Abstract
A cross-sectional study examined the occurrence of Tritrichomonas foetus, and other intestinal parasites, in feral and shelter cats in Prince Edward Island (PEI). Fecal samples were collected from 100 feral cats, 100 cats from the PEI Humane Society, and 5 cats from a private residence. The occurrence of T. foetus, based on fecal culture, was 0% in feral and shelter cats. A single positive sample was obtained from an owned Abyssinian cat that was imported to PEI. Intestinal parasites were identified via fecal flotation in 76% of feral cats and 39% of cats from the humane society. Feral cats had a higher incidence of Toxocara cati than cats from the humane society (P < 0.001), conversely, shelter cats had a higher incidence of Cystoisospora spp. (P < 0.001). These results suggest that while T. foetus is not of importance in feral and shelter cats in PEI, imported cats could serve as reservoirs.
Résumé
Étude transversale de l’infection par Tritrichomonas foetus chez les chats féraux et de refuge à l’Île-du-Prince-Édouard, au Canada. Une étude transversale a examiné l’occurrence de Tritrichomonas foetus, et d’autres parasites intestinaux, chez les chats féraux et de refuge à l’Île-du-Prince-Édouard (Î.-P.-É.). Des échantillons de fèces ont été prélevés auprès de 100 chats féraux, de 100 chats de la PEI Humane Society et de cinq chats d’une résidence privée. En se basant sur la culture de fèces, l’occurrence de T. foetus était de 0 % chez les chats féraux et les chats de refuge. Un seul échantillon positif a été obtenu auprès d’un chat abyssinien qui avait été importé à l’Île-du-Prince-Édouard et appartenait à un propriétaire. Les parasites intestinaux ont été identifiés par flottaison fécale chez 76 % des chats féraux et 39 % des chats de la société de protection des animaux. Les chats féraux présentaient une incidence supérieure de Toxocara cati à celle des chats de la société de protection des animaux (P < 0,001), par contre, les chats de refuge avaient une incidence supérieure de Cystoisospora spp. (P < 0,001). Ces résultats suggèrent que même si T. foetus n’a pas une présence importante chez les chats féraux et les chats de refuge de l’Île-du-Prince-Édouard, les chats importés pourraient servir de réservoirs.
(Traduit par Isabelle Vallières)
Introduction
Tritrichomonas foetus, a parasitic protozoan within the Order Trichomonadida, has long been recognized as a venereal pathogen of naturally bred cattle and was identified as an enteric pathogen of domestic cats in 2003 (1). Cross-infection studies, DNA sequencing, phylogenetic analysis, and the assessment of morphological data have led researchers to conclude that the causative agent of feline trichomonosis is distinct (2–4). This organism has been referred to as T. foetus cat genotype and most recently as Tritrichomonas blagburni nova species (2–4). For this manuscript we will continue to use T. foetus.
Tritrichomonas foetus is an obligate mucosoflagellate that lives in the alimentary tract of the feline host (1). The parasite is variable in size, measuring 5.7 to 13.0 μm long × 3.9 to 5.5 μm wide and has been described as pyriform, fusiform, or rounded in shape (4). Tritrichomonas foetus possesses 4 flagella; 3 are located near the anterior of the cell, and the 4th forms an undulating membrane and extends posteriorly (1).
Tritrichomonas foetus reproduces asexually by binary fission, producing 2 daughter cells, each of which becomes an individual trophozoite (5). The parasite does not form resistant cysts and transmission of trophozoites from host to host occurs via the fecal-oral route (6).
Trichomonads possess adhesive surface proteins and have cytotoxic and proteolytic activity. These features play important roles in tissue invasion and host cell destruction (7,8). In the feline host, T. foetus inhabits the ileum, cecum, and colon resulting in a lymphoplasmacytic and neutrophilic colitis (9). Microscopic features such as attenuation of the superficial colonic epithelium, hypertrophy of crypt epithelial cells, increased mitotic activity, goblet cell loss, and crypt microabscessation have been identified in cats infected with T. foetus (9).
Dense housing conditions are believed to be the most significant risk factor for T. foetus infections in cats (10). Presumably, cats living in close proximity, as in catteries, are more likely to acquire the parasite through mutual grooming and shared litter boxes.
