Path analysis segregates duloxetine's direct and indirect effects on pain and depression. The direct and indirect effects on depressive symptoms are the inverse of pain effects.
Keywords: Duloxetine, Depression, Pain, Path analysis
Abstract
In treating Major Depressive Disorder with associated painful physical symptoms (PPS), the effect of duloxetine on PPS has been shown to decompose into a direct effect on PPS and an indirect effect on PPS via depressive symptoms (DS) improvement. To evaluate the changes in relative contributions of the direct and indirect effects over time, we analyzed pooled data from 3 randomized double-blind studies comparing duloxetine 60 mg/d with placebo in patients with major depressive disorder and PPS. Changes from baseline in Montgomery–Åsberg Depression Rating Scale total and Brief Pain Inventory-Short Form average pain score were assessed over 8 weeks. Path analysis examined the (1) direct effect of treatment on PPS and/or indirect effect on PPS via DS improvement and (2) direct effect of treatment on DS and/or indirect effect on DS via PPS improvement. At week 1, the direct effect of duloxetine on PPS (75.3%) was greater than the indirect effect through DS improvement (24.7%) but became less (22.6%) than the indirect effect (77.4%) by week 8. Initially, the direct effect of duloxetine on PPS was markedly greater than its indirect effect, whereas later the indirect effect predominated. Conversely, at week 1, the direct effect of treatment on DS (46.4%) was less than the indirect effect (53.6%), and by week 8 it superseded (62.6%) the indirect effect (37.4%). Thus, duloxetine would relieve PPS directly in the initial phase and indirectly via improving DS in the later phase.
1. Introduction
Major depressive disorder (MDD), primarily described in terms of emotional features such as depressed mood,2,30 also includes physical symptoms (eg, idiopathic pain, changes in weight, sleep, appetite)20,22,35,45 that are the major complaints driving patients seeking medical care.16,21,35,52 Painful physical symptoms (PPS), generally present as unexplained headache, pain in the joints or abdomen, or pains in the chest, neck, or shoulder areas, are frequently encountered physical symptoms.6,20,21,26 PPS is linked with poorer clinical outcomes and contribute to the overall burden of MDD.8,12,29,34,37
Some studies suggest that the depressive symptoms (DS) and PPS present different trajectories.22,40,48 For example, MDD patients with PPS receiving serotonin-selective reuptake inhibitors for 9 months initially showed similar improvements in both DS and PPS. However, while DS continued to improve, PPS did not improve further after 1 month.28
Duloxetine is balanced in its affinity of binding to serotonin and norepinephrine transporter sites and potently inhibits serotonin and norepinephrine reuptake, thus enhancing monoaminergic tone.11,32,60 Randomized, double-blind placebo-controlled trials with MDD patients with PPS showed that duloxetine is effective in improving both DS and PPS.7,8 However, the extent of interactions between DS and PPS improvement in MDD is not well understood, and the reciprocal roles of pain relief and relief of DS on each other in MDD patients are currently under investigation.40
For example, in a 9-week treatment of MDD patients with duloxetine, 51% of duloxetine's total effect on overall pain was due to a direct effect and 49% was the result of an indirect effect mediated through change in depression severity.22 However, because not all the patients necessarily had PPS associated with MDD, these results may not be applied to the specific population of patients with MDD and associated PPS.
In a different study,50 the effect of an 8-week treatment with duloxetine in a population screened for MDD with PPS indicated that 59% of the likelihood of remission of MDD was because of the direct effect of treatment while indirect effect through pain reduction accounted for 41% of the effect. Although this study clarified the attribution of PPS improvement toward remission in patients with MDD, the path analysis was performed only at a single time point after treatment initiation; therefore, the time course of depression improvement indirectly attributed to PPS improvement over time remained unexamined.
To the best of our knowledge, time-related changes in the relative contributions of the direct effect of duloxetine on PPS and indirect effect on PPS via DS improvement, or of the reverse situation, have not been studied. Therefore, the present investigation was undertaken to ascertain the relative contributions of PPS and of DS at several time points after beginning duloxetine treatment for patients with MDD using path analysis.
2. Methods
2.1. Data sources and study selection
The Eli Lilly clinical trial database contains all clinical trials of duloxetine for patients with MDD that were conducted by Eli Lilly and Company or its partners outside Japan. We reviewed this database and selected randomized, double-blind parallel-group clinical trials that included duloxetine (≤60 mg/d) or placebo for the acute treatment of MDD with associated PPS. Studies using duloxetine 60 mg/d were chosen because this dose has been confirmed as effective in the treatment of MDD and is commonly used globally.8,17 As a result, a total of the following 4 studies were initially identified: HMDH,10 HMGR,25 HMGU,24 and HMCB.9 No studies meeting these criteria were excluded. For the evaluation of pain severity, all studies used the same scale, Brief Pain Inventory-Short Form (BPI-SF).13–15 However, for the evaluation of DS, 3 studies, namely HMDH, HMGR, and HMGU, used the Montgomery–Åsberg Depression Rating Scale (MADRS)43 and 1 study, HMCB, used the Hamilton Rating Scale for Depression. Because our aim was to conduct patient-level integrated analysis using the same analysis method and scales across studies, we included the former 3 studies (ie, HMDH, HMGR, and HMGU) in the analysis and excluded the latter 1 study (ie, HMCB) from the analysis.
