Abstract
This study reports a retrospective evaluation of epidemiological data from cystine stones of dogs submitted to the Urinary Stone Analysis Center Bonn, Germany, over a period of 35 years. Of the 20 316 uroliths submitted from 1979 to 2013, 1760 were cystine stones. In total, 109 breeds were affected with 16 breeds having an odds ratio > 1.0. Most of the cystine uroliths were retrieved from male dogs, with only 19 female dogs (1.1%) being affected. Percentage of submitted cystine stones amongst all stones decreased significantly over 35 years from 38.9% to 4.4%.
Résumé
Urolithiase de cystine canine : compte rendu de 1760 soumissions pendant 35 ans (1979–2013). Cette étude présente un rapport sur une évaluation rétrospective des données épidémiologiques provenant des calculs de cystine de chiens soumis à l’Urinary Stone Analysis Center, à Bonn, en Allemagne, pendant une période de 35 ans. Parmi les 20 316 urolithes soumis de 1979 à 2013, 1760 étaient des calculs de cystine. Au total, 109 races ont été touchées et 16 races avaient un rapport de cote > 1,0. La plupart des urolithes de cystine provenaient de chiens, et seulement 19 chiennes (1,1 %) étaient affectées. Le pourcentage des calculs de cystine soumis parmi tous les calculs a significativement baissé au cours des 35 années, passant de 38,9 % à 4,4 %.
(Traduit par Isabelle Vallières)
Introduction
Cystinuria with possible development of cystine urolithiasis is a well-known genetic disease in humans (1,2) and reported in various animal species including dogs, cats (3–5), and wolves (6). Due to the low solubility of cystine in urine having a pH < 7.0 (typical dog urine has a pH range of 5.5 to 7.5), there is a high risk of lifelong stone formation. The relative occurrence of cystine stones in dogs is variable, depending on the country of origin (4,5,7–15). The present retrospective study reports on epidemiological data of cystine uroliths in dogs from 1979 to January 2013.
Materials and methods
A computer-assisted search of data from analyses of uroliths submitted to the Urinary Stone Analysis Center Bonn, Germany, was used to compile information about the composition of all urinary stones and the signalment of submissions from 1979 to January 2013. Uroliths were submitted mainly from Germany (99.1%), but a small percentage was received from other European countries (Finland, Switzerland, Italy, and France). Uroliths from dogs were analyzed with infrared spectrometry, as previously described (1,16,17). Epidemiological data such as breed, age, gender, and location of stone were recorded when available.
If necessary, prior to analysis, the uroliths were dried at 37°C. The dried material was then homogenized in an agate grinder, with a sample of less than 1 mg required for analysis. Analysis was performed by an infrared FT-IR spectrometer (Spectrum one; Perkin Elmer, Baesweiler, Germany). Evaluation of the graphs was performed with an atlas of infrared spectra for the analysis of uroliths (17) as well as with a computerized library of personally created reference spectra.
A urolith containing at least 70% of a single mineral was classified as that mineral type (18). Uroliths containing < 70% of a single mineral component were classified as mixed.
The frequency of cystine uroliths amongst all submitted stones was calculated for each year. Epidemiological data of cystine stones submitted from 1979 to 1996 were compared to data for cystine stones submitted from 1997 to 2013 by either Pearson Chi-square test/Fisher exact test (discrete values), or t-test (age). Change in frequency in the 12 most frequently affected breeds was assessed during 5 time periods, each lasting 7 y (1979 to 1985, 1986 to 1992, 1993 to 1999, 2000 to 2007, 2007 to 2013) and significant changes over time were assessed by Chi-square tests for linear trend. For all breeds, odds ratios (OR) were calculated by using other breeds than the considered breed as well as mixed breeds as reference group. Odds ratios and 95% confidence intervals (CI) were calculated using the exact method with a statistical software package (StatXact Ver. 9.0.0; Cytel Corporation, Cambridge, Massachusetts, USA). A P-value < 0.05 was considered significant for all comparisons.