Affected cats can display variable clinical signs. Most infected cats experience signs of chronic large bowel diarrhea such as tenesmus, hematochezia, and mucus in the stool, but cats may have no obvious clinical signs (1,10–13). The diarrhea can wax and wane and the character of the feces can vary from semi-formed to soft or, less likely, liquid (11). Affected cats often maintain good body condition without signs of systemic illness; however, depression, hyporexia or anorexia, weight loss, vomiting, and abdominal pain have been reported (10,11).
In recent years, there have been increased numbers of reports of clinical feline trichomonosis. Trichomonads have been isolated from cats in the United States, the United Kingdom, Germany, Italy, Switzerland, Greece, Australia, Korea, and Canada (14–20). Several epidemiologic studies have been conducted in the United States and the United Kingdom. Gookin et al (10) reported a prevalence of 31% [36 out of 117; 95% confidence interval (95% CI): 23% to 40%] in a sample population of domestic cats from an international cat show. Tritrichomonas foetus positive cats represented 12% of the total cats at the show. Stockdale et al (21) reported T. foetus infections in 10% (17/173; 95% CI: 6% to 15%) of sampled cats throughout the United States while Gunn-Moore et al (14) found a prevalence of 14% (16/111; 95% CI: 8% to 22%) in a sample population of domestic cats in the United Kingdom. Most recently, T. foetus was isolated from 23.6% of cats sampled at cat shows in Ontario, Canada; infection rates of 0.7% of cats at a veterinary clinic and 0% of cats at a local humane society in Ontario were also reported (20).
The prevalence of T. foetus in cat populations in Prince Edward Island (PEI), Canada, has not been previously reported. Understanding of infection rates in PEI can help to determine whether increased testing for this parasite is warranted in cats with compatible clinical signs. Therefore, the primary objective of this study was to conduct a coprological survey to compare the prevalence of T. foetus between feral and shelter cat populations in PEI. A secondary objective was to determine the extent of co-infections of T. foetus with common intestinal parasites in these cat populations.
Materials and methods
A prospective, cross-sectional study, involving 200 cats, was conducted between July 2011 and February 2013. The first 100 cats that were presented to the Atlantic Veterinary College (AVC) feral cat spay and neuter program and the first 100 cats housed at the PEI Humane Society that produced a fecal sample were included in the study. Feral cats were defined as free-roaming, non-owned, intact cats. Cats housed at the humane society included intact and neutered cats that were surrendered by their owners or were picked up by animal control — their length of stay in the shelter was not recorded. Fecal samples were also obtained from 5 purebred cats living together in a private residence in PEI. The study was approved by the Animal Care Committee of the University of Prince Edward Island.
Feral cats were trapped humanely the night before presentation to the AVC as part of the spay and neuter program. The gender and approximate age of each feral cat were recorded. Cats were classified as juvenile or adult based on the appearance of their teeth. Cats with deciduous teeth (< 6 mo of age) were classified as juveniles; all others were classified as adults. All feral cats were pre-medicated for the sterilization procedure. Following administration of the sedatives, a fecal sample was obtained digitally from each cat via the rectum and placed into a sterile fecal collection cup. After complete recovery from anesthesia the cats were released to the area where they had been trapped.
The gender, age, vaccine, and anti-parasitic treatment (if known) were also recorded for each cat housed at the PEI Humane Society. If the age of the cat was not known, cats were classified as juvenile or adult based on their weight and appearance of their teeth as previously described. Cats at the humane society were mostly housed singly, but occasionally were housed in groups of up to 4 cats in 1 enclosure. Fecal samples were collected within 6 h of voiding from litter boxes and placed into sterile fecal collection cups.
The character of all fecal samples from both populations of cats was assessed and recorded as liquid, soft, normal, or firm. A small amount of feces, the size of a grain of rice (approximately 0.03 g) taken from the surface of the fecal sample, was used to inoculate a commercial culture test kit (InPouch TF-Feline; Biomed Diagnostics, White City, Oregon, USA) for the cultivation of T. foetus as previously described (22). Pouches were inoculated with feces within 6 h of samples being obtained or voided, as recommended, and then incubated at 37°C (23). Prior to microscopic examination, the medium in the pouches was examined for clarity and presence of gas bubbles. Pouches were examined daily for 10 d unless the clarity was poor or the presence of gas bubbles hindered evaluation. Each pouch was examined at 200× to 400× total magnification, taking care to examine the sides of the pouch where the material settles, as previously described (22). The presence of T. foetus was confirmed using polymerase chain reaction (PCR).