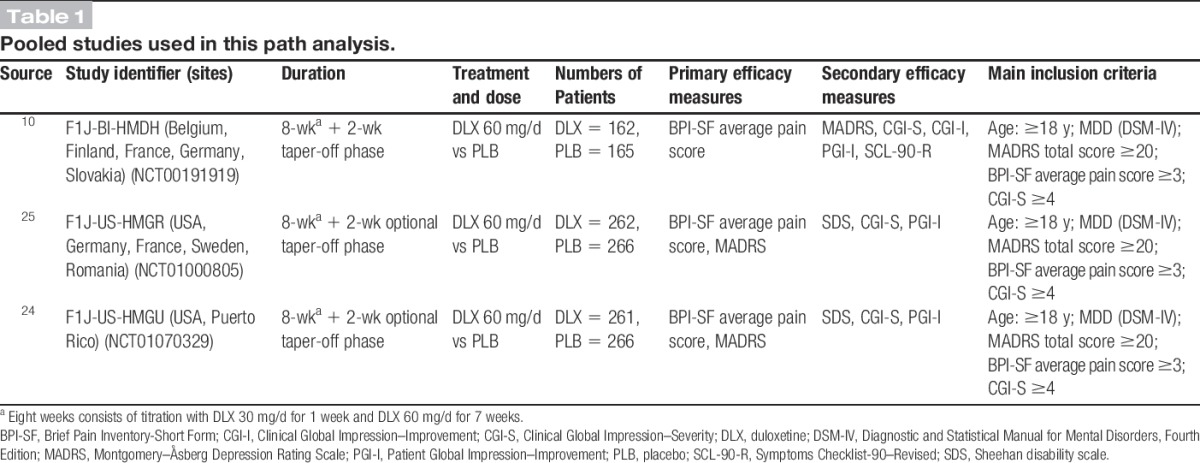
The common inclusion criteria for the 3 included studies HMDH, HMGR, and HMGU (Table 1) were male or female outpatients aged at least 18 years who met the Diagnostic and Statistical Manual for Mental Disorders, Fourth Edition1 criteria for MDD and had MADRS ≥20, at least moderate pain based on BPI-SF average pain score ≥3, and a Clinical Global Impression of Severity score ≥4. Exclusion criteria included the following: current Axis I disorder (other than MDD), history of bipolar disorder, schizophrenia, or other psychotic disorders; any diagnosed pain syndrome as per medical history (for HMDH) or presence of pain of a known origin (for HMGR and HMGU). The primary efficacy outcome measure for HMDH was BPI-SF average pain score, and the primary efficacy outcome measures for HMGR and HMGU were BPI-SF average pain score and MADRS total score. For all studies, data were collected at baseline and on weeks 1, 2, 4, and 8. Trial HMDH also included data collection on week 6. The studies are summarized in Table 1.
Table 1.
Pooled studies used in this path analysis.
The protocol was approved by the appropriate ethical review boards for each of the study centers. Patients gave written informed consent before participating in the studies. The studies were all conducted in accordance with the regulatory standards in each country and conformed to the standards dictated by Good Clinical Practice and the Declaration of Helsinki.4 The studies were registered at clinicaltrials.gov (NCT00191919, NCT01070329, and NCT01000805).
2.2. Treatment
Across 3 studies, all the enrolled patients were randomized to an oral dose of 60 mg/d of duloxetine or placebo. Patients randomly assigned to duloxetine 60 mg/d started on duloxetine 30 mg/d for 1 week and then were titrated up to duloxetine 60 mg/d for 7 weeks, followed by a 2-week taper period for HMDH or an optional taper-off period for HMGR and HMGU.
2.3. Assessments
To evaluate improvement in DS, change in MADRS total score from baseline was assessed. The MADRS is a rating scale for depression, and it uses 10 core symptoms of depression that are rated on a scale of 0 to 6 for a total maximal score of 60.
To evaluate improvement in PPS, change in BPI-SF average pain score from baseline was assessed. This inventory was designed to assess pain intensity and its interference with activities of daily living. The questionnaire uses a 0 (no pain or does not interfere) to 10 (pain as severe as you can imagine or completely interferes) scale and is a self-assessment tool used by the patient.13–15 For the purposes of the present investigation, only responses to BPI-SF item 5, 24-hour average pain score, were considered, because this was the primary outcome measure for pain used in studies HMDH, HMGR, and HMGU.10,24,25 Moreover, the single item, average pain score, is commonly used in randomized clinical trials for the assessment of pain relief,5,27,51,53,57 and its use is endorsed by the IMMPACT recommendations for clinical pain trials18,19,53,58 as well as by the FDA Draft Guidance for Industry: Patient-Reported Outcome Measures.23 Depression (MADRS) and average pain score (BPI-SF) were assessed at baseline (Day 0) and at 1, 2, 4, and 8 weeks of treatment.
Path analysis is related to multiple regression methods and is a means to decompose correlations to determine the correlations among variables on an effect. In the present investigation, path analysis was first performed to determine the direct effect of duloxetine treatment on PPS and the indirect effects on PPS due to DS improvement over time. An alternate path analysis was performed to determine the direct effect of duloxetine treatment on DS improvement and the indirect effect on DS through PPS improvement over time. The analyses were performed at 1, 2, 4, and 8 weeks.
2.4. Statistical methods
All randomized patients with MADRS total score ≥20 and with BPI-SF average pain score ≥3 who received placebo or duloxetine (60 mg/d) were included in the analyses. Patients for whom there were no baseline MADRS data, or no baseline BPI-SF baseline data, or for whom there were no post–baseline MADRS or BPI-SF data were not included in the analyses. The measures of efficacy were based on a mixed-effects model repeated-measures approach. The model included the fixed effects of “study,” “treatment,” “week,” “baseline,” “treatment-by-week interaction,” and “baseline-by-week interaction.” All statistical tests were conducted at the significance level of 2-sided 5%, and no adjustments for multiplicity were conducted.
The path analyses used in this analysis are based on previously described studies.40,50 Path analyses were performed with 2 path analysis models. The primary path analysis was performed to examine the direct and indirect treatment effect on PPS improvement. In this case, the prespecified causal relationships were defined as treatment having a direct effect on PPS improvement and an indirect effect on PPS improvement due to DS improvement. In this path analysis model (Figure 1A), PPS improvement and DS improvement were described with the following 2 linear regression models:
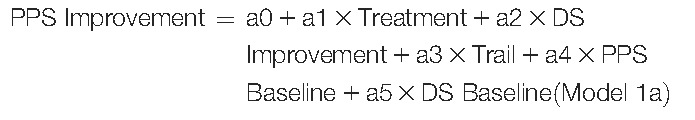 |
and
 |
Figure 1.
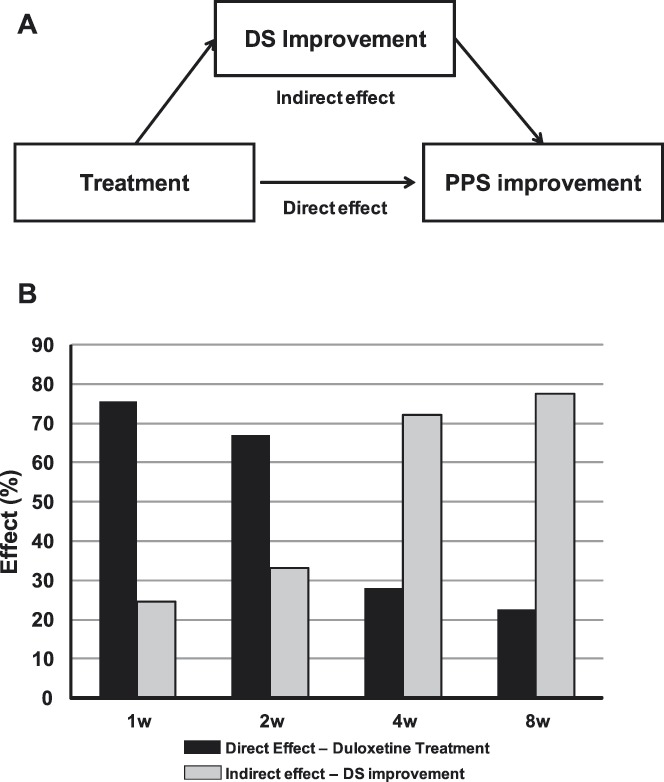
(A) Path analysis diagram that illustrates direct and indirect effects of treatment on PPS improvement. (B) Path analysis results showed a marked direct effect of treatment on PPS improvement at 1 and 2 weeks of treatment. The relationship was reversed for 4 and 8 weeks, indicated by the marked indirect effect by way of DS improvement. DS, depressive symptoms; PPS, physical pain symptoms; w, week(s).
