Abstract
During their colonization of plants, human enteric pathogens, such as Salmonella enterica, are known to benefit from interactions with phytopathogens. At least in part, benefits derived by Salmonella from the association with a soft rot caused by Pectobacterium carotovorum were shown to be dependent on Salmonella KdgR, a regulator of genes involved in the uptake and utilization of carbon sources derived from the degradation of plant polymers. A Salmonella kdgR mutant was more fit in soft rots but not in the lesions caused by Xanthomonas spp. and Pseudomonas spp. Bioinformatic, phenotypic, and gene expression analyses demonstrated that the KdgR regulon included genes involved in uptake and metabolism of molecules resulting from pectin degradation as well as those central to the utilization of a number of other carbon sources. Mutant analyses indicated that the Entner-Doudoroff pathway, in part controlled by KdgR, was critical for the persistence within soft rots and likely was responsible for the kdgR phenotype.
INTRODUCTION
Interactions within the phytopathogen-plant host-microbiome triangle are coming to the forefront of scientific research, as it is becoming clear that these interactions are central to crop disease resistance, productivity, and food safety (1–3). Recent studies suggest that the persistence of human enteric pathogens, such as Salmonella and pathogenic Escherichia coli, in plants, soil, and water represent an important component of their life cycle outside the animal host (4, 5). The ability of human pathogens, such as pathogenic E. coli and nontyphoidal strains of Salmonella enterica, to persist in the environment is determined, to a great extent, by their interactions with native microbial communities (3, 4, 6–8). In fact, supermarket surveys established that human pathogens are significantly more likely to be found in produce affected by bacterial soft rot than in lesions caused by mechanical damage or fungal pathogens (9). Follow-up laboratory studies supported conclusions of supermarket surveys and demonstrated that within soft rots, S. enterica and E. coli reach cell numbers that are 10 to 1,000 times higher than those in intact plants (7, 10–13). The effects of phytobacteria on human pathogens occupying the same niche are significant not only in the magnitude of their growth increase but also in the breadth of physiological adaptation to the cohabitation. For example, the growth of S. enterica on lettuce leaf macerate caused by another soft-rot pathogen, Dickeya dadantii, affected ∼18% of the transcriptome (compared to a rich laboratory medium) and included genes involved in growth under oxygen-limiting conditions, carbohydrate, iron, and zinc acquisition, and responses to inhibitory compounds and toxins (12). Nevertheless, mechanisms leading to the increased proliferation of human pathogens in soft rots remain only partially understood.
Grosskopf and Soyer generalized that interactions within microbial communities are driven by either social or metabolic interactions (14). However, there is no evidence that Salmonella's SdiA- and LuxS-mediated quorum-sensing systems are involved in the interactions with phytopathogens in planta (11, 15, 16). Therefore, it is reasonable to hypothesize that metabolic interactions between S. enterica and phytopathogens are responsible for its increased proliferation within lesions caused by soft rotters. However, mechanisms of these metabolic interactions currently are not known.
Previously, it was hypothesized that human pathogens benefit from pectinolytic activities of phytophathogens in soft rots (17). Unlike phytophathogens, neither S. enterica nor E. coli has the ability to degrade pectin; however, they can take up pectin oligomers and monomers. In gammaproteobacteria, all of these functions are mediated by the transcriptional regulator KdgR (18, 19). In phytophathogens, KdgR regulates plant cell wall degradation and the uptake of the degradation products across the Enterobacteriaceae (20, 21). Therefore, this study focused on elucidating the role of KdgR and its regulon in the persistence of S. enterica within soft rots.
KdgR is an IclR family transcriptional regulator which functions as a repressor, although other members of the family can function both as repressors and activators (20, 22, 23). In vitro, Erwinia chrysanthemi KdgR binds to different operators, with KD (equilibrium dissociation constant) values between 0.1 and 10 nM (21). However, in vitro binding affinity generally did not correlate with the degree of repression of the corresponding gene. As a dimer, it binds a 17-bp KdgR box, containing two half sites. Binding of 2-keto-3-deoxygluconate (KDG) to KdgR releases this repressor protein from its binding sites (20, 21). The characterization of the DNA binding site for KdgR in E. chrysanthemi (20, 21) led to comparative genomics studies that outlined KdgR regulons in a number of gammaproteobacteria (18). In S. enterica, initial predictions identified kduI-kduD (involved in 5-keto-4-deoxyuronate catabolism), kdgM (rhamnogalacturonan catabolism), and kdgK (2-keto-3-deoxygluconate kinase) among the 11-member putative regulon (18). E. coli KdgR was shown to contribute to the regulation of the Entner-Doudoroff (ED) pathway, which catabolizes glucose to pyruvate and then feeds into glycolysis (24, 25). The Entner-Doudoroff pathway also is involved in the utilization of gluconate, idonate, galacturonate, glucuronate, β-glucuronide, fructuronate, tagaturonate, and 2-keto-3-deoxy-gluconate and is hypothesized to contribute to the fitness of E. coli in the large intestine (25). In addition to their metabolic functions in the Entner-Doudoroff pathway, eda (2-keto-3-deoxy-6-phosphogluconate aldolase) and edd (6-phosphogluconate dehydratase) were shown to contribute to phosphate and nitrogen starvation responses as well as stress responses (25).
With this study, we tested the overarching hypothesis that the S. enterica KdgR regulon contributes to the fitness of this human pathogen in tomato soft rots caused by an aggressive strain of Pectobacterium carotovorum. This study sought to better define the Salmonella KdgR regulon and further investigated potential mechanisms by which KdgR contributes to the fitness of Salmonella within soft rots.
MATERIALS AND METHODS
Bacterial culture.
Salmonella mutants were grown overnight in a shaker at 37°C in Luria-Bertani (LB) medium supplemented with 50 μg/ml kanamycin or 10 μg/ml tetracycline as needed. Wild-type S. enterica serovar Typhimurium 14028 was grown without selection at 37°C. P. carotovorum SR38 was grown overnight in a shaker at 30°C in LB.
The ability of Salmonella to grow on specific carbon sources was tested using M9 minimal medium supplemented with 0.2% (wt/vol) of a carbon source. Prior to dilution in M9, cultures were grown overnight in LB. They then were washed in phosphate-buffered saline, pH 7.0 (PBS). Suspensions were dilution plated onto LB agar.
Strain construction.