Results
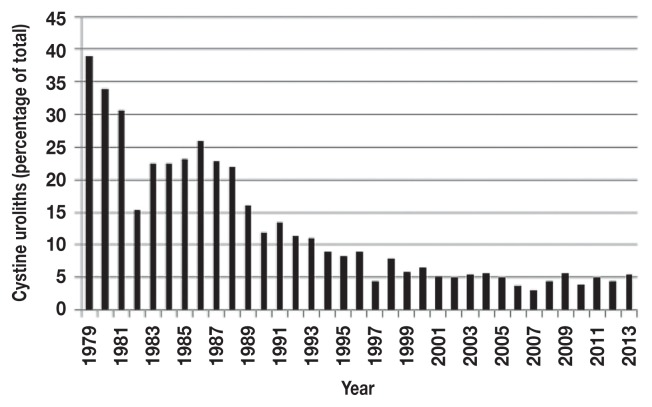
Mineral composition of all uroliths is shown in Table 1. Of the 20 316 canine uroliths analyzed, 1760 (8.7%) were composed of > 70% cystine. In 8 mixed stones which contained between 40% and 65% cystine, the other main components were struvite (n = 3), calcium oxalate monohydrate (whewellite) (n = 3), and carbonate apatite (n = 2). Frequency of cystine stones amongst all submitted stones decreased from 38.9% (1979) to 4.4% (2012) (Figure 1). From 1979 to 1996, 16.2% of uroliths were composed of cystine, while from 1997 to 2013, 5.1% of all submitted stones were of cystine origin (P < 0.001).
Table 1.
Mineral composition of 20 316 uroliths of dogs submitted from 1979 to 2013
| Mineral type | Number | Percent |
|---|---|---|
| Struvite | 1267 | 50.5 |
| Calcium oxalate | 5783 | 28.5 |
| Cystine | 1760 | 8.7 |
| Ammonium urate | 1231 | 6.1 |
| Carbonate apatite | 221 | 1.1 |
| Brushite | 211 | 1.0 |
| Sodium urate | 98 | 0.5 |
| Xanthine | 73 | 0.4 |
| Silicate | 69 | 0.3 |
| Protein | 39 | 0.2 |
| Potassium urate | 24 | 0.1 |
| Drugs | 12 | 0.1 |
| Uric acid | 12 | 0.1 |
| Calcite | 11 | 0.1 |
| Others | 33 | 0.2 |
| Mixed stones | 472 | 2.2 |
| Total | 20 316 | 100.0 |
Figure 1.
Frequency of canine cystine uroliths over time, expressed as a percentage of total uroliths.
Location
Four stones were submitted from either of the kidneys and 7 from either ureter, 650 (36.9%) stones were found in the bladder, 383 (21.8%) in the urethra, and 554 (31.5%) in both bladder and urethra. In the remaining 162 (9.2%) cases location was unknown. There was no significant difference in cystine uroliths location (upper versus lower urinary tract) between 1979 to 1996 and 1997 to 2013.
Gender
More than 94% of all cystine uroliths were from intact male dogs (Table 2) and only 16 (0.9%) were from intact female dogs (male to female ratio 88:1). The male-to-female ratio in all other types of uroliths over the same time period was 1.5:1. There was no significant difference in gender of dogs from which cystine uroliths were submitted between 1979 to 1996 and 1997 to 2013.
Table 2.
Gender distribution of dogs with cystine urolithiasis (n = 1760) compared to dogs with other urinary stones (n = 18 556) (P < 0.001)
| Gender | Other stones N (%) |
Cystine stones N (%) |
|---|---|---|
| Male | 7773 (41.9%) | 1597 (90.7%) |
| Male neutered | 2385 (12.9%) | 77 (4.4%) |
| Female | 5037 (27.1%) | 16 (0.9%) |
| Female spayed | 2677 (14.4%) | 3 (0.2%) |
| Gender unknown | 684 (3.7%) | 67 (3.8%) |
| Total | 18 556 (100%) | 1760 (100%) |
Stones from dogs with unknown gender were excluded from analysis.