The direct and indirect effects were calculated as % of the total from models 1 and 2 such that:
and
For each week where the analyses were performed, PPS or DS improvement was defined as the “Change from baseline in BPI-SF average pain score/MADRS total score” at that week.
An alternate path analysis model was evaluated where the prespecified causal relationships were defined as treatment having a direct effect on DS improvement and an indirect effect on DS improvement due to PPS improvement. In this path analysis model (Figure 2A), DS improvement and PPS improvement were described with the following 2 linear regression models:
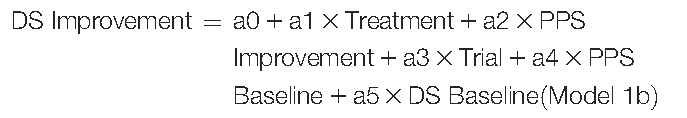 |
and
 |
Figure 2.
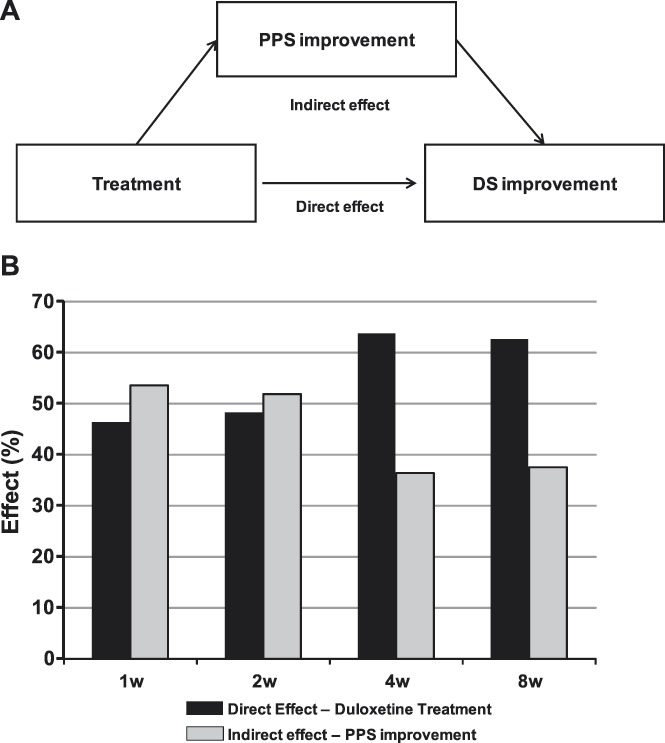
(A) Path analysis diagram that illustrates direct and indirect effects of treatment on DS improvement. (B) Path analysis results showed a slightly greater indirect effect by way of PPS improvement at 1 and 2 weeks of treatment. The relationship is reversed for 4 and 8 weeks, indicated by the increased direct effect of treatment on DS improvement relative to the indirect effect by way of PPS improvement. DS, depressive symptoms; PPS, physical pain symptoms; w, week(s).
The %direct and %indirect effects and the PPS or DS improvement determinations were made in the same manner as described above.
3. Results
3.1. Patient baseline characteristics
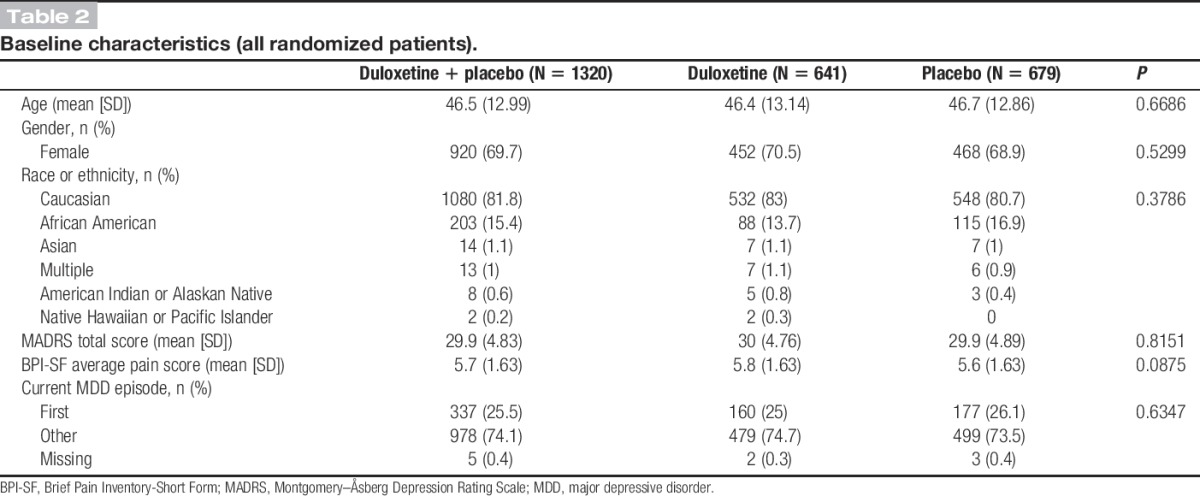
A total of 1320 patients were randomly assigned to the treatment groups, resulting in 641 receiving treatment with duloxetine and 679 receiving placebo (Table 2). Most of the patients were female (69.7%) and Caucasian (81.8%). The mean age (SD) of the patients was 46.5 (12.99) years. The mean baseline MADRS total score (SD) and BPI-SF average pain score (SD) were 29.9 (2.83) and 5.7 (1.63), respectively. Within this population, 25.5% were undergoing their first MDD episode. Gender and ethnicity distributions, age, and baseline illness characteristics were similar between duloxetine and placebo groups (Table 2).
Table 2.
Baseline characteristics (all randomized patients).
