Abstract
Forty-four dogs with multicentric lymphoma were treated using a cyclophosphamide, doxorubicin, vincristine, and prednisolone (CHOP) induction protocol or treated using a cyclophosphamide, mitoxantrone, vincristine, and prednisolone (CMOP) induction protocol. There was no statistical difference in signalment and the presence of historical negative prognostic factors between the groups. The median progression-free survival (PFS) in the CHOP and CMOP groups were 222 d and 162 d, respectively (P = 0.75). The median survival time (MST) of dogs in CHOP and CMOP groups were 318 d and 242 d, respectively (P = 0.63). Anorexia and diarrhea episodes were significantly higher in the CHOP group than in the CMOP group (P = 0.02 and P = 0.01, respectively). These results suggest that the CMOP protocol provides similar PFS, MST and causes fewer side effects compared to the CHOP protocol. Therefore, the CMOP protocol may be another treatment choice for canine multicentric lymphoma.
Résumé
Comparaison de l’efficacité et de la toxicité de la doxorubicine et du mitoxantrone dans la chimiothérapie combinée pour le lymphome canin. Quarante-quatre chiens atteints d’un lymphome multicentrique ont été traités à l’aide d’un protocole d’induction au cyclophosphamide, à la doxorubicine, à la vincristine et à la prednisolone (CHOP) ou traités en utilisant un protocole d’induction à la cyclophosphamide, au mitoxantrone, à la vincristine et à la prednisolone (CMOP). Il n’y a eu aucune différence statistique dans le signalement et la présence des facteurs de pronostic négatifs historiques entre les groupes. La survie sans progression (SSP) médiane des groupes CHOP et CMOP était de 222 jours et de 162 jours, respectivement (P = 0,75). La durée de survie moyenne (DSM) des chiens des groupes CHOP et CMOP a été de 318 jours et de 242 jours, respectivement (P = 0,63). Les épisodes d’anorexie et de diarrhée ont été significativement plus élevés dans le groupe CHOP comparativement au groupe CMOP (P = 0,02 et P = 0,01, respectivement). Ces résultats suggèrent que le protocole CMOP offre une SSP et une DSM semblables tout en causant moins d’effets secondaires comparativement au protocole CHOP. Par conséquent, le protocole CMOP peut représenter un autre choix de traitement pour les lymphomes multicentriques canins.
(Traduit par Isabelle Vallières)
Introduction
Lymphoma is the most common hematopoietic neoplasm in dogs. The annual incidence rate is estimated to be between 24 and 114 cases per 100 000 dogs (1,2). The disease predominantly involves the lymphoreticular system, which includes the lymph nodes, spleen, liver, blood, and bone marrow. Alimentary tract, skin, central nervous system, mediastinum, and kidney involvement have also been reported (3). Untreated dogs usually die within 4 to 6 wk (4). Reported negative prognostic factors include stage V, substage b, hypercalcemia, T-cell origin, and anemia (5,6).
Combination chemotherapy is the most common treatment for canine lymphoma, and numerous combination chemotherapy protocols have been developed. The most common drugs used in these protocols are prednisone, vincristine, cyclophosphamide, doxorubicin, and L-asparaginase. The median progression-free survival (PFS) is approximately 9 mo, and median survival time (MST) is 7 to 15 mo (7–11). Since doxorubicin-containing protocols yield favorable therapeutic outcomes, doxorubicin is used in almost all therapeutic protocols (12). However, doxorubicin can cause severe adverse effects, including bone marrow suppression, gastroenteritis, and, particularly, cardiac toxicity (13,14). In 1 study, clinical cardiac adverse effects including electrocardiographic abnormalities and congestive heart failure developed in 18.3% of dogs after doxorubicin treatment (14).
Mitoxantrone is a synthetic antitumor antibiotic with similar action to doxorubicin, but it induces less cardiotoxicity in humans and animals (15,16). It can be used to treat multiple tumor types including lymphoma (17). Mitoxantrone has been used as an alternative in treating cancer patients who cannot tolerate the adverse effects of doxorubicin. Mitoxantrone is also used to replace doxorubicin after the life time dose of doxorubicin has been reached. For canine lymphoma patients, mitoxantrone exhibits therapeutic activity and low toxicity when used as single-agent therapy (18). Mitoxantrone was administered at a dose of 5.0 mg/m2, IV, every 3 wk in that study, and the response rate was 41% (18). However, combination therapy that includes mitoxantrone has not been evaluated widely. Daters et al (19) evaluated a mitoxantrone-containing chemotherapy protocol for treating canine lymphoma, but that study did not directly compare the efficacy of mitoxantrone and doxorubicin.
