 and focal adhesion complex activation within proteinuric glomerular diseases suggest that the signaling mechanisms involved are not homogeneous.
Our study provides valuable mechanistic insights into the signaling mechanisms that come into play during podocyte foot process structural changes following injury. The observation that nephrin is tyrosine phosphorylated following injury has been reported previously (6, 11). Furthermore, it has been established that the Src kinase Fyn is responsible for phosphorylation of nephrin tyrosine residues Y1193 and Y1208, which are necessary for the nephrin-Nck interaction. Kurihara et al. observed a correlation between increased tyrosine phosphorylation of podocyte junctional proteins and induction of foot process effacement in a rat model (64). The functional significance of nephrin phosphorylation, as well as the mechanism by which Fyn activation was induced in this setting, was undefined. The data presented here suggest that in the absence of Shp2 following injury, activation of Src kinases, as well as nephrin tyrosine phosphorylation, is attenuated. Furthermore, Shp2 deletion or inhibition of Shp2 activity in mouse podocytes resulted in lack of podocyte effacement. In the NTS model, the absence of foot process spreading in mouse podocytes with Shp2 deleted also resulted in reduced proteinuria, indicating preserved filter integrity. These results suggest that Shp2 is necessary for activation of the Src kinase Fyn, which is needed for nephrin tyrosine phosphorylation in response to a stimulus that results in podocyte foot process effacement and albuminuria. It is tempting to speculate that there is a causal relationship between nephrin phosphorylation and foot process spreading. However, this remains to be proven, since deletion of Shp2 could affect the podocyte structure through an effect on other signaling events within the cell. Our observation that both genetic deletion and chemical inhibition of Shp2 prevent foot process effacement following injury suggests that altering Shp2-dependent signaling events may attenuate foot process spreading in some human glomerular diseases. Since global inhibition of Shp2 activity is likely to be detrimental, further studies to develop either cell-specific inhibition of Shp2 or targeting other components of the signaling cascade are needed.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by grants to P. Garg from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) (DK097027, DK081403, and DK096053) and an American Society of Nephrology Carl Gottshalk Research Scholar Award (P. Garg). The work was also supported by grants to D. J. Salant (DK090029) and S. R. Patel (DK082409). Support was also obtained from the University of Michigan's George M. O'Brien Translational Research Core Center Grant P30 DK0801943 and MDRTC Cell and Molecular Biology Core Grant P60 DK020572.
Funding Statement
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of manuscript.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/MCB.00956-15.
REFERENCES
- 1.Holzman LB, St John PL, Kovari IA, Verma R, Holthofer H, Abrahamson DR. 1999. Nephrin localizes to the slit pore of the glomerular epithelial cell. Kidney Int 56:1481–1491. doi: 10.1046/j.1523-1755.1999.00719.x. [DOI] [PubMed] [Google Scholar]