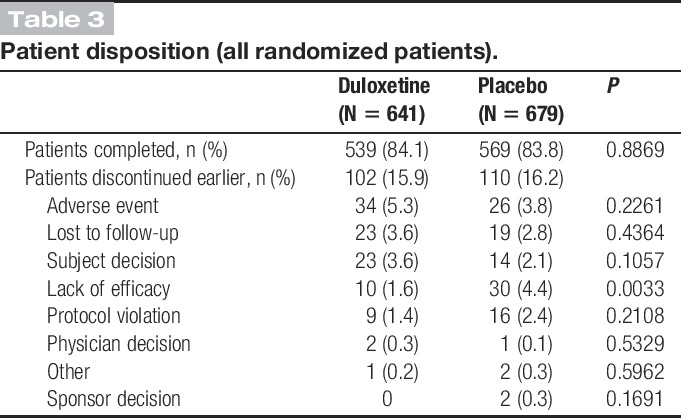
3.1.1. Patient disposition
At the end of the 8-week treatment, there was no significant difference in the numbers of patients discontinued early between duloxetine and placebo groups (duloxetine: 15.9% vs placebo: 16.2%). A significantly (P = 0.0033) greater number of patients discontinued treatment for lack of efficacy in the placebo group (4.4%) compared with that in the duloxetine group (1.6%). There was no significant difference (P = 0.2261) between the duloxetine (5.3%) and placebo (3.8%) groups in discontinuation due to adverse events (Table 3).
Table 3.
Patient disposition (all randomized patients).
3.1.2. Depressive symptoms' improvement over time
Depressive symptoms measured by MADRS total score continuously decreased over the 8-week study period for both duloxetine and placebo groups (Figure 3A). At each time point, the reduction in MADRS total score for patients receiving duloxetine was significantly greater than the decrease in MADRS total score experienced by the placebo group (Figure 3A). In addition, the difference in MADRS score reduction between the duloxetine group and the placebo group increased from a least-squares mean difference (SE) of −0.72 (0.27; P = 0.0074) at week 1 to a mean difference (SE) of −4.46 (0.54; P < 0.0001) at week 8 (Figure 3A).
Figure 3.
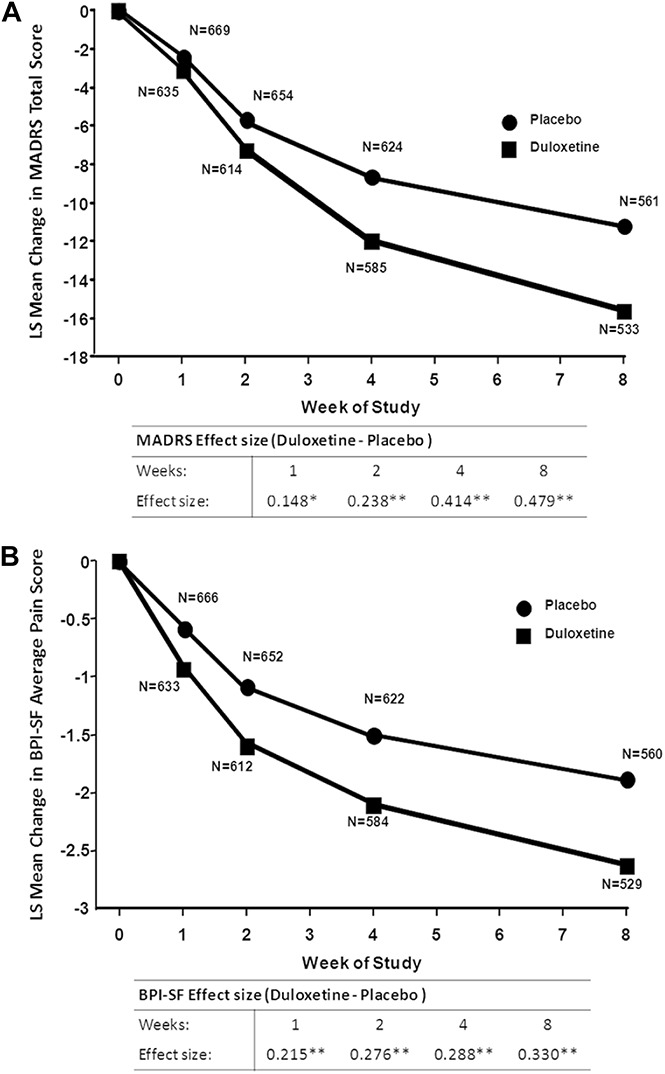
Change from baseline in MADRS total score (A) and BPI-SF average pain score. (B) Effect size is the LS mean estimate divided by the square root of the residual variance (*P ≤ 0.01; **P ≤ 0.001 MMRM). BPI-SF, Brief Pain Inventory-Short Form; LS mean, least-squares mean; MADRS, Montgomery–Åsberg Depression Rating Scale; MMRM, mixed-effects model repeated-measures.
3.1.3. Painful physical symptoms' improvement over time
Likewise, a continuous significant (P < 0.001) PPS improvement as measured by BPI-SF average pain score was observed in duloxetine and placebo groups throughout the 8-week trial period (Figure 3B). The difference in BPI-SF scores between duloxetine and placebo groups increased over time from a least-squares mean difference (SE) of −0.35 (0.09; P = 0.0001) at week 1 to a mean difference (SE) of −0.76 (0.14; P < 0.0001) (Figure 3B).
3.1.4. Path analyses—direct and indirect effects on painful physical symptoms
In the first path analysis model proposed for path analyses, the causal relationships were defined as treatment having a direct effect on PPS reduction and an indirect effect on PPS reduction due to DS improvement (Figure 1A). The path analysis was applied to the PPS and DS scores collected at weeks 1, 2, 4, and 8 (Figure 1B). At week 1, path analysis indicated that 75.3% of the improvement in PPS was attributed to a direct effect of the duloxetine treatment and the remaining 24.7% of the improvement was attributed to an indirect effect through DS improvement (Figure 1B). However, over time, the percentage of the direct effect on PPS improvement was reduced such that at week 8 only 22.6% of the PPS improvement was due to a direct effect of the treatment and 77.4% of the PPS improvement was attributed to the indirect effect through DS improvement (Figure 1B).
3.1.5. Path analyses—direct and indirect effects on depressive symptoms
The alternate path analysis model defined the causal relationships as treatment having a direct effect on DS improvement and an indirect effect on DS improvement by reducing PPS (Figure 2A). At week 1, the direct effect of treatment on DS improvement was 46.4% and the indirect effect through PPS improvement was 53.6% (Figure 2B). Over time, the direct effect of treatment on DS improvement increased somewhat such that at week 8, 62.6% of the DS improvement was attributed to a direct effect of treatment and 37.4% of the DS improvement was attributed to an indirect effect by way of PPS improvement (Figure 2B).