Deletion mutants were constructed using Datsenko-Wanner mutagenesis (26), and entire open reading frames were replaced with frt-kan-frt cassettes (Table 1). Deletions were confirmed by PCR with primers listed in Table S1 in the supplemental material. RIVET (recombinase-based in vivo expression technology) reporters were constructed by first removing a kanamycin resistance marker (26) and then mating the plasmid pCE70 or pCE71, containing a tnpR-lacZ fusion, to the mutant strain (27). The orientation of the reporter fusion was confirmed by PCR. Phage P22 then was used to transduce a res1-tetRA-res1 cassette into the strain. Resulting constructs then were purified on LB EGTA (10 mM) with kanamycin and tetracycline and then screened on Evans-blue uranium (EBU) agar.
TABLE 1.
Strains used in this studya
| Strain or plasmid | Genotype or description | Source, construction, or reference |
|---|---|---|
| Strain | ||
| Escherichia coli | ||
| DH5α | F− Δ(lacZYA-argF)U169 recA1 endA1 hsdR17 supE44 thi-1 gyrA96 relA2 | Life Technologies |
| Pectobacterium carotovorum subsp. carotovorum | ||
| SR38 | Aggressive soft-rot pathogen isolated from a shipment of Florida tomatoes | Laboratory collection, from J. Bartz |
| Salmonella enterica serovar Typhimurium | ||
| 14028 | Wild type | American Type Culture Collection |
| JS246 | 14028 yjeP::res1-tetRA-res1 | 26 |
| JTN215 | 14028 kdgR27::kan | Made by Datsenko-Wanner (26) mutagenesis using primers JTN177 and JTN178; verified with primers JTN179 and JTN180 |
| AG32 | 14028 kdgR27::frt res1-tetRA-res1 | AG1 × P22/JS246 |
| AG1 | 14028 kdgR27::frt | Made by electroporating pCP20 into JTN215 |
| AG2 | 14028 kdgR27::tnpR-lacZY | Made by electroporating pCE70 into AG1 |
| AG3 | 14028 kdgR2::tnpR-lacZY res1-tetRA-res1 | AG2 × P22/JS246 |
| AG15 | 14028 kdgK15::frt-kan-frt | Made by Datsenko-Wanner (26) mutagenesis using primers JTN163 and JTN164; verified with primers JTN165 and JTN166 |
| AG19 | 14028 kdgK15::frt | Made by electroporating pCP20 into AG15 |
| AG27 | 14028 kdgK15-tnpR-lacZY | Made by electroporating pCE70 into AG19 |
| AG28 | 14028 kdgK15-tnpR-lacZY res1-tetRA-res1 | AG27 × P22/JS246 |
| AG38 | 14028 kdgR27::frt kdgK15::tnpR-lacZY res1-tetRA-res1 | AG32 × P22/AG27 |
| AG25 | 14028 kduID45::frt-kan-frt | Made by Datsenko-Wanner (26) mutagenesis using primers JTN167 and JTN168; verified with primers JTN169 and JTN170 |
| AG31 | 14028 kduID45::frt | Made by electroporating pCP20 into AG25 |
| AG33 | 14028 kduID45::tnpR-lacZY | Made by electroporating pCE70 into AG31 |
| AG36 | 14028 kduID45::tnpR-lacZY res1-tetRA-res1 | AG33 × P22/JS246 |
| AG39 | 14028 kdgR27::frt kduID45::tnpR-lacZY res1-tetRA-res1 | AG32 × P22/AG33 |
| AG26 | 14028 kdgM32::frt-kan-frt | Made by Datsenko-Wanner (26) mutagenesis using primers AG7 and AG8; verified with primers AG33 and AG34 |
| AG30 | 14028 kdgM32::frt | Made by electroporating pCP20 into AG26 |
| AG34 | 14028 kdgM32::tnpR-lacZY | Made by electroporating pCE70 into AG31 |
| AG35 | 14028 kdgM32::tnpR-lacZY res1-tetRA-res1 | AG34 × P22/JS246 |
| AG41 | 14028 kdgR27::frt kdgM::tnpR-lacZY res1-tetRA-res1 | Ag32 × P22/AG34 |
| KI1 | 14028 eda42::frt-kan-frt | Made by Datsenko-Wanner (26) mutagenesis using primers AG92 and AG93; verified with primers AG94 and AG95 |
| AG42 | 14028 eda42::frt | Made by electroporating pCP20 into KI1 |
| AG45 | 14028 eda42::tnpR-lacZY | Made by electroporating pCE71 into AG42 |
| AG46 | 14028 eda42::tnpR-lacZY res1-tetRA-res1 | AG45 × P22/JS246 |
| AG47 | 14028 kdgR27::frt eda42::tnpR-lacZY res1-tetRA-res1 | AG32 × P22/AG45 |
| AG44 | 14028 kdgT44::frt-kan-frt | Made by Datsenko-Wanner (26) mutagenesis using primers AG64 and AG65; verified with primers AG66 and AG67 |
| AG47 | 14028 kdgK15::frt kduID::frt-kan-frt | AG19 × P22/AG25 |
| AG48 | 14028 kdgK15::frt kduID::frt | Made by electroporating pCP20 into AG47 |
| AG49 | 14028 kdgK15::frt kduID45::frt kdgM32::frt-kan-frt | AG48 × P22/AG26 |
| Plasmid | ||
| pKD4 | frt-kan-frt template | 26 |
| pCP20 | FLP+ l cI857+ l PR Repts Ampr Cmr | 26 |
| pCE70 | FRT-tnpR-lacZY oriR6K (kan); contains wild-type tnpR promoter, FRT orientation A | 27 |
| pCE71 | FRT-tnpR-lacZY oriR6K (kan); contains wild-type tnpR promoter, FRT orientation B | 27 |
| pKD46 | λ Red+ (Ampr pSC101 oriTS) | 26 |
FRT, FLP recombination target.
Vegetable inoculations.
For competitive fitness experiments, overnight cultures of mutant and wild-type strains were washed in PBS and diluted to a concentration of ∼104 CFU/ml. These suspensions were combined in an approximately 1:1 ratio. Three shallow wounds were made on the surface of each green tomato. Three microliters of the inoculum was spotted per wound. For the assays in soft rots, an additional 3 μl of an undiluted (109 CFU/ml) but washed P. carotovorum overnight culture was inoculated into the tomatoes concurrently with Salmonella. Tomatoes were sampled 3 days later, which corresponds to the development of a soft-rot lesion covering at least three-quarters of the fruit. Samples were plated on xylose-lysine deoxycholate agar (XLD; Oxoid) for the quantification of Salmonella. XLD plates were incubated at 42°C to prevent growth of Pectobacterium essentially as described previously (11). Salmonella colonies then were patched onto selective media with kanamycin, and the ratio of the wild type (kanamycin sensitive) to the mutant (kanamycin resistant) was calculated to determine how much it changed from 1:1. Competitive fitness indices were calculated as described before using the formula (Mout/WTout)/(Min/WTin), where M is the proportion of mutant cells and WTin or WTout is the proportion of the wild-type cells in the inocula or in the recovered samples, respectively (11). Competitive fitness of the kdgR mutant in green bell peppers and romaine lettuce was assayed similarly. Vegetables were purchased from a local grocery store.