Trichomonad PCR and DNA sequencing
Polymerase chain reaction testing was performed in duplicate. A 1-mL aliquot of the original parasite culture was used to extract DNA using the QIAamp DNA Mini Kit (QIAGEN, Toronto, Ontario) as per the manufacturer’s instructions. The DNA amplification of the Trichomonas spp. internal transcribed spacer region (ITS1/5.8S rDNA/ITS2 region) ~300 bp was performed using trichomonad-specific primers TFR1 (5′-TGCTTCAGT TCAGCGGGTCTTCC-3′) and TFR2 (5′-CGGTAGGTG AACCTGCCGTTGG-3′) (24). Polymerase chain reaction components were 12.5 μL Amplitaq Gold Master Mix (Applied Biosystems, Life Technologies, Burlington, Ontario), 4.5 μL nuclease-free water, 2.5 μL forward primer (10 μM), 2.5 μL reverse primer (10 μM), and 3 μL of undiluted target DNA. Negative controls substituted 3 μL of nuclease-free water for target DNA and positive controls used 3 μL of T. gallinae DNA (purple finch isolate species confirmed by sequencing the ITS region). The PCR parameters for the ITS region amplification were 94°C for 2 min, followed by 40 cycles of 94°C for 30 s, 67°C for 30 s, 72°C for 2 min, and a final extension at 72°C for 15 min. The PCR amplicons were examined following electrophoresis in1% agarose gel with ethidium bromide and were sequenced directly in both directions at the McGill University and Genome Quebec Innovation Centre, Montreal, Quebec. Sequences were aligned with published trichomonad sequences from GenBank using BioEdit (25).
A portion of each fecal sample was used for detection of Giardia spp. using a point-of-care enzyme-linked immunosorbent assay (ELISA, SNAP Giardia; IDEXX Laboratories, Westbrook, Maine, USA). The manufacturer reports a sensitivity of 92% and specificity of 99.8% (26).
The remainder of each fecal sample was stored at 4°C and fecal analysis was performed within 24 to 72 h of collection by the AVC Diagnostic Services parasitology laboratory using a centrifugal zinc sulfate flotation technique (27). Samples were positive if eggs, cysts, or oocysts were present.
Fecal samples were also collected from 5 purebred cats living in a private residence. One of the cats was experiencing chronic diarrhea while the other 4 cats had no clinical signs. The cats’ primary care veterinarian contacted the AVC Diagnostic Laboratory for testing of T. foetus. Samples were collected from litter boxes as described for the humane society cats and then evaluated for T. foetus, Giardia spp., and other gastrointestinal parasites as previously described.
Statistical analyses
Based on previous published studies of feline T. foetus prevalence, the sample size of 100 animals per population was chosen, as it was sufficient to detect a difference in prevalence of 14% versus 31%, 19 times out of 20, with a power set at 0.8 (10,14). The numbers of feral and shelter cats with various intestinal parasites were compared using Fisher’s exact test and a significance level of 0.05. All statistical methods were carried out with the R language and environment for statistical computing (28), and the confidence intervals used for the proportions were calculated with Pearson-Klopper’s exact method (package ‘binom,’ version 1.1-1). Given the In Pouch test had reported imperfect diagnostic sensitivities, the lowest sensitivity (83%) was used with the Rogan-Gladen estimator to approximate upper limits for predicted true prevalences (23,29).
Results
Fecal samples were obtained from 100 mixed-breed feral cats and 100 mixed-breed cats housed at the PEI Humane Society. Twenty-four feral cats were classified as juvenile and 76 as adults. There were 43 females and 57 males. Twenty humane society cats were classified as juvenile and 80 as adults. There were 52 females and 48 males. None of the feral cats produced liquid stool. Fourteen samples were characterized as soft, 1 was considered normal, and the remaining 85 samples were firm. Of the fecal samples obtained from the cats at the humane society, 5 were liquid, 35 were soft, 45 were normal, and the remaining 15 were firm.
Tritrichomonas foetus was not identified in any of the samples obtained from the feral or shelter cats enrolled in the study. Based on the negative In Pouch results of all cats sampled (assuming a diagnostic sensitivity of 83% and specificity of 100%), the prevalence of T. foetus infection in feral and shelter cats (treated as 2 separate populations) in PEI would be < 3.5% [based on the 95th percentile from a Beta (1101) distribution, as explained by Vose (30)] if we assumed the pathogen to be present in these 2 populations.