4. Discussion
The results of this study show that treatment of patients who have MDD with associated PPS with 60 mg/d of duloxetine for 8 weeks results in a progressive improvement of both DS and PPS, as indicated by a reduction in MADRS total scores and BPI-SF average pain scores. At week 1, the direct effect of treatment on PPS reduction was 75% and only 25% of the PPS relief was attributed to DS improvement. The large direct component on PPS relief was also present at week 2, but the balance shifted somewhat abruptly such that the indirect effect outweighed the direct effect beginning at week 4. After 8 weeks of treatment, the PPS improvement in depressed patients with PPS was largely due to the indirect effect resulting from the DS improvement (77%) and only 23% was attributed to a direct effect of duloxetine. Accordingly, the observations made in the present investigation are consistent with earlier studies on the interactions between MDD and PPS.22,28,40,48,50 Importantly, this study extends these observations to show that there is a marked shift in the direct effect of duloxetine on pain relief in patients with MDD over time.
First, when assessing the attribution of the direct effect of treatment on PPS and the indirect effect via DS improvement during the acute phase treatment as a whole (eg, 8-9 weeks), this study showed a direct effect of duloxetine of only 23% and an indirect effect of 77%. When compared with a previous report by Fava et al22 where the direct effect of duloxetine on PPS reduction attributed 51% and the indirect effect 49%, our results indicate a lower attribution of direct effect on PPS and higher attribution on indirect effect via DS improvement. This inconsistency may be explained by the difference in population in the study. In the study by Fava et al,22 the patient population was not initially screened for PPS, resulting in a diverse range of pain severity, whereas our study used a specific population: patients with MDD and associated PPS. From a clinical perspective, these results suggest that, for the patients with MDD and with associated PPS, although the patients explicitly exhibit PPS, it is rather important to improve DS to achieve PPS improvement in the end.
Second, when assessing the attribution of the direct effect of treatment on PPS and indirect effect via DS improvement during the very beginning of acute phase treatment (ie, 1-2 weeks), this study reveals a new clinical perspective. This study showed that until 2 weeks of treatment the direct effect of treatment on PPS dominated over indirect effect via DS improvement. From a clinical perspective, this result suggests that, to quickly relieve the patients with MDD with associated PPS from pain symptoms in the initial phase of treatment, it is important to count on the effect of pharmacological therapy rather than to expect DS to indirectly improve PPS. This clinical implication is underscored by the larger effect size elicited by duloxetine with regard to PPS improvement and in contrast to DS improvement at weeks 1 and 2, as shown in Figure 1.
Third, the shift in dominance of the direct effect of duloxetine on PPS to the indirect effect over time is consistent with the known neurobiology of serotonin and norepinephrine reuptake inhibitors with respect to pain and depression. The pain relief obtained with duloxetine is of an immediate nature. Duloxetine engages descending pain modulation that is likely to be impaired among patients with MDD.39 Duloxetine is also clinically effective in several persistent painful conditions, such as chronic musculoskeletal pain of the back, osteoarthritis, diabetic neuropathy, and fibromyalgia.54 These conditions are associated with dysfunction of endogenous pain modulation, and it is highly likely that duloxetine, by enhancing noradrenergic transmission, engages descending pain modulatory systems.41,46,47,54,55 In contrast, the antidepressant action of these drugs is not immediate in onset but occurs after a considerable latency of 3 to 6 weeks.42 This latency to effect indicates that the clinical benefit is not due to an immediate elevation in the synaptic availability of norepinephrine and serotonin, but rather to neuroplastic changes that take place over time in response to the elevation in basal levels of these transmitters.44,49 Accordingly, the predominance of the direct effect of duloxetine during the initial trial period is likely due to engagement of noradrenergic pain modulatory systems, whereas the later indirect effects are due to the antidepressant mechanisms coming online.48 The important clinical implication here is that pain relief with a serotonin and norepinephrine reuptake inhibitor is not simply due to alleviation of depression but due to possible engagement of descending pain modulation.
Finally, when the alternate path analyses was performed, it was found that after 8 weeks of treatment the direct effect (62.6%) of duloxetine on depression predominated over the indirect effect (37.4%). The results of this study are consistent with those of Robinson et al.50 In that study, which also used a pain-rich population, the MADRS remission score was used as a measure of reversal of depression, and path analysis indicated that the direct effect of duloxetine accounted for 59% of the treatment on remission and the indirect effect through pain reduction accounted for 41% after 8 weeks of treatment.50 This study extends these observations by revealing a shift in the predominance of indirect vs direct effects, in the opposite direction to our primary analysis. The indirect effect of duloxetine on DS through reduction of PPS predominated during the early time points, and the direct effects predominated at the later time points. Moreover, unlike the effects on pain, the difference in direct and indirect effects was much lower and the shift occurred more gradually.
We acknowledge that there are some limitations of the study that should be taken into consideration. Despite attempts to enhance diversity in the population sample, most of the patient population is Caucasian (82%) and female (70%). Potential differences due to gender, ethnicity, or cultural considerations3,36 may be underrepresented in this sample. For example, patients with MDD in Asian cultures tend to emphasize physical symptoms over emotional ones and may report greater incidences of PPS.3,38 In addition, there are some additional considerations regarding path analysis. Although this analysis is based on assumptions of causal relationships, in this study only PPS and DS were considered. Thus, the effects of treatment on other symptoms that may be associated with either DS or PPS but were not included in the MADRS or BPI-SF scale, such as anxiety, fatigue, or other non-PPS, were not considered separately. Accordingly, the possible contribution of other factors to the direct effect should at least be kept in mind. In addition, BPI average pain may not fully represent the character and severity of PPS, although it is commonly used for evaluation of PPS9,10,24,25 and of other types of chronic pain5,27,51,53,57 in many randomized clinical trials. Another limitation of the study is that only a single dose, 60 mg/d, of duloxetine was included, and higher doses of duloxetine may have different effects on both DS and PPS. However, it should be noted that a number of randomized clinical trials that included direct comparisons between 60 and 120 mg/d of duloxetine for MDD33 and painful diabetic neuropathy or fibromyalgia5,27,31,56,59 lead us to suggest that the higher (ie, 120 mg/d) dose of duloxetine is not likely to lead to different results in this study. We also believe that by conducting patient-level integrated analysis for 3 studies with the same analysis method and scales, it is expected that this would produce higher internal validity and reproducibility.
