Abstract
We developed a counterselectable deletion system for Thermus thermophilus HB27 based on cytosine deaminase (encoded by codA) from Thermaerobacter marianensis DSM 12885 and the sensitivity of T. thermophilus HB27 to the antimetabolite 5-fluorocytosine (5-FC). The deletion vector comprises the pUC18 origin of replication, a thermostable kanamycin resistance marker functional in T. thermophilus HB27, and codA under the control of a constitutive putative trehalose promoter from T. thermophilus HB27. The functionality of the system was demonstrated by deletion of the bglT gene, encoding a β-glycosidase, and three carotenoid biosynthesis genes, CYP175A1, crtY, and crtI, from the genome of T. thermophilus HB27.
INTRODUCTION
Thermus thermophilus HB27 is a Gram-negative, yellow-pigmented, aerobic bacterium growing at temperatures up to 85°C (1). It belongs to the phylum Deinococcus-Thermus and has a GC content of 69%. Due to its specific amenable characteristics, like high growth rates, cell yields, constitutive natural competence, and, not least, the availability of its genome sequence comprising the 1.90-Mb chromosome and a 0.23-Mb megaplasmid (2), T. thermophilus emerged as a laboratory model for studying the molecular basis of thermophilia (3, 4). The small genome of T. thermophilus contains few functional paralogues, and consequently, studying knockout mutants is one appropriate approach to elucidate specific gene functions in the organism (3).
A number of genetic tools have been developed for genetic manipulation of T. thermophilus HB27, including various T. thermophilus-Escherichia coli shuttle vectors and different deletion or integration systems (5–9). For plasmid maintenance or identification of desired mutants, only a few engineered thermostable antibiotic resistance markers, namely, kanamycin nucleotidyltransferase (10, 11), bleomycin binding protein (12), and hygromycin B phosphotransferase (13), are available. An alternative positive-selection strategy is complementation of gene defects of auxotrophic host strains by supplying gene functions, such as tryptophan synthetase (trpB) (14) or malate dehydrogenase (mdh) (15), in trans.
The limited number of selection markers and the demand for mutants whose construction does not irreversibly consume these rare markers pushed the development of alternative, counterselectable systems for markerless genome manipulation in T. thermophilus, including negative-selection systems based on pyrE (6, 16), the rpsL1 allele (17), bgl-lacZ (18), or the pheS allele (5). These systems allow clearance of any positive-selection marker, thus enabling its reuse in the next step of sequential strain construction or maintenance of expression plasmids. The general principle of a counterselection strategy follows two steps: The first step includes the targeted chromosomal integration of a suicide plasmid carrying the desired allele to be exchanged, an antibiotic resistance marker, and a counterselectable marker by homologous recombination. Clones with integrated plasmids are identified by their antibiotic resistance. The second step employs the counterselectable trait, allowing excision of the plasmid, together with the selection markers, via homologous recombination, thereby leaving the allele to be exchanged in the chromosome.
A reliable counterselection strategy is inhibition of thymidylate synthetase by the uracil analog 5-fluorouracil (5-FU). The most commonly used marker of this kind is uracil phosphoribosyltransferase (upp) (UPRTase, Upp) (EC 2.4.2.9), which converts uracil to UMP (see Fig. S1 in the supplemental material). Transformation of the uracil analog 5-FU to 5-fluorouridine monophosphate (5-FUMP) by Upp and further conversion of 5-FUMP results in irreversible inhibition of thymidylate synthetase (19, 20). An alternative counterselectable marker is cytosine deaminase (codA) (EC 3.5.4.1), which has been applied for various bacteria (21–23). CodA (EC 3.5.4.1) catalyzes the deamination of cytosine and its analog, 5-fluorocytosine (5-FC), to uracil and 5-FU, respectively, which are subsequently converted to UMP and 5-FUMP by Upp (24). A deletion system developed for T. thermophilus employs pyrE, encoding orotic acid phosphoribosyltransferase (EC 2.4.2.10), as a negative-selection marker (6). In UMP de novo synthesis, PyrE catalyzes the synthesis of orotidine monophosphate (OMP) from orotic acid, which is then converted to UMP by PyrF. Conversion of 5-fluoroorotic acid (5-FO) finally also results in synthesis of 5-FUMP (see Fig. S1 in the supplemental material).
In T. thermophilus HB27, a pyrE gene (TT_C1380) and an upp gene (TT_C0946), but no codA gene or orthologs, have been identified. In contrast to pyrE and upp, application of heterologous codA as a counterselectable marker for T. thermophilus HB27 has the great advantage that the wild type can be used directly because prior construction of a codA deletion strain is not required. We constructed a new markerless deletion system for T. thermophilus HB27 using codA (Tmar_1477) from Thermaerobacter marianensis DSM 12885. T. marianensis DSM 12885 has a GC content of 72.5%, an optimal growth temperature from 74°C to 76°C, and a pH optimum of 7.0 to 7.5 (25), properties that are comparable to the optimal growth parameters of T. thermophilus HB27. The codA deletion system was used to delete the bglT gene (TT_P0042), encoding a β-glycosidase, and three carotenoid biosynthesis genes, CYP175A1, crtY, and crtI (TT_P0059, TT_P0060, and TT_P0066), encoding a β-carotene hydroxylase of the P450 superfamily (26, 27), a lycopene β-cyclase, and a phytoene desaturase (2, 28) (see Fig. S2 in the supplemental material), on the megaplasmid pTT27 from the genome of T. thermophilus HB27.