Trichomonads were observed microscopically within the In Pouch culture system from 1 of the 5 fecal samples obtained from a private residence. The positive fecal sample was from a 6-year-old castrated male Abyssinian cat. The positive trichomonad culture isolate was confirmed as being identical to T. foetus cat genotype (GenBank accession number GU170217) by ITS-PCR and DNA sequencing.
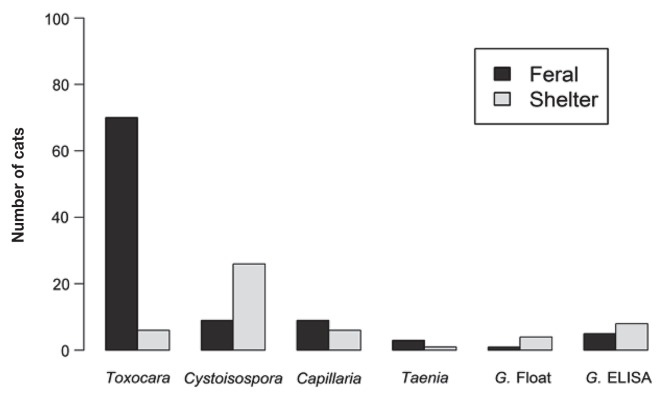
Intestinal parasites were identified via fecal flotation in 76 of 100 feral cats (76%; 95% CI: 66% to 84%) (Figure 1). Toxocara cati was identified in 70 of 100 fecal samples (70%; 95% CI: 60% to 79%) from feral cats. Cystoisospora spp. were identified in 9 cats (9%; 95% CI: 4% to 16%), Capillaria spp. were identified in 9 cats (9%; 95% CI: 4% to 16%) and Taenia spp. were identified in 3 cats (3%; 95% CI: < 1% to 9%). Fourteen of the 100 feral cats (14%; 95% CI: 8% to 22%) had co-infections (representing 18% of the 76 feral cats that tested positive for parasites): 5 with Cystoisospora spp. and Toxocara cati, 5 with Capillaria spp. and T. cati, 2 with Taenia spp. and T. cati, and 2 with Capillaria spp., Cystoisospora spp. and T. cati. Giardia cysts were identified in 1 of 100 fecal samples. Five of 100 (5%; 95% CI: 2% to 11%) feral cats tested positive for Giardia spp. using the point-of-care ELISA. All feral cats tested negative for FeLV and FIV.
Figure 1.
Comparison of intestinal parasites identified in feral cats versus shelter cats in PEI.
G. Float — Giardia fecal float; G. ELISA — Giardia ELISA SNAP test.
Intestinal parasites were identified via fecal flotation in 39 of 100 cats housed at the PEI Humane Society (39%; 95% CI: 29% to 49%) (Figure 1). Cystoisospora species was identified in 26 of 100 humane society cats (26%; 95% CI: 18% to 36%). Toxocara cati was identified in 6 cats (6%; 95% CI: 2% to 13%), Capillaria spp. were identified in 6 cats (6%; 95% CI: 2% to 13%) and Taenia spp. were identified in 1 cat (1%; 95% CI: < 1% to 5%). Five of 100 cats (5%; 95% CI: 2% to 11%) had co-infections (representing 12% of the humane society cats that tested positive for parasites): 2 with Cystoisospora and Capillaria spp., 1 with Capillaria spp. and T. cati, 1 with Taenia spp. and T. cati, and 1 with Giardia spp. and Cystoisospora spp. Giardia cysts were identified via fecal flotation in 4 of 100 (4%; 95% CI: 1% to 10%) fecal samples from humane society cats. Eight of 100 (8%; 95% CI: 4% to 15%) humane society cats tested positive for Giardia using the point of care ELISA.
There were statistically significant differences in the number of cats with Toxocara cati and Cystoisospora spp. between feral and shelter cats, where there were more feral cats with Toxocara cati than shelter cats (70 versus 6, respectively; P < 0.001), and more shelter cats with Cystoisospora spp. than feral cats (26 versus 9, respectively; P = 0.002).