The purpose of this study was to compare the response rate, disease-free interval, overall survival time, and side effects of doxorubicin and mitoxantrone in combination chemotherapy administered to canine multicentric lymphoma patients. The hypothesis is that mitoxantrone can provide a similar therapeutic effect as doxorubicin in combination chemotherapy for canine lymphoma.
Materials and methods
Patient selection and evaluation
Dogs diagnosed with multicentric lymphoma, according to either histopathology or cytology, at the National Taiwan University Veterinary Hospital from December 1, 2009 to December 31, 2013, were enrolled in this prospective study. Patients which had life expectancy less than 6 wk or had previously received any chemotherapeutic drugs or corticosteroids were excluded from the study.
Pretreatment evaluation included physical examination, a complete blood (cell) count (CBC), a biochemistry panel, thoracic and abdominal radiographs, an abdominal ultrasound, and immunophenotype identification. The immunophenotype was determined using flow cytometry or immunohistochemistry. Clinical staging was performed according to the World Health Organization (WHO) staging criteria for canine lymphoma (20). Bone marrow aspiration was not routinely performed unless the CBC indicated bone marrow involvement.
Treatment protocol
The enrolled dogs were divided into 2 groups by random allocation. We administered a modified 25-week Madison-Wisconsin chemotherapy protocol that excluded L-asparaginase in the cyclophosphamide, doxorubicin, vincristine, and prednisolone (CHOP) group, and administered mitoxantrone instead of doxorubicin to the cyclophosphamide, mitoxantrone, vincristine, and prednisolone (CMOP) group (Table 1). Doxorubicin and mitoxantrone were diluted with normal saline and administered intravenously over 2 h. Treatment was delayed 1 wk if neutropenia (< 3000 cells/μL) or thrombocytopenia (< 100 000 cells/μL) was identified in the pretreatment assessment, or if a clinical condition indicated that chemotherapy was contraindicated. We delayed treatment for 1 wk rather than reducing the dose in order to maintain the same dosage during the entire course of therapy. Patients that vomited were treated with metoclopramide (Siuguan, Chiayi City, Taiwan), 0.5 mg/kg body weight (BW), PO, q12h and patients with diarrhea were treated by oral anti-diarrhea compound prescription (each tablet contained kaolin 0.167 g, bismuth subcarbonate 0.167 g, and albumin tannate 0.167 g), 1 tablet per 10 kg BW, q12h.
Table 1.
Modified Madison-Wisconsin protocol without maintenance
| Week | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 11 | 13 | 15 | 17 | 19 | 21 | 23 | 25 | |
| Vincristine (0.7 mg/m2 IV) | • | • | • | • | • | • | • | • | |||||||||
| Cyclophosphamide (250 mg/m2 PO, single dose) | • | • | • | • | |||||||||||||
| Doxorubicin (30 mg/m2 IV) or Mitoxantrone (6 mg/m2 IV) | • | • | • | • | |||||||||||||
| Prednisolone (PO q24h) | 2 mg/kg BW × 7 d, then 1.5 mg/kg BW × 7 d, then 1.0 mg/kg BW × 7 d, then 0.5 mg/kg BW × 7 d, then stop | ||||||||||||||||
Response and toxicity criteria
Treatment response was determined by conducting physical examinations and taking lymph node measurements. The size of each lymph node was obtained with caliper measurements of tumor diameter in the largest dimension and then perpendicular to that diameter before each treatment. Lymph node sizes were compared with previously recorded sizes. Response evaluation criteria for peripheral nodal lymphoma in dogs (v1.0) were used to evaluate the therapeutic response (21). Complete response was characterized by a complete disappearance of all measurable disease; partial response was characterized by a > 30% decrease but < 100% decrease in the Mean Sum longest diameter of target lesions; stable disease was characterized by a < 30% decrease or < 20% increase in target lesions; and progressive disease was characterized by a > 20% increase in target lesions or the development of a new lesion. The PFS was calculated from initiation of treatment to the time of disease progression. The MST was calculated from initiation of treatment to the time of patient death. Dogs were censored in analysis of the duration of remission if they were alive in remission or lost to follow-up in remission. Dogs were censored in survival analysis if they died from causes other than lymphoma or were alive at the end of the study.
A CBC and physical examination were performed before each chemotherapy. After doxorubicin and mitoxantrone administration, treatment-associated hematologic and gastrointestinal toxicities were recorded from grades 1 to 5, according to the Veterinary Cooperative Oncology Group Common Terminology Criteria for Adverse Events (22). Gastrointestinal toxicity was recorded by the owner at home and reported to the veterinarian for scoring. Echocardiographic examination was performed prior to each dose of antitumor antibiotic to measure fraction shortening (FS) in order to monitor cardiac contractility. The formula of FS is as follows: FS (%) = (left ventricular internal dimension, diastole) — (left ventricular internal dimension, systole)]/(left ventricular internal dimension, diastole) × 100.