In conclusion, this study advances our understanding of the potential interaction between DS and PPS in MDD. To the best of our knowledge, there are no other published studies available where the attribution of DS and of PPS in MDD was examined over time. Moreover, the patient population selected included only patients with PPS of at least moderate severity, allowing a more rigorous examination of the interaction between pain and DS. The results of this study showed that duloxetine produced a large direct effect on pain relief during the initial 2 weeks of treatment. During this stage, the improvement in PPS in patients with MDD was likely not due to a reduction in DS, but rather to engagement of endogenous noradrenergic pain modulatory systems. In contrast, the reduction in PPS during the latter stage of treatment was likely due more to DS improvement. This dynamic shift provides context for proposing that both the DS and PPS in patients with MDD should be addressed simultaneously at treatment initiation, rather than focusing only on the DS with the idea that improvement in pain will spontaneously follow.
Conflict of interest statement
E. Harada contributed to this work as a former full-time employee of Eli Lilly Japan K.K.. The opinions expressed in this work are solely his and do not represent his current affiliation; the Japanese Ministry of Health, Labour and Welfare. S. Fujikoshi, H. Tokuoka, and J. Funai are employees of Eli Lilly Japan K.K. M. M. Wohlreich is an employee of Eli Lilly and Company and owns Eli Lilly stock. N. Iwata has been a consultant for Eli Lilly, Janssen, Otsuka, Shionogi, GSK, Tanabe-Mitsubishi, Dainippon-Sumitomo, and Mebix and has received grants from Eli Lilly, Otsuka, Dainippon-Sumitomo, GSK, and Daiichi-Sankyo. M. H. Ossipov is employed by inVentiv Health Clinical, LLC and owns Eli Lilly stock.
Acknowledgements
The authors thank the investigators and staff who conducted these clinical trials. The authors also thank the patients who volunteered to take part in these important studies. The authors also thank Ralf Jaeger, full-time employee of Accovion GmbH, for statistical support; and Angela Lorio, full-time employee of inVentiv Health Clinical, LLC, for editorial support. These trials and the preparation of the article were sponsored by Eli Lilly and Company, Indianapolis, IN, USA. Eli Lilly and Company contracted Accovion for statistical support and inVentiv Health Clinical for writing and editorial support.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
References
- [1].American Psychiatric Association. DSM-IV-TR: diagnostic and statistical manual of mental disorders. Washington: American Psychiatric Press, 2000. [Google Scholar]
- [2].American Psychiatric Association. Diagnostic and statistical manual of mental disorders. Arlington: American Psychiatric Publishing, 2013. [Google Scholar]
- [3].Ang QQ, Wing YK, He Y, Sulaiman AH, Chiu NY, Shen YC, Wang G, Zhang C, Lee KH, Singh P, Granger RE, Raskin J, Dossenbach M. Association between painful physical symptoms and clinical outcomes in East Asian patients with major depressive disorder: a 3-month prospective observational study. Int J Clin Pract 2009;63:1041–9. [DOI] [PubMed] [Google Scholar]
- [4].Anonymous. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. J Int Bioethique 2004;15:124–9. [PubMed] [Google Scholar]
- [5].Arnold LM, Rosen A, Pritchett YL, D'Souza DN, Goldstein DJ, Iyengar S, Wernicke JF. A randomized, double-blind, placebo-controlled trial of duloxetine in the treatment of women with fibromyalgia with or without major depressive disorder. PAIN 2005;119:5–15. [DOI] [PubMed] [Google Scholar]
- [6].Bair MJ, Robinson RL, Katon W, Kroenke K. Depression and pain comorbidity: a literature review. Arch Intern Med 2003;163:2433–45. [DOI] [PubMed] [Google Scholar]
- [7].Ball SG, Desaiah D, Spann ME, Zhang Q, Russell JM, Robinson MJ, Demyttenaere K. Efficacy of duloxetine on painful physical symptoms in major depressive disorder for patients with clinically significant painful physical symptoms at baseline: a meta-analysis of 11 double-blind, placebo-controlled clinical trials. Prim Care Companion CNS Disord 2011;13:PCC.11r01181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ball SG, Desaiah D, Zhang Q, Thase ME, Perahia DG. Efficacy and safety of duloxetine 60 mg once daily in major depressive disorder: a review with expert commentary. Drugs Context 2013;2013:212245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Brannan SK, Mallinckrodt CH, Brown EB, Wohlreich MM, Watkin JG, Schatzberg AF. Duloxetine 60 mg once-daily in the treatment of painful physical symptoms in patients with major depressive disorder. J Psychiatr Res 2005;39:43–53. [DOI] [PubMed] [Google Scholar]
- [10].Brecht S, Courtecuisse C, Debieuvre C, Croenlein J, Desaiah D, Raskin J, Petit C, Demyttenaere K. Efficacy and safety of duloxetine 60 mg once daily in the treatment of pain in patients with major depressive disorder and at least moderate pain of unknown etiology: a randomized controlled trial. J Clin Psychiatry 2007;68:1707–16. [DOI] [PubMed] [Google Scholar]
- [11].Bymaster FP, Beedle EE, Findlay J, Gallagher PT, Krushinski JH, Mitchell S, Robertson DW, Thompson DC, Wallace L, Wong DT. Duloxetine (Cymbalta), a dual inhibitor of serotonin and norepinephrine reuptake. Bioorg Med Chem Lett 2003;13:4477–80. [DOI] [PubMed] [Google Scholar]
- [12].Chopra K, Arora V. An intricate relationship between pain and depression: clinical correlates, coactivation factors and therapeutic targets. Expert Opin Ther Targets 2014;18:159–76. [DOI] [PubMed] [Google Scholar]
- [13].Cleeland CS. Measurement of pain by subjective report. In: Chapmen CR, Loeser JD, editors. Issues in pain measurement, Vol. 12 New York: Raven Press, 1989. p. 391–403. [Google Scholar]