Discussion
Tritrichomonas foetus was not identified in any of the fecal samples from feral cats or cats housed at the humane society. A single positive sample was obtained from a purebred Abyssinian cat that lived in a multi-cat household. The infected cat was originally purchased from a cattery in Italy and shipped to a breeder in Ontario, Canada. An individual living in PEI subsequently purchased the cat. The cat had suffered from diarrhea since the owner acquired it 1 year earlier. None of the other 4 cats in the household were experiencing any clinical signs and none tested positive for T. foetus. Information was not obtained on the number of litter boxes in the household, the cats’ behavior towards each other (e.g., if the cats routinely groomed one another), and how much space the cats had. While it is possible that 1 or more of the fecal samples were falsely negative, it is also possible that there were low levels of environmental contamination in the household and that the cats did not display behaviors such as mutual grooming that would have led to the spread of infection.
Given that if T. foetus was present in either of the 2 populations (feral and shelter cats), it would have an estimated prevalence of < 0.035 (19 times out of 20), and that the prevalence in endemic populations is reported to be > 3.5% (> 14%), it is likely that the feral and shelter cats in PEI are free from T. foetus infections (10,14). However, there were several limitations to this study, which may have led to false negative results in the 2 sample populations.
Of the 200 fecal samples from the feral and humane society cats evaluated in this study, only 54 (27%) were classified as soft or liquid. The remaining 146 samples (73%) were normal to firm. Trichomonads have been isolated from normal feces, and in a recent study, no significant association was found between the presence of T. foetus and diarrhea at the time of, or in the 6 mo prior to, sampling (20). However, while T. foetus infected cats may not have any clinical signs, a strong association has been previously demonstrated between infection and the presence of diarrhea (10,17). Stockdale et al (31) surveyed fecal samples from 173 pet cats and found that all infected cats had diarrhea while Xenoulis et al (17) documented diarrhea in 98% of cats (101 out of 103 cats) with T. foetus. The 1 cat we identified with intestinal trichomonosis was experiencing chronic diarrhea and had soft stool at the time the feces were evaluated. Freshly voided, diarrheic samples are considered ideal for inoculating the culture medium (32). Samples obtained from normal or dry stools are believed to be less suitable for testing and are reported to uncommonly test positive even if infection is present (32). We cannot exclude the possibility that fecal sample collection using a saline flush of the colon may have resulted in superior quality samples for the detection of trichomonads (32).
Previous studies have shown a propensity for T. foetus infection in purebred cats (10,12,14,16). In a survey of pet cats in the United States, 12 of 17 cats identified with T. foetus were pure-bred cats less than 1 year of age (31). In the United Kingdom, pedigreed cats were significantly more likely to be PCR-positive for T. foetus than domestic crossbred cats (14). All of the feral and shelter cats included in our study were of mixed breeding, with 44 out of 200 cats (22%; 95% CI: 16% to 28%) classified as juvenile. Mixed breed cats that live in crowded situations have also been documented to have T. foetus infections. Holliday et al (15) examined fecal samples from 74 cats with chronic large bowel diarrhea that were living in a rescue colony in Italy. All 24 T. foetus infected cats were neutered, non-pedigreed domestic cats. The only positive fecal sample we observed was obtained from a 6-year-old purebred Abyssinian originally from a cattery. While purebred cats may be at increased risk for infection, it is more likely that the management practices within most catteries (e.g., high-density housing) provide a setting with high levels of environmental contamination. In our study, feral and shelter cats were chosen as they represent populations of cats that may be living in close proximity to one another thereby increasing the risk of acquiring infection. Although humane society cats were kept in groups of up to 4 cats, most fecal samples were obtained from cats that were caged singly. Feral cats were humanely trapped from colonies of various sizes; however, the size and setup within the colonies (e.g., number of feeding stations and shelter areas) was unknown. Ultimately, it is likely that neither group of cats represented intensively housed populations that would be required to promote maintenance and spread of T. foetus infection.
