ABSTRACT
Rhodobacter capsulatus is capable of synthesizing two nitrogenases, a molybdenum-dependent nitrogenase and an alternative Mo-free iron-only nitrogenase, enabling this diazotroph to grow with molecular dinitrogen (N2) as the sole nitrogen source. Here, the Mo responses of the wild type and of a mutant lacking ModABC, the high-affinity molybdate transporter, were examined by proteome profiling, Western analysis, epitope tagging, and lacZ reporter fusions. Many Mo-controlled proteins identified in this study have documented or presumed roles in nitrogen fixation, demonstrating the relevance of Mo control in this highly ATP-demanding process. The levels of Mo-nitrogenase, NifHDK, and the Mo storage protein, Mop, increased with increasing Mo concentrations. In contrast, Fe-nitrogenase, AnfHDGK, and ModABC, the Mo transporter, were expressed only under Mo-limiting conditions. IscN was identified as a novel Mo-repressed protein. Mo control of Mop, AnfHDGK, and ModABC corresponded to transcriptional regulation of their genes by the Mo-responsive regulators MopA and MopB. Mo control of NifHDK and IscN appeared to be more complex, involving different posttranscriptional mechanisms. In line with the simultaneous control of IscN and Fe-nitrogenase by Mo, IscN was found to be important for Fe-nitrogenase-dependent diazotrophic growth. The possible role of IscN as an A-type carrier providing Fe-nitrogenase with Fe-S clusters is discussed.
IMPORTANCE Biological nitrogen fixation is a central process in the global nitrogen cycle by which the abundant but chemically inert dinitrogen (N2) is reduced to ammonia (NH3), a bioavailable form of nitrogen. Nitrogen reduction is catalyzed by nitrogenases found in diazotrophic bacteria and archaea but not in eukaryotes. All diazotrophs synthesize molybdenum-dependent nitrogenases. In addition, some diazotrophs, including Rhodobacter capsulatus, possess catalytically less efficient alternative Mo-free nitrogenases, whose expression is repressed by Mo. Despite the importance of Mo in biological nitrogen fixation, this is the first study analyzing the proteome-wide Mo response in a diazotroph. IscN was recognized as a novel member of the molybdoproteome in R. capsulatus. It was dispensable for Mo-nitrogenase activity but supported diazotrophic growth under Mo-limiting conditions.
INTRODUCTION
Biological nitrogen fixation (BNF) is a central process in the global nitrogen cycle, which converts chemically inert atmospheric dinitrogen to ammonia, a bioavailable form of nitrogen. BNF is catalyzed by complex metalloenzymes called nitrogenases, which are exclusively found in diazotrophic bacteria and archaea but not in eukaryotes (1). All diazotrophs synthesize a molybdenum-dependent nitrogenase containing an iron-molybdenum cofactor, FeMoco. In addition to Mo-nitrogenases, some bacteria synthesize alternative Mo-free nitrogenases, which contain either an iron-vanadium cofactor, FeVco, or an iron-only cofactor, FeFeco (2). Alternative nitrogenases are less efficient than Mo-nitrogenases in terms of consumption of reducing power and ATP per molecule of N2 fixed (3, 4). Consequently, diazotrophs preferentially utilize Mo-nitrogenase as long as sufficient molybdate, the only bioavailable form of molybdenum, is available. To support Mo-nitrogenase activity under Mo-limiting conditions, most diazotrophs synthesize a high-affinity molybdate transporter, ModABC (1).
The purple nonsulfur alphaproteobacterium Rhodobacter capsulatus is known for its metabolic versatility, and it has been used for decades as a model organism to study photosynthesis, hydrogen production, and nitrogen fixation (5–9). In particular, it is capable of using light energy to generate the ATP required for the energetically demanding nitrogen fixation process. R. capsulatus synthesizes two nitrogenases, namely, a Mo-nitrogenase and a Fe-nitrogenase but no V-nitrogenase (10, 11). The synthesis and activity of the two nitrogenases are controlled at the transcriptional, translational, and posttranslational levels by a regulatory cascade responding to ammonium and Mo availability (8, 9). Upon ammonium limitation, the nitrogen regulatory protein NtrC becomes activated by phosphorylation. In turn, NtrC-P activates transcription of nifA and anfA, which encode the transcription activators of Mo-nitrogenase and Fe-nitrogenase genes, respectively. At high Mo concentrations, anfA transcription is repressed by two structurally and functionally related Mo-responsive regulators, MopA and MopB, thus restricting synthesis of Fe-nitrogenase to Mo-limiting conditions (12–14). In addition, MopA and MopB repress transcription of the mopA-modABC and morABC genes coding for the regulator MopA, for the high-affinity Mo uptake system ModABC, and for a putative MorABC transporter of unknown function (14). Besides its function as a repressor, MopA activates transcription of the mop gene, which codes for a putative Mo storage protein (14). Last but not least, the levels of Mo-nitrogenase reductase, NifH, and of FdxD, a ferredoxin thought to protect Mo-nitrogenase against oxygen damage, were recently found to increase with increasing Mo concentrations (15).
To better understand the contrasting effects of Mo on Fe-nitrogenases and Mo-nitrogenases and to identify previously unrecognized Mo-controlled proteins, we examined the proteome of R. capsulatus under Mo-limiting and Mo-replete conditions. Besides known members of the molybdoproteome, IscN (corresponding to gene locus identifier rcc03272) was identified as a novel, previously unrecognized Mo-repressed protein. IscN belongs to a distinct cluster in the family of HesB-like proteins related to iron-sulfur cluster formation (16). R. capsulatus IscN was found to be important for diazotrophic growth when Fe-nitrogenase is used but dispensable for N2 reduction by Mo-nitrogenase.
MATERIALS AND METHODS
Strains, plasmids, and growth conditions.
The bacterial strains and plasmids used in this study are listed in Table 1. Peptone-yeast (PY) complex medium used for R. capsulatus, selection of R. capsulatus mutant strains, and RCV (Rhodobacter capsulatus V) chemically defined (minimal) medium lacking molybdate and a fixed-nitrogen source were previously described (15, 17–20). When required, appropriate concentrations of Na2MoO4, 10 mM l-serine, or 10 mM (NH4)2SO4 were added. For diazotrophic growth, 3-ml cultures were placed in screw-cap 17-ml Hungate tubes, prior to flushing the headspace with N2 gas and incubation in the light.
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid | Relevant characteristic(s)/descriptiona | Source or reference |
|---|---|---|
| Strains | ||
| E. coli JM83 | Host for plasmid amplification | 40 |
| E. coli S17-1 | Donor for conjugational plasmid transfer | 41 |
| R. capsulatus B10S | Spontaneous Smr mutant of wild-type strain B10 | 17 |
| R. capsulatus B10S::pEW10 | B10S carrying a chromosomal mop-FLAG fusion | This study |
| R. capsulatus B10S::pEW13 | B10S carrying a chromosomal iscN-FLAG fusion | This study |
| R. capsulatus B10S::pEW18 | B10S carrying a chromosomal mop-lacZ fusion | This study |
| R. capsulatus B10S::pEW51 | B10S carrying a chromosomal nifU1-lacZ fusion | This study |
| R. capsulatus B10S::pEW53 | B10S carrying a chromosomal nifU1-FLAG fusion | This study |
| R. capsulatus B10S::pEW58 | B10S carrying a chromosomal iscN-lacZ fusion | This study |
| R. capsulatus B10S::pYP348 | B10S carrying a chromosomal nifK-lacZ fusion | This study |
| R. capsulatus BS85 | Polar nifD::Sp mutant (ΔnifDK) of B10S | 15 |
| R. capsulatus BS85-YP340 | nifDK-iscN double mutant of B10S; Spr | This study |
| R. capsulatus R438 | Polar modA::Gm mutant (ΔmodABC) of B10S | 13 |
| R. capsulatus YP340 | Markerless iscN mutant (ΔiscN) of B10S | This study |
| Plasmids | ||
| pBO2337 | pK19mobsacB derivative carrying a triple FLAG tag | 42 |
| pEW10 | pYP168 derivative carrying mop-FLAG; Kmr oriT | This study |
| pEW13 | pYP168 derivative carrying iscN-FLAG; Kmr oriT | This study |
| pEW18 | pYP168 derivative carrying mop-lacZ; Tcr oriT | This study |
| pEW51 | pYP168 derivative carrying nifU1-lacZ; Tcr oriT | This study |
| pEW53 | pYP168 derivative carrying nifU1-FLAG; Kmr oriT | This study |
| pEW58 | pYP168 derivative carrying iscN-lacZ; Tcr oriT | This study |
| pK19mobsacB | Mobilizable narrow-host-range vector; Kmr oriT | 43 |
| pUC18 | Narrow-host-range vector; Apr | 40 |
| pYP35 | pBSL15 derivative carrying lacTeT; Tcr oriT | 24 |
| pYP168 | pUC18 derivative with reduced MCS (SmaI only) | This study |
| pYP190 | pYP168 derivative; Smr Spr oriT | This study |
| pYP247 | pYP190 derivative carrying FLAG-KaT; Kmr oriT | This study |
| pYP340 | pK19mobsacB derivative carrying DNA flanking iscN | This study |
| pYP348 | pYP168 derivative carrying nifK-lacZ; Tcr oriT | This study |
Ap, ampicillin; Gm, gentamicin; Km, kanamycin; Sp, spectinomycin; Sm, streptomycin; Tc, tetracycline.