- 2.Garg P, Verma R, Cook L, Soofi A, Venkatareddy M, George B, Mizuno K, Gurniak C, Witke W, Holzman LB. 2010. Actin-depolymerizing factor cofilin-1 is necessary in maintaining mature podocyte architecture. J Biol Chem 285:22676–22688. doi: 10.1074/jbc.M110.122929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garg P, Verma R, Nihalani D, Johnstone DB, Holzman LB. 2007. Neph1 cooperates with nephrin to transduce a signal that induces actin polymerization. Mol Cell Biol 27:8698–8712. doi: 10.1128/MCB.00948-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jones N, Blasutig IM, Eremina V, Ruston JM, Bladt F, Li H, Huang H, Larose L, Li SS, Takano T, Quaggin SE, Pawson T. 2006. Nck adaptor proteins link nephrin to the actin cytoskeleton of kidney podocytes. Nature 440:818–823. doi: 10.1038/nature04662. [DOI] [PubMed] [Google Scholar]
- 5.Venkatareddy M, Cook L, Abuarquob K, Verma R, Garg P. 2011. Nephrin regulates lamellipodia formation by assembling a protein complex that includes ship2, filamin and lamellipodin. PLoS One 6:e28710. doi: 10.1371/journal.pone.0028710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Verma R, Kovari I, Soofi A, Nihalani D, Patrie K, Holzman LB. 2006. Nephrin ectodomain engagement results in Src kinase activation, nephrin phosphorylation, Nck recruitment, and actin polymerization. J Clin Invest 116:1346–1359. doi: 10.1172/JCI27414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kestila M, Lenkkeri U, Mannikko M, Lamerdin J, McCready P, Putaala H, Ruotsalainen V, Morita T, Nissinen M, Herva R, Kashtan CE, Peltonen L, Holmberg C, Olsen A, Tryggvason K. 1998. Positionally cloned gene for a novel glomerular protein—nephrin—is mutated in congenital nephrotic syndrome. Mol Cell 1:575–582. doi: 10.1016/S1097-2765(00)80057-X. [DOI] [PubMed] [Google Scholar]
- 8.Hamano Y, Grunkemeyer JA, Sudhakar A, Zeisberg M, Cosgrove D, Morello R, Lee B, Sugimoto H, Kalluri R. 2002. Determinants of vascular permeability in the kidney glomerulus. J Biol Chem 277:31154–31162. doi: 10.1074/jbc.M204806200. [DOI] [PubMed] [Google Scholar]
- 9.Li H, Lemay S, Aoudjit L, Kawachi H, Takano T. 2004. SRC-family kinase Fyn phosphorylates the cytoplasmic domain of nephrin and modulates its interaction with podocin. J Am Soc Nephrol 15:3006–3015. doi: 10.1097/01.ASN.0000146689.88078.80. [DOI] [PubMed] [Google Scholar]
- 10.Verma R, Wharram B, Kovari I, Kunkel R, Nihalani D, Wary KK, Wiggins RC, Killen P, Holzman LB. 2003. Fyn binds to and phosphorylates the kidney slit diaphragm component nephrin. J Biol Chem 278:20716–20723. doi: 10.1074/jbc.M301689200. [DOI] [PubMed] [Google Scholar]
- 11.George BVR, Soofi A, Garg P, Zhang J, Park TJ, Giardino L, Ryzhova L, Johnstone DB, Wong H, Nihalani D, Salant DJ, Hanks SK, Curran T, Rastaldi MP, Holzman LB. 2012. Crk1/2-dependent signaling is necessary for podocyte foot process spreading in mouse models of glomerular disease. J Clin Invest 122:674–692. doi: 10.1172/JCI60070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garg P, Rabelink T. 2011. Glomerular proteinuria: a complex interplay between unique players. Adv Chronic Kidney Dis 18:233–242. doi: 10.1053/j.ackd.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garg P, Verma R, Holzman LB. 2007. Slit diaphragm junctional complex and regulation of the cytoskeleton. Nephron Exp Nephrol 106:e67–e72. doi: 10.1159/000101795. [DOI] [PubMed] [Google Scholar]
- 14.Stein PL, Vogel H, Soriano P. 1994. Combined deficiencies of Src, Fyn, and Yes tyrosine kinases in mutant mice. Genes Dev 8:1999–2007. doi: 10.1101/gad.8.17.1999. [DOI] [PubMed] [Google Scholar]