To determine the growth of Salmonella in tomatoes, washed, diluted bacterial cultures were seeded into tomatoes as monocultures starting with approximately 100 CFU per wound. Following a 3-day incubation at 22°C, tomatoes were stomached in a Whirl-Pak bag (with an equal volume of PBS) using a Stomacher 400 circulator (Seward). Aliquots of the homogenate then were plated onto XLD plates and incubated at 42°C.
RIVET assays were conducted in a similar fashion. After the homogenate was plated on XLD, individual colonies were patched onto an LB plate with tetracycline. Percent resolution was taken as the ratio of patched colonies that grew (tetracycline resistant, in which the reporter was not activated) to those that did not (and lost the tetracycline resistance due to the activation of the reporter). Percent resolution was used as a measure of gene expression.
Biolog phenotype microarray.
Phenotype microarrays were performed using Biolog phenotype microarrays (PM1 and PM2). Cells were prepared according to the manufacturer's protocols with the following modification: cultures were grown overnight in LB and then starved in M9 minimal medium overnight. The plates then were read using a Victor3 plate reader (PerkinElmer). The function heatmap.2 from the R package gplots (https://cran.r-project.org/web/packages/gplots/index.html) was used to plot the data using the mean absorbance values (two replicates) per growth condition for each bacterium following 24 h of incubation. The dendrogram imposed over the rows and columns was constructed with a complete-linkage hierarchical clustering using the Euclidean distance between the mean absorbance values.
RT-qPCR.
Cultures were grown, washed, and inoculated into tomatoes as described above, only without the dilution. RNA was extracted following a 24-h incubation. To extract RNA, 4 ml of diethyl pyrocarbonate (DEPC)-treated water and 1 ml of 95:5 absolute ethanol-phenol was added to a 15-ml Falcon tube. Approximately 0.5 g of RNase-free 0.1-mm glass beads then were added to the tubes. A 1.5-g aliquot of inoculated tomato tissue was added to the tube and ground using a glass rod. The tubes then were vortexed, and 500 μl of the resulting liquid was aliquoted into microcentrifuge tubes. The Ambion RNAqueous kit was used for the remainder of the extraction by following the manufacturer's instructions. Samples were treated using an Ambion Turbo DNAfree kit. cDNA was synthesized using the OriGene cDNA synthesis kit. Quantitative PCR (qPCR) was performed using Applied Biosystems SYBR green reaction mix and measured using an Applied Biosystems thermal cycler. Primers were designed to amplify small (∼200-bp) fragments and subjected to a BLAST search against the P. carotovorum and S. enterica serovar Typhimurium genomes to check for nonspecific binding. To ascertain that nonspecific binding of primer to the P. carotovorum template did not occur, controls of soft rot (without S. enterica) were tested at least once for each primer pair. qPCR statistics were performed using the Livak method (28). As a control, the housekeeping gene rpoD was used after establishing that its expression did not differ regardless of the presence of kdgR or the growth in the soft rot. The expression of a particular gene in the wild-type background in intact tomatoes was used as the calibrator. Error was determined using the propagation of errors from the standard deviations.
Bioinformatics prediction of KdgR binding sites.
The program Retrieve-Sequence from Regulatory Sequence Analysis Tools (RSAT) was used to obtain the upstream regions (−400; +100 in relation to the start codon) of all coding sequences (CDS) in 28 RefSeq Salmonella genomes and their plasmids (29). Using 40 experimentally validated KdgR binding sites, a position-specific scoring matrix (PSSM) was constructed using the program Consensus with default values (29). For each of the 28 genomes utilized, an oligonucleotide frequency background file was created with the program Convert-Background from RSAT using Markov Model Order 4 (30). The upstream regions of each Salmonella CDS then were scanned with the constructed PSSM using Matrix-Scan with an upper threshold value of 1e−5 and inputting a previously generated genome-specific background file (30).
Salmonella orthologous genes from 40 experimentally validated KdgR regulons were downloaded from the Biocyc database. An ad hoc Perl script recovered 211 sequences corresponding to the predicted binding sites that matched the upstream regions of the orthologous genes previously retrieved. The Salmonella-fitted PSSM was constructed with an input of 211 sequences to the program consensus. The genome of S. enterica serovar Typhimurium LT2 was rescanned using this final PSSM with the program Matrix Scan with an upper threshold value of 1e−5 and inputting a previously generated genome-specific background file (30). The PSSM kdgR logo was created using Weblogo interface (31).
RESULTS AND DISCUSSION
kdgR contributes to fitness within soft rots.
To determine whether kdgR contributes to the persistence of Salmonella within soft rots, we first tested the expression of the kdgR RIVET reporter in intact tomatoes and those with the soft rot caused by Pectobacterium carotovorum. After 24 h of incubation within intact tomatoes and within soft rots, the activation of the reporter reached ∼90% with no significant differences in the expression of the reporter within soft rots and intact tomatoes (see Fig. S1 in the supplemental material). Within 3 days, the reporter was fully activated inside tomatoes, regardless of the presence of the soft rot (see Fig. S1 in the supplemental material). It is important to note, however, that RIVET reporters, once resolved, are not useful for the detection of downregulation of gene expression.
However, when the fitness of the kdgR mutant was compared with that of the wild type in intact tomatoes and those affected by soft rot or bacterial spot (associated with Xanthomonas spp. and Pseudomonas spp.), a significant increase in the fitness of the kdgR mutant was observed in soft-rotted tomatoes only (Fig. 1). While the kdgR mutant was more competitive than the wild type in the soft-rotted tomatoes, there were no significant differences in fitness between the wild type and mutant in intact tomatoes and those infected with necrotizing pathogens leading to the bacterial spot (Fig. 1). In green bell peppers and romaine lettuce coinoculated with S. Typhimurium and P. carotovorum, a trend for increased fitness of the kdgR mutant also was observed, although the differences in fitness were not statistically significant (data not shown). The increased fitness of the mutant is consistent with the function of KdgR as a repressor of gene expression. With the follow-up experiments, we focused on defining the mechanisms by which kdgR affects the fitness of Salmonella within tomato soft rots. Because Salmonella KdgR is not well characterized, initially we carried out two high-throughput studies to delineate potential members of the KdgR regulon and to identify differences in metabolic capabilities of the mutant and the wild type that allow it to persist better in soft rots.