Age
Mean [± standard deviation (SD)] age of dogs from which cystine uroliths were submitted was 5.9 ± 2.5 y with dogs aged 3 to 6 y most commonly affected. In 44 dogs age was unknown. Male dogs were on average somewhat older than female dogs (m:f = 5.9:5.5 y). There was no significant age difference between intact and castrated male dogs. Mean age of all dogs from which uroliths except cystine stones were submitted was significantly higher (7.5 ± 3.1 y; P < 0.001). The age was unknown in 504 dogs with non-cystine uroliths. Age of dogs from which cystine stones were submitted from 1979 to 1996 was significantly (P < 0.001) older (6.1 ± 2.5 y) than for dogs from which cystine stones were submitted from 1997 to 2013 (5.6 ± 2.6 y).
Breed
Cystine stones were submitted from 109 breeds of dogs (in 8 submissions, breed was not recorded), while other stone types were submitted from 209 breeds of dogs (in 18 submissions, breed was not recorded). The highest odds ratios were found in the Saluki, mastiff, and puli, with 14 breeds being significantly at risk of cystine urolithiasis compared to other stones (Table 3). Cystine uroliths were most commonly diagnosed in dachshunds and basset hounds. Throughout the study period, there was a significant change in relative cystine stone occurrence amongst some predisposed breeds including mixed breed dogs (Table 4).
Table 3.
Odds ratios (95% CI) for 23 breeds from which cystine uroliths were submitted in comparison to all other stones submitted from the same breeds combined as well as mixed breeds
| Breeds | Cystine uroliths (N) | Other uroliths (N) | Odds ratio (95% CI) relative to all other breeds combined | Odds ratio (95% CI) relative to mixed breeds |
|---|---|---|---|---|
| Saluki | 13 | 2 | 69.2 (15.6; 632) | 75.8 (17.0; 693) |
| Mastiff | 24 | 8 | 32.1 (13.9; 82.8) | 35.0 (15.0; 90.8) |
| Puli | 50 | 17 | 32.0 (18.1; 59.2) | 34.3 (19.1; 64.2) |
| Basset hound | 97 | 110 | 9.81 (7.35; 13.1) | 10.3 (7.52; 14.0) |
| Munster lander | 44 | 57 | 8.34 (5.48; 12.6) | 9.00 (5.81; 13.9) |
| English bulldog | 50 | 86 | 6.30 (4.33; 9.06) | 6.78 (4.58; 9.94) |
| Dachshund | 594 | 1500 | 5.82 (5.19; 6.52) | 4.62 (3.94; 5.42) |
| French bulldog | 34 | 64 | 5.71 (3.64; 8.81) | 6.20 (3.89; 9.72) |
| Australian terrier | 25 | 48 | 5.57 (3.28; 9.24) | 6.07 (3.53; 10.2) |
| Rottweiler | 58 | 122 | 5.16 (3.69; 7.14) | 5.55 (3.89; 7.84) |
| Toy pincher | 22 | 69 | 3.40 (2.00; 5.58) | 3.72 (2.15; 6.19) |
| Afghan | 12 | 39 | 3.27 (1.55; 6.38) | 3.59 (1.69; 7.09) |
| Chihuahua | 22 | 90 | 2.60 (1.55; 4.20) | 2.85 (1.67; 4.67) |
| Irish terrier | 18 | 112 | 1.71 (0.97; 2.83) | 1.87 (1.06; 3.16) |
| German braque | 13 | 122 | 1.13 (0.58; 2.01) | 1.24 (0.63; 2.24) |
| Doberman | 32 | 323 | 1.05 (0.70; 1.52) | 0.09 (0.76; 1.71) |
| Mixed breed | 273 | 3184 | 0.89 (0.77; 1.02) | — |
| Welsh corgi | 12 | 165 | 0.77 (0.39; 1.38) | 0.85 (0.42; 1.55) |
| Poodle | 42 | 641 | 0.68 (0.49; 0.94) | 0.76 (0.53; 1.07) |
| Cavalier King Charles spaniel | 10 | 314 | 0.33 (0.16; 0.62) | 0.37 (0.17; 0.70) |
| Cocker spaniel | 17 | 610 | 0.29 (0.17; 0.47) | 0.33 (0.19; 0.54) |
| Yorkshire terrier | 42 | 1542 | 0.27 (0.19; 0.37) | 0.32 (0.22; 0.44) |
| Jack Russell terrier | 13 | 560 | 0.24 (0.13; 0.41) | 0.27 (0.14; 0.48) |
Table 4.