Proteome profiling in response to molybdenum.
R. capsulatus wild-type and ΔmodABC strains were grown under nitrogenase-derepressing conditions in RCV minimal medium with or without addition of 10 μM Na2MoO4. Media contained 10 mM serine, which (in contrast to ammonium) does not repress nitrogen fixation (17). Cultures (10 ml) were grown to early logarithmic phase prior to harvest of cells by centrifugation (10 min; 16,000 × g; 20°C). Cells were washed three times with 50 mM triethylammonium bicarbonate (TEAB) buffer (Sigma-Aldrich, Taufkirchen, Germany) and resuspended in 0.5 ml 50 mM TEAB buffer. Cells were disrupted by ultrasonication in a Vial Tweeter instrument (Hielscher, Teltow, Germany). Subsequently, samples were centrifuged (30 min; 16,000 × g; 4°C) to remove cell debris before 0.4-ml volumes of the supernatants were subjected to methanol-chloroform precipitation. For this purpose, 0.8 ml methanol, 0.2 ml chloroform, and 0.3 ml water were added to each sample prior to vortex mixing and centrifugation (2 min; 16,000 × g; 20°C). After the upper phases were discarded, 0.8 ml methanol was added. Subsequently, samples were subjected to vortex mixing and centrifuged (2 min; 16,000 × g; 20°C). After methanol was removed, protein pellets were air-dried and resuspended in 70 μl 50 mM TEAB buffer. Protein concentrations were determined by the Bradford assay (21). After addition of 0.1% RapiGest (Waters, Milford, MA), cysteines were reduced and alkylated by addition of 2.5 mM Tris(2-carboxyethyl)phosphine (Sigma-Aldrich) for 60 min at 60°C and 5 mM iodoacetamide (Sigma-Aldrich) for 25 min at 25°C in the dark, respectively. Trypsin (sequencing grade) (Promega, Madison, WI) was added with a protease-to-substrate ratio of 1:100, and tryptic digestion was carried out for 5 h at 37°C with mild shaking. For precipitation of RapiGest, 2 μl of trifluoroacetic acid (Biosolve; Valkenswaard, The Netherlands) was added and the samples were centrifuged twice at 4°C and 16,000 × g. A total amount of 750 ng of each sample containing 50 fmol PhosB peptides as an Hi3 quantitation standard (Waters) was injected on a nanoAcquity ultraperformance liquid chromatography (nanoUPLC) system (Waters). Samples were loaded and eluted from a reversed-phase C18 column (column length, 250 mm; inner diameter, 75 μm; particle size, 1.7 μm; pore size, 130 Å; heated to 40°C) using the following gradient of solvent A (0.1% formic acid [FA] in distilled water) and solvent B (0.1% formic acid–acetonitrile) with a flow of 350 nl/min: beginning of procedure to 36 min, constant 1% solvent B; 36 to 120 min, linear to 20% solvent B; 120 to 180 min, linear to 30% solvent B; 180 to 240 min, linear to 60% solvent B. FA and acetonitrile were purchased from Biosolve. The nanoUPLC column was coupled online to a Synapt G2-S high-definition mass spectrometry (HDMS) electrospray ionization-time of flight (ESI/ToF) mass spectrometer (Waters). Spectra were recorded in positive ionization mode and resolution mode over a mass range of 50 to 1,800 m/z with 1.5 s/scan using elevated-energy mass spectrometry (MSE) technology and a trap collision energy ramp of 14 to 45 V. The following parameters were used for the NanoLockSpray source: capillary voltage, 1.9 kV; sampling cone voltage, 20 V; source temperature, 100°C; desolvation temperature, 200°C; cone gas flow, 50 liters/h; desolvation gas flow, 600 liters/h. Leucine enkephalin, serving as a lock mass analyte, was fed through the lock spray channel (lock mass capillary voltage, 3.0 kV).
Data were analyzed using ProteinLynxGlobalServer 2.5.2 software (Waters). Mass spectra were processed using the following parameters: chromatographic peak width, automatic; MS ToF resolution, automatic; lock mass window, 0.25 Da; low-energy threshold, 50 counts; elevated-energy threshold, 15 counts; intensity threshold, 750 counts. A nonredundant version of the Rhodobacter capsulatus database (NCBI BioProject accession no. PRJNA47509) containing 3,637 protein entries (including sequences of the PhosB standard, trypsin, and keratin) was used for protein identification using the following parameters: peptide tolerance, automatic; fragment tolerance, automatic; minimum fragment ion matches per peptide, 2; minimum fragment ion matches per protein, 6; minimum peptide matches per protein, 3; maximum protein mass, 300 kDa; primary digest reagent, trypsin; secondary digest reagent, none; missed cleavages, 1; fixed modifications, carbamidomethyl C; variable modifications, deamidation, N and Q, and oxidation, M; false-positive rate, 4%; calibration protein, PhosB standard; calibration protein concentration for Hi3 quantitation, 50 fmol. For each run, the measured total protein load on column was calculated and used for normalization. Up- and downregulated proteins in response to Mo-replete (+Mo) conditions were selected using a confidence interval of 95% and P values below 0.05. For the wild-type and ΔmodABC strains, proteins with log2 ratios below −0.857 or −1.346 and above 1.079 or 1.945 were considered significantly down- or upregulated, respectively. Proteins present in three replicates under one set of conditions and in none of the respective other conditions were considered “unique” for conditions without Mo addition (−Mo) or +Mo conditions.
Construction of R. capsulatus iscN mutant strains.
DNA fragments flanking iscN (rcc03272) were PCR amplified using primer pairs UP-Rc3272-2/LP-Rc3272-2 (UP/LP-Rc3272-2) and UP/LP-Rc3272-3 and genomic R. capsulatus DNA as a template (Table 2). The resulting iscN upstream and downstream fragments were digested with PstI plus BamHI and BamHI plus HindIII, respectively, prior to ligation into the mobilizable narrow-host-range (suicide) vector pK19mobsacB (which does not replicate in R. capsulatus), digested with PstI plus HindIII (Table 1). The resulting pYP340 plasmid was conjugationally transferred into R. capsulatus strains B10S (wild-type strain) and BS85 (ΔnifDK mutant). Selection for the vector-encoded kanamycin (Km) resistance indicated plasmid integration into the chromosome by single-recombination events. Subsequently, Km-resistant B10S and BS85 exconjugants were grown without selective pressure before cells were spread on PY plates containing 10% sucrose. Growth on sucrose plates indicated loss of the vector-encoded sacB gene and deletion of the iscN gene. The identities of markerless iscN mutants YP340 and BS85-YP340 were verified by PCR (data not shown). In these mutants, the iscN coding region is in-frame deleted except for the first and last five codons.
TABLE 2.
Primers used for PCR amplification of selected DNA fragments
| Primer | Oligonucleotide sequence (5′ → 3′)a | Amplified region |
|---|---|---|
| UP-FLAG-1 | GGGGACTACAAAGACCATGACGGTG | Triple FLAG tag |
| UP-Rc570-4 | CCGTCTGGTCGACGCGATCG | nifK coding region |
| UP-Rc3261-1 | GCGCGGATCCGGTTGAACCCGCGTCCCGCC | mop coding region |
| UP-Rc3271-1 | CGGGCCGCCGGGCAGGCCGC | nifU1 coding region |
| UP-Rc3271-30 | GGCCGCCGGGCAGGCCGCCC | nifU1 coding region |
| UP-Rc3272-1 | CTCTGTCGCAAATCCCTCAATG | iscN coding region |
| UP-Rc3272-2 | GGCTTTATCTGCAGGCGCTGATC | iscN upstream region |
| UP-Rc3272-3 | CGCGGGATCCGGCAAATCCTTCTGCTGAGGG | iscN downstream region |
| LP-FLAG-1 | AGATCTTATCATTTATCGTCGTCATCTTTG | Triple FLAG tag |
| LP-Rc570-5 | GCAAGCTTTCAGCGGGTCAGATCGAAGCTGATG | nifK coding region |
| LP-Rc3261-1 | GGGGTTCTTGCCGACGATGACGTCAG | mop coding region |
| LP-Rc3271-1 | GGGGTGCCGGATGTCGGGGATGAC | nifU1 coding region |
| LP-Rc3271-2 | CGAAGCTTTCAGTGCCGGATGTCGGGGATGAC | nifU1 coding region |
| LP-Rc3272-1 | GGGGCAGAAGGATTTGCCGCAGCCGC | iscN coding region |
| LP-Rc3272-2 | CGCGGGATCCGGTGATCTCGATCATGACGC | iscN upstream region |
| LP-Rc3272-3 | GCGCGAATTCACGATCACCTTGGCGCCCTC | iscN downstream region |
| LP-Rc3272-5 | GCGGATCCTCAGCAGAAGGATTTGCC | iscN coding region |
The BamHI, BglII, EcoRI, HindIII, and PstI restriction sites used for cloning purposes are underlined.