Statistical analysis
Continuous variables (age and body weight) were compared using Student’s t-test. The proportion of dogs in each group with known prognostic factors (clinical stage, substage, immunophenotype, and hypercalcemia), the proportion of dogs experiencing toxicity, and the response rate were compared using Fisher’s exact test. Fraction shortening was compared using linear regression equations. The median PFS and MST were determined using Kaplan-Meier analysis, and differences between the 2 groups were assessed using the log-rank test. All statistical analyses were considered significant at P < 0.05.
Results
Forty-four canine lymphoma patients met the inclusion criteria during the period of study. Most dogs in the study were mixed breed (n = 11), followed by golden retrievers (n = 10), Labrador retrievers (n = 4), beagles (n = 4), Maltese terriers (n = 3), schnauzers (n = 2), shih tzus (n = 2), and 1 each of bull terrier, cocker spaniel, Chihuahua, dachshund, Welsh corgi, Rottweiler, West Highland white terrier, and Yorkshire terrier. The continuous variables were normally distributed (P = 0.20). Ages (mean ± SD) of the dogs in the CHOP and CMOP groups were 7.6 ± 2.9 y (median: 7 y; range: 1 to 14 y) and 8.3 ± 2.6 y (median: 8 y; range: 4 to 13 y), respectively (P = 0.42). Body weights of dogs in the CHOP and CMOP groups were 19.0 ± 12.0 kg (median: 17.5 kg; range: 1.9 to 44.5 kg) and 18.2 ± 10.1 kg (median: 17.5 kg; range: 2.2 to 28.3 kg), respectively (P = 0.82). The CHOP group comprised 13 female (4 intact) and 9 male (6 intact) dogs, and the CMOP group comprised 8 female (3 intact) and 14 male (8 intact) dogs (P = 0.13). Thirteen, 7, and 2 dogs in the CHOP group and 12, 8, and 2 dogs in the CMOP group had WHO stage III, WHO stage IV, and WHO stage V lymphomas, respectively. There were 20 and 17 dogs classified as substage a, 2 and 5 dogs classified as substage b in the CHOP and CMOP groups, respectively. One and 21 dogs in each group were diagnosed as T-cell and B-cell lymphoma, respectively. There was no significant difference in the proportions of historical negative factors such as clinical stage (P = 1), substage (P = 0.22), immunophenotype (P = 1), and presence of hypercalcemia (P = 0.31) between the 2 groups (Table 2). Then, we used multivariate analysis to assess the prognostic factors listed. No significant difference was observed between the CHOP and CMOP groups (P = 0.42).
Table 2.
Patient characteristics
| CHOP (n = 22) | CMOP (n = 22) | P-value | |
|---|---|---|---|
| Age (years) | 7.6 | 8.3 | 0.42 |
| Body weight (kg) | 19.0 | 18.2 | 0.82 |
| Gender | |||
| Female | 13 (59.1%) | 8 (36.4%) | 0.13 |
| Male | 9 (40.9%) | 14 (63.6%) | |
| Clinical stage | |||
| III | 13 (59.1%) | 12 (54.5%) | 0.50 |
| IV | 7 (31.8%) | 8 (36.4%) | 0.73 |
| V | 2 (9.1%) | 2 (9.1%) | 1 |
| Substage | |||
| a | 20 (90.9%) | 17 (77.3%) | 0.22 |
| b | 2 (9.1%) | 5 (22.7%) | 0.22 |