Percentage of stones that were cystine stones in 12 breeds during 5 time periods for stones submitted from dogs with urolithiasis
| Breed | 1979 to 1985 | 1986 to 1992 | 1993 to 1999 | 2000 to 2006 | 2007 to 2013 | Test for linear trend |
|---|---|---|---|---|---|---|
| Dachshund | 42.1 | 37.1 | 22.3 | 14.5 | 12.5 | P < 0.0001 |
| Mixed breed | 24.7 | 16.3 | 7.6 | 6.0 | 2.8 | P < 0.0001 |
| Basset hound | 70.9 | 68.3 | 40.0 | 22.5 | 19.4 | P < 0.0001 |
| Rottweiler | 57.1 | 31.6 | 30.8 | 31.9 | 29.2 | P = 0.47 |
| Puli | 100 | 89.3 | 68.4 | 62.5 | 0 | P < 0.0001 |
| English bulldog | 0 | 50 | 0 | 47.2 | 32.7 | P = 0.14 |
| Munster lander | 81.2 | 50 | 36.8 | 46.4 | 10 | P = 0.0001 |
| Poodle | 10.5 | 8.3 | 3.8 | 4.8 | 0 | P = 0.003 |
| Yorkshire terrier | 10.3 | 2.8 | 2.2 | 1.9 | 2.2 | P = 0.002 |
| French bulldog | 100 | 0 | 14.3 | 36 | 35.9 | P = 0.79 |
| Doberman | 34.3 | 3.7 | 4.9 | 4.2 | 0 | P < 0.0001 |
| Chihuahua | 0 | 0 | 0 | 17.2 | 20.5 | P < 0.0001 |
Discussion
The recognition of cystine urolithiasis can be traced back to 1810 when W. H. Wollaston recovered 2 glistening yellowish bladder stones from an 8-year-old human patient (19). He named the material “cystic oxide” (from the Greek — kystis — for bladder). Thirteen years later, the same substance was found by Lassaigne in a bladder stone from a dog (20). The exact chemical structure was eventually elucidated in 1902 by Neuberg (21). Infrared spectrometry is considered the gold standard technique to analyze uroliths in Germany as it gives a fingerprint of any substance present. Other recognized techniques such as optical crystallography or X-ray diffraction will not analyze all stones, e.g., non-crystalline substances or they are not as accurate (20).
Frequency of cystinuria with urolith formation in human patients is around 0.5% without large variations over time. In 1972, 0.5% and almost 40 years later in 2011, 0.52% of submitted stones from human patients were of cystine origin (1,2). This contrasts with the relative occurrence of cystine urolithiasis in dogs with a large variation over time and from various geographical locations. The present study showed a decreased relative occurrence over time from almost 40% (1979) to around 5% (2013). Studies from the American continent reported a relative frequency between 0.3% to 0.8% in North America and 2% in South America (4,10,18,22–24). Reports from Europe described a higher percentage of cystine uroliths amongst submitted stones, varying between 3.0% and 5.6% (5,13,14). A marked change in relative frequency of cystine stones over time was reported by Sosnar et al (13), with 10.3% in 1997 and 3.5% in 2002. No explanation was given. A study from Australia-Oceana in 2009/2010 reported a relative frequency of 3.8% cystine uroliths (10). In 1974, there was a relative occurrence of 22% for cystine uroliths reported from 110 stones by Clark in the UK (25).
The reason for the marked drop in relative frequency of cystine uroliths in the present retrospective study is not clear. Due to genetic predisposition, risk of recurrent stone formation is high. There have been reports of dogs with up to 6 episodes of cystine urolithiasis (22). In Germany, stone analysis for prophylactic measures in veterinary medicine became widely accepted as good medicine only after 1990. This partly explains the high frequency of cystine stones in the early days of stone analysis. Nowadays, appropriate medical therapy and prophylactic measures potentially prevent recurrent stone formation at an early stage. Furthermore, results of stone analysis are always reported to the veterinarian with the advice to exclude the animal in question from further breeding (20). It is to be expected that the nadir of relative frequency has been reached since a leveling off was observed in the last 15 y with cystine uroliths seen at a relative occurrence of about 5% (1997 to 2013).