MATERIALS AND METHODS
Plasmids, bacterial strains, and growth conditions.
The bacterial strains and plasmids used in this study are listed in Table S1 in the supplemental material. E. coli DH5α was grown at 37°C with shaking in LB medium (10 g liter−1 tryptone, 5 g liter−1 yeast extract, 5 g liter−1 NaCl) supplemented with 50 μg ml−1 kanamycin (Km), as appropriate. T. thermophilus strains were cultured in Thermus broth (TB) medium (8 g liter−1 tryptone, 4 g liter−1 yeast extract, 3 g liter−1 NaCl, pH 7.5) (29) at 70°C with shaking. For selection, the medium was supplemented with 25 μg ml−1 kanamycin, as appropriate. T. thermophilus strains were also grown in minimal medium 162 (M162) (30) with a minor modification [100 mg liter−1 nitrilotriacetic acid, 0.4 mg liter−1 Bacto nutrient broth, 0.005 mM iron(III) citrate, 40 mg liter−1 CaSO4 · 2H2O, 0.2 g liter−1 MgCl2 · 6H2O, 0.1 g liter−1 (NH4)2SO4, 15 mM Na2HPO4, 5 mM KH2PO4, pH 7.5], supplemented with 0.1 mg liter−1 biotin, 1 mg liter−1 thiamine, and 2.0 g liter−1 d-glucose. Negative selection was carried out on M162 agar plates containing 1.5% (wt/vol) agar with 15 μg ml−1 5-FC (Sigma-Aldrich, Steinheim, Germany).
Transformation procedures.
The electroporation method (31) was used to transform competent E. coli DH5α (32) with plasmids. The transformation method used for naturally competent T. thermophilus HB27 was principally derived from two methods described previously (4, 33, 34). The cells were first grown in TB medium at 70°C overnight. The culture was diluted 1:100 with fresh TB medium and incubated at 70°C until the optical density at 550 nm (OD550) reached a value of 0.8. Next, 500 μl of cell culture was mixed with 50 to 100 ng of plasmid DNA and incubated further at 70°C. After 2 h of incubation, 150 μl of the cultures was plated on TB agar medium supplemented with 25 μg ml−1 kanamycin. The plates were incubated at 65°C overnight.
Plasmid construction.
Standard protocols were used for recombinant DNA techniques (31). Plasmid DNA was prepared with the innuPrep Plasmid minikit (Analytik Jena, Jena, Germany). To isolate genomic DNA of T. thermophilus strains, 4 × 109 cells were harvested from an overnight culture by centrifugation (5 min; 4,500 × g) and resuspended in 180 μl lysis buffer (25 mM Tris-HCl, pH 8.0, 25 mM EDTA, 10% [wt/vol] sucrose, 0.5 mM glycine, 20 mg ml−1 lysozyme). Genomic DNA was extracted with the DNeasy blood and tissue kit (Qiagen, Hilden, Germany) using the protocol “pretreatment for Gram-positive bacteria.” Oligonucleotides (see Table S2 in the supplemental material) were synthesized by Eurofins MWG (Ebersberg, Germany). DNA sequencing was performed by GATC Biotech (Constance, Germany).
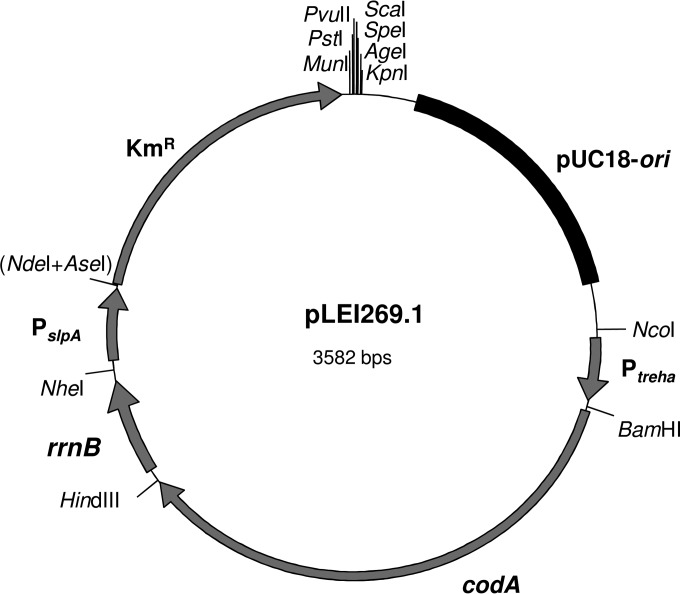
The basic integrative vector pLEI269.1 (Fig. 1) is based on pUC18 (35) and the Thermus vector pMY1 (33, 36). It was constructed by ligation of several PCR fragments carrying a different restriction site on each end. The pUC18 origin of replication was amplified with primers s10346 and s9162b. The PCR fragment contains an NcoI site and a multiple cloning site (MCS) comprising restriction sites for PstI, PvuII, ScaI, SpeI, AgeI, and KpnI. The promoter region (Ptreha) (see Table S3 in the supplemental material) of the putative trehalose operon (TT_C0611 to TT_C0615) (2) was amplified using primers s9549 (NcoI) and s9551 (BamHI) and genomic DNA of T. thermophilus HB27 as the template. The codA gene (Tmar_1477) of T. marianensis was synthesized by GenScript (Piscataway, NJ, USA). Eleven undesirable restriction sites within the gene were removed in consideration of codon usage (codA) (see Table S4 in the supplemental material), and BamHI and HindIII sites were added on both ends for cloning. In pLEI269.1, codA is constitutively expressed under the control of Ptreha. In order to prevent transcriptional readthrough from Ptreha, the transcriptional terminator rrnB of E. coli was amplified with primers s9088 and s9436 from pJOE5751.1 (37) as the template and positioned downstream of codA using HindIII and NheI restriction sites. For plasmid maintenance in both E. coli and T. thermophilus HB27, pLEI269.1 carries a thermostable kanamycin resistance marker (38) under the control of the S-layer protein A promoter (PslpA) (39). PslpA, together with the thermostable kanamycin resistance (Kmr) gene, was amplified with s9289 and s9290 (NheI and PstI) from pMY1. The NdeI restriction site between PslpA and the Kmr gene was deleted by PCR using primers s9289 and s9400 (NheI and AseI).