- [14].Cleeland CS. The brief pain inventory user guide. Available at: http://www.mdanderson.org/education-and-research/departments-programs-and-labs/departments-and-divisions/symptom-research/symptom-assessment-tools/BPI_UserGuide.pdf 2009. Accessed November 25, 2015. [Google Scholar]
- [15].Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore 1994;23:129–38. [PubMed] [Google Scholar]
- [16].Corruble E, Guelfi JD. Pain complaints in depressed inpatients. Psychopathology 2000;33:307–9. [DOI] [PubMed] [Google Scholar]
- [17].Cowen PJ, Ogilvie AD, Gama J. Efficacy, safety and tolerability of duloxetine 60 mg once daily in major depression. Curr Med Res Opin 2005;21:345–56. [DOI] [PubMed] [Google Scholar]
- [18].Dworkin RH, Turk DC, Farrar JT, Haythornthwaite JA, Jensen MP, Katz NP, Kerns RD, Stucki G, Allen RR, Bellamy N, Carr DB, Chandler J, Cowan P, Dionne R, Galer BS, Hertz S, Jadad AR, Kramer LD, Manning DC, Martin S, McCormick CG, McDermott MP, McGrath P, Quessy S, Rappaport BA, Robbins W, Robinson JP, Rothman M, Royal MA, Simon L, Stauffer JW, Stein W, Tollett J, Wernicke J, Witter J. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. PAIN 2005;113:9–19. [DOI] [PubMed] [Google Scholar]
- [19].Dworkin RH, Turk DC, Wyrwich KW, Beaton D, Cleeland CS, Farrar JT, Haythornthwaite JA, Jensen MP, Kerns RD, Ader DN, Brandenburg N, Burke LB, Cella D, Chandler J, Cowan P, Dimitrova R, Dionne R, Hertz S, Jadad AR, Katz NP, Kehlet H, Kramer LD, Manning DC, McCormick C, McDermott MP, McQuay HJ, Patel S, Porter L, Quessy S, Rappaport BA, Rauschkolb C, Revicki DA, Rothman M, Schmader KE, Stacey BR, Stauffer JW, von Stein T, White RE, Witter J, Zavisic S. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain 2008;9:105–21. [DOI] [PubMed] [Google Scholar]
- [20].Escobar JI, Interian A, Diaz-Martinez A, Gara M. Idiopathic physical symptoms: a common manifestation of psychiatric disorders in primary care. CNS Spectr 2006;11:201–10. [DOI] [PubMed] [Google Scholar]
- [21].Fava M. Somatic symptoms, depression, and antidepressant treatment. J Clin Psychiatry 2002;63:305–7. [DOI] [PubMed] [Google Scholar]
- [22].Fava M, Mallinckrodt CH, Detke MJ, Watkin JG, Wohlreich MM. The effect of duloxetine on painful physical symptoms in depressed patients: do improvements in these symptoms result in higher remission rates? J Clin Psychiatry 2004;65:521–30. [DOI] [PubMed] [Google Scholar]
- [23].Food and Drug Administration. Guidelines for industry. Patient-reported outcome measures: use in medical product development to support labeling claims. Rockville: U.S. Department of Health and Human Services, 2006. [Google Scholar]
- [24].Gaynor PJ, Gopal M, Zheng W, Martinez JM, Robinson MJ, Hann D, Marangell LB. Duloxetine versus placebo in the treatment of major depressive disorder and associated painful physical symptoms: a replication study. Curr Med Res Opin 2011;27:1859–67. [DOI] [PubMed] [Google Scholar]
- [25].Gaynor PJ, Gopal M, Zheng W, Martinez JM, Robinson MJ, Marangell LB. A randomized placebo-controlled trial of duloxetine in patients with major depressive disorder and associated painful physical symptoms. Curr Med Res Opin 2011;27:1849–58. [DOI] [PubMed] [Google Scholar]
- [26].Goesling J, Clauw DJ, Hassett AL. Pain and depression: an integrative review of neurobiological and psychological factors. Curr Psychiatry Rep 2013;15:421. [DOI] [PubMed] [Google Scholar]
- [27].Goldstein DJ, Lu Y, Detke MJ, Lee TC, Iyengar S. Duloxetine vs. placebo in patients with painful diabetic neuropathy. PAIN 2005;116:109–18. [DOI] [PubMed] [Google Scholar]
- [28].Greco T, Eckert G, Kroenke K. The outcome of physical symptoms with treatment of depression. J Gen Intern Med 2004;19:813–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Greenberg PE, Leong SA, Birnbaum HG, Robinson RL. The economic burden of depression with painful symptoms. J Clin Psychiatry 2003;64(suppl 7):17–23. [PubMed] [Google Scholar]
- [30].Hyman S, Chisholm D, Kessler R, Patel V, Whiteford H. Chapter 31 Mental Disorders' in Disease Control Priorities in Developing Countries, 2nd edition Jamison DT, et al. (eds). Washington, DC: The World Bank; 2006. [Google Scholar]
- [31].Kajdasz DK, Iyengar S, Desaiah D, Backonja MM, Farrar JT, Fishbain DA, Jensen TS, Rowbotham MC, Sang CN, Ziegler D, McQuay HJ. Duloxetine for the management of diabetic peripheral neuropathic pain: evidence-based findings from post hoc analysis of three multicenter, randomized, double-blind, placebo-controlled, parallel-group studies. Clin Ther 2007;29:2536–46. [DOI] [PubMed] [Google Scholar]
- [32].Karpa KD, Cavanaugh JE, Lakoski JM. Duloxetine pharmacology: profile of a dual monoamine modulator. CNS Drug Rev 2002;8:361–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Kornstein SG, Dunner DL, Meyers AL, Whitmyer VG, Mallinckrodt CH, Wohlreich MM, Detke MJ, Hollandbeck MS, Greist JH. A randomized, double-blind study of increasing or maintaining duloxetine dose in patients without remission of major depressive disorder after initial duloxetine therapy. J Clin Psychiatry 2008;69:1383–92. [DOI] [PubMed] [Google Scholar]
- [34].Kroenke K, Jackson JL. Outcome in general medical patients presenting with common symptoms: a prospective study with a 2-week and a 3-month follow-up. Fam Pract 1998;15:398–403. [DOI] [PubMed] [Google Scholar]
- [35].Kroenke K, Price RK. Symptoms in the community. Prevalence, classification, and psychiatric comorbidity. Arch Intern Med 1993;153:2474–80. [PubMed] [Google Scholar]
- [36].Lee P, Zhang M, Hong JP, Chua HC, Chen KP, Tang SW, Chan BT, Lee MS, Lee B, Gallagher GL, Dossenbach M. Frequency of painful physical symptoms with major depressive disorder in asia: relationship with disease severity and quality of life. J Clin Psychiatry 2009;70:83–91. [DOI] [PubMed] [Google Scholar]
- [37].Leuchter AF, Husain MM, Cook IA, Trivedi MH, Wisniewski SR, Gilmer WS, Luther JF, Fava M, Rush AJ. Painful physical symptoms and treatment outcome in major depressive disorder: a STAR*D (Sequenced Treatment Alternatives to Relieve Depression) report. Psychol Med 2010;40:239–51. [DOI] [PubMed] [Google Scholar]