Culturing of feces for T. foetus was performed using a commercially available culture kit, which is easy to use and relatively inexpensive. A single fecal culture using this system has a reported sensitivity of 83% compared with examination of a single direct fecal smear, which has a low sensitivity of 14% (10,23). Like direct fecal smear, culture relies on the presence of viable organisms within the sample. Tritrichomonas foetus does not exhibit encystment and therefore the organism is considered to be fragile outside of the host. Although delayed inoculation of pouches could result in a loss of viable organisms, a study by Hale et al (23) found that the organisms are more resilient than previously believed — surviving up to 7 d in fecal samples stored at room temperature. Fecal samples kept at room temperature for up to 6 h are suitable for diagnostic investigation (23). The main challenge associated with this diagnostic tool is the skill of the individual examining the pouch in identifying motile trichomonads. The same investigator (OR) examined all of the pouches and identified the single positive pouch; however, it is possible that small numbers of organisms may have been overlooked. Overgrowth of fecal flora and subsequent accumulation of gas within the pouches was a common problem in this study, occurring within 3 to 7 d of inoculation. This may have been the result of inoculation of too much fecal material into the pouch. The presence of gas can impair visualization and may be another reason for false negative results. Clothier et al (33) found that bacterial contamination severely impaired the culture of T. foetus from bull preputial samples. The overgrowth of enteric bacteria in our study, therefore, may have reduced the sensitivity of the InPouch culture system.
Extraction and amplification of T. foetus DNA from feces via polymerase chain reaction (PCR) is the most sensitive method of testing (10). Unlike fecal cultures, PCR allows for the detection of live and dead trichomonads. Compared with other methods of testing, PCR requires highly trained personnel and specialized equipment. The use of PCR in conjunction with culturing of the parasite or performing multiple fecal cultures on each sample may have resulted in improved detection of T. foetus.
A secondary objective of this study was to determine the extent of co-infections of T. foetus with common intestinal parasites. No co-infections were detected; however, several different intestinal parasites were identified amongst both populations of cats. There were similar low infection rates of Giardia spp., Taenia spp., and Capillaria spp. among the 2 groups. Feral cats had a statistically significant higher incidence of T. cati infections than cats housed at the PEI Humane Society, while shelter cats had a statistically significant higher incidence of Cystoisospora spp. infections than feral cats. These results are in agreement with other studies investigating intestinal parasites in cats (34). Toxocara cati is common among feral cats in which transmission of the parasite can occur via predation (ingestion of the infected tissues of paratenic hosts such as mice and other vertebrates). Other modes of transmission include the fecal-oral route or transmammary transmission of larvae from the queen to her kittens (35). Similarly, Cystoisospora is common among intensively housed cats. Because of the resistant nature of coccidian oocysts, shelter environments may permanently harbor some oocyst load. Environmental contamination is greater due to high-density housing, presence of kittens and young cats, increased litter box use (less outdoor access), and higher levels of stress (36). A proportion of cats housed at the humane society also received a dose of selamectin (Revolution; Zoetis, Kirkland Quebec) and nitenpyram (Capstar; Novartis Animal Health US, Greensboro, North Carolina, USA), which may have affected the parasite burden, specifically T. cati, in this population of cats.
This study is the first investigation of T. foetus in domestic cats in PEI. Because T. foetus was not isolated from any of the 200 feral and shelter cats that were tested, it appears unlikely that this parasite is of importance in these cats. The single positive sample we obtained suggests that cats imported to the island, particularly those from catteries, could serve as reservoirs of infection. As such, testing for the parasite in purebred household cats with compatible clinical signs is recommended. Further studies investigating the presence of T. foetus in cats, particularly those with diarrhea, are needed to determine whether the parasite is more prevalent amongst cattery-raised purebred cats or household mixed breed cats.