FIG 1.
The basic integrative vector pLEI269.1 for targeted gene deletion in T. thermophilus HB27. The vector consists of the pUC18 origin of replication, the promoter region of a putative trehalose operon (Ptreha) responsible for constitutive expression of codA (encoding cytosine deaminase from T. marianensis), a transcription terminator (rrnB), a Kmr cassette under the control of the S-layer protein A promoter (PslpA), and a multiple cloning site for integration of flanking regions of the gene to be deleted. All the fragments were amplified by PCR and ligated with each other by their terminal restriction sites via several cloning steps (see Materials and Methods).
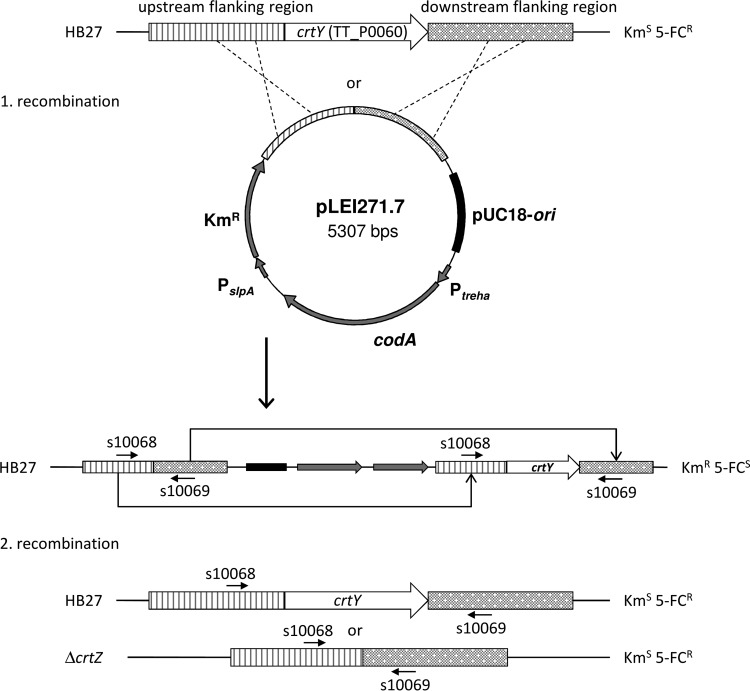
For the construction of the different plasmids carrying deletion cassettes, flanking sequences (each about 800 bp) of the genes to be deleted were amplified by PCR and integrated into pLEI269.1 using its MCS. The up- and downstream regions of the carotenoid biosynthesis genes CYP175A1, crtY, and crtI were amplified with the primers listed in Table S2 in the supplemental material and genomic DNA of T. thermophilus HB27 as the template. The PCR fragments were fused by their PstI (CYP175A1 and crtY) or SalI (crtI) restriction sites and inserted into pLEI269.1 via MunI and SpeI restriction sites, thereby creating pLEI270.5, pLEI271.7 (Fig. 2), and pLEI273.2, respectively. The flanking regions of bglT were amplified with primers s9978, s10236, s9980, and s9981, fused by their PstI sites, and integrated into the basic vector by MunI and KpnI restriction sites to create pLEI257.1. All the plasmids were verified by DNA sequencing.
FIG 2.
Principle of the counterselection system for targeted gene deletion in T. thermophilus HB27 demonstrated with pLEI271.7. The binding sites of the primers s10068 and s10069 used for analytical PCR of the mutant strains are indicated. The integrative vector pLEI271.7 contains the flanking regions of crtY (encoding lycopene β-cyclase), a kanamycin resistance determinant, and codA (encoding cytosine deaminase) as a counterselectable trait. The plasmid integrates into the genome by homologous recombination with the upstream or downstream flanking region of crtY. The transformants are resistant to Km and sensitive to 5-FC. The cell loses the integrated plasmid by a second homologous recombination. Depending on where the recombination occurs, crtY is cut out, together with the plasmid, or the wild-type situation is restored. The ΔcrtY strain, as well as the reconstituted wild type, is resistant to 5-FC and sensitive to Km.