| T-cell | 1 (4.5%) | 1 (4.5%) | 1 |
| B-cell | 21 (95.5%) | 21 (95.5%) | 1 |
| Hypercalcemia | 0 (0%) | 1 (4.5%) | 0.31 |
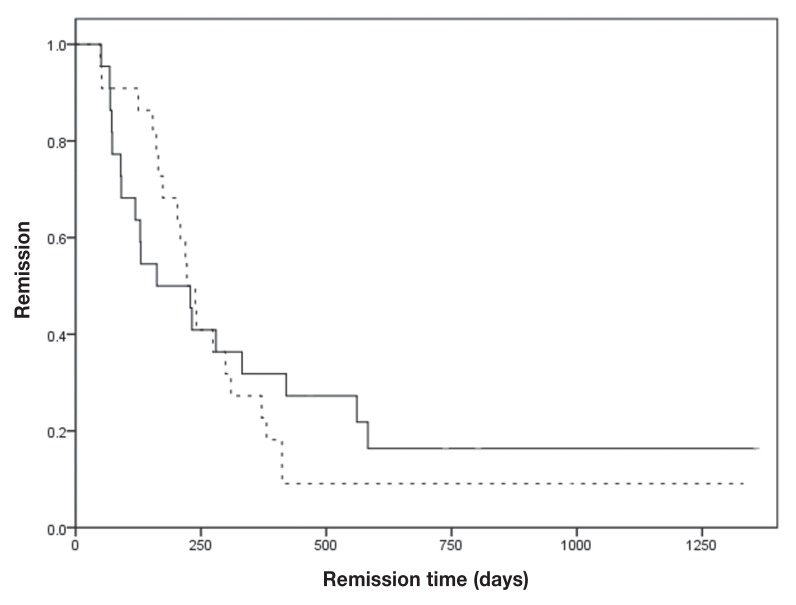
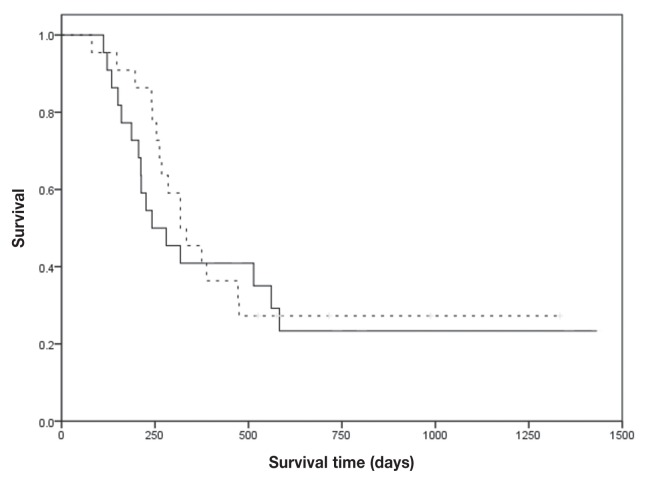
The response rates of the 2 groups were similar. The overall response rate was 90.9% in the each group (P = 1). Eighteen dogs (81.8%) exhibited complete remission (CR) and 2 dogs (9.1%) exhibited partial remission (PR) in the CHOP group, whereas 17 dogs (77.3%) exhibited CR and 3 dogs (13.6%) exhibited PR in the CMOP group (Table 3). There was no difference between the 2 groups regarding the PFS and survival time (ST). The median PFS and ST of the CHOP group were 222 d [95% confidence interval (CI): 185 to 259 d] and 318 d (95% CI: 215 to 421 d), respectively, and those of the CMOP group were 162 d (95% CI: 44 to 280 d) and 242 d (95% CI: 121 to 363 d) (P = 0.75 and 0.63, respectively; Figures 1, 2). The power of this study was 6%.
Table 3.
Outcome characteristics
| CHOP (n = 22) | CMOP (n = 22) | P-value | |
|---|---|---|---|
| Response rate | |||
| Overall (CR + PR) | 20 (90.9%) | 20 (90.9%) | 1 |
| Complete response | 18 (81.8%) | 17 (77.3%) | 0.71 |
| Partial remission | 2 (9.1%) | 3 (13.6) | 0.63 |
| Stable disease | 2 (9.1%) | 2 (9.1%) | 1 |
| Progression free survival | 222 days | 162 days | 0.75 |
| Median survival time | 318 days | 242 days | 0.63 |
CR — complete remission; PR — partial remission.
Figure 1.
Kaplan-Meier curves for the first remission duration for lymphoma dogs. Dogs were treated with CHOP (dashed line) or CMOP (solid line) protocol.
Figure 2.
Kaplan-Meier curves for the survival times for lymphoma dogs. Dogs were treated with CHOP (dashed line) or CMOP (solid line) protocol.
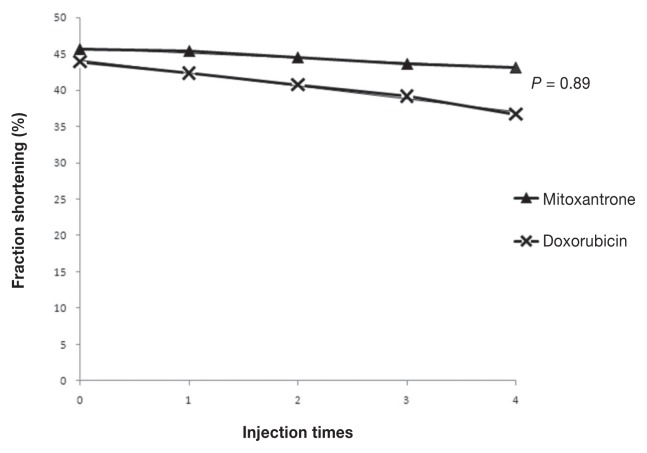
Only 1 episode of neutropenia (P = 1) and no episode of thrombocytopenia (P = 1) occurred in each group, and there was no significant difference between the groups. Sixteen dogs in the CHOP group experienced gastrointestinal toxicity, consisting of 21 episodes of anorexia, 19 episodes of diarrhea, and 12 episodes of vomiting. Details of the grading of gastrointestinal toxicity are listed in Table 4. Seven dogs in the CMOP group experienced gastrointestinal toxicity, consisting of 6 episodes of anorexia, 2 episodes of diarrhea, and 1 episode of vomiting. All gastrointestinal toxicity episodes were classified as grade 1. Anorexia and diarrhea episodes were significantly higher in the CHOP group than in the CMOP group (P = 0.02 and P = 0.01, respectively). Vomiting occurred in 8 (36.4%) dogs in the CHOP group and 1 (4.6%) dog in the CMOP group. Vomiting episodes were not significantly different between groups (P = 0.08; Table 4). No significant differences between the 2 groups existed in the linear regression equations of the FS over time (P = 0.89; Figure 3). No dog died or was euthanized as a result of toxicity.