There was a marked change in breed predisposition during this long time period. Ideally, breed distribution would be compared to a national registry for dog breeds, but such a registry does not exist in Germany. Since dog health insurance is uncommon in Germany, comparing breeds with an insurance company database would generate bias relating to which breeds of dogs are more likely to be insured. Thus the relative OR was calculated for dog breeds with cystine calculi compared to all breeds with other stone types submitted for analysis as well as to mixed breed dogs. Interestingly, OR and 95% CI were very similar if all breeds with other uroliths or mixed breed dogs were used as reference populations. In the 1980s, cystine stones were mainly submitted from dachshunds and basset hounds, while nowadays mainly bulldogs and Chihuahuas are affected. Breed predisposition for cystinuria and breed distribution in various countries play an important role. In the USA, commonly affected breeds are bull mastiff, Scottish deerhound, English bulldog, Newfoundland, and dachshund (10,18,22,24). Similar breeds are commonly affected in Canada with increased prevalence seen in English bulldog, Newfoundland, and Chihuahua (26). In contrast, in the UK, terrier breeds are mainly affected (Yorkshire, Jack Russell, and West Highland white) (5,12), while in Spain/Portugal, mainly bulldogs are affected (14) and in the Czech Republic, it is almost exclusively dachshunds (13). Dachshunds, bulldogs, and basset hounds are also the most commonly affected breeds in Sweden (9,15). For some breeds (Newfoundland, Landseer, Labrador) the gene (SLC3A1) encoding for the defect is known and DNA-based genetic tests are available to diagnose abnormal cystine excretion. This has led to a classification scheme for dogs with cystinuria depending on mode of inheritance and other variables (27,28). Screening programs for the defect have markedly decreased the incidence of cystinuria in these breeds worldwide (27).
Cystine stones were rarely found in female dogs in the present study (1.1%) and in previous reports (5,11). Only recently, a gender-limited/androgen-dependent inheritance of cystinuria has been reported in the mastiff, Scottish deerhound, and Irish terrier (28). Castration leads to markedly decreased cystine excretion resulting in abolished risk for urolith formation. It is not known whether this testosterone-dependent cystine urolith formation is present in other breeds. However, it might explain the marked skewing seen in the present study in which only 4.6% of male dogs with cystine stones had been neutered. Since castration is now commonly performed in Germany in male dogs not intended to be used for breeding, this might also explain the decrease in relative frequency of cystine stones over time.
Dogs with cystine uroliths are younger than dogs with other urinary stones with an age distribution of 4.3 to 4.5 y (22,26) compared to dogs with calcium oxalate uroliths, which have a mean age of 8.1 y. This observation was confirmed in the present study, which found that dogs having cystine stones were significantly younger than dogs with other stone types. The younger age can be explained by the genetic nature of the stone formation, as for example reported in Newfoundland dogs (28). Dogs with testosterone-dependent cystine urolithiasis were shown to be older (28), thus a bi-modal age distribution might have been expected, but this was not seen.
Like other uroliths (8), almost all cystine stones (99%) were retrieved from the lower urinary tract. In more than 58% of cases, the urethra was affected which could be explained by the almost exclusive occurrence in male dogs. Only 1.1% were submitted from female dogs from 9 breeds, mainly mixed breed dogs (n = 6) and dachshunds (n = 5). In 3 previous studies, female dogs rarely had cystine uroliths, with 0, 1 English bulldog and 1 Newfoundland out of 134, 102 and 59 cases, respectively (15,22,26). Lulich et al (2013) reported that 98.1% of dogs with cystine stones were male (10). Cystine uroliths grow very slowly and have a waxen, slippery surface which causes them to be easily washed out with urine unseen in female dogs. Earlier reports found 67.5% of cystine uroliths to be less than 5 mm in size (13).