- 15.Shen SH, Bastien L, Posner BI, Chretien P. 1991. A protein-tyrosine phosphatase with sequence similarity to the SH2 domain of the protein-tyrosine kinases. Nature 352:736–739. doi: 10.1038/352736a0. [DOI] [PubMed] [Google Scholar]
- 16.Denhez B, Lizotte F, Guimond MO, Jones N, Takano T, Geraldes P. 2015. Increased SHP-1 protein expression by high glucose levels reduces nephrin phosphorylation in podocytes. J Biol Chem 290:350–358. doi: 10.1074/jbc.M114.612721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feng GS. 1999. Shp-2 tyrosine phosphatase: signaling one cell or many. Exp Cell Res 253:47–54. doi: 10.1006/excr.1999.4668. [DOI] [PubMed] [Google Scholar]
- 18.Neel BG, Gu H, Pao L. 2003. The ‘Shp'ing news: SH2 domain-containing tyrosine phosphatases in cell signaling. Trends Biochem Sci 28:284–293. doi: 10.1016/S0968-0004(03)00091-4. [DOI] [PubMed] [Google Scholar]
- 19.Saxton TM, Henkemeyer M, Gasca S, Shen R, Rossi DJ, Shalaby F, Feng GS, Pawson T. 1997. Abnormal mesoderm patterning in mouse embryos mutant for the SH2 tyrosine phosphatase Shp-2. EMBO J 16:2352–2364. doi: 10.1093/emboj/16.9.2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang W, Klaman LD, Chen B, Araki T, Harada H, Thomas SM, George EL, Neel BG. 2006. An Shp2/SFK/Ras/Erk signaling pathway controls trophoblast stem cell survival. Dev Cell 10:317–327. doi: 10.1016/j.devcel.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 21.Dance M, Montagner A, Salles JP, Yart A, Raynal P. 2008. The molecular functions of Shp2 in the Ras/Mitogen-activated protein kinase (ERK1/2) pathway. Cell Signal 20:453–459. doi: 10.1016/j.cellsig.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 22.Zhang SQ, Yang W, Kontaridis MI, Bivona TG, Wen G, Araki T, Luo J, Thompson JA, Schraven BL, Philips MR, Neel BG. 2004. Shp2 regulates SRC family kinase activity and Ras/Erk activation by controlling Csk recruitment. Mol Cell 13:341–355. doi: 10.1016/S1097-2765(04)00050-4. [DOI] [PubMed] [Google Scholar]
- 23.Pawson T. 1994. Tyrosine kinase signalling pathways. Princess Takamatsu Symp 24:303–322. [PubMed] [Google Scholar]
- 24.Yang X, Dutta U, Shaw LM. 2010. SHP2 mediates the localized activation of Fyn downstream of the alpha6beta4 integrin to promote carcinoma invasion. Mol Cell Biol 30:5306–5317. doi: 10.1128/MCB.00326-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Noel LA, Arts FA, Montano-Almendras CP, Cox L, Gielen O, Toffalini F, Marbehant CY, Cools J, Demoulin JB. 2014. The tyrosine phosphatase SHP2 is required for cell transformation by the receptor tyrosine kinase mutants FIP1L1-PDGFRalpha and PDGFRalpha D842V. Mol Oncol 8:728–740. doi: 10.1016/j.molonc.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oh ES, Gu H, Saxton TM, Timms JF, Hausdorff S, Frevert EU, Kahn BB, Pawson T, Neel BG, Thomas SM. 1999. Regulation of early events in integrin signaling by protein tyrosine phosphatase SHP-2. Mol Cell Biol 19:3205–3215. doi: 10.1128/MCB.19.4.3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qu CK, Yu WM, Azzarelli B, Feng GS. 1999. Genetic evidence that Shp-2 tyrosine phosphatase is a signal enhancer of the epidermal growth factor receptor in mammals. Proc Natl Acad Sci U S A 96:8528–8533. doi: 10.1073/pnas.96.15.8528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O'Meara YM, Natori Y, Minto AW, Goldstein DJ, Manning EC, Salant DJ. 1992. Nephrotoxic antiserum identifies a beta 1-integrin on rat glomerular epithelial cells. Am J Physiol 262:F1083–F1091. [DOI] [PubMed] [Google Scholar]