- [38].Li HC, Zhang MY, Wang G, Zhang HG, Zhang HY, Liu Y, Li M, Zhang CP, Tang JS, Wu WY, Singh P, Granger RE, Raskin J, Ang QQ. Association between painful physical symptoms and clinical outcomes in Chinese patients with major depressive disorder: a three-month observational study. Chin Med J (Engl) 2010;123:2063–9. [PubMed] [Google Scholar]
- [39].Maletic V, Raison CL. Neurobiology of depression, fibromyalgia and neuropathic pain. Front Biosci (Landmark Ed) 2009;14:5291–338. [DOI] [PubMed] [Google Scholar]
- [40].Marangell LB, Clauw DJ, Choy E, Wang F, Shoemaker S, Bradley L, Mease P, Wohlreich MM. Comparative pain and mood effects in patients with comorbid fibromyalgia and major depressive disorder: secondary analyses of four pooled randomized controlled trials of duloxetine. PAIN 2011;152:31–7. [DOI] [PubMed] [Google Scholar]
- [41].Meske DS, Xie JY, Oyarzo J, Badghisi H, Ossipov MH, Porreca F. Opioid and noradrenergic contributions of tapentadol in experimental neuropathic pain. Neurosci Lett 2014;562:91–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Millan MJ. Multi-target strategies for the improved treatment of depressive states: conceptual foundations and neuronal substrates, drug discovery and therapeutic application. Pharmacol Ther 2006;110:135–370. [DOI] [PubMed] [Google Scholar]
- [43].Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry 1979;134:382–9. [DOI] [PubMed] [Google Scholar]
- [44].Nestler EJ, Barrot M, DiLeone RJ, Eisch AJ, Gold SJ, Monteggia LM. Neurobiology of depression. Neuron 2002;34:13–25. [DOI] [PubMed] [Google Scholar]
- [45].Ohayon MM, Schatzberg AF. Using chronic pain to predict depressive morbidity in the general population. Arch Gen Psychiatry 2003;60:39–47. [DOI] [PubMed] [Google Scholar]
- [46].Ossipov MH, Dussor GO, Porreca F. Central modulation of pain. J Clin Invest 2010;120:3779–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Ossipov MH, Morimura K, Porreca F. Descending pain modulation and chronification of pain. Curr Opin Support Palliat Care 2014;8:143–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Perahia DG, Pritchett YL, Desaiah D, Raskin J. Efficacy of duloxetine in painful symptoms: an analgesic or antidepressant effect? Int Clin Psychopharmacol 2006;21:311–17. [DOI] [PubMed] [Google Scholar]
- [49].Pittenger C, Duman RS. Stress, depression, and neuroplasticity: a convergence of mechanisms. Neuropsychopharmacology 2008;33:88–109. [DOI] [PubMed] [Google Scholar]
- [50].Robinson MJ, Sheehan D, Gaynor PJ, Marangell LB, Tanaka Y, Lipsius S, Ohara F, Namiki C. Relationship between major depressive disorder and associated painful physical symptoms: analysis of data from two pooled placebo-controlled, randomized studies of duloxetine. Int Clin Psychopharmacol 2013;28:330–8. [DOI] [PubMed] [Google Scholar]
- [51].Russell IJ, Mease PJ, Smith TR, Kajdasz DK, Wohlreich MM, Detke MJ, Walker DJ, Chappell AS, Arnold LM. Efficacy and safety of duloxetine for treatment of fibromyalgia in patients with or without major depressive disorder: results from a 6-month, randomized, double-blind, placebo-controlled, fixed-dose trial. PAIN 2008;136:432–44. [DOI] [PubMed] [Google Scholar]
- [52].Simon GE, VonKorff M, Piccinelli M, Fullerton C, Ormel J. An international study of the relation between somatic symptoms and depression. N Engl J Med 1999;341:1329–35. [DOI] [PubMed] [Google Scholar]
- [53].Skljarevski V, Zhang S, Desaiah D, Alaka KJ, Palacios S, Miazgowski T, Patrick K. Duloxetine versus placebo in patients with chronic low back pain: a 12-week, fixed-dose, randomized, double-blind trial. J Pain 2010;11:1282–90. [DOI] [PubMed] [Google Scholar]
- [54].Skljarevski V, Zhang S, Iyengar S, D'Souza D, Alaka K, Chappell A, Wernicke J. Efficacy of duloxetine in patients with chronic pain conditions. Curr Drug Ther 2011;6:296–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Staud R. Abnormal endogenous pain modulation is a shared characteristic of many chronic pain conditions. Expert Rev Neurother 2012;12:577–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Sultan A, Gaskell H, Derry S, Moore RA. Duloxetine for painful diabetic neuropathy and fibromyalgia pain: systematic review of randomised trials. BMC Neurol 2008;8:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Tesfaye S, Wilhelm S, Lledo A, Schacht A, Tolle T, Bouhassira D, Cruccu G, Skljarevski V, Freynhagen R. Duloxetine and pregabalin: high-dose monotherapy or their combination? The “COMBO-DN study”—a multinational, randomized, double-blind, parallel-group study in patients with diabetic peripheral neuropathic pain. PAIN 2013;154:2616–25. [DOI] [PubMed] [Google Scholar]
- [58].Turk DC, Dworkin RH, Burke LB, Gershon R, Rothman M, Scott J, Allen RR, Atkinson JH, Chandler J, Cleeland C, Cowan P, Dimitrova R, Dionne R, Farrar JT, Haythornthwaite JA, Hertz S, Jadad AR, Jensen MP, Kellstein D, Kerns RD, Manning DC, Martin S, Max MB, McDermott MP, McGrath P, Moulin DE, Nurmikko T, Quessy S, Raja S, Rappaport BA, Rauschkolb C, Robinson JP, Royal MA, Simon L, Stauffer JW, Stucki G, Tollett J, von ST, Wallace MS, Wernicke J, White RE, Williams AC, Witter J, Wyrwich KW. Developing patient-reported outcome measures for pain clinical trials: IMMPACT recommendations. PAIN 2006;125:208–15. [DOI] [PubMed] [Google Scholar]
- [59].Wernicke JF, Pritchett YL, D'Souza DN, Waninger A, Tran P, Iyengar S, Raskin J. A randomized controlled trial of duloxetine in diabetic peripheral neuropathic pain. Neurology 2006;67:1411–20. [DOI] [PubMed] [Google Scholar]
- [60].Wong DT, Bymaster FP. Dual serotonin and noradrenaline uptake inhibitor class of antidepressants potential for greater efficacy or just hype? Prog Drug Res 2002;58:169–222. [DOI] [PubMed] [Google Scholar]