Acknowledgments
The authors thank the Companion Animal Trust Fund, Atlantic Veterinary College (AVC) for financial support for this project. We are grateful to Whitney Kelly-Clark for her assistance with culture techniques and PCR confirmation and to Nicole Murphy for her assistance with parasite identification. We also thank the PEI Humane Society and the Cat Action Team (AVC feral cat spay and neuter program) for their assistance with sample collection. CVJ
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Levy MG, Gookin JL, Poore M, Birkenheuer AJ, Dykstra MJ, Litaker R. Tritrichomonas foetus and not Pentatrichomonas hominis is the etiologic agent of feline trichomonal diarrhea. J Parasitol. 2003;89:99–104. doi: 10.1645/0022-3395(2003)089[0099:TFANPH]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 2.Stockdale HD, Dillon AR, Newton JC, et al. Experimental infection of cats (Felis catus) with Tritrichomonas foetus isolated from cattle. Vet Parasitol. 2008;154:156–161. doi: 10.1016/j.vetpar.2008.02.024. [DOI] [PubMed] [Google Scholar]
- 3.Slapeta J, Craig S, McDonell D, Emery D. Tritrichomonas foetus from domestic cats and cattle are genetically distinct. Exp Parasitol. 2010;126:209–213. doi: 10.1016/j.exppara.2010.04.024. [DOI] [PubMed] [Google Scholar]
- 4.Walden HS, Dykstra C, Dillon A, et al. A new species of Tritrichomonas (Sarcomastigophora: Trichomonida) from the domestic cat (Felis catus) Parasitol Res. 2013;112:2227–2235. doi: 10.1007/s00436-013-3381-8. [DOI] [PubMed] [Google Scholar]
- 5.Schwebke JR, Burgess D. Trichomoniasis. Clin Microbiol Rev. 2004;17:794–803. doi: 10.1128/CMR.17.4.794-803.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Manning K. Update on the diagnosis and management of Tritrichomonas foetus infection in cats. Top Companion Anim Med. 2010;25:145–148. doi: 10.1053/j.tcam.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 7.Burgess DE, Knoblock KF, Daugherty T, Robertson NP. Cytotoxic and hemolytic effects of Tritrichomonas foetus on mammalian cells. Infect Immun. 1990;58:3627–3632. doi: 10.1128/iai.58.11.3627-3632.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burgess DE, McDonald CM. Analysis of adhesion and cytotoxicity of Tritrichomonas foetus to mammalian cells by use of monoclonal antibodies. Infect Immun. 1992;60:4253–4259. doi: 10.1128/iai.60.10.4253-4259.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yaeger MJ, Gookin JL. Histologic features associated with Tritrichomonas foetus-induced colitis in domestic cats. Vet Pathol. 2005;42:797–804. doi: 10.1354/vp.42-6-797. [DOI] [PubMed] [Google Scholar]
- 10.Gookin JL, Stebbins ME, Hunt E, et al. Prevalence of and risk factors for feline Tritrichomonas foetus and Giardia infection. J Clin Microbiol. 2004;42:2707–2710. doi: 10.1128/JCM.42.6.2707-2710.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xenoulis P, Lopinski DJ, Read SA, Schodolski JS, Steiner JM. Intestinal Tritrichomonas foetus infection in cats: A retrospective study of 104 cases. J Feline Med Surg. 2013;15:1098–1103. doi: 10.1177/1098612X13495024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Foster DM, Gookin JL, Poore MF, Stebbins ME, Levy MG. Outcome of cats with diarrhea and Tritrichomonas foetus infection. J Am Vet Med Assoc. 2004;225:888–892. doi: 10.2460/javma.2004.225.888. [DOI] [PubMed] [Google Scholar]
- 13.Gookin JL, Levy MG, Law JM, Papich MG, Poore MF, Breitschwerdt EB. Experimental infection of cats with Tritrichomonas foetus. Am J Vet Res. 2001;62:1690–1697. doi: 10.2460/ajvr.2001.62.1690. [DOI] [PubMed] [Google Scholar]
- 14.Gunn-Moore DA, McCann TM, Reed N, Simpson KE, Tennant B. Prevalence of Tritrichomonas foetus infection in cats with diarrhoea in the UK. J Feline Med Surg. 2007;9:214–218. doi: 10.1016/j.jfms.2007.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holliday M, Deni D, Gunn-Moore DA. Tritrichomonas foetus infection in cats with diarrhoea in a rescue colony in Italy. J Feline Med Surg. 2009;11:131–134. doi: 10.1016/j.jfms.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frey CF, Schild M, Hemphill A, et al. Intestinal Tritrichomonas foetus infection in cats in Switzerland detected by in vitro cultivation and PCR. Parasitol Res. 2009;104:783–788. doi: 10.1007/s00436-008-1255-2. [DOI] [PubMed] [Google Scholar]