- 29.Lu W, Gong D, Bar-Sagi D, Cole PA. 2001. Site-specific incorporation of a phosphotyrosine mimetic reveals a role for tyrosine phosphorylation of SHP-2 in cell signaling. Mol Cell 8:759–769. doi: 10.1016/S1097-2765(01)00369-0. [DOI] [PubMed] [Google Scholar]
- 30.Harita Y, Kurihara H, Kosako H, Tezuka T, Sekine T, Igarashi T, Ohsawa I, Ohta S, Hattori S. 2009. Phosphorylation of nephrin triggers Ca2+ signaling by recruitment and activation of phospholipase C-{gamma}1. J Biol Chem 284:8951–8962. doi: 10.1074/jbc.M806851200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moeller MJ, Sanden SK, Soofi A, Wiggins RC, Holzman LB. 2003. Podocyte-specific expression of Cre recombinase in transgenic mice. Genesis 35:39–42. doi: 10.1002/gene.10164. [DOI] [PubMed] [Google Scholar]
- 32.Kerppola TK. 2006. Design and implementation of bimolecular fluorescence complementation (BiFC) assays for the visualization of protein interactions in living cells. Nat Protoc 1:1278–1286. doi: 10.1038/nprot.2006.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kerppola TK. 2008. Bimolecular fluorescence complementation (BiFC) analysis as a probe of protein interactions in living cells. Annu Rev Biophys 37:465–487. doi: 10.1146/annurev.biophys.37.032807.125842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fornaro M, Burch PM, Yang W, Zhang L, Hamilton CE, Kim JH, Neel BG, Bennett AM. 2006. SHP-2 activates signaling of the nuclear factor of activated T cells to promote skeletal muscle growth. J Cell Biol 175:87–97. doi: 10.1083/jcb.200602029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.New LA, Keyvani Chahi A, Jones N. 2013. Direct regulation of nephrin tyrosine phosphorylation by Nck adaptor proteins. J Biol Chem 288:1500–1510. doi: 10.1074/jbc.M112.439463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aoudjit L, Jiang R, Lee TH, New LA, Jones N, Takano T. 2011. Podocyte protein, nephrin, is a substrate of protein tyrosine phosphatase 1B. J Signal Transduct 2011:376543. doi: 10.1155/2011/376543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Topham PS, Csizmadia V, Soler D, Hines D, Gerard CJ, Salant DJ, Hancock WW. 1999. Lack of chemokine receptor CCR1 enhances Th1 responses and glomerular injury during nephrotoxic nephritis. J Clin Invest 104:1549–1557. doi: 10.1172/JCI7707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yanagita M, Ishimoto Y, Arai H, Nagai K, Ito T, Nakano T, Salant DJ, Fukatsu A, Doi T, Kita T. 2002. Essential role of Gas6 for glomerular injury in nephrotoxic nephritis. J Clin Invest 110:239–246. doi: 10.1172/JCI0214861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Takemoto M, Asker N, Gerhardt H, Lundkvist A, Johansson BR, Saito Y, Betsholtz C. 2002. A new method for large scale isolation of kidney glomeruli from mice. Am J Pathol 161:799–805. doi: 10.1016/S0002-9440(10)64239-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kerppola TK. 2008. Bimolecular fluorescence complementation: visualization of molecular interactions in living cells. Methods Cell Biol 85:431–470. doi: 10.1016/S0091-679X(08)85019-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu D, Pang Y, Ke Y, Yu J, He Z, Tautz L, Mustelin T, Ding S, Huang Z, Feng GS. 