FIG 1.
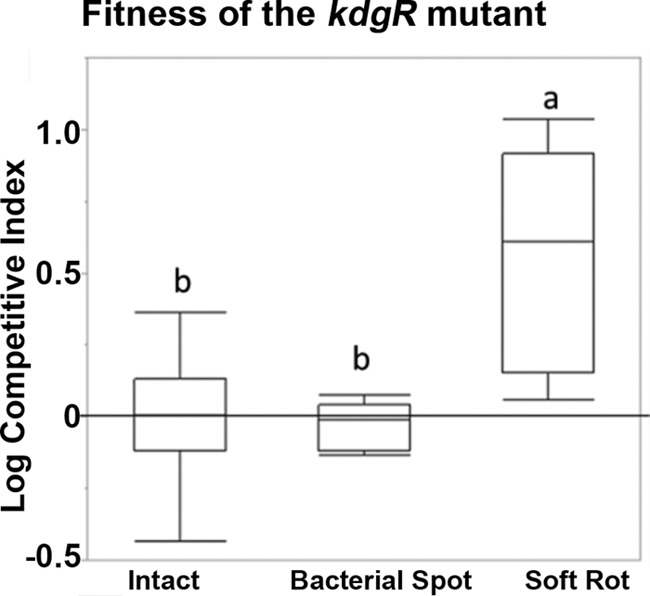
Fitness of the kdgR mutant in soft rots. S. enterica serovar Typhimurium 14028 and the isogenic kdgR mutant were coinoculated at a 1:1 ratio into intact green tomatoes or into tomatoes with P. carotovorum SR38 or Xanthomonas species. The ratio of the wild-type Salmonella to the mutant was assessed by plating and patching onto selective media after a 3-day incubation. Box plots indicate 25 to 75% quantiles, and lines within box plots are medians. Whiskers indicate the first or third quantile plus 1.5 multiplied by the inner quantile range. The zero line indicates no difference in competitive fitness. Competitive indices were compared using Student's t test, and different letters indicate statistically significant differences (P < 0.05).
Bioinformatic predictions identify novel members of the KdgR regulon.
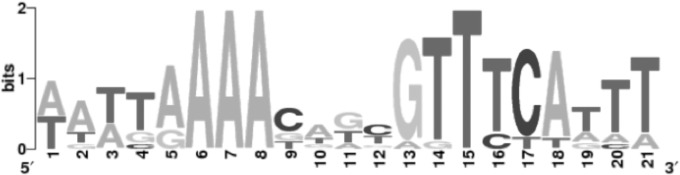
A whole-genome scan was performed on 28 complete Salmonella genomes (see Table S2 in the supplemental material) using a PSSM derived from the KdgR binding sequences experimentally validated in other gammaproteobacteria (18, 30). A PSSM was generated from the targets identified in the first scan based on 211 sites found in the upstream regions of the Salmonella genes that are orthologous to the previously validated KdgR regulon members (Fig. 2). This Salmonella KdgR PSSM shared some features with those of Vibrio spp. and E. chrysanthemi (18); however, it was distinct from both.
FIG 2.
Sequence logo for KdgR binding sites in Salmonella enterica. The position-specific scoring matrix (PSSM) was derived from the KdgR binding sequences experimentally validated in other gammaproteobacteria. A PSSM was generated from the targets identified in the first scan based on 211 sites found in the upstream regions of the Salmonella genes that are orthologous to the previously validated KdgR regulon members and 40 experimentally validated targets of KdgR. The PSSM kdgR logo was created using the Weblogo interface.
The Salmonella enterica serovar Typhimurium LT2 genome then was rescanned with this new PSSM, recovering a total of 38 putative KdgR binding sites (see Table S3 in the supplemental material). This two-step methodology was chosen to reduce bias generated by utilizing the original interspecies KdgR PSSM (32). The list of putative KdgR targets (see Table S3) included 8 out of 12 putative KdgR targets previously suggested (18), as well as over 30 additional targets, including those with predicted functions in iron acquisition, uptake and/or utilization of arabinose, ketoglutarate, rhamnose, fructose, ribose, myoinositol, and lyxose, aspartate and pyruvate metabolism, as well as several regulators with unknown functions (see Table S3). This significantly expands the putative Salmonella KdgR regulon beyond controlling functions involved in the uptake and utilization of dimers and monomers resulting from pectin degradation (18). Some of these predicted functions of the KdgR regulon members were tested in subsequent experiments to determine to what extent they contribute to the fitness of Salmonella in soft rots.
Biolog profiling defines metabolic contributions of KdgR.
In parallel with the comparative genomics analyses to identify targets of KdgR, we carried out Biolog profiling of the S. enterica serovar Typhimurium wild type, its isogenic kdgR mutant, and the strains of Pectobacterium and Xanthomonas that were used in the competitive fitness experiments presented in Fig. 1. The goal of this experiment was 3-fold: (i) to define metabolic consequences of the disruption of kdgR, (ii) to determine the level of concordance between predicted targets of KdgR and metabolic consequences of the kdgR mutation, and (iii) to characterize metabolic differences between the wild-type Salmonella strain, its kdgR mutant, and the two phytopathogens used in this study.
Based on the results of the Biolog test, there were over 40 substrates that the kdgR mutant was able to utilize better than the wild type (Fig. 3; also see Fig. S2 in the supplemental material). Compared to the wild type, the kdgR mutant was better able to utilize l-arabinose, d-ribose, l- and d-aspartic acids, α-ketoglutarate, myoinositol, l-rhamnose, l-lyxose, and pyruvate, which is consistent with the bioinformatic identification of the KdgR binding sites upstream of araH (arabinose permease), STM1933 (ribose 5-phosphate isomerase), STM4510 (aspartate racemase), kdgK (ketodeoxygluconokinase), yifZ (ketodeoxyglutarate permease), STM4435 (2-keto-myoinositol isomerase), STM1911 (rhamnogalacturonyl hydrolase), yiaK (2,3-diketo-l-gulonate reductase), and pykF (pyruvate kinase). Biolog tests were followed up with retesting the ability of the wild type and the kdgR mutant to utilize carbon sources such as rhamnose, fructose, and pyruvate. The results of the retesting (see Table S4) are consistent with the high-throughput Biolog test and indicated an increased ability of the kdgR mutant to utilize these carbon sources.