Deletion strain construction.
T. thermophilus HB27 was transformed with the plasmids carrying the constructed deletion cassettes as described above. Due to their inability to autonomously replicate in T. thermophilus HB27, the plasmids can be maintained only by integration into the megaplasmid pTT27 via homologous recombination (Fig. 2). Cells with integrated plasmids were selected on TB agar with kanamycin at 65°C overnight. Several clones were isolated and streaked out three times on TB agar plates supplemented with kanamycin. From the last plate, kanamycin-resistant clones were checked for 5-FC sensitivity on M162 agar plates at 65°C for 72 h. The presence of codA causes sensitivity to 5-FC. Next, a kanamycin-resistant and 5-FC-sensitive clone was incubated in TB medium without antibiotics at 70°C overnight. The overnight culture was diluted 1:104 with TB medium containing 15 μg ml−1 5-FC and incubated further at 70°C for 6 h. Meanwhile, the integrated plasmid should be excised via a second homologous recombination causing either deletion of the desired marker or reconstitution of the wild type (Fig. 2). In both cases, loss of the plasmid causes resistance to 5-FC and sensitivity to kanamycin. The culture was diluted 1:10 or 1:102 with H2O, and 100 μl was plated on M162 agar with 5-FC and incubated at 65°C for 72 h. M162 media were necessary, as TB media obviously contain pyrimidines competing with 5-FC uptake and use and allowing Thermus, despite containing the integrative deletion vector, to grow on TB plates supplemented with 5-FC. Several 5-FC-resistant clones were isolated, streaked out three times on M162 plates with 5-FC, and finally transferred onto TB plates to obtain quick growth at 65°C. Sensitivity to kanamycin and resistance to 5-FC of the clones were tested on TB agar with kanamycin and M162 agar with 5-FC. Clones carrying the desired deletions were identified by PCR (see below). The numbers of colonies used to delete the four genes and the numbers of mutants obtained are summarized in Table S5 in the supplemental material.
Identification of deletion mutants by PCR.
The deletion mutants grown on TB plates were scraped off and treated with the DNeasy blood and tissue kit as described above. The prepared genomic DNAs were applied as templates for PCRs with primers s10068/s10069, s10363/s10364, s10365/s10366, and s10369/s10370 (see Table S2 in the supplemental material) for verification of bglT, CYP175A1, crtY, and crtI deletion, respectively. The primers were designed with AT-rich sequences of the flanking regions in order to minimize nonspecific binding with the GC-rich genomic DNA of T. thermophilus HB27. Genomic DNA of the T. thermophilus HB27 wild type and the plasmid DNAs used for deletion strain construction served as controls.
β-Glycosidase assay.
The bglT gene from T. thermophilus HB27 encodes a β-glycosidase with a broad substrate specificity for the β-anomeric linkage and catalyzes the hydrolysis of β-galacto-, β-gluco-, and β-fucopyranosides (40). We used p-nitrophenyl β-galactopyranoside (pNPGal) as the substrate to measure its enzyme activity.
Cell cultures grown in TB medium at 70°C for 6 h were used for the assay. The cells were harvested by centrifugation (5 min; 4,500 × g), washed with 0.1 M potassium phosphate buffer (pH 6.5), and then resuspended in the same buffer. An ultrasonic homogenizer (Sonopuls HD2070; Bandelin, Berlin, Germany) was applied for cell disruption. The supernatant containing soluble proteins was assayed after centrifugation (15 min; 16,100 × g). The reaction mixture, containing 25 μl of the supernatant and 450 μl of 0.1 M potassium phosphate buffer (pH 6.5), was preincubated at 70°C for 5 min. Then, 25 μl pNPGal (4 mg ml−1; Sigma-Aldrich, Steinheim, Germany) was added to the mixture. After 2 to 5 min incubation at 70°C, the reaction was stopped by adding 1 ml 400 mM sodium borate buffer (pH 9.4). The release of p-nitrophenol (pNP) was measured at 405 nm. One unit of enzyme activity was defined as the amount of enzyme catalyzing the liberation of 1 μmol of pNP per minute, using an extinction coefficient (ε405; pH 10) of 18.5 × 103 M−1 cm−1 for pNP. The amount of total soluble protein was determined by the Bradford method (41). Specific activity was expressed as units per milligram of protein.
TLC of carotenoid extracts from T. thermophilus strains.
T. thermophilus HB27 wild type and mutants were cultivated overnight in TB medium at 70°C. The next day, 2.6 × 1010 cells were harvested by centrifugation (5 min; 4,500 × g), washed with 1 ml H2O, and stored at −20°C until use. For carotenoid extraction, the cells were resuspended in 1 ml potassium phosphate buffer (0.1 M; pH 6.5) with 10 mg ml−1 lysozyme and incubated at 37°C for 1 h. The suspensions were centrifuged (5 min; 16,100 × g), and the cells were washed with 1 ml H2O and extracted with 600 μl acetone. The acetone extracts were mixed with 200 μl H2O and 200 μl hexane for reextraction. The samples were mixed and centrifuged (5 min; 16,100 × g), and the upper hexane phase was isolated. Reextraction with 200-μl portions of hexane was repeated until the hexane phase remained colorless. The hexane extracts were pooled and dried in a vacuum centrifuge. The extracts were dissolved in 20 μl hexane and applied for thin-layer chromatography (TLC) (silicagel 60 F254; Merck, Darmstadt, Germany) with petroleum-diethyl ether-acetone (40:10:10) as the mobile phase. β-Carotene (Sigma-Aldrich, Steinheim, Germany) and lycopene (extracted from tomato puree) were used as authentic standards.