2009. A conserved mechanism for control of human and mouse embryonic stem cell pluripotency and differentiation by Shp2 tyrosine phosphatase. PLoS One 4:e4914. doi: 10.1371/journal.pone.0004914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li J, Kang Y, Wei L, Liu W, Tian Y, Chen B, Lin X, Li Y, Feng GS, Lu Z. 2014. Tyrosine phosphatase Shp2 mediates the estrogen biological action in breast cancer via interaction with the estrogen extranuclear receptor. PLoS One 9:e102847. doi: 10.1371/journal.pone.0102847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen L, Sung SS, Yip ML, Lawrence HR, Ren Y, Guida WC, Sebti SM, Lawrence NJ, Wu J. 2006. Discovery of a novel shp2 protein tyrosine phosphatase inhibitor. Mol Pharmacol 70:562–570. doi: 10.1124/mol.106.025536. [DOI] [PubMed] [Google Scholar]
- 44.Buday L, Wunderlich L, Tamas P. 2002. The Nck family of adapter proteins: regulators of actin cytoskeleton. Cell Signal 14:723–731. doi: 10.1016/S0898-6568(02)00027-X. [DOI] [PubMed] [Google Scholar]
- 45.Li X, Chuang PY, D'Agati VD, Dai Y, Yacoub R, Fu J, Xu J, Taku O, Premsrirut PK, Holzman LB, He JC. 2015. Nephrin preserves podocyte viability and glomerular structure and function in adult kidneys. J Am Soc Nephrol 26:2361–2377. doi: 10.1681/ASN.2014040405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Uchida K, Suzuki K, Iwamoto M, Kawachi H, Ohno M, Horita S, Nitta K. 2008. Decreased tyrosine phosphorylation of nephrin in rat and human nephrosis. Kidney Int 73:926–932. doi: 10.1038/ki.2008.19. [DOI] [PubMed] [Google Scholar]
- 47.Marshall CJ. 1995. Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell 80:179–185. doi: 10.1016/0092-8674(95)90401-8. [DOI] [PubMed] [Google Scholar]
- 48.Sugimoto S, Wandless TJ, Shoelson SE, Neel BG, Walsh CT. 1994. Activation of the SH2-containing protein tyrosine phosphatase, SH-PTP2, by phosphotyrosine-containing peptides derived from insulin receptor substrate-1. J Biol Chem 269:13614–13622. [PubMed] [Google Scholar]
- 49.Lechleider RJ, Sugimoto S, Bennett AM, Kashishian AS, Cooper JA, Shoelson SE, Walsh CT, Neel BG. 1993. Activation of the SH2-containing phosphotyrosine phosphatase SH-PTP2 by its binding site, phosphotyrosine 1009, on the human platelet-derived growth factor receptor. J Biol Chem 268:21478–21481. [PubMed] [Google Scholar]
- 50.Pluskey S, Wandless TJ, Walsh CT, Shoelson SE. 1995. Potent stimulation of SH-PTP2 phosphatase activity by simultaneous occupancy of both SH2 domains. J Biol Chem 270:2897–2900. doi: 10.1074/jbc.270.7.2897. [DOI] [PubMed] [Google Scholar]
- 51.Eck MJ, Pluskey S, Trub T, Harrison SC, Shoelson SE. 1996. Spatial constraints on the recognition of phosphoproteins by the tandem SH2 domains of the phosphatase SH-PTP2. Nature 379:277–280. doi: 10.1038/379277a0. [DOI] [PubMed] [Google Scholar]
- 52.Barford D, Neel BG. 1998. Revealing mechanisms for SH2 domain mediated regulation of the protein tyrosine phosphatase SHP-2. Structure 6:249–254. doi: 10.1016/S0969-2126(98)00027-6. [DOI] [PubMed] [Google Scholar]
- 53.Barua D, Faeder JR, Haugh JM. 2007. Structure-based kinetic models of modular signaling protein function: focus on Shp2. Biophys J 92:2290–2300. doi: 10.1529/biophysj.106.093484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Turlo KA, Gallaher SD, Vora R, Laski FA, Iruela-Arispe ML. 2010. When Cre-mediated recombination in mice does not result in protein loss. Genetics 186:959–967. doi: 10.1534/genetics.110.121608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nagy A. 2000. Cre recombinase: the universal reagent for genome tailoring. Genesis 26:99–109. doi:. [DOI] [PubMed] [Google Scholar]