Construction of R. capsulatus lacZ reporter strains and β-galactosidase assays.
Primer pairs UP-Rc570-4/LP-Rc570-5, UP/LP-3261-1, UP-Rc3271-1/LP-Rc3271-2, and UP-Rc3272-1/LP-Rc3272-5 were used to PCR amplify the R. capsulatus nifK (rcc00570), mop (rcc03261), nifU1 (rcc03271), and iscN (rcc03272) genes, respectively (Table 2). In each case, a HindIII or a BamHI site was added immediately downstream of the respective stop codon. The amplification products were blunt-end cloned into the SmaI site of suicide vector pYP168 (Table 1). Subsequently, a lacTeT cassette (containing a promoterless lacZ gene, the tetRA tetracycline resistance genes, and the oriT transfer origin) from plasmid pYP35 was cloned into the above-mentioned HindIII and BamHI sites. The resulting pYP348 (nifK-lacZ), pEW18 (mop-lacZ), pEW51 (nifU1-lacZ), and pEW58 (iscN-lacZ) hybrid plasmids were conjugationally transferred into R. capsulatus wild-type strain B10S. Selection for tetracycline resistance indicated plasmid integration by single-recombination events, placing the lacZ reporters under the control of the respective R. capsulatus promoters. The resulting B10S::pYP348 (nifK-lacZ), B10S::pEW18 (mop-lacZ), B10S::pEW51 (nifU1-lacZ), and B10S::pEW58 (iscN-lacZ) reporter strains were grown phototrophically until the late exponential phase prior to determination of LacZ (β-galactosidase) activity (Table 1), as described previously (23).
Construction of a mobilizable resistance cassette carrying a FLAG tag.
To simplify construction of mobilizable FLAG fusions, we constructed a DNA cassette (designated FLAG-KaT) consisting of a triple-FLAG tag, a selectable marker (kanamycin resistance), and a conjugational transfer origin (oriT), flanked by SmaI sites. For this purpose, a triple-FLAG tag was PCR amplified using primer pair UP/LP-FLAG-1 and plasmid pBO2337 as a template (Tables 1 and 2). The amplification product was blunt-end cloned into the SmaI site of vector pYP190, prior to insertion of Km and oriT sequences into the BglII site downstream of the triple-FLAG stop codon. The resulting hybrid plasmid pYP247 served as a source for the FLAG-KaT cassette to create in-frame FLAG reporter fusions.
Construction of R. capsulatus FLAG reporter strains and epitope tagging.
Primer pairs UP/LP-Rc3261-1, UP-Rc3271-30/LP-Rc3271-1, and UP/LP-Rc3272-1 were used to PCR amplify the R. capsulatus mop (rcc03261), nifU1 (rcc03271), and iscN (rcc03272) genes, respectively (Table 2). In each case, the stop codon was exchanged against three cytidine residues (CCC). The amplification products were blunt-end cloned into the SmaI site of pYP168 (Table 1). The SmaI sites downstream of the respective coding regions were recovered due to the CCC extensions. These SmaI sites were used for insertion of a SmaI fragment carrying the FLAG-KaT cassette from plasmid pYP247 (Table 1). The resulting pEW10 (mop-FLAG), pEW53 (nifU1-FLAG), and pEW13 (iscN-FLAG) hybrid plasmids were conjugationally transferred into R. capsulatus wild-type strain B10S. Selection for kanamycin resistance indicated plasmid integration by single recombination. The resulting B10S::pEW10 (mop-FLAG), B10S::pEW53 (nifU1-FLAG), and B10S::pEW13 (iscN-FLAG) reporter strains were examined for Mop, NifU1, and IscN accumulation, respectively, by Western analysis using FLAG-specific antibodies (see below).
Detection of NifH, NifDK, and FLAG-tagged proteins by Western analysis.
R. capsulatus strains were grown under nitrogenase-derepressing conditions in RCV minimal medium at different molybdate concentrations. Protein isolation, gel-electrophoretic separation, Western blot, and immunodetection analyses were carried out as described recently (15). Detection of Mo-nitrogenase proteins was performed using antisera raised against R. capsulatus NifH (Eurogentec, Cologne, Germany) and NifDK (the kind gift of Yves Jouanneau) and goat anti-rabbit IgG–horseradish peroxidase (HRP) conjugate (Bio-Rad, Munich, Germany). Detection of FLAG-tagged proteins was done using monoclonal anti-FLAG M2 antibodies (Sigma-Aldrich, Taufkirchen, Germany) and goat anti-mouse IgG (H+L)–HRP conjugate (Bio-Rad). After addition of Luminata Forte Western HRP substrate (Millipore, Billerica, MA), Mo-nitrogenase and FLAG-tagged proteins were detected using Amersham Hyperfilm ECL (GE Healthcare, Buckinghamshire, United Kingdom).
Accession numbers.
The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium (22) via the PRIDE partner repository with the data set identifier PXD003123. The DNA sequence of the FLAG-KaT cassette is available under GenBank accession number KT756677.
RESULTS
Proteome profiling of Rhodobacter capsulatus in response to molybdenum availability.
R. capsulatus synthesizes a high-affinity molybdate transporter, ModABC, which supports Mo-nitrogenase activity in minimal medium without Mo addition (–Mo) by making use of the Mo present in trace amounts in the chemicals used (13, 15, 24). A ΔmodABC strain lacking the Mo transporter no longer exhibits Mo-nitrogenase activity in –Mo medium, indicating that the wild-type strain faces moderate Mo limitation in –Mo medium, whereas the ΔmodABC strain encounters severe Mo limitation (Mo starvation). In addition to ModABC, R. capsulatus synthesizes an oxyanion permease, PerO, which functionally substitutes for ModABC at Mo concentrations of 10 μM and above (+Mo; Mo-replete conditions) (24). Apparently, both transporters contribute to Mo uptake at concentrations around 1 μM, whereas PerO is no longer involved in Mo uptake at concentrations below 100 nM. To achieve a comprehensive view of the Mo response of R. capsulatus, we compared the proteome profiles of wild-type and ΔmodABC strains grown in medium without (–Mo) or with (+Mo) 10 μM molybdate. Total soluble proteins from these cultures were identified and quantified by label-free mass spectrometry (see Materials and Methods). For wild-type and ΔmodABC cultures, 1,012 and 920 proteins, respectively, were identified in at least two of three replicates under both –Mo and +Mo conditions. In the wild-type strain, 32 proteins were unique to –Mo conditions or significantly downregulated by Mo, while 14 proteins were unique to +Mo conditions or upregulated by Mo. In the ΔmodABC strain, 15 proteins were unique to –Mo conditions or repressed by Mo, while 3 proteins were induced by Mo. Identified proteins and their abundances are listed in Tables S1 (wild-type strain; –Mo/+Mo) and S2 (ΔmodABC mutant; –Mo/+Mo) in the supplemental material.
Mo induces accumulation of Mo-nitrogenase proteins.