FIG 3.
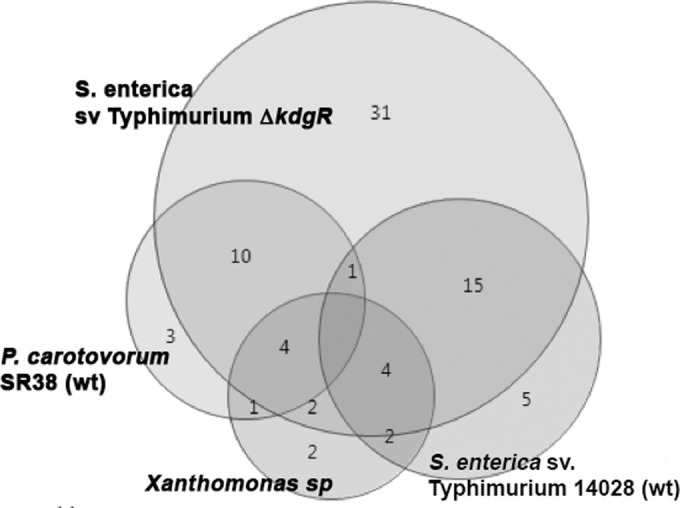
Overlap in utilization of carbon sources by S. enterica serovar Typhimurium, S. enterica serovar Typhimurium kdgR, P. carotovorum SR38, and Xanthomonas species. The ability of the bacteria to respire on substrates in Biolog plates PM1 and PM2 was documented (see Fig. S2 in the supplemental material).
In general, the kdgR mutant was able to respire better than the isogenic wild type on more than 40 substrates. Of them, 31 substrates were more efficiently utilized by the kdgR mutant than by the parental wild type, P. carotovorum or Xanthomonas species (Fig. 3). Of the substrates that were differentially utilized by S. enterica serovar Typhimurium and its kdgR mutant, the mutant also was more proficient in the utilization of nutrients than Pectobacterium (with the exception of N-acetylglucosamine, asparagine, serine, rhamnose, arabinose, ribose, fructose, and glucose). Compared with the kdgR mutant, Xanthomonas species appear to be able to better utilize only N-acetylglucosamine. Even though the kdgR mutant was better than the wild type at the utilization of the common plant sugars (rhamnose, arabinose, glucose, and fructose), P. carotovorum was significantly more efficient at respiring on these substrates (Fig. 3; also see Fig. S2 in the supplemental material). Nevertheless, to more robustly test this hypothesis, fitness of the mutants in the KdgR regulon members, which represent a diversity of KdgR-controlled functions, was tested in subsequent experiments.
Contribution of members of the KdgR regulon to fitness in soft rots.
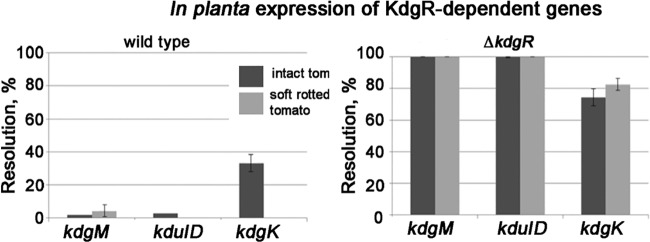
To test which of the members of the KdgR regulon contribute to fitness within soft rots, their regulation was tested using RT-qPCR and RIVET as well as fitness tests of the corresponding mutants. Results of the RT-qPCR experiments and/or RIVET reporter assays indicate that kdgK, kduI, araH, and fruF are subject to kdgR regulation in green tomatoes and in soft rots (Fig. 4 and Table 2). kdgR-dependent regulation of sodA and iroN was observed only in the soft rot (Table 2); however, the magnitude of the response was essentially the same when the accumulation of these transcripts was tested in the kdgR mutant in intact tomatoes versus that in the kdgR mutant in a soft rot (Table 2). Genes kduI (encoding 4-deoxy-l-threo-5-hexosulose-uronate ketol-isomerase) and kdgM (encoding oligogalacturonate-specific porin) appear to be most strongly controlled by KdgR, while kdgK (2-keto-3-deoxygluconate kinase), araH, and fruF likely are controlled by additional regulators (Table 2 and Fig. 4).
FIG 4.
In planta expression of KdgR-regulated genes. RIVET reporters in kdgM, kduID, and kdgK were constructed in the wild-type and ΔkdgR backgrounds. The resolution of the reporters was tested in soft rots and in intact green tomatoes (tom) following a 3-day incubation. Soft rots were initiated by coinoculating P. carotovorum SR38 with S. enterica RIVET reporters.
TABLE 2.
RT-qPCR analysis of KdgR-regulated genes
| Gene | Expression level |
|||
|---|---|---|---|---|
| kdgR mutant vs wild type in green tomatoes | Wild type in soft rot vs wild type in tomatoes | kdgR mutant in soft rot vs kdgR mutant in green tomatoes | kdgR mutant in soft rot vs wild type in soft rot | |
| kdgK | 26.9 ± 0.7 | 59.6 ± 3.3 | 4.3 ± 2.2 | 1.9 ± 3.9 |
| kduI | 342.5 ± 2.0 | 148.1 ± 2.1 | 2.1 ± 0.3 | 4.86 ± 3.1 |
| araH | 9.4 ± 0.8 | 19.3 ± 1.6 | 6.8 ± 2.2 | 3.3 ± 2.7 |
| fruF | 2.1 ± 0.6 | 15.8 ± 1.5 | 9.8 ± 2.1 | 1.3 ± 2.5 |
| edd | 4.2 ± 1.0 | 26.6 ± 1.7 | 5.4 ± 2.1 | 0.8 ± 2.5 |
| eda | 6.5 ± 3.0 | 8.1 ± 3.4 | 1.2 ± 2.1 | 0.9 ± 2.6 |
| sodA | 0.86 ± 1.7 | 0.97 ± 0.2 | 8.5 ± 0.6 | 7.5 ± 0.6 |
| iroN | 0.59 ± 0.9 | 1.1 ± 1.4 | 5.4 ± 0.4 | 2.9 ± 1.2 |
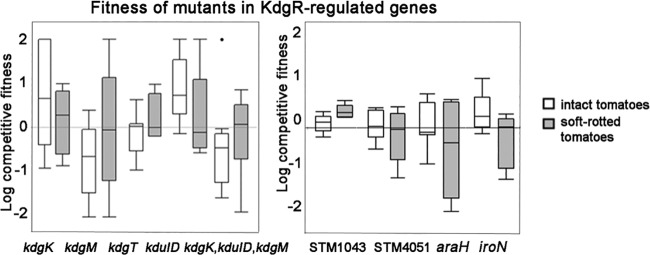
The fitness of mutants (kdgK, kdgM, kdgT [2-keto-3-deoxygluconate permease], STM1043 [putative attachment and invasion protein], STM4051 [hypothetical protein], araH, and iroN) was tested in intact tomatoes and those with soft rot (Fig. 5). Mutations in any of these genes, or a quadruple mutation in kdgK, kduID, and kdgM, did not decrease the competitive fitness of the strains (Fig. 5). These data indicate that by themselves these genes are not responsible for the kdgR phenotype in soft rot.