There are several limitations to this study. Due to the retrospective nature, it is unknown how many cystine uroliths were submitted from the same dog. If multiple stones had been submitted from the same dog of a rare breed, this could affect the OR for that breed. Multiple cystine stones submitted from female or very young or old dogs would also affect the statistical analysis. These limitations are present in all epidemiological studies on urolithiasis based on submitted stones. Other limitations are that not all epidemiological data were available for each stone and inaccuracies in data submission might exist. The size of the data pool should mitigate the significance of any errors in data recording from individual cases. Stones voided and not retrieved, those dissolved medically or with dietary measures and stones not submitted or submitted to other laboratories will affect the present epidemiologic analysis of cystine urolithiasis in the German dog population. The authors hope the knowledge of breed predisposition to cystine urolith formation and developing knowledge as to which classification of cystinuria each breed falls into, will ultimately be useful in early diagnosis, treatment, and prevention of cystine urolithiasis in dogs.
Acknowledgments
Collection of data for this study was kindly supported by Royal Canin, Germany. Statistical assistance was received from Dr. K. Failing, Giessen, Germany. CVJ
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Hesse A, Schneider HJ, Hienzsch E. Die Infrarotspektroskopische Harnsteinanalyse. Dtsch Med Wschr. 1972;97:1694–1701. doi: 10.1055/s-0028-1107632. [DOI] [PubMed] [Google Scholar]
- 2.Knoll T, Schubert AB, Fahlenkamp D, Leusmann DB, Wendt-Nordahl G, Schubert G. Urolithiasis through the ages: Data on more than 200,000 urinary stone analysis. J Urol. 2011;185:1304–1311. doi: 10.1016/j.juro.2010.11.073. [DOI] [PubMed] [Google Scholar]
- 3.Hesse A, Orzekowsky H, Frenk M, Neiger R. Epidemiologische Daten zur Harnsteinerkrankung bei Katzen im Zeitraum 1981–2008. Tierärztl Praxis. 2012;40:95–101. [PubMed] [Google Scholar]
- 4.Houston DM, Moore AEP. Canine and feline urolithiasis: Examination of over 50 000 submissions to the Canadian veterinary urolith centre from 1998 to 2008. Can Vet J. 2009;50:1263–1268. [PMC free article] [PubMed] [Google Scholar]
- 5.Roe K, Pratt A, Lulich JP, Osborne C, Syme HM. Analysis of 14,008 uroliths from dogs in the UK over a 10-year period. J Small Anim Pract. 2012;53:634–640. doi: 10.1111/j.1748-5827.2012.01275.x. [DOI] [PubMed] [Google Scholar]
- 6.Wenkel R, Berg W, Prange H. Harnsteine bei Kleintieren und anderen Tierarten — eine retrospektive Studie aus den Jahren 1980–1989. Dtsch Tierärztliche Wschr. 1998;105:182–186. [PubMed] [Google Scholar]
- 7.Hesse A. Canine urolithiasis, epidemiology and analysis of urinary calculi. J Small Anim Pract. 1990;31:599–604. [Google Scholar]
- 8.Hesse A, Orzekowsky H, Neiger R. Urolithiasis beim Hund–15495 Analyseergebnisse und anamnestische Daten aus dem Zeitraum 1979–2007. Kleintierpraxis. 2012;7:633–639. [Google Scholar]
- 9.Hoppe A, Denneberg T. Cystinuria in the dog: Clinical studies during 14 years of medical treatment. J Vet Intern Med. 2001;15:361–367. [PubMed] [Google Scholar]
- 10.Lulich JP, Osborne CA, Albasan H, Koehler LA, Ulrich LM, Lekcharoensuk C. Recent shifts in the global proportions of canine uroliths. Vet Record. 2013;172:363–369. doi: 10.1136/vr.101056. [DOI] [PubMed] [Google Scholar]
- 11.Osborne CA, Sanderson SL, Lulich JP, et al. Canine cystine urolithiasis — Cause, detection, treatment and prevention. Vet Clin North Am Small Anim Pract. 1999;29:193–213. doi: 10.1016/s0195-5616(99)50011-9. [DOI] [PubMed] [Google Scholar]