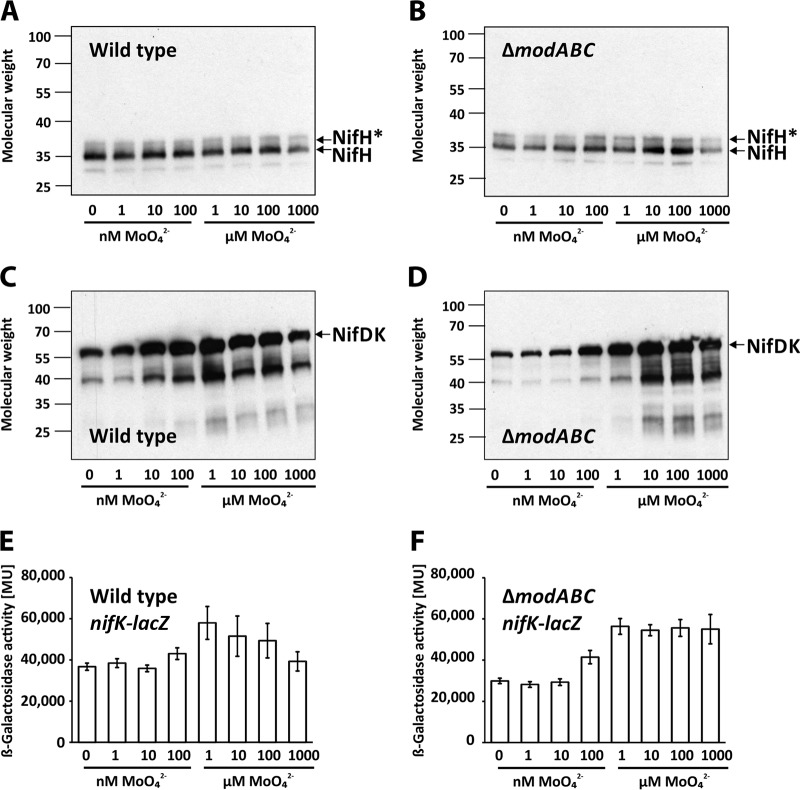
As expected, Mo induced NifD and NifK accumulation in wild-type and ΔmodABC strains (Table 3). In contrast, Mo-responsive differences in NifH levels were obvious only in the ΔmodABC mutant and not in the wild type (Table 3). To examine the Mo responses of NifH, NifD, and NifK in more detail, wild-type and ΔmodABC strains were grown at different Mo concentrations (0, 1 nM, 10 nM, 100 nM, 1 μM, 10 μM, 100 μM, and 1 mM) prior to Western analysis using antisera raised against nitrogenase reductase (NifH) and dinitrogenase (NifDK) (Fig. 1). The NifH antiserum detected a strong 32-kDa band and a weaker slower-migrating band which correspond to the unmodified (active) and the ADP-ribosylated (inactive) forms of NifH, respectively (Fig. 1A and B) (25, 26). Consistent with the proteome data, NifH levels in the wild type remained almost constant at all Mo concentrations tested, while in the ΔmodABC strain, lower NifH levels were observed at nanomolar Mo concentrations (Fig. 1A and B). NifH levels dropped off at the highest Mo concentration (1 mM), likely as a result of toxic effects previously described for millimolar Mo concentrations (24). The NifDK antiserum identified two bands, at 56 kDa and 40 kDa, whose intensities gradually increased with increasing Mo concentrations (Fig. 1C and D). The 56-kDa band probably represents two proteins, NifD and NifK, of similar masses (NifD, 55.7 kDa; NifK, 56.3 kDa). The identical Mo-responsive patterns of the 40-kDa and 56-kDa bands suggest that the faster-migrating band represents degradation products of NifD or NifK or both.
TABLE 3.
Levels of R. capsulatus nitrogen fixation proteins in response to Mo availability
| Protein | Description or function | Gene locus IDa | Level of protein (fmol)b |
|||
|---|---|---|---|---|---|---|
| Wild-type strain |
ΔmodABC mutant |
|||||
| −Mo | +Mo | −Mo | +Mo | |||
| Mo-nitrogenase and Mo storage | ||||||
| NifH | Mo-nitrogenase | rcc00572 | 207.8 | 232.1 | 82.4 | 326.0 |
| NifD | Mo-nitrogenase | rcc00571 | 53.7 | 130.8 | 8.1 | 201.6 |
| NifK | Mo-nitrogenase | rcc00570 | 84.2 | 185.8 | 15.7 | 226.4 |
| FdxD | Shetna ferredoxin | rcc00573 | 13.6 | 11.5 | 5.3 | 17.6 |
| Mop | Mo storage | rcc03261 | 52.0 | NF | 32.3 | 20.8 |
| Fe-nitrogenase | ||||||
| AnfA | Transcription activator | rcc00584 | 2.4 | NF | 4.2 | NF |
| AnfH | Fe-nitrogenase | rcc00585 | 112.4 | NF | 447.3 | 0.7 |
| AnfD | Fe-nitrogenase | rcc00586 | 20.4 | NF | 80.5 | NF |
| AnfG | Fe-nitrogenase | rcc00587 | 6.8 | NF | 36.8 | NF |
| AnfK | Fe-nitrogenase | rcc00588 | 32.2 | NF | 91.6 | 2.1 |
| AnfO | Fe-nitrogenase accessory | rcc00589 | 2.3 | NF | 6.5 | NF |
| AnfR | Fe-nitrogenase accessory | rcc00590 | NF | NF | 3.5 | NF |
| Anf3 | Fe-nitrogenase accessory | rcc00591 | 11.5 | NF | 35.0 | NF |
| Molybdate transport and Mo-responsive regulation | ||||||
| MopB | Mo-responsive regulator | rcc00560 | 1.1 | 1.4 | 0.4 | 0.8 |
| MopA | Mo-responsive regulator | rcc00561 | 8.9 | NF | 4.5 | 5.2 |
| ModA | Mo transport | rcc00562 | 176.6 | NF | 1.3 | NF |
| MorA | Mo transport | rcc02219 | 16.6 | NF | 24.3 | 11.1 |
| Fe-S cluster and FeMoco biosynthesis | ||||||
| IscN | Fe-S processing | rcc03272 | 4.9 | NF | 12.3 | 5.2 |
| NifU1 | Fe-S biosynthesis | rcc03271 | 3.1 | 4.0 | 4.4 | 3.0 |
| NifS | Cysteine desulfurase | rcc03270 | 2.6 | 1.3 | 1.2 | 1.6 |
| NifW | Nitrogenase stabilization | rcc03268 | 1.9 | 1.5 | 3.5 | 2.1 |
| NifE | FeMoco scaffold | rcc03280 | 3.1 | 1.0 | 1.8 | 1.6 |
| NifN | FeMoco scaffold | rcc03279 | 3.8 | 1.8 | 2.4 | 2.9 |
| NifX | FeMoco biosynthesis | rcc03278 | 25.7 | 20.3 | 27.1 | 26.1 |
| FdxB | Ferredoxin | rcc03275 | 4.0 | 3.8 | 5.8 | 4.2 |
| Electron transfer to nitrogenase | ||||||
| RnfC | Ferredoxin reduction | rcc03289 | 2.6 | 1.8 | 2.8 | 4.2 |
| RnfG | Ferredoxin reduction | rcc03291 | 3.0 | 2.7 | 2.8 | 3.0 |
ID, identifier.
“+Mo” indicates addition of 10 μM Na2MoO4. Values with standard deviations of <25% are in bold. NF, protein never found in any of the replicates. Values for proteins identified in one replicate only are in italics.
FIG 1.
Accumulation of Mo-nitrogenase proteins and nifK-lacZ expression. R. capsulatus strains were grown in RCV minimal medium with the indicated molybdate concentrations. The strains used were as follows: B10S, the wild-type strain (A and C); R438, the ΔmodABC mutant (B and D); B10S::pYP348, the wild-type strain carrying nifK-lacZ (E); and R438::pYP348, the ΔmodABC mutant carrying nifK-lacZ (F). (A to D) Western analyses were performed using antisera against NifH (A and B) or NifDK (C and D). “NifH*” marks the ADP-ribosylated form of NifH. Molecular weight data are given in thousands. (E and F) LacZ (β-galactosidase) activity is given in Miller units (MU) (23). The results represent the means and standard deviations of the results of at least three independent measurements.
To determine whether expression of the nifHDK operon responds to the cellular Mo status, we constructed reporter strains carrying transcriptional nifK-lacZ fusions chromosomally integrated into the wild-type and ΔmodABC strain backgrounds. These strains carry a promoterless lacZ reporter immediately downstream of the nifK stop codon, placing the reporter under the control of the nifHDK promoter but leaving the nifHDK coding regions intact. LacZ activity was induced roughly 2-fold by Mo in the wild-type and ΔmodABC strains (Fig. 1E and F). Likewise, NifD and NifK levels increased about 2-fold in the wild type upon Mo addition as revealed by proteome profiling (Table 3). These findings suggest that nifK-lacZ transcription levels in the wild type correlate with NifDK levels. However, NifD and NifK levels differed 25- and 14-fold in the ΔmodABC strain in response to Mo, respectively (Table 3). These differences cannot solely be explained by transcriptional regulation but suggest additional posttranscriptional control of NifDK levels (see Discussion).
As described above for NifH, Mo-responsive differences in FdxD levels were observed in the ΔmodABC mutant but not in the wild type (Table 3). This correlates with earlier findings demonstrating coordinated expression of nifH and fdxD genes (15). FdxD promotes diazotrophic growth by Mo-nitrogenase (but not by Fe-nitrogenase) in the presence of oxygen (15).
Mo induces accumulation of the Mo-binding Mop protein.