- 56.Ma H, Togawa A, Soda K, Zhang J, Lee S, Ma M, Yu Z, Ardito T, Czyzyk J, Diggs L, Joly D, Hatakeyama S, Kawahara E, Holzman L, Guan JL, Ishibe S. 2010. Inhibition of podocyte FAK protects against proteinuria and foot process effacement. J Am Soc Nephrol 21:1145–1156. doi: 10.1681/ASN.2009090991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wei C, Moller CC, Altintas MM, Li J, Schwarz K, Zacchigna S, Xie L, Henger A, Schmid H, Rastaldi MP, Cowan P, Kretzler M, Parrilla R, Bendayan M, Gupta V, Nikolic B, Kalluri R, Carmeliet P, Mundel P, Reiser J. 2008. Modification of kidney barrier function by the urokinase receptor. Nat Med 14:55–63. doi: 10.1038/nm1696. [DOI] [PubMed] [Google Scholar]
- 58.Yu DH, Qu CK, Henegariu O, Lu X, Feng GS. 1998. Protein-tyrosine phosphatase Shp-2 regulates cell spreading, migration, and focal adhesion. J Biol Chem 273:21125–21131. doi: 10.1074/jbc.273.33.21125. [DOI] [PubMed] [Google Scholar]
- 59.Schlaepfer DD, Mitra SK. 2004. Multiple connections link FAK to cell motility and invasion. Curr Opin Genet Dev 14:92–101. doi: 10.1016/j.gde.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 60.Hsia DA, Mitra SK, Hauck CR, Streblow DN, Nelson JA, Ilic D, Huang S, Li E, Nemerow GR, Leng J, Spencer KS, Cheresh DA, Schlaepfer DD. 2003. Differential regulation of cell motility and invasion by FAK. J Cell Biol 160:753–767. doi: 10.1083/jcb.200212114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mitra SK, Mikolon D, Molina JE, Hsia DA, Hanson DA, Chi A, Lim ST, Bernard-Trifilo JA, Ilic D, Stupack DG, Cheresh DA, Schlaepfer DD. 2006. Intrinsic FAK activity and Y925 phosphorylation facilitate an angiogenic switch in tumors. Oncogene 25:5969–5984. doi: 10.1038/sj.onc.1209588. [DOI] [PubMed] [Google Scholar]
- 62.Wu L, Bernard-Trifilo JA, Lim Y, Lim ST, Mitra SK, Uryu S, Chen M, Pallen CJ, Cheung NK, Mikolon D, Mielgo A, Stupack DG, Schlaepfer DD. 2008. Distinct FAK-Src activation events promote alpha5beta1 and alpha4beta1 integrin-stimulated neuroblastoma cell motility. Oncogene 27:1439–1448. doi: 10.1038/sj.onc.1210770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Reiser J, Pixley FJ, Hug A, Kriz W, Smoyer WE, Stanley ER, Mundel P. 2000. Regulation of mouse podocyte process dynamics by protein tyrosine phosphatases rapid communication. Kidney Int 57:2035–2042. doi: 10.1046/j.1523-1755.2000.00070.x. [DOI] [PubMed] [Google Scholar]
- 64.Kurihara H, Anderson JM, Farquhar MG. 1995. Increased Tyr phosphorylation of ZO-1 during modification of tight junctions between glomerular foot processes. Am J Physiol 268:F514–F524. [DOI] [PubMed] [Google Scholar]
- 65.Lahdenperä J, Kilpeläinen P, Liu XL, Pikkarainen T, Reponen P, Ruotsalainen V, Tryggvason K. 2003. Clustering-induced tyrosine phosphorylation of nephrin by Src family kinases. Kidney Int 64:404–413. doi: 10.1046/j.1523-1755.2003.00097.x. [DOI] [PubMed] [Google Scholar]
- 66.Rivera GM, Briceno CA, Takeshima F, Snapper SB, Mayer BJ. 2004. Inducible clustering of membrane-targeted SH3 domains of the adaptor protein Nck triggers localized actin polymerization. Curr Biol 14:11–22. doi: 10.1016/j.cub.2003.12.033. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.