Table 4.
Toxicity characteristics
| CHOP (n = 22) | CMOP (n = 22) | P-value | |
|---|---|---|---|
| Neutropenia | 1 (4.6%) | 1 (4.6%) | 1 |
| Grade 1 episode | 1 | 1 | |
| Mean grade | 0.02 | 0.02 | |
| Thrombocytopenia | 0 (0%) | 0 (0%) | 1 |
| Anorexia | 12 (54.6%) | 6 (27.3%) | 0.02 |
| Grade 1 episode | 12 | 6 | |
| Grade 2 episode | 6 | 0 | |
| Grade 3 episode | 2 | 0 | |
| Grade 4 episode | 1 | 0 | |
| Mean grade | 0.43 | 0.08 | |
| Diarrhea | 11 (50%) | 2 (9.1%) | 0.01 |
| Grade 1 episode | 10 | 2 | |
| Grade 2 episode | 7 | 0 | |
| Grade 3 episode | 2 | 0 | |
| Mean grade | 0.38 | 0.02 | |
| Vomiting | 8 (36.4%) | 1 (4.6%) | 0.08 |
| Grade 1 episode | 7 | 1 | |
| Grade 2 episode | 2 | 0 | |
| Grade 3 episode | 3 | 0 | |
| Mean grade | 0.23 | 0.01 |
Figure 3.
Fraction shortening changes after each treatment.
Fifteen dogs in the CHOP group and 11 in the CMOP group completed the 25-week chemotherapy protocol. Two dogs in the CHOP group and 4 dogs in the CMOP group did not relapse until the study closed. Four dogs in the CHOP group and 3 dogs in the CMOP group that completed the 25-week protocol in CR were treated with the same protocol at the time of relapse, and all dogs achieved a second CR. The dogs which did not complete the 25-week protocol or did not respond to re-induction therapy when relapsed were treated with rescue therapy. Rescue protocol was administered with L-asparaginase, lomustine, and prednisolone. Two and 4 dogs in the CHOP and CMOP groups that were still in CR were censored from remission analysis. Six dogs in each group were still alive when the study closed and were censored from survival analysis. Thirty-two dogs died of lymphoma progression. The median follow-up of the censored dogs were 895 d and 727 d in remission and survival analysis, respectively.
Discussion
Combination chemotherapy protocols often include drugs with various mechanisms of action, thereby potentially delaying the onset of multidrug resistance. Each drug in a combination should be known to be effective as a single agent, should not share resistance mechanisms, and, ideally, should not result in overlapping toxicities (23). Most combination chemotherapy protocols currently used in veterinary medicine for canine lymphoma are CHOP-based. A previous study indicated that patients treated using combination chemotherapy without doxorubicin were at an approximately two-fold higher risk for relapse and death than patients treated with doxorubicin (12). That study compared CHOP and COAP (A, cytosine arabinoside) protocols for evaluating the PFS and ST. Since doxorubicin is an antitumor antibiotic and cytosine arabinoside is an antimetabolite, the reason for the different results with the CHOP and COAP protocols is unclear. In this study, mitoxantrone was used to replace doxorubicin in a combination chemotherapy protocol. These drugs belong to the same category and share similar mechanisms of action. Our results indicate that the responses to both drugs in combination protocols for induction therapy were similar. Since antitumor antibiotics should be considered the main drug in multiagent chemotherapeutic protocols for canine lymphoma, we believe that mitoxantrone-containing combination chemotherapy protocol is an alternative therapeutic choice for canine lymphoma.