FIG 5.
Fitness of the mutants in kdgR-regulated genes in soft rots and intact tomatoes. Mutants were coinoculated with the isogenic wild type in a 1:1 ratio in green tomatoes (empty boxes) or in tomatoes that also were seeded with P. carotovorum SR38 (shaded boxes). Ratios of the mutant to the wild type were assessed by patching onto selective media, and log competitive indices were determined. Box plots indicate 25 to 75% quantiles, and lines within box plots are medians. Whiskers indicate the first or third quantile plus 1.5 multiplied by the inner quantile range. The fitness of mutants in intact tomatoes and in soft rots was compared using Student's t test, and none were significant.
The Entner-Doudoroff pathway is important for growth in soft rots.
Because none of the tested genes within the KdgR regulon appeared to be responsible for the observed phenotype of the kdgR mutant in soft rots, we focused on an alternative hypothesis. We hypothesized that the increased fitness of the kdgR mutant within soft rots is due to the regulatory effects of KdgR on the Entner-Doudoroff pathway. The rationale for this hypothesis is based on the observation that the kdgR mutant was better able to utilize carbon sources (gluconic acid, glucuronic acid, and glucose) that feed into the Entner-Doudoroff pathway (see Fig. S2 in the supplemental material) and based on the report that E. coli KdgR contributes to the regulation of eda (Entner-Doudoroff aldolase), which is central to the metabolism of sugar acids (24). Eda is involved in the conversion of the phosphorylated KDG molecule (KDPG) into pyruvate and glyceraldehyde-3-phosphate, which feeds into the tricarboxylic acid (TCA) cycle. It has been reported that eda is important for growth of Salmonella on d-gluconate and d-glucuronate (33). The latter is a monomer which results from the breakdown of pectic polymers (34). Furthermore, Goudeau et al. reported that genes involved in the uptake of gluconate and idonate (metabolized via the Entner-Doudoroff pathway) were upregulated in Salmonella grown on lettuce leaf macerate caused by D. dadantii (12, 33–35).
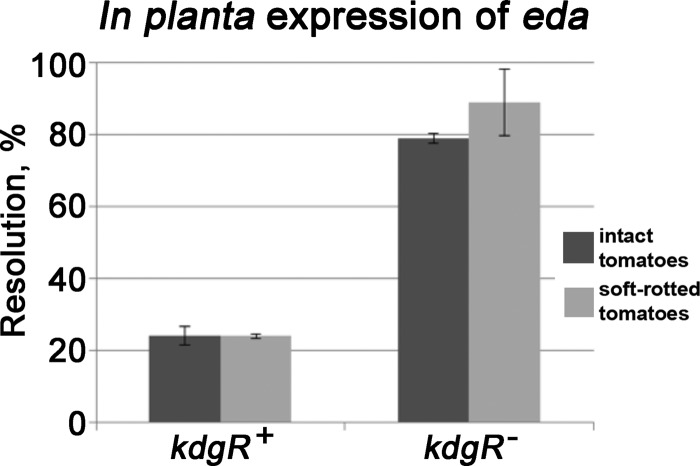
Consistent with this hypothesis, RT-qPCR and RIVET experiments demonstrated that the expression of eda and edd (6-phosphogluconate dehydratase) was significantly derepressed when kdgR was deleted (Table 1 and Fig. 6). The magnitude of the derepression was higher in the tomatoes with soft rot than in intact tomatoes (Table 2 and Fig. 6). In competition assays, the fitness of the eda mutant in the intact tomato did not differ significantly from that of the wild-type parental strain. However, in soft rot the eda mutant is almost completely uncompetitive against the wild type, and phenotype of the edd mutant is similar although not as severe (Fig. 7). To ensure that the eda mutation is not generally deleterious, the growth of the mutant and the wild type in LB broth shake cultures was compared. Both strains had the same growth rate and reached the same final population densities (data not shown). Collectively, these observations indicate that the Entner-Doudoroff pathway is important for the fitness of Salmonella within soft rots. The increased fitness of the kdgR mutant is consistent with the reduced fitness of the eda mutant in this model.
FIG 6.
In planta expression of eda. Expression of eda::tnpR-lacZY reporters in S. enterica serovar Typhimurium 14028 was determined in wild-type and kdgR backgrounds. Reporters were inoculated into tomatoes with or without Pectobacterium carotovorum SR38. Samples were plated following 3 days of incubation and scored for the loss of the tetracycline marker.
FIG 7.
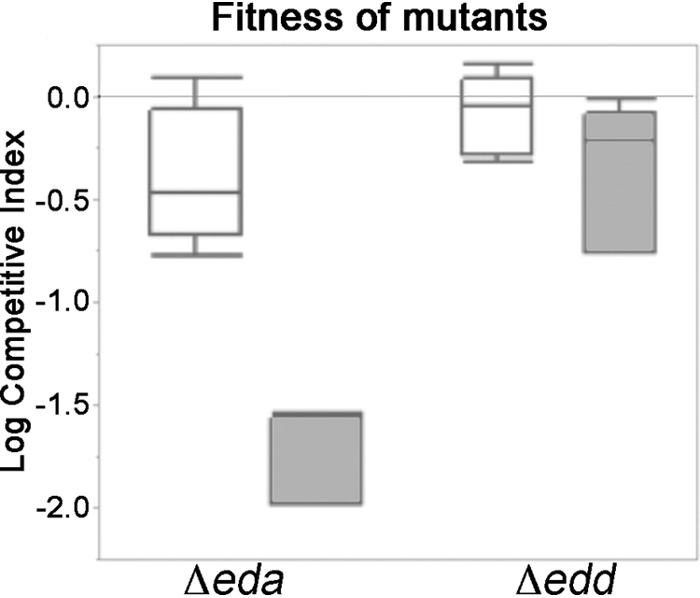
Fitness of the eda and edd mutants in tomatoes. The mutants were coinoculated with the isogenic wild type in a 1:1 ratio in green tomatoes or into tomatoes that also were seeded with P. carotovorum SR38. Ratios of the mutants to the wild type were assessed by patching onto selective media, and log competitive indices were determined. Box plots indicate 25 to 75% quantiles, and lines within box plots are medians. Whiskers indicate the first or third quantile plus 1.5 multiplied by the inner quantile range.