RESULTS
The integrative deletion vector.
The basis of the presented codA counterselection system for T. thermophilus HB27 is an integrative plasmid derived from pUC18 and the Thermus vector pMY1 (33). Only a few constitutive and even fewer regulated promoters are available for gene expression in T. thermophilus HB27. We have observed that the promoter region of the putative trehalose operon (Ptreha) shows a constitutive promoter activity together with bglT as a reporter gene in T. thermophilus HB27 (data not shown). Thus, Ptreha was applied for codA expression on the integrative deletion vector. Expression of codA from Ptreha in T. thermophilus HB27 was verified on M162 agar supplemented with 5-FC. The presence of the genomically integrated vector caused sensitivity to 5-FC when a concentration of 15 μg ml−1 was applied.
Deletion of selected genes from the T. thermophilus HB27 genome.
In order to demonstrate the functionality of the new codA counterselection system, we chose bglT as the first target for deletion. BglT is a widely used reporter protein for studying promoter activities in T. thermophilus HB27 (16, 42). The additional copy of bglT on the megaplasmid pTT27 of T. thermophilus HB27 interferes with these reporter assays. Therefore, a bglT-deficient strain is a desirable host for promoter studies using bglT as a reporter gene. We deleted bglT with pLEI257.1 carrying the homologous up- and downstream regions of bglT following the procedure described in Materials and Methods. In order to control the substeps of the deletion procedure, analytical PCRs were performed using primers s10068/s10069 and genomic DNA of T. thermophilus HB27 (wild type), T. thermophilus LW14 (with pLEI257.1 integrated into the genome after the first recombination), T. thermophilus LW19 (ΔbglT), or pLEI257.1 (deletion plasmid) as the template (Fig. 3). The primers used bind within the up- and downstream regions of bglT. The wild type should show a PCR fragment (1,723 bp) that contains bglT between these flanking sequences. The PCR fragment of the bglT deletion strain should have a size reduced by 1,308 bp, based on the size of bglT. The strain with the integrated plasmid should show both PCR fragments and an additional fragment corresponding to the whole plasmid (Fig. 2).
FIG 3.
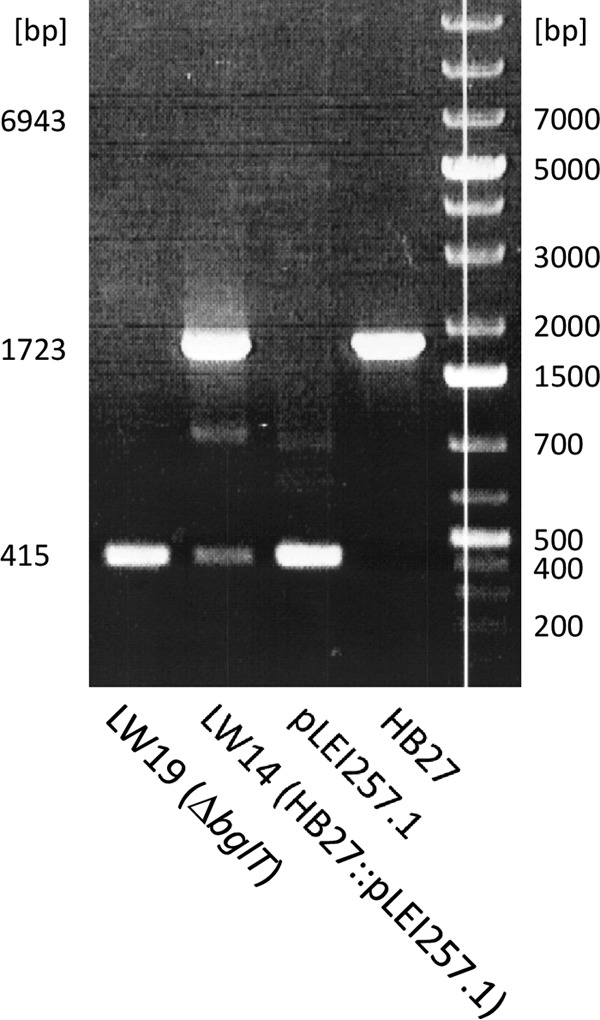
PCR analysis with primers s10068/s10069 and genomic DNA of T. thermophilus HB27 (wild type), T. thermophilus LW14 (with pLEI257.1 integrated into the genome), T. thermophilus LW19 (ΔbglT), or pLEI257.1. The fragments were separated by 0.7% agarose gel electrophoresis and stained with ethidium bromide. The 415-bp, 1,723-bp, and 6,943-bp markers on the left correspond to the expected fragment sizes.