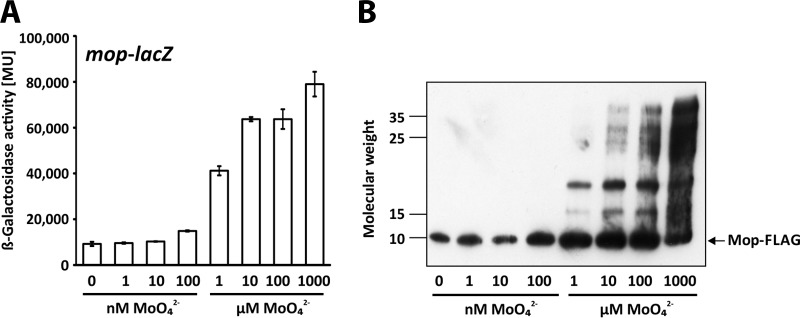
The proteome analysis showed that the putative Mo storage protein, Mop, is less abundant or not even found in the presence of 10 μM Mo (Table 3). This counterintuitive finding is not consistent with previous studies, in which an episomal mop-lacZ fusion was activated by Mo (14). To clarify this discrepancy, we constructed reporter strains carrying chromosomally integrated mop-lacZ and mop-FLAG fusions (Fig. 2). As described above for the nifK-lacZ fusion, the lacZ reporter was fused downstream of the mop stop codon, placing the reporter under the control of the mop promoter but leaving the mop coding region intact. In the mop-FLAG strain, a triple-FLAG tag was fused in-frame to the last codon of the mop gene.
FIG 2.
Expression of mop-lacZ and Mop-FLAG accumulation. R. capsulatus strains were grown in RCV minimal medium with the indicated molybdate concentrations. (A and B) The strains used were B10S::pEW18, the wild-type strain carrying mop-lacZ (A), and B10S::pEW10, the wild-type strain carrying mop-FLAG (B). (A) LacZ (β-galactosidase) activity is given in Miller units (23). The results represent the means and standard deviations of the results of at least three independent measurements. (B) Western analysis was performed using FLAG antibodies. Molecular weight data are given in thousands.
Expression of the chromosomal mop-lacZ fusion increased with increasing Mo concentrations, indicating that mop transcription is indeed induced by Mo (Fig. 2A). Hence, the chromosomal mop-lacZ fusion used in this study and the episomal fusion used in the earlier study gave essentially the same results, indicating that mop transcription is not affected by reporter copy numbers (this study and reference 14). Essentially the same response was also observed with the Mop-FLAG protein, as shown by Western analysis using FLAG-specific antibodies (Fig. 2B). In addition to the 9.5-kDa band representing the Mop-FLAG monomer, slower-migrating bands were observed at higher Mo concentrations. Both the numbers of bands and their intensities increased gradually with increasing Mo concentrations. Most likely, the slower-migrating bands represent Mop-molybdate complexes. In line with this assumption, recombinant Mop protein purified from Escherichia coli formed highly stable Mo-binding hexamers (27). Such Mop-molybdate complexes might have been lost during protein preparation for proteome profiling, because the protein preparation protocol for proteome profiling included two precipitation steps (see Materials and Methods). When the sample preparation protocol for proteome analysis was applied to prepare protein from the mop-FLAG strain for Western blot analysis, the upper phase obtained after the first precipitation step contained considerable amounts of Mop-FLAG protein (data not shown), suggesting that Mop-molybdate complexes indeed might have been discarded in our proteome profiling approach. In conclusion, our findings suggest that mop expression is induced when Mo is abundant.
Mo inhibits accumulation of Fe-nitrogenase and Mo transport proteins.
The structural proteins of Fe-nitrogenase, AnfHDGK, were found at large amounts under –Mo conditions, while +Mo conditions prevented AnfHDGK accumulation (Table 3). AnfHDGK proteins were present at much larger amounts in the ΔmodABC strain than in the wild type, indicating that maximal Fe-nitrogenase accumulation requires severe Mo starvation. Similarly, accumulation of AnfOR-Anf3 Fe-nitrogenase accessory proteins and of AnfA, the transcriptional activator of the anfHDGKOR-anf3 operon, occurred only under –Mo conditions. Previous studies revealed that anfA transcription is effectively repressed by the Mo-responsive MopA and MopB regulators, which directly bind to the anfA promoter (12, 14). Together, these findings suggest that Mo repression of Fe-nitrogenase is a consequence of the absence of AnfA under these conditions.
MopA and MopB are encoded by two divergently transcribed operons, mopA-modABC and mopB (14). Transcription of the mopA-modABC operon is repressed by MopA and MopB, while mopB is constitutively expressed. In line with those earlier findings, MopA and ModA levels were repressed by Mo, while the amount of MopB remained fairly constant (Table 3). MorA, a ModA-like protein of unknown function, also accumulated under Mo-limiting conditions, albeit at lower levels than ModA (Table 3). As is the case for the anfA and mopA-modABC genes, morABC transcription is directly repressed by MopA and MopB (14).
Mo inhibits accumulation of IscN.
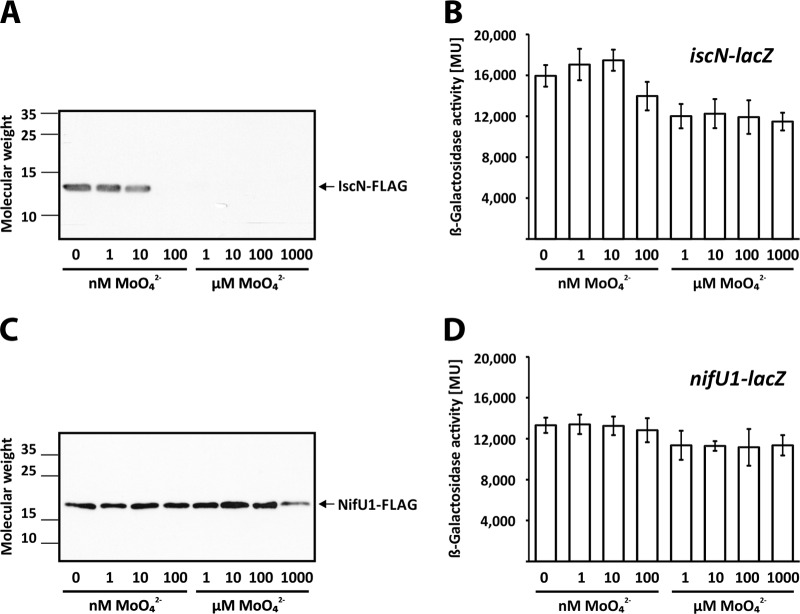
The proteome analysis uncovered a previously unknown Mo-responsive protein, IscN. The R. capsulatus iscN gene forms part of the iscN-nifU1-nifSVW operon, whose products have been implicated in iron-sulfur (Fe-S) cluster and iron-molybdenum cofactor (FeMoco) biosynthesis (28). Mo strictly inhibited IscN accumulation in the wild-type strain and, to lesser extent, in the ΔmodABC strain, while the levels of NifU1, NifS, and NifW remained more or less constant under –Mo and +Mo conditions (Table 3). IscN accumulated to larger amounts in the ΔmodABC strain than in the wild type, suggesting that maximal IscN accumulation requires Mo starvation as described above for the Fe-nitrogenase proteins.
To validate Mo inhibition of IscN accumulation and to examine iscN transcription, we constructed reporter strains carrying chromosomally integrated iscN-FLAG and iscN-lacZ fusions (Fig. 3A and B). For comparison, we generated strains with FLAG and lacZ fusions to nifU1, the second gene in the iscN-nifU1-nifSVW operon (Fig. 3C and D). Consistent with the proteome data, IscN-FLAG accumulated at low-nanomolar Mo concentrations but was repressed at Mo concentrations of 100 nM and above (Fig. 3A). In contrast, levels of NifU1-FLAG were comparably high at all Mo concentrations (Fig. 3C). These findings suggest that IscN accumulation is exclusively controlled by Mo, whereas expression of the downstream genes of the iscN-nifU1-nifSVW operon is not affected by Mo. LacZ activity in the iscN-lacZ reporter strain was slightly (1.5-fold) inhibited by Mo, while LacZ activity in the nifU1-lacZ strain did not respond to Mo at all (Fig. 3B and D). The poor transcriptional response suggests that Mo control of IscN levels depends mainly on posttranscriptional mechanisms (see Discussion).
FIG 3.
Expression of iscN-FLAG, iscN-lacZ, nifU1-FLAG, and nifU1-lacZ reporter fusions. R. capsulatus strains were grown in RCV minimal medium with the indicated molybdate concentrations. The strains used were as follows: B10S::pEW13, the wild-type strain carrying iscN-FLAG (A); B10S::pEW58, the wild-type strain carrying iscN-lacZ (B); B10S::pEW51, the wild-type strain carrying nifU1-FLAG (C); and B10S::pEW53, the wild-type strain carrying nifU1-lacZ (D). (A and C) Western analysis was performed using FLAG antibodies. Molecular weight data are given in thousands. (B and D) LacZ (β-galactosidase) activity is given in Miller units (23). The results represent the means and standard deviations of the results of at least three independent measurements.