Conclusions.
Interactions of pathogens with native host-associated microbiota are complex, and their mechanisms only now are beginning to be understood (7, 11, 12, 36–39). Salmonella appears to be able to repurpose the strategies it uses to obtain nutrients in the intestine in order to gain 1,2-propanediol and ethanolamine from soft rots (12). Nevertheless, mechanisms by which these enteric pathogens take advantage of the presence of phytopathogens only now are beginning to be understood (7, 12, 38).
Several models could be tested to define mechanisms behind these interactions. Six basic motifs (from commensalism to amensalism) generally are recognized in microbe-microbe interactions (14). Under the conditions that were optimal for soft-rot development, the presence of Salmonella reduced growth rates and final population densities reached by P. carotovorum in planta while S. enterica benefited from this relationship (11, 13), suggesting that these interactions are either predatory or amensalistic (14). At least in part, this could be due to the ability of S. enterica to manipulate the pH of the soft rot and inhibit growth of P. carotovorum in microaerophilic, but not aerobic, conditions (13).
None of the KdgR-regulated genes directly involved in the uptake or metabolism of 2-keto-3-deoxygluconate (KDG) or pectin oligomers seem to play a role in this interaction. However, the decreased fitness of eda in soft rots points to a role of this Salmonella gene during interactions with P. carotovorum. The mechanism by which Salmonella KdgR-regulated genes are derepressed within the soft rot remains unclear. In soft-rot bacteria, binding of KdgR to KDG releases the protein from its operator sites (20); however, in our experiments the addition of KDG to lacZ reporter fusions in kdgR-regulated genes did not relieve the repression (data not shown). Metabolic interactions clearly play a role in the interactions of enterics with the soft-rot phytopathogens. The full complement and the exact mechanisms governing these interactions have yet to be fully understood, although it seems likely that an alternative glycolytic pathway, as well as pathways involved in 1,2-propanediol and ethanolamine utilization, are critical to the persistence of enterics within soft rots.
Supplementary Material
ACKNOWLEDGMENT
We are grateful to Jason T. Noel for the construction of JTN215.
Funding Statement
This research was also supported by a Florida Department of Agriculture and Consumer Services block grant.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.03355-15.
REFERENCES
- 1.Berg G, Grube M, Schloter M, Smalla K. 2014. Unraveling the plant microbiome: looking back and future perspectives. Front Microbiol 5:148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bulgarelli D, Schlaeppi K, Spaepen S, Ver Loren van Themaat E, Schulze-Lefert P. 2013. Structure and functions of the bacterial microbiota of plants. Annu Rev Plant Biol 64:807–838. doi: 10.1146/annurev-arplant-050312-120106. [DOI] [PubMed] [Google Scholar]
- 3.Brandl MT, Cox CE, Teplitski M. 2013. Salmonella interactions with plants and their associated microbiota. Phytopathology 103:316–325. doi: 10.1094/PHYTO-11-12-0295-RVW. [DOI] [PubMed] [Google Scholar]
- 4.Martinez-Vaz BM, Fink RC, Diez-Gonzalez F, Sadowsky MJ. 2014. Enteric pathogen-plant interactions: molecular connections leading to colonization and growth and implications for food safety. Microb Environ 29:123–135. doi: 10.1264/jsme2.ME13139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brandl MT. 2006. Fitness of human enteric pathogens on plants and implications for food safety. Annu Rev Phytopathol 44:367–392. doi: 10.1146/annurev.phyto.44.070505.143359. [DOI] [PubMed] [Google Scholar]
- 6.Carter MQ, Xue K, Brandl MT, Liu F, Wu L, Louie JW, Mandrell RE, Zhou J. 2012. Functional metagenomics of Escherichia coli O157:H7 interactions with spinach indigenous microorganisms during biofilm formation. PLoS One 7:e44186. doi: 10.1371/journal.pone.0044186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aruscavage D, Phelan PL, Lee K, LeJeune JT. 2010. Impact of changes in sugar exudate created by biological damage to tomato plants on the persistence of Escherichia coli O157:H7. J Food Sci 75:M187–M192. doi: 10.1111/j.1750-3841.2010.01593.x. [DOI] [PubMed] [Google Scholar]
- 8.Teplitski M, Warriner K, Bartz J, Schneider KR. 2011. Untangling metabolic and communication networks: interactions of enterics with phytobacteria and their implications in produce safety. Trends Microbiol 19:121–127. doi: 10.1016/j.tim.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 9.Wells JM, Butterfield JE. 1997. Salmonella contamination associated with bacterial soft rot of fresh fruits and vegetables in the marketplace. Plant Disease 81:867–872. doi: 10.1094/PDIS.1997.81.8.867. [DOI] [PubMed] [Google Scholar]
- 10.Brandl MT. 2008. Plant lesions promote the rapid multiplication of Escherichia coli O157:H7 on postharvest lettuce. Appl Environ Microbiol 74:5285–5289. doi: 10.1128/AEM.01073-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Noel JT, Joy J, Smith JN, Fatica M, Schneider KR, Ahmer BM, Teplitski M. 2010. Salmonella SdiA recognizes N-acyl homoserine lactone signals from Pectobacterium carotovorum in vitro, but not in a bacterial soft rot. Mol Plant Microbe Interact 23:273–282. doi: 10.1094/MPMI-23-3-0273. [DOI] [PubMed] [Google Scholar]
- 12.Goudeau DM, Parker CT, Zhou Y, Sela S, Kroupitski Y, Brandl MT. 2013. The Salmonella transcriptome in lettuce and cilantro soft rot reveals a niche overlap with the animal host intestine. Appl Environ Microbiol 79:250–262. doi: 10.1128/AEM.02290-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kwan G, Charkowski AO, Barak JD. 2013. Salmonella enterica suppresses Pectobacterium carotovorum subsp. carotovorum population and soft rot progression by acidifying the microaerophilic environment. mBio 4:e00557-12. doi: 10.1128/mBio.00557-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grosskopf T, Soyer OS. 2014. Synthetic microbial communities. Curr Opin Microbiol 18:72–77. doi: 10.1016/j.mib.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brandl MT, Miller WG, Bates AH, Mandrell RE. 2005. Production of autoinducer 2 in Salmonella enterica serovar Thompson contributes to its fitness in chickens but not on cilantro leaf surfaces. Appl Environ Microbiol 71:2653–2662. doi: 10.1128/AEM.71.5.2653-2662.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cox CE, McClelland M, Teplitski M. 2013. Consequences of disrupting Salmonella AI-2 signaling on interactions within soft rots. Phytopathology 103:352–361. [DOI] [PubMed] [Google Scholar]