As expected, the PCR with T. thermophilus HB27 wild-type DNA resulted in a fragment of 1,723 bp, and the PCR with pLEI257.1 yielded a fragment of 415 bp (Fig. 3). The PCR with T. thermophilus LW14 DNA showed both fragments. The large fragment (6,943 bp) corresponding to the whole integrated plasmid could not be detected under the PCR conditions used. PCR with DNA of the deletion strain T. thermophilus LW19 exhibited only the small fragment of 415 bp, suggesting the deficiency of bglT. The β-glycosidase assay with crude extracts of T. thermophilus HB27 and LW19 with pNPGal as the substrate revealed reduced β-glycosidase activity of the ΔbglT strain (3 mU mg−1 of total soluble proteins) compared to the wild type (8 mU mg−1). This result is in agreement with the data published previously (42) and further substantiates the loss of bglT.
T. thermophilus HB27 produces carotenoids (43), especially thermozeaxanthin (44, 45). The carotenoid biosynthesis genes are located on the megaplasmid pTT27. Deletion of genes of the carotenoid-biosynthetic pathway is a very colorful way to demonstrate the applicability of the codA counterselection system, because removal of a gene involved in the multistep process of carotenoid biosynthesis results in change of color of the mutant strain.
Similar to the bglT deletion, the three genes CYP175A1, crtY, and crtI participating in the carotenoid synthesis pathway were deleted with the three plasmids pLEI270.5, pLEI271.7, and pLEI273.2 carrying the appropriate deletion cassettes. The ΔCYP175A1, ΔcrtY, and ΔcrtI strains are referred to as T. thermophilus LW35, LW37, and LW40, respectively. For carotenoid analyses, the strains were incubated in TB medium at 70°C for 24 h. Cells were collected by centrifugation and suspended in potassium phosphate buffer. All of the cell suspensions had the same cell concentration (see Fig. S3 in the supplemental material). T. thermophilus HB27 showed the typical yellow color, whereas T. thermophilus LW35 (ΔCYP175A1) lacking β-carotene hydroxylase presented an orange color due to the enrichment of β-carotene in the cell membrane. The deletion of crtY, coding for lycopene β-cyclase, led to an accumulation of red lycopene (T. thermophilus LW37), which could be observed as a light-rose color of the cell suspension. The phytoene desaturase CrtI converts colorless phytoene to lycopene. Therefore, the cells of T. thermophilus LW40 (ΔcrtI) appeared white. The development of cell colors caused by the gene deletions corresponds to those of the accumulated intermediates and is summarized in Fig. S2 in the supplemental material. The carotenoids of T. thermophilus HB27, LW35, and LW37 were extracted as described in Materials and Methods and analyzed by thin-layer chromatography, together with authentic standards of β-carotene and lycopene (see Fig. S4 in the supplemental material). T. thermophilus LW37 (ΔcrtY) accumulated lycopene, as expected. β-Carotene and smaller amounts of lycopene could be detected in the extract of T. thermophilus LW35 (ΔCYP175A1). The obtained Rf values of the samples were 0 (T. thermophilus HB27), 0.97 (β-carotene, authentic standard), 0.97 (T. thermophilus LW35 ΔCYP175A1), 0.91 (lycopene, authentic standard), and 0.91 (T. thermophilus LW37 ΔcrtY).
DISCUSSION
Commonly used reporter genes of expression vectors for T. thermophilus encode thermostable α-galactosidase and β-galactosidase (42, 46, 47), β-glycosidase (bglT) (16), or phytoene synthase (crtB) (9, 48). All the named reporter genes originate from T. thermophilus HB27 or a closely related species whose native enzyme activities interfere with reporter assays. Hence, mutant strains deficient in these interfering enzymes would facilitate promoter studies employing glucosidases or galactosidases as reporter enzymes. In addition, clarifying specific gene functions of T. thermophilus is another issue of great interest at this time, as it is an indispensable part of uncovering the molecular mechanisms underlying thermophilia.
Deletion systems allowing markerless genome manipulation provide powerful tools for genetic manipulation, enabling the construction of mutant strains for biotechnology or the study of specific gene functions with knockout mutants. Compared to other reported deletion methods for T. thermophilus, the codA-based deletion system described here has some advantages. (i) As a markerless and negative deletion system, the antibiotic resistance gene used for selection is excised from the genome. This allows the generation of multiple gene deletions/mutations in the parental strain to produce a final strain unmarked by an antibiotic resistance gene. (ii) The previously reported deletion systems based on pyrE (6), bgl (18), or upp need a pyrE-, bgl-, or upp-deficient host strain. In contrast, no codA ortholog exists in T. thermophilus HB27, and the wild type can be used directly as a parental strain in the reported deletion system. An additional disadvantage of the pyrE deletion system is the uracil auxotrophic feature of the pyrE-deficient host strain. (iii) Furthermore, the codA sequence used in the integrative plasmid should not cause unexpected homologous recombination with the genome sequence. However, pheS (5), rpsL1 (17), or bgl-lacZ (18), as well as the two promoters PslpA and Ptreha that are also part of the integrative vector, originate from T. thermophlilus and could potentially recombine with the native genomic genes, leading to false-positive clones after the first recombination step. Using pheS as a counterselectable marker, a spontaneous large-scale deletion, including the carotenoid synthesis genes and the β-glycosidase gene in the megaplasmid, was observed, apparently mediated by insertion sequence (IS) elements (5). However, unexpected recombination or large-scale deletions have not been observed so far with the codA deletion system.