- 17.Xenoulis PG, Saridomichelakis MN, Read SA, Suchodolski JS, Steiner JM. Detection of Tritrichomonas foetus in cats in Greece. J Feline Med Surg. 2010;12:831–833. doi: 10.1016/j.jfms.2010.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bell ET, Gowan RA, Lingard AE, McCoy RJ, Slapeta J, Malik R. Naturally occurring Tritrichomonas foetus infections in Australian cats: 38 cases. J Feline Med Surg. 2010;12:889–898. doi: 10.1016/j.jfms.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lim S, Park SI, Ahn KS, Oh DS, Ryu JS, Shin SS. First report of feline intestinal Trichomoniasis caused by Tritrichomonas foetus in Korea. Korean J Parasitol. 2010;48:247–251. doi: 10.3347/kjp.2010.48.3.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hosein A, Kruth SA, Pearl DL, et al. Isolation of Tritrichomonas foetus from cats sampled at a cat clinic, cat shows and a humane society in southern Ontario. J Feline Med Surg. 2013;15:706–711. doi: 10.1177/1098612X13475617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stockdale HD, Givens MD, Dykstra CC, Blagburn BL. Tritrichomonas foetus infections in surveyed pet cats. Vet Parasitol. 2009;160:13–17. doi: 10.1016/j.vetpar.2008.10.091. [DOI] [PubMed] [Google Scholar]
- 22.Gookin JL, Foster DM, Poore MF, Stebbins ME, Levy MG. Use of a commercially available culture system for diagnosis of Tritrichomonas foetus infection in cats. J Am Vet Med Assoc. 2003;222:1376–1379. doi: 10.2460/javma.2003.222.1376. [DOI] [PubMed] [Google Scholar]
- 23.Hale S, Norris JM, Slapeta J. Prolonged resilience of Tritrichomonas foetus in cat faeces at ambient temperature. Vet Parasitol. 2009;166:60–65. doi: 10.1016/j.vetpar.2009.07.032. [DOI] [PubMed] [Google Scholar]
- 24.Felleisen RS. Comparative sequence analysis of 5.8S rDNA genes and internal transcribed spacer (ITS) regions of trichomonadid protozoa. Parasitology. 1997;115:111–119. doi: 10.1017/s0031182097001212. [DOI] [PubMed] [Google Scholar]
- 25.Hall T. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. 1999;41:95–98. [Google Scholar]
- 26.Groat R, Monn M, Flynn L, Curato J. Survey of clinic practices and testing for diagnosis of Giardia infections in dogs and cats (poster presentation) Proceedings ACVIM Forum. 2003 [Google Scholar]
- 27.Conboy GA, Zajac AM. Veterinary Clinical Parasitology. 8th ed. West Sussex, United Kingdom: Wiley-Blackwell; 2012. pp. 7–8. [Google Scholar]
- 28.R Development Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2014. [Last accessed December 9, 2015]. Available from: http://www.R-project.org. [Google Scholar]
- 29.Greiner M, Gardner IA. Epidemiologic issues in the validation of veterinary diagnostic tests. Prev Vet Med. 2000;45:43–59. doi: 10.1016/s0167-5877(00)00114-8. [DOI] [PubMed] [Google Scholar]
- 30.Vose D. Risk Analysis: A Quantitative Guide. 3rd ed. Chichester, England: John Wiley & Sons; 2008. [Google Scholar]
- 31.Stockdale HD, Givens MD, Dykstra CC, Blagburn BL. Tritrichomonas foetus infections in surveyed pet cats. Vet Parasitol. 2009;160:13–17. doi: 10.1016/j.vetpar.2008.10.091. [DOI] [PubMed] [Google Scholar]
- 32.Tolbert MK, Gookin JL. Tritrichomonas foetus: A new agent of feline diarrhea. Compend Contin Educ Vet. 2009;31:374–381. [PubMed] [Google Scholar]
- 33.Clothier KA, Villanueva M, Torain A, Hult C, Wallace R. Effects of bacterial contamination of media on the diagnosis of Tritrichomonas foetus by culture and real-time PCR. Vet Parasitol. 2015;208:143–149. doi: 10.1016/j.vetpar.2015.01.006. [DOI] [PubMed] [Google Scholar]
- 34.Lucio-Forster A, Bowman DD. Prevalence of fecal-borne parasites detected by centrifugal flotation in feline samples from two shelters in upstate New York. J Feline Med Surg. 2011;13:300–303. doi: 10.1016/j.jfms.2010.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bowman DD. Georgis’ Parasitology for Veterinarians. 6th ed. Philadelphia, Pennsylvania: WB Saunders; 1995. pp. 203–207. [Google Scholar]
- 36.Lister AL, Nichols J, Hall K, Camp J, Mohamed AS. Use of ponazuril paste to treat coccidiosis in shelter-housed cats and dogs. Vet Parasitol. 2014;202:319–325. doi: 10.1016/j.vetpar.2014.03.003. [DOI] [PubMed] [Google Scholar]