IscN is important for Fe-nitrogenase-dependent diazotrophic growth.
To examine the role of IscN in nitrogen fixation, nonpolar iscN deletions were introduced into the R. capsulatus B10S wild-type strain and the BS85 ΔnifDK strain (see Materials and Methods). B10S is capable of fixing N2 using either Mo- or Fe-nitrogenase, while BS85 is no longer able to synthesize Mo-nitrogenase but can still grow diazotrophically using Fe-nitrogenase (15, 29). Parental and ΔiscN strains grew comparably well with a fixed nitrogen source, ammonium, indicating that deletion of iscN does not cause general growth defects (data not shown).
To test diazotrophic growth properties, strains were grown in RCV minimal medium lacking a fixed nitrogen source under N2 gas (see Materials and Methods). The ΔiscN and ΔnifDK strains grew with N2 nitrogen at levels equal to those seen with the wild type (Fig. 4A and B). In contrast, diazotrophic growth of the ΔiscN-ΔnifDK double mutant was delayed for at least 3 days (Fig. 4B). Reinoculation in fresh medium and incubation under diazotrophic conditions reproduced the delayed growth phenotype, suggesting that the ΔiscN-ΔnifDK strain adapts its metabolism over time rather than acquiring growth-promoting mutations. Together, these findings show that IscN is critical for Fe-nitrogenase-dependent growth but dispensable for growth via Mo-nitrogenase.
FIG 4.
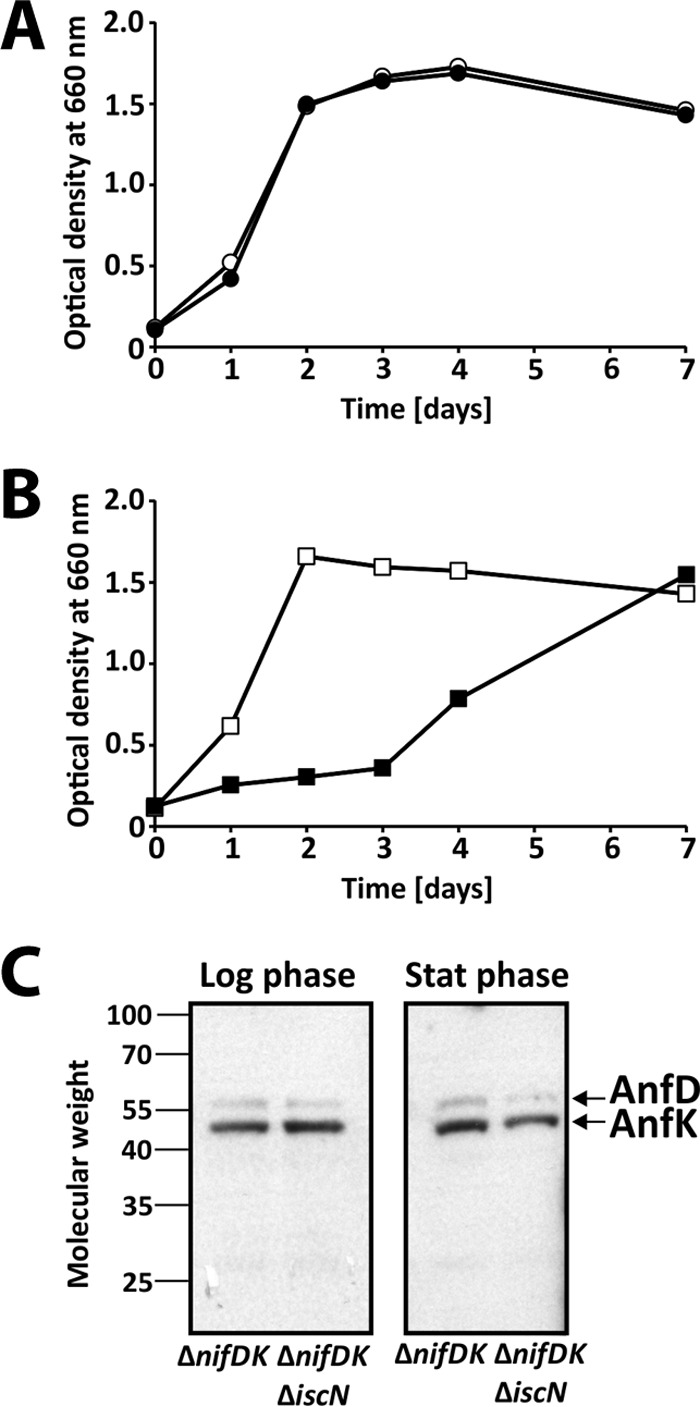
Diazotrophic growth of iscN mutant strains. (A and B) To examine diazotrophic growth, R. capsulatus strains were grown in RCV minimal medium under a pure N2 atmosphere. The strains used in the experiments represented by panel A were B10S (wild type; open circles) and YP340 (ΔiscN mutant; filled circles). The strains used in the experiments represented by panel B were BS85 (ΔnifDK mutant; open squares) and BS85-YP340 (ΔnifDK-ΔiscN mutant; filled squares). Growth experiments were done three times, and one representative data set is shown. In addition, the ΔnifDK and ΔnifDK-ΔiscN strains were grown in RCV medium containing serine. (C) Samples taken at the logarithmic phase (Log phase) and the stationary phase (Stat phase) were examined by Western analysis. Antibodies raised against R. capsulatus NifDK were used to detect AnfD and AnfK proteins. Molecular weight data are given in thousands.
To determine whether iscN disruption affects Fe-nitrogenase expression, we examined AnfDK accumulation. For this purpose, ΔnifDK and ΔiscN-ΔnifDK strains were grown in RCV minimal medium containing serine as a fixed nitrogen source to achieve comparable levels of growth of the two mutants. As mentioned above, serine does not repress nitrogen fixation (17). Samples were taken at the logarithmic and stationary phases and adjusted to the same cell density. Western analysis using antibodies raised against R. capsulatus NifDK identified two bands likely corresponding to AnfD (59.1 kDa) and AnfK (50.7 kDa) (Fig. 4C). Levels of AnfD and AnfK were comparable in the two strains at both growth phases, suggesting that IscN is dispensable for Fe-nitrogenase expression but is instead important for Fe-nitrogenase maturation.
DISCUSSION
Despite the importance of molybdenum in biological nitrogen fixation, our knowledge about Mo regulation of this process is fragmentary. This is the first approach analyzing the proteome-wide response to Mo availability in a diazotrophic bacterium. In this context, R. capsulatus is of special interest since this diazotroph provides the opportunity to examine the Mo-controlled interplay between a Mo-dependent nitrogenase and an alternative Mo-free Fe-only nitrogenase. Many Mo-controlled proteins identified in this study are involved in nitrogen fixation, indicating the importance of Mo regulation in this process (Table 3). Mo control of selected proteins was validated by Western analysis, epitope tagging, and lacZ reporter fusion studies.
The most prominent Mo-controlled proteins were NifHDK (Mo-nitrogenase), AnfHDGK (Fe-nitrogenase), ModA (Mo transport), and Mop (Mo storage), all of which reached extreme abundances under certain conditions (Table 3). Maximal levels of NifHDK and Mop were observed under Mo-replete conditions, while AnfHDGK and ModA were exclusively synthesized under Mo-limiting conditions. In addition, AnfA (the anfHDGK activator), MopA (a Mo-responsive regulater), MorA (a ModA-like protein), and IscN were Mo repressed. In contrast, the levels of other nitrogen fixation proteins involved in Fe-S cluster formation (NifU1-NifSW), FeMoco biosynthesis (NifENX), and electron transfer (RnfCG) remained more or less constant under –Mo and +Mo conditions (Table 3). This indicates that a subset of abundant nitrogen fixation proteins and certain regulators in R. capsulatus are controlled in a manner dependent on the presence of Mo. The regulatory mechanisms underlying Mo-controlled protein accumulation are discussed below.
Besides Mo-nitrogenase containing FeMoco, the iron-molybdenum cofactor, R. capsulatus is capable of synthesizing the molybdoenzymes xanthine dehydrogenase (XDH), formate dehydrogenase (FDH), and aldehyde dehydrogenase (ALDH), containing the Moco molybdopterin cofactor (30–32). The XDH, FDH, ALDH, and Moco biosynthesis enzymes were expressed at very low levels or were not even identified in our data set (see Tables S1 and S2 in the supplemental material). With one exception, ALDH2 (corresponding to gene locus identifier rcc02448), none of the detected enzymes responded to Mo availability. However, ALDH2 expression was too low to prove Mo regulation of this enzyme by epitope tagging (data not shown).