The ΔbglT strain T. thermophilus LW19 shows considerable β-galactosidase activity despite the lack of bglT. This is in agreement with the ΔbglT strain of T. thermophilus HB27 (T. thermophiles PPKU) constructed by Park and Kilbane (42), with a measured reduction of 50% of the β-galactosidase level compared to the wild type. Furthermore, inactivation of the identical bglT gene in the T. thermophilus strain TH125 led to 55% reduction of the pNP–β-galactoside-hydrolyzing activity (49). This background activity was probably due to the existence of two putative β-galactosidase genes (TT_P0220 and TT_P0222) (2). Despite the reduced β-galactosidase activity of T. thermophilus LW19, the deletion strain should allow the use of the β-galactosidase gene as a reporter gene. The purified BglT protein exhibits the highest catalytic efficiency for the substrate β-NP-glucopyranoside (40). Additionally, we used this substrate to measure the BglT activity and observed loss of activity in the deletion strain T. thermophilus LW19, but still with some background level, which is in agreement with the data published by Ohta et al. (16).
Until now, the genes crtY (TT_P0060) and crtI (TT_P0066) of T. thermophilus have been identified exclusively by genome sequence analysis. TLC analysis of carotenoid extracts revealed that lycopene is the main carotenoid produced by the ΔcrtY strain. This result supports the sequence annotation of TT_P0060 as crtY (encoding lycopene β-cyclase). The colorless ΔcrtI strain strongly suggests an interruption of the carotenoid synthesis pathway at a step before lycopene synthesis. In accordance with the sequence analysis, it is likely caused by a deficiency of phytoene desaturase. In this respect, crtI codes for phytoene desaturase of T. thermophilus HB27. The accumulation of β-carotene in the T. thermophilus ΔCYP175A1 (TT_P0059) strain reflects the results obtained with enzyme assays with the Thermus β-carotene hydroxylase described previously (26). Interestingly, the carotenoid extract of the ΔCYP175A1 strain revealed considerable amounts of lycopene, in addition to β-carotene, in TLC (see Fig. S4 in the supplemental material). This is most likely the result of an incomplete conversion of lycopene by CrtY.
Supplementary Material
ACKNOWLEDGMENT
The kanamycin resistance plasmid pMY1 was a gift from J. Berenguer (Universidad Autonoma de Madrid, Madrid, Spain) (33, 36).
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.03524-15.
REFERENCES
- 1.Oshima T, Imahori K. 1974. Description of Thermus thermophilus (Yoshida and Oshima) comb. nov., a nonsporulating thermophilic bacterium from a Japanese thermal spa. Int J Syst Bacteriol 24:102–112. doi: 10.1099/00207713-24-1-102. [DOI] [Google Scholar]
- 2.Henne A, Brüggemann H, Raasch C, Wiezer A, Hartsch T, Liesegang H, Johann A, Lienard T, Gohl O, Martinez-Arias R, Jacobi C, Starkuviene V, Schlenczeck S, Dencker S, Huber R, Klenk H-P, Kramer W, Merkl R, Gottschalk G, Fritz H-J. 2004. The genome sequence of the extreme thermophile Thermus thermophilus. Nat Biotechnol 22:547–553. doi: 10.1038/nbt956. [DOI] [PubMed] [Google Scholar]
- 3.Cava F, Hidalgo A, Berenguer J. 2009. Thermus thermophilus as biological model. Extremophiles 13:213–231. doi: 10.1007/s00792-009-0226-6. [DOI] [PubMed] [Google Scholar]
- 4.Koyama Y, Hoshino T, Tomizuka N, Furukawa K. 1986. Genetic transformation of the extreme thermophile Thermus thermophilus and of other Thermus spp. J Bacteriol 166:338–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carr JF, Danziger ME, Huang AL, Dahlberg AE, Gregory ST. 2015. Engineering the genome of Thermus thermophilus using a counterselectable marker. J Bacteriol 197:1135–1144. doi: 10.1128/JB.02384-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tamakoshi M, Yaoi T, Oshima T, Yamagishi A. 1999. An efficient gene replacement and deletion system for an extreme thermophile, Thermus thermophilus. FEMS Microbiol Lett 173:431–437. doi: 10.1111/j.1574-6968.1999.tb13535.x. [DOI] [PubMed] [Google Scholar]
- 7.Tamakoshi M, Uchida M, Tanabe K, Fukuyama S, Yamagishi A, Oshima T. 1997. A new Thermus-Escherichia coli shuttle integration vector system. J Bacteriol 179:4811–4814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fujita A, Misumi Y, Koyama Y. 2012. Two versatile shuttle vectors for Thermus thermophilus-Escherichia coli containing multiple cloning sites, lacZα gene and kanamycin or hygromycin resistance marker. Plasmid 67:272–275. doi: 10.1016/j.plasmid.2011.12.013. [DOI] [PubMed] [Google Scholar]