- 17.Teplitski M, Barak JD, Schneider KR. 2009. Human enteric pathogens in produce: un-answered ecological questions with direct implications for food safety. Curr Opin Biotechnol 20:166–171. doi: 10.1016/j.copbio.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 18.Rodionov DA, Gelfand MS, Hugouvieux-Cotte-Pattat N. 2004. Comparative genomics of the KdgR regulon in Erwinia chrysanthemi 3937 and other gamma-proteobacteria. Microbiology 150:3571–3590. doi: 10.1099/mic.0.27041-0. [DOI] [PubMed] [Google Scholar]
- 19.Suvorova IA, Tutukina MN, Ravcheev DA, Rodionov DA, Ozoline ON, Gelfand MS. 2011. Comparative genomic analysis of the hexuronate metabolism genes and their regulation in gammaproteobacteria. J Bacteriol 193:3956–3963. doi: 10.1128/JB.00277-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nasser W, Reverchon S, Robert-Baudouy J. 1992. Purification and functional characterization of the KdgR protein, a major repressor of pectinolysis genes of Erwinia chrysanthemi. Mol Microbiol 6:257–265. doi: 10.1111/j.1365-2958.1992.tb02007.x. [DOI] [PubMed] [Google Scholar]
- 21.Nasser W, Reverchon S, Condemine G, Robert-Baudouy J. 1994. Specific interactions of Erwinia chrysanthemi KdgR repressor with different operators of genes involved in pectinolysis. J Mol Biol 236:427–440. doi: 10.1006/jmbi.1994.1155. [DOI] [PubMed] [Google Scholar]
- 22.Pouyssegur J, Stoeber F. 1974. Genetic control of the 2-keto-3-deoxy-d-gluconate metabolism in Escherichia coli K-12: kdg regulon. J Bacteriol 117:641–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Molina-Henares AJ, Krell T, Eugenia Guazzaroni M, Segura A, Ramos JL. 2006. Members of the IclR family of bacterial transcriptional regulators function as activators and/or repressors. FEMS Microbiol Rev 30:157–186. doi: 10.1111/j.1574-6976.2005.00008.x. [DOI] [PubMed] [Google Scholar]
- 24.Murray EL, Conway T. 2005. Multiple regulators control expression of the Entner-Doudoroff aldolase (Eda) of Escherichia coli. J Bacteriol 187:991–1000. doi: 10.1128/JB.187.3.991-1000.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peekhaus N, Conway T. 1998. What's for dinner? Entner-Doudoroff metabolism in Escherichia coli. J Bacteriol 180:3495–3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Datsenko KA, Wanner BL. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci U S A 97:6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Merighi M, Ellermeier CD, Slauch JM, Gunn JS. 2005. Resolvase-in vivo expression technology analysis of the Salmonella enterica serovar Typhimurium PhoP and PmrA regulons in BALB/c mice. J Bacteriol 187:7407–7416. doi: 10.1128/JB.187.21.7407-7416.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(T)(−delta delta C) method. Methods 25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 29.Thomas-Chollier M, Defrance M, Medina-Rivera A, Sand O, Herrmann C, Thieffry D, van Helden J. 2011. RSAT 2011: regulatory sequence analysis tools. Nucleic Acids Res 39:W86–W91. doi: 10.1093/nar/gkr377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Turatsinze JV, Thomas-Chollier M, Defrance M, van Helden J. 2008. Using RSAT to scan genome sequences for transcription factor binding sites and cis-regulatory modules. Nat Protoc 3:1578–1588. doi: 10.1038/nprot.2008.97. [DOI] [PubMed] [Google Scholar]
- 31.Crooks GE, Hon G, Chandonia JM, Brenner SE. 2004. WebLogo: a sequence logo generator. Genome Res 14:1188–1190. doi: 10.1101/gr.849004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Frandi A, Mengoni A, Brilli M. 2010. Comparative genomics of VirR regulons in Clostridium perfringens strains. BMC Microbiol 10:65. doi: 10.1186/1471-2180-10-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miller KA, Phillips RS, Mrazek J, Hoover TR. 2013. Salmonella utilizes d-glucosaminate via a mannose family phosphotransferase system permease and associated enzymes. J Bacteriol 195:4057–4066. doi: 10.1128/JB.00290-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hugouvieux-Cotte-Pattat N, Condemine G, Nasser W, Reverchon S. 1996. Regulation of pectinolysis in Erwinia chrysanthemi. Annu Rev Microbiol 50:213–257. doi: 10.1146/annurev.micro.50.1.213. [DOI] [PubMed] [Google Scholar]
- 35.Patra T, Koley H, Ramamurthy T, Ghose AC, Nandy RK. 2012. The Entner-Doudoroff pathway is obligatory for gluconate utilization and contributes to the pathogenicity of Vibrio cholerae. J Bacteriol 194:3377–3385. doi: 10.1128/JB.06379-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brandl MT, Mandrell RE. 2002. Fitness of Salmonella enterica serovar Thompson in the cilantro phyllosphere. Appl Environ Microbiol 68:3614–3621. doi: 10.1128/AEM.68.7.3614-3621.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barak JD, Liang AS. 2008. Role of soil, crop debris, and a plant pathogen in Salmonella enterica contamination of tomato plants. PLoS One 3:e1657. doi: 10.1371/journal.pone.0001657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aruscavage D, Miller SA, Ivey ML, Lee K, LeJeune JT. 2008. Survival and dissemination of Escherichia coli O157:H7 on physically and biologically damaged lettuce plants. J Food Prot 71:2384–2388. [DOI] [PubMed] [Google Scholar]
- 39.Brandl MT, Carter MQ, Parker CT, Chapman MR, Huynh S, Zhou Y. 2011. Salmonella biofilm formation on Aspergillus niger involves cellulose–chitin interactions. PLoS One 6:e25553. doi: 10.1371/journal.pone.0025553. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.