R. capsulatus synthesizes two Mo-responsive regulators, MopA and MopB, each of which is sufficient to repress transcription of the anfA, mopA-modABC, and morABC genes by binding to the respective promoters (14). As a consequence of anfA repression, AnfA-dependent anfHDGK transcription is repressed under Mo-replete conditions. In addition to its repressor function, MopA acts as an activator of mop transcription (14). As outlined above, AnfA, MopA, ModA, MorA, and AnfHDGK were expressed only under Mo-limiting conditions, while Mop levels increased with increasing Mo concentrations (Table 3 and Fig. 2). We thus conclude that Mo-controlled transcriptional regulation of members of the MopA-MopB regulon determines the levels of their products.
Mo-induced LacZ activity in a nifK-lacZ reporter strain suggests that nifHDK transcription responds to the cellular Mo status and, consequently, determines Mo-induced accumulation of the NifHDK proteins (Table 3 and Fig. 1). However, Mo responses of NifD and NifK levels were much more pronounced than those of NifH, indicating the existence of one or more posttranscriptional mechanisms controlling accumulation of Mo-nitrogenase proteins. Indeed, the primary transcript of the nifHDK operon was found to be processed into separate nifH and nifDK mRNAs as shown by Northern analysis (33). Cleavage of nifHDK mRNA involves a guanine-cytidine-rich hairpin structure encoded by the nifH-nifD intergenic region. Hence, one could speculate that nifHDK mRNA processing contributes to Mo-responsive control of NifHDK levels. Irrespective of a role in Mo control, nifHDK mRNA cleavage likely controls the ratio between nitrogenase reductase (NifH) and dinitrogenase (NifDK) subunits.
IscN was the only product of the iscN-nifU1-nifSVW operon whose accumulation was repressed by Mo (Table 3 and Fig. 3A and C). LacZ activity in an iscN-lacZ reporter strain was only slightly inhibited by Mo (Fig. 3B), suggesting that IscN levels are mainly controlled posttranscriptionally. At present, we cannot rule out the possibility that Mo inhibition of LacZ activity results from Mo repression of iscN transcription. However, this seems unlikely, since nifU1-lacZ expression was not affected by Mo at all (Fig. 3D). Alternatively, Mo inhibition of LacZ activity in the iscN-lacZ reporter strain may have resulted from Mo control of iscN-lacZ mRNA stability, or translational coupling of iscN-lacZ genes, when iscN translation was Mo repressed. Since NifHDK accumulation was Mo induced, while IscN accumulation was Mo repressed, NifHDK and IscN levels are probably controlled by different mechanisms. Future studies will have to clarify whether Mo specifically affects stability of nifHDK and iscN mRNAs, translation efficiency, or stability of NifHDK and IscN proteins.
IscN was shown to be important for Fe-nitrogenase-mediated diazotrophic growth (Fig. 4B) but dispensable for expression of Fe-nitrogenase (Fig. 4C), suggesting that IscN is involved in Fe-nitrogenase maturation. The R. capsulatus iscN-nifU1-nifSVW products exhibit strong similarity to their well-characterized Azotobacter vinelandii counterparts (28). A. vinelandii NifS and NifU act as sulfur donor and scaffold for Fe-S biosynthesis, respectively (34–36). Three different roles have been assigned to the A. vinelandii IscN counterpart, NifIscA. First, NifIscA was shown to bind Fe(II) and Fe(III) and to act as an iron donor for Fe-S biosynthesis (37). Second, NifIscA was proposed to function as an alternate scaffold for Fe-S biosynthesis (38). Third, NifIscA was shown to bind 2Fe-2S and 4Fe-4S clusters and to act as an A-type carrier transferring 4Fe-4S clusters to apo-nitrogenase reductase, NifH (39). Since R. capsulatus IscN was important for Fe-nitrogenase activity but dispensable for Mo-nitrogenase function (Fig. 4A and B), a major role as an iron donor or alternate scaffold in Fe-S biosynthesis is unlikely, as the two nitrogenases should have been equally affected. More likely, IscN may act as an A-type carrier transferring 4Fe-4S clusters to AnfH but not to NifH. Future studies will have to address the interaction and Fe-S cluster transfer between IscN and AnfH. Furthermore, it will be interesting to determine whether the IscN-like proteins IscA (with gene locus identifier rcc01599) and ErpA (with gene locus identifier rcc02325) are involved in nitrogen fixation, possibly by transferring 4Fe-4S clusters to NifH.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to Yves Jouanneau (Grenoble, France) for providing antisera raised against R. capsulatus NifDK. We thank Pascal Prochnow (Bochum, Germany) for help with proteome profiling.
Funding Statement
This work was supported by a grant from the Deutsche Forschungsgemeinschaft (DFG) (Ma 1814/4-1 to B.M.). The mass spectrometer was financed by a grant (Forschungsgroßgeräte der Länder) by the German Federal State of North Rhine-Westphalia (to J.E.B.).
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JB.00750-15.
REFERENCES
- 1.Zhang Y, Gladyshev VN. 2008. Molybdoproteomes and evolution of molybdenum utilization. J Mol Biol 379:881–899. doi: 10.1016/j.jmb.2008.03.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McGlynn SE, Boyd ES, Peters JW, Orphan VJ. 2012. Classifying the metal dependence of uncharacterized nitrogenases. Front Microbiol 3:419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hu Y, Lee CC, Ribbe MW. 2012. Vanadium nitrogenase: a two-hit wonder? Dalton Trans 41:1118–1127. doi: 10.1039/C1DT11535A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schneider K, Gollan U, Dröttboom M, Selsemeier-Voigt S, Müller A. 1997. Comparative biochemical characterization of the iron-only nitrogenase and the molybdenum nitrogenase from Rhodobacter capsulatus. Eur J Biochem 244:789–800. doi: 10.1111/j.1432-1033.1997.t01-1-00789.x. [DOI] [PubMed] [Google Scholar]
- 5.Bauer C, Elsen S, Swem LR, Swem DL, Masuda S. 2003. Redox and light regulation of gene expression in photosynthetic prokaryotes. Philos Trans R Soc Lond B Biol Sci 358:147–153; discussion 153–144. doi: 10.1098/rstb.2002.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Friedrich B, Schwartz E. 1993. Molecular biology of hydrogen utilization in aerobic chemolithotrophs. Annu Rev Microbiol 47:351–383. doi: 10.1146/annurev.mi.47.100193.002031. [DOI] [PubMed] [Google Scholar]
- 7.Klug G. 1993. The role of mRNA degradation in the regulated expression of bacterial photosynthesis genes. Mol Microbiol 9:1–7. doi: 10.1111/j.1365-2958.1993.tb01663.x. [DOI] [PubMed] [Google Scholar]
- 8.Masepohl B, Klipp W. 1996. Organization and regulation of genes encoding the molybdenum nitrogenase and the alternative nitrogenase in Rhodobacter capsulatus. Arch Microbiol 165:80–90. doi: 10.1007/s002030050301. [DOI] [Google Scholar]
- 9.Masepohl B, Hallenbeck PC. 2010. Nitrogen and molybdenum control of nitrogen fixation in the phototrophic bacterium Rhodobacter capsulatus. Adv Exp Med Biol 675:49–70. doi: 10.1007/978-1-4419-1528-3_4. [DOI] [PubMed] [Google Scholar]
- 10.Schneider K, Müller A, Schramm U, Klipp W. 1991. Demonstration of a molybdenum- and vanadium-independent nitrogenase in a nifHDK-deletion mutant of Rhodobacter capsulatus. Eur J Biochem 195:653–661. doi: 10.1111/j.1432-1033.1991.tb15750.x. [DOI] [PubMed] [Google Scholar]