- 9.Maseda H, Hoshino T. 1998. Development of expression vectors for Thermus thermophilus. J Ferment Bioeng 86:121–124. doi: 10.1016/S0922-338X(98)80044-5. [DOI] [Google Scholar]
- 10.Hashimoto Y, Yano T, Kuramitsu S, Kagamiyama H. 2001. Disruption of Thermus thermophilus genes by homologous recombination using a thermostable kanamycin-resistant marker. FEBS Lett 506:231–234. doi: 10.1016/S0014-5793(01)02926-X. [DOI] [PubMed] [Google Scholar]
- 11.Hoseki J, Yano T, Koyama Y, Kuramitsu S, Kagamiyama H. 1999. Directed evolution of thermostable kanamycin-resistance gene: a convenient selection marker for Thermus thermophilus. J Biochem 126:951–956. doi: 10.1093/oxfordjournals.jbchem.a022539. [DOI] [PubMed] [Google Scholar]
- 12.Brouns SJJ, Wu H, Akerboom J, Turnbull AP, de Vos WM, van der Oost J. 2005. Engineering a selectable marker for hyperthermophiles. J Biol Chem 280:11422–11431. doi: 10.1074/jbc.M413623200. [DOI] [PubMed] [Google Scholar]
- 13.Nakamura A, Takakura Y, Kobayashi H, Hoshino T. 2005. In vivo directed evolution for thermostabilization of Escherichia coli hygromycin B phosphotransferase and the use of the gene as a selection marker in the host-vector system of Thermus thermophilus. J Biosci Bioeng 100:158–163. doi: 10.1263/jbb.100.158. [DOI] [PubMed] [Google Scholar]
- 14.Koyama Y, Arikawa Y, Furukawa K. 1990. A plasmid vector for an extreme thermophile, Thermus thermophilus. FEMS Microbiol Lett 60:97–101. [DOI] [PubMed] [Google Scholar]
- 15.Kayser KJ, Kilbane JJ. 2001. New host-vector system for Thermus spp. based on the malate dehydrogenase gene. J Bacteriol 183:1792–1795. doi: 10.1128/JB.183.5.1792-1795.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ohta T, Tokishita S- I, Imazuka R, Mori I, Okamura J, Yamagata H. 2006. β-Glucosidase as a reporter for the gene expression studies in Thermus thermophilus and constitutive expression of DNA repair genes. Mutagenesis 21:255–260. doi: 10.1093/mutage/gel025. [DOI] [PubMed] [Google Scholar]
- 17.Blas-Galindo E, Cava F, López-Viñas E, Mendieta J, Berenguer J. 2007. Use of a dominant rpsL allele conferring streptomycin dependence for positive and negative selection in Thermus thermophilus. Appl Environ Microbiol 73:5138–5145. doi: 10.1128/AEM.00751-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Angelov A, Li H, Geissler A, Leis B, Liebl W. 2013. Toxicity of indoxyl derivative accumulation in bacteria and its use as a new counterselection principle. Syst Appl Microbiol 36:585–592. doi: 10.1016/j.syapm.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 19.Cohen SS, Flaks JG, Barner HD, Loeb MR, Lichtenstein J. 1958. The mode of action of 5-fluorouracil and its derivatives. Proc Natl Acad Sci U S A 44:1004–1012. doi: 10.1073/pnas.44.10.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Andersen PS, Smith JM, Mygind B. 1992. Characterization of the upp gene encoding uracil phosphoribosyltransferase of Escherichia coli K12. Eur J Biochem 204:51–56. doi: 10.1111/j.1432-1033.1992.tb16604.x. [DOI] [PubMed] [Google Scholar]
- 21.Dubeau M-P, Ghinet MG, Jacques P-E, Clermont N, Beaulieu C, Brzezinski R. 2009. Cytosine deaminase as a negative selection marker for gene disruption and replacement in the genus Streptomyces and other actinobacteria. Appl Environ Microbiol 75:1211–1214. doi: 10.1128/AEM.02139-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kostner D, Peters B, Mientus M, Liebl W, Ehrenreich A. 2013. Importance of codB for new codA-based markerless gene deletion in Gluconobacter strains. Appl Microbiol Biotechnol 97:8341–8349. doi: 10.1007/s00253-013-5164-7. [DOI] [PubMed] [Google Scholar]
- 23.van der Geize R, de Jong W, Hessels GI, Grommen AWF, Jacobs AAC, Dijkhuizen L. 2008. A novel method to generate unmarked gene deletions in the intracellular pathogen Rhodococcus equi using 5-fluorocytosine conditional lethality. Nucleic Acids Res 36:e151. doi: 10.1093/nar/gkn811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O'Donovan GA, Neuhard J. 1970. Pyrimidine metabolism in microorganisms. Bacteriol Rev 34:278–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takai K, Inoue A, Horikoshi K. 1999. Thermaerobacter marianensis gen. nov., sp. nov., an aerobic extremely thermophilic marine bacterium from the 11,000 m deep Mariana Trench. Int J Syst Bacteriol 49:619–628. doi: 10.1099/00207713-49-2-619. [DOI] [PubMed] [Google Scholar]
- 26.Blasco F, Kauffmann I, Schmid RD. 2004. CYP175A1 from Thermus thermophilus HB27, the first beta-carotene hydroxylase of the P