Daters et al (19) investigated CHOP and mitoxantrone-based maintenance chemotherapy protocols to treat canine lymphoma. The PFS and MST in that study were 302 and 622 d, respectively, which were longer than our results. One possible reason was that the cumulative doses of doxorubicin and mitoxantrone in that study were higher than in our study. The cumulative dose of antitumor antibiotics may play an important part in combination chemotherapy. Another potential reason was that we used maintenance-free and consolidation-free protocols. Discontinuous protocols have the possibility of reducing the development of drug resistance and improving the chances of second remission following relapse. Although a recent report (24) indicated that short discontinuous chemotherapy was better than longer protocols that included maintenance, further prospective, randomized, well-controlled studies are needed to verify this.
During this study, the patients in the CHOP group had more episodes of anorexia and diarrhea than did those in the CMOP group. Two patients in the CHOP group experienced severe gastrointestinal toxicity and required hospitalization for intensive care, increasing therapeutic costs. All episodes of gastrointestinal toxicity in patients in the CMOP group were self-limited and did not require medication. Therefore, we believe the CMOP protocol is a safer and less expensive therapy for canine lymphoma than is the CHOP protocol.
The incidence of hematologic toxicity after doxorubicin or mitoxantrone administration was low in both groups. The possible reason was that nadir checks were not performed, and blood tests were conducted before subsequent treatments, which occurred 2 wk after the previous administration. Therefore, the actual hematologic toxicity may have been underestimated. The other reason was the use of delayed chemotherapy. In our experience, many patients with neutrophil counts between 2000 and 3000 cells/μL had side effects and infection after chemotherapy. Therefore, the criterion for delayed chemotherapy in our study was a neutrophil count < 3000 cells/μL, which is higher than most oncologists would use.
The dose of doxorubicin was 30 mg/m2 for all dogs in order to avoid the bias of size of dog. Previous studies showed that small dogs had higher levels of neutropenia than large dogs when administered cytotoxic drugs at doses based on body surface area (25). Similarly, studies in humans revealed that chemotherapy doses based on body surface area calculations are higher in children than in adults (26). In this study, there was no significant difference in body weight between the dogs with and without gastrointestinal toxicity. Small sample sizes of small dogs may be a possible reason for this. Mitoxantrone was used with 6 mg/m2 dosage in our clinic for many types of tumors and caused no obvious side effects including myelosuppression and gastrointestinal toxicity. Chemotherapeutic drugs should be used at their maximally tolerated dosages. Therefore, we used mitoxantrone at 6 mg/m2, which is higher than other oncologists used.
In our study, the CMOP protocol provided similar efficacy and less side effects than the CHOP protocol. Therefore, when the CMOP protocol is applied in induction therapy for canine lymphoma, doxorubicin can be saved as part of combination rescue therapy. Although tumor cells that are resistant to mitoxantrone may develop cross-resistance to doxorubicin, the mechanism of mitoxantrone resistance may be different than that for doxorubicin resistance (17,27).
Dogs of large breeds are at a higher risk of developing lymphoma and dilated cardiomyopathy (28,29). Doxorubicin is the most common drug used to treat canine lymphoma, but it potentially induces cardiomyopathy (30). No evidence of drug-induced cardiotoxicity caused by mitoxantrone was observed. In our study, the trends in FS after each treatment in both groups were not statistically different. A possible reason for this was the short observation period (approximately 27 wk). Since the cardiotoxicity of doxorubicin is cumulative and irreversible (31), future studies should address whether the cardiotoxicity of the 2 drugs changes after a longer period. Another possible reason is that cardiotoxicity is related to the peak plasma doxorubicin concentration (32). Infusion of diluted doxorubicin over several hours in our study could have lowered the peak plasma concentration, potentially reducing the amount of doxorubicin entering myocardial cells (33).
This study had some limitations. The number of patients is small, making it difficult to draw broad conclusions regarding efficacy and toxicity. The number of dogs required for an 80% power for PFS was 810 for a difference of 1 mo, and 90 per group for a 3-month difference. A longer and more rigorously controlled prospective study may be needed to clarify our results. Clinical staging was typically minimal because bone marrow aspiration, spleen, and liver fine-needle aspiration were not performed on every dog. Stage migration may lead some dogs to have been falsely classified into lower clinical stages than warranted. Relapsed patients did not uniformly respond to the re-induction protocols; additional rescue protocols were used according to patient response. The different rescue protocols may have influenced the ST. Gastrointestinal toxicity scoring was assessed by owners at home rather than by clinicians; this may have overestimated or underestimated the scores.