- 12.Rogers KD, Jones B, Roberts L, Montalto N, Beckett S. Composition of uroliths in small domestic animals in the United Kingdom. Vet J. 2011;188:228–230. doi: 10.1016/j.tvjl.2010.04.022. [DOI] [PubMed] [Google Scholar]
- 13.Sosnar M, Bulkovs T, Ruzicka M. Epidemiology of canine urolithiasis in the Czech Republic from 1997 to 2002. J Small Anim Pract. 2005;46:177–184. doi: 10.1111/j.1748-5827.2005.tb00308.x. [DOI] [PubMed] [Google Scholar]
- 14.Vrabelova D, Silvestrini P, Ciudad J, et al. Analysis of 2735 canine uroliths in Spain and Portugal. A retrospective study: 2004–2006. Res Vet Science. 2011;91:208–211. doi: 10.1016/j.rvsc.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 15.Wallerström BI, Wägberg TI, Lagergren CH. Cystine calculi in the dog: An epidemiological retrospective study. J Small Anim Pract. 1990;33:78–84. [Google Scholar]
- 16.Hesse A, Sanders G, Leusmann DB. Analysis of canine urinary stones using infrared spectroscopy and scanning electron microscopy. Scan Electron Micr. 1986;1986:1705–1712. [PubMed] [Google Scholar]
- 17.Hesse A, Sanders G. Atlas of Infrared Spectra for the Analysis of Urinary Concrements. Stuttgart, Germany: Georg Thieme; 1988. [Google Scholar]
- 18.Osborne CA, Lulich JP, Polzin DJ, et al. Analysis of 77,000 canine uroliths. Perspectives from the Minnesota urolith center. Vet Clin North Am Small Anim Pract. 1999;29:17–38. doi: 10.1016/s0195-5616(99)50002-8. [DOI] [PubMed] [Google Scholar]
- 19.Wollaston WH. On cystic oxide, a new species of urinary calculus. Philos Trans Royal Soc London. 1810;100:223–230. [Google Scholar]
- 20.Hesse A, Neiger R. Urinary Stones in Small Animal Medicine. London, UK: Manson Publishing Ltd; 2009. [Google Scholar]
- 21.Neuberg C, Über Cystein I. Ber Dtsch Chem Ges. 1902;35:3161–3164. [Google Scholar]
- 22.Case LC, Ling GV, Franti CF, Ruby AL, Stevens F, Johnson DL. Cystine-containing urinary calculi in dogs: 102 cases (1981–1989) J Am Vet Med Assoc. 1992;201:129–133. [PubMed] [Google Scholar]
- 23.Del Angel-Caraza J, Diez-Prieto I, Perez-García CC, García-Rodríguez MB. Composition of lower urinary stones in canines in Mexico City. Urol Res. 2010;38:201–204. doi: 10.1007/s00240-009-0248-7. [DOI] [PubMed] [Google Scholar]
- 24.Low WW, Uhl JM, Kass PH, Ruby A, Westropp JL. Evaluation of trends in urolith composition and characteristics of dogs with urolithiasis: 25,499 cases (1985–2006) J Am Vet Med Assoc. 2010;236:193–200. doi: 10.2460/javma.236.2.193. [DOI] [PubMed] [Google Scholar]
- 25.Clark WT. The distribution of canine urinary calculi and their recurrence following treatment. J Small Anim Pract. 1974;15:437–444. doi: 10.1111/j.1748-5827.1974.tb06521.x. [DOI] [PubMed] [Google Scholar]
- 26.Houston DM, Moore AEP, Favrin MG, Hoff B. Canine urolithiasis: A look at over 16 000 urolith submissions to the Canadian Veterinary Urolith Centre from February 1998 to April 2003. Can Vet J. 2004;45:225–230. [PMC free article] [PubMed] [Google Scholar]
- 27.Brons AK, Henthorn PS, Raj K, et al. SLC3A1 and SLC7A9 mutations in autosomal recessive or dominant canine cystinuria: A new classification system. J Vet Intern Med. 2013;27:1400–1408. doi: 10.1111/jvim.12176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Henthorn PS, Liu J, Gidalevich T, Fang J, Casal ML, Patterson DF. Canine cystinuria: Polymorphism in the canine SLC3A1 gene and identification of a nonsense mutation in cystinuric Newfoundland dogs. Hum Genet. 2000;107:295–303. doi: 10.1007/s004390000392. [DOI] [PubMed] [Google Scholar]