- 11.Strnad H, Lapidus A, Paces J, Ulbrich P, Vlcek C, Paces V, Haselkorn R. 2010. Complete genome sequence of the photosynthetic purple nonsulfur bacterium Rhodobacter capsulatus SB 1003. J Bacteriol 192:3545–3546. doi: 10.1128/JB.00366-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kutsche M, Leimkühler S, Angermüller S, Klipp W. 1996. Promoters controlling expression of the alternative nitrogenase and the molybdenum uptake system in Rhodobacter capsulatus are activated by NtrC, independent of σ54, and repressed by molybdenum. J Bacteriol 178:2010–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang G, Angermüller S, Klipp W. 1993. Characterization of Rhodobacter capsulatus genes encoding a molybdenum transport system and putative molybdenum-pterin-binding proteins. J Bacteriol 175:3031–3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wiethaus J, Wirsing A, Narberhaus F, Masepohl B. 2006. Overlapping and specialized functions of the molybdenum-dependent regulators MopA and MopB in Rhodobacter capsulatus. J Bacteriol 188:8441–8451. doi: 10.1128/JB.01188-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoffmann MC, Müller A, Fehringer M, Pfänder Y, Narberhaus F, Masepohl B. 2014. Coordinated expression of fdxD and molybdenum nitrogenase genes promotes nitrogen fixation by Rhodobacter capsulatus in the presence of oxygen. J Bacteriol 196:633–640. doi: 10.1128/JB.01235-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dombrecht B, Tesfay MZ, Verreth C, Heusdens C, Nápoles MC, Vanderleyden J, Michiels J. 2002. The Rhizobium etli gene iscN is highly expressed in bacteroids and required for nitrogen fixation. Mol Genet Genomics 267:820–828. doi: 10.1007/s00438-002-0715-0. [DOI] [PubMed] [Google Scholar]
- 17.Klipp W, Masepohl B, Pühler A. 1988. Identification and mapping of nitrogen fixation genes of Rhodobacter capsulatus: duplication of a nifA-nifB region. J Bacteriol 170:693–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Masepohl B, Kaiser B, Isakovic N, Richard CL, Kranz RG, Klipp W. 2001. Urea utilization in the phototrophic bacterium Rhodobacter capsulatus is regulated by the transcriptional activator NtrC. J Bacteriol 183:637–643. doi: 10.1128/JB.183.2.637-643.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sicking C, Brusch M, Lindackers A, Riedel KU, Schubert B, Isakovic N, Krall C, Klipp W, Drepper T, Schneider K, Masepohl B. 2005. Identification of two new genes involved in diazotrophic growth via the alternative Fe-only nitrogenase in the phototrophic purple bacterium Rhodobacter capsulatus. J Bacteriol 187:92–98. doi: 10.1128/JB.187.1.92-98.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weaver PF, Wall JD, Gest H. 1975. Characterization of Rhodopseudomonas capsulata. Arch Microbiol 105:207–216. doi: 10.1007/BF00447139. [DOI] [PubMed] [Google Scholar]
- 21.Bradford MM. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 22.Vizcaíno JA, Deutsch EW, Wang R, Csordas A, Reisinger F, Ríos D, Dianes JA, Sun Z, Farrah T, Bandeira N, Binz PA, Xenarios I, Eisenacher M, Mayer G, Gatto L, Campos A, Chalkley RJ, Kraus HJ, Albar JP, Martinez-Bartolomé S, Apweiler R, Omenn GS, Martens L, Jones AR, Hermjakob H. 2014. ProteomeXchange provides globally co-ordinated proteomics data submission and dissemination. Nat Biotechnol 32:223–226. doi: 10.1038/nbt.2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller JH. 1972. Experiments in molecular genetics, p 352–355. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- 24.Gisin J, Müller A, Pfänder Y, Leimkühler S, Narberhaus F, Masepohl B. 2010. A Rhodobacter capsulatus member of a universal permease family imports molybdate and other oxyanions. J Bacteriol 192:5943–5952. doi: 10.1128/JB.00742-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jouanneau Y, Meyer CM, Vignais PM. 1983. Regulation of nitrogenase activity through iron protein interconversion into an active and an inactive form in Rhodopseudomonas capsulata. Biochim Biophys Acta 749:318–328. doi: 10.1016/0167-4838(83)90242-X. [DOI] [Google Scholar]
- 26.Masepohl B, Krey R, Klipp W. 1993. The draTG gene region of Rhodobacter capsulatus is required for post-translational regulation of both the molybdenum and the alternative nitrogenase. J Gen Microbiol 139:2667–2675. doi: 10.1099/00221287-139-11-2667. [DOI] [PubMed] [Google Scholar]
- 27.Wiethaus J, Müller A, Neumann M, Neumann S, Leimkühler S, Narberhaus F, Masepohl B. 2009. Specific interactions between four molybdenum-binding proteins contribute to Mo-dependent gene regulation in Rhodobacter capsulatus. J Bacteriol 191:5205–5215. doi: 10.1128/JB.00526-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Masepohl B, Angermüller S, Hennecke S, Hübner P, Moreno-Vivian C, Klipp W. 1993. Nucleotide sequence and genetic analysis of the Rhodobacter capsulatus ORF6-nifUI SVW gene region: possible role of NifW in homocitrate processing. Mol Gen Genet 238:369–382. doi: 10.1007/BF00291996. [DOI] [PubMed] [Google Scholar]
- 29.Hoffmann MC, Pfänder Y, Fehringer M, Narberhaus F, Masepohl B. 2014. NifA- and CooA-coordinated cowN expression sustains nitrogen fixation by Rhodobacter capsulatus in the presence of carbon monoxide. J Bacteriol 196:3494–3502. doi: 10.1128/JB.01754-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hartmann T, Leimkühler S. 2013. The oxygen-tolerant and NAD+-dependent formate dehydrogenase from Rhodobacter capsulatus is able to catalyze the reduction of CO2 to formate. FEBS J 280:6083–6096. doi: 10.1111/febs.12528. [DOI] [PubMed] [Google Scholar]
- 31.Leimkühler S, Wuebbens MM, Rajagopalan KV. 2011. The history of the discovery of the molybdenum cofactor and novel aspects of its biosynthesis in bacteria. Coord Chem Rev 255:1129–1144. doi: 10.1016/j.ccr.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mendel RR, Leimkühler S. 2015. The biosynthesis of the molybdenum cofactors. J Biol Inorg Chem 20:337–347. doi: 10.1007/s00775-014-1173-y. [DOI] [PubMed] [Google Scholar]
- 33.Willison JC, Pierrard J, Hübner P. 1993. Sequence and transcript analysis of the nitrogenase structural gene operon (nifHDK) of Rhodobacter capsulatus: evidence for intramolecular processing of nifHDK mRNA. Gene 133:39–46. doi: 10.1016/0378-1119(93)90222-O. [DOI] [PubMed] [Google Scholar]
- 34.Dos Santos PC, Smith AD, Frazzon J, Cash VL, Johnson MK, Dean DR. 2004. Iron-sulfur cluster assembly: NifU-directed activation of the nitrogenase Fe protein. J Biol Chem 279:19705–19711. doi: 10.1074/jbc.M400278200. [DOI] [PubMed] [Google Scholar]
- 35.Smith AD, Jameson GN, Dos Santos PC, Agar JN, Naik S, Krebs C, Frazzon J, Dean DR, Huynh BH, Johnson MK. 2005. NifS-mediated assembly of [4Fe-4S] clusters in the N- and C-terminal domains of the NifU scaffold protein. Biochemistry 44:12955–12969. doi: 10.1021/bi051257i. [DOI] [PubMed] [Google Scholar]
- 36.Yuvaniyama P, Agar JN, Cash VL, Johnson MK, Dean DR. 2000. NifS-directed assembly of a transient [2Fe-2S] cluster within the NifU protein. Proc Natl Acad Sci U S A 97:599–604. doi: 10.1073/pnas.97.2.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mapolelo DT, Zhang B, Naik SG, Huynh BH, Johnson MK. 2012. Spectroscopic and functional characterization of iron-bound forms of Azotobacter vinelandii NifIscA. Biochemistry 51:8056–8070. doi: 10.1021/bi300664j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Krebs C, Agar JN, Smith AD, Frazzon J, Dean DR, Huynh BH, Johnson MK. 2001. IscA, an alternate scaffold for Fe-S cluster biosynthesis. Biochemistry 40:14069–14080. doi: 10.1021/bi015656z. [DOI] [PubMed] [Google Scholar]
- 39.Mapolelo DT, Zhang B, Naik SG, Huynh BH, Johnson MK. 2012. Spectroscopic and functional characterization of iron-sulfur cluster-bound forms of Azotobacter vinelandii NifIscA. Biochemistry 51:8071–8084. doi: 10.1021/bi3006658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vieira J, Messing J. 1982. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene 19:259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- 41.Simon R, Priefer U, Pühler A. 1983. A broad host range mobilization system for in vivo genetic-engineering: transposon mutagenesis in gram negative bacteria. Nat Biotechnol 1:784–791. doi: 10.1038/nbt1183-784. [DOI] [Google Scholar]
- 42.Möller P, Overlöper A, Förstner KU, Wen TN, Sharma CM, Lai EM, Narberhaus F. 2014. Profound impact of Hfq on nutrient acquisition, metabolism and motility in the plant pathogen Agrobacterium tumefaciens. PLoS One 9:e110427. doi: 10.1371/journal.pone.0110427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schäfer A, Tauch A, Jäger W, Kalinowski J, Thierbach G, Pühler A. 1994. Small mobilizable multi-purpose cloning vectors derived from the Escherichia coli plasmids pK18 and pK19: selection of defined deletions in the chromosome of Corynebacterium glutamicum. Gene 145:69–73. doi: 10.1016/0378-1119(94)90324-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.