In conclusion, the present study indicated that the CMOP protocol was effective for treating canine lymphoma. There was no significant difference in response rate, PFS and MST between the 2 protocols. Gastrointestinal toxicity was less prominent in the CMOP group than in the CHOP group; hematotoxicity and cardiotoxicity were not significant during the study period. CVJ
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Dorn CR, Taylor DO, Schneider R. The epidemiology of canine leukemia and lymphoma. Bibl Haematol. 1969;36:403–415. doi: 10.1159/000391733. [DOI] [PubMed] [Google Scholar]
- 2.Dobson JM, Samuel S, Milstein H, Rogers K, Wood JLN. Canine neoplasia in the UK: Estimates of incidence rates from a population of insured dogs. J Small Anim Pract. 2002;43:240–246. doi: 10.1111/j.1748-5827.2002.tb00066.x. [DOI] [PubMed] [Google Scholar]
- 3.Leifert CE, Matus RE. Canine lymphosarcoma: Clinical considerations. Semin Vet Med/Surg. 1986;1:43–50. [PubMed] [Google Scholar]
- 4.MacEwen EG, Patnaik AK, Wilkins RJ. Diagnosis and treatment of canine hematopoietic neoplasms. Vet Clin North Am Small Anim Pract. 1977;7:105–118. doi: 10.1016/s0091-0279(77)50009-3. [DOI] [PubMed] [Google Scholar]
- 5.Teske E, Van Heerde P, Rutteman GR, Kurzman ID, Moore PF, MacEwen EG. Prognostic factors for treatment of malignant lymphoma in dogs. J Am Vet Med Assoc. 1994;205:1722–1728. [PubMed] [Google Scholar]
- 6.Miller AG, Morley PS, Rao S, Avery AC, Lana SE, Olver CS. Anemia is associated with decreased survival time in dogs with lymphoma. J Vet Intern Med. 2009;23:116–122. doi: 10.1111/j.1939-1676.2008.00210.x. [DOI] [PubMed] [Google Scholar]
- 7.Garrett LD, Thamm DH, Chun R, Dudley R, Vail DM. Evaluation of a 6-month chemotherapy protocol with no maintenance therapy for dogs with lymphoma. J Vet Intern Med. 2002;16:704–709. doi: 10.1892/0891-6640(2002)016<0704:eoacpw>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 8.Flory AB, Rassnick KM, Al-Sarraf R, et al. Combination of CCNU and DTIC chemotherapy for treatment of resistant lymphoma in dogs. J Vet Intern Med. 2008;22:164–171. doi: 10.1111/j.1939-1676.2007.0005.x. [DOI] [PubMed] [Google Scholar]
- 9.Keller ET, MacEwen EG, Rosenthal RC, Helfand SC, Fox LE. Evaluation of prognostic factors and sequential combination chemotherapy for canine lymphoma. J Vet Intern Med. 1993;7:289–295. doi: 10.1111/j.1939-1676.1993.tb01021.x. [DOI] [PubMed] [Google Scholar]
- 10.Rassnick KM, McEntee MC, Erb HN, et al. Comparison of 3 protocols for treatment after induction of remission in dogs with lymphoma. J Vet Intern Med. 2007;21:1364–1373. doi: 10.1892/07-057.1. [DOI] [PubMed] [Google Scholar]
- 11.Yamazaki J, Takahashi M, Setoguchi A, Fujino Y, Ohno K, Tsujimoto H. Monitoring of minimal residual disease (MRD) after multidrug chemotherapy and its correlation to outcome in dogs with lymphoma: A proof-of-concept pilot study. J Vet Intern Med. 2010;24:897–903. doi: 10.1111/j.1939-1676.2010.0536.x. [DOI] [PubMed] [Google Scholar]
- 12.Hosoya K, Kisseberth WC, Lord LK, et al. Comparison of COAP and UW-19 protocols for dogs with multicentric lymphoma. J Vet Intern Med. 2007;21:1355–1363. doi: 10.1892/06-284.1. [DOI] [PubMed] [Google Scholar]
- 13.Postorino NC, Susaneck SJ, Withrow SJ, Macy DW, Harris C. Single agent therapy with adriamycin for canine lymphosarcoma. J Am Anim Hosp Assoc. 1989;25:221–225. [Google Scholar]
- 14.Mauldin GE, Fox PR, Patnaik AK, Bond BR, Mooney SC, Matus RE. Doxorubicin-induced cardiotoxicosis clinical features in 32 dogs. J Vet Intern Med. 2008;6:82–88. doi: 10.1111/j.1939-1676.1992.tb03156.x. [DOI] [PubMed] [Google Scholar]
- 15.Sparano BM, Gordon G, Hall C, Iatropoulos MJ, Noble JF. Safety assessment of new anticancer compound, mitoxantrone, in beagle dogs: Comparison with doxorubicin. II. Histologic and ultrastructural pathology. Cancer Treat Rep. 1982;66:1145–1158. [PubMed] [Google Scholar]
- 16.Tham P, Dougherty W, Iatropoulos MJ, et al. The effect of mitoxantrone treatment in beagle dogs previously treated with minimally cardiotoxic doses of doxorubicin. Am J Pathol. 1987;128:121–130. [PMC free article] [PubMed] [Google Scholar]
- 17.Henry CJ. Toxicity and efficacy of mitoxantrone for treatment of various malignant tumors in companion animals. Canine Pract. 1999;24:10–12. [Google Scholar]
- 18.Moore AS, Ogilvie GK, Ruslander D, et al. Evaluation of mitoxantrone for the treatment of lymphoma in dogs. J Am Vet Med Assoc. 1994;204:1903–1903. [PubMed] [Google Scholar]
- 19.Daters AT, Mauldin GE, Mauldin GN, Brodsky EM, Post GS. Evaluation of a multidrug chemotherapy protocol with mitoxantrone based maintenance (CHOP-MA) for the treatment of canine lymphoma. Vet Comp Oncol. 2010;8:11–22. doi: 10.1111/j.1476-5829.2009.00199.x. [DOI] [PubMed] [Google Scholar]
- 20.Owen LN. TNM classification of tumours in domestic animals. 1st ed. Geneva, Switzerland: World Health Organization; 1980. pp. 46–47. [Google Scholar]
- 21.Vail DM, Michels GM, Khanna C, Selting KA, London CA. Response evaluation criteria for peripheral nodal lymphoma in dogs (v1.0) — A veterinary cooperative oncology group (VCOG) consensus document. Vet Comp Oncol. 2010;8:28–37. doi: 10.1111/j.1476-5829.2009.00200.x. [DOI] [PubMed] [Google Scholar]
- 22.Veterinary Co-operative Oncology Group. Common terminology criteria for adverse events (VCOG-CTCAE) following chemotherapy or biological antineoplastic therapy in dogs and cats v1.0. Vet Comp Oncol. 2004;2:195–213. doi: 10.1111/j.1476-5810.2004.0053b.x. [DOI] [PubMed] [Google Scholar]
- 23.Chu E, DeVita VT. Principles of medical oncology. In: DeVita VT, Hellman S, Rosenberg SA, editors. Cancer, Principles and Practice of Oncology. 7th ed. Philadelphia, Pennsylvania: Lippincott Williams & Wilkins; 2005. pp. 295–306. [Google Scholar]
- 24.Moore AS, Cotter SM, Rand WM, et al. Evaluation of a discontinuous treatment protocol (VELCAP-S) for canine lymphoma. J Vet Intern Med. 2001;15:348–354. [PubMed] [Google Scholar]
- 25.Arrington KA, Legendre AM, Tabeling GS, Frazier DL. Comparison of body surface area-based and weight-based dosage protocols for doxorubicin administration in dogs. Am J Vet Res. 1994;55:1587–1592. [PubMed] [Google Scholar]
- 26.Gurney H. Dose calculation of anticancer drugs: A review of the current practice and introduction of an alternative. J Clin Oncol. 1996;14:2590–2611. doi: 10.1200/JCO.1996.14.9.2590. [DOI] [PubMed] [Google Scholar]
- 27.Harker WG, Slade DL, Dalton WS, Meltzer PS, Trent JM. Multidrug resistance in mitoxantrone-selected HL-60 leukemia cells in the absence of P-glycoprotein overexpression. Cancer Res. 1989;49:4542–4549. [PubMed] [Google Scholar]
- 28.Detweiler DK. Genetic aspects of cardiovascular diseases in animals. Circulation. 1964;30:114–127. doi: 10.1161/01.cir.30.1.114. [DOI] [PubMed] [Google Scholar]
- 29.Villamil JA, Henry CJ, Hahn AW, Bryan JN, Tyler JW, Caldwell CW. Hormonal and sex impact on the epidemiology of canine lymphoma. J Cancer Epidemiol. 2009;591753 doi: 10.1155/2009/59175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lefrak EA, Pit̓ha J, Rosenheim S, Gottlieb JA. A clinicopathologic analysis of adriamycin cardiotoxicity. Cancer. 1973;32:302–314. doi: 10.1002/1097-0142(197308)32:2<302::aid-cncr2820320205>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 31.Jaenke RS. Delayed and progressive myocardial lesions after adriamycin administration in the rabbit. Cancer Res. 1976;36:2958–2966. [PubMed] [Google Scholar]
- 32.Legha SS, Benjamin RS, Mackay B, et al. Reduction of doxorubicin cardiotoxicity by prolonged continuous intravenous infusion. Ann Intern Med. 1982;96:133–139. doi: 10.7326/0003-4819-96-2-133. [DOI] [PubMed] [Google Scholar]
- 33.Pacciarini MA, Barbieri B, Colombo T, Broggini M, Garattini S, Donelli MG. Distribution and antitumor activity of adriamycin given in a high-dose and a repeated low-dose schedule to mice. Cancer Treat Rep. 1978;62:791–800. [PubMed] [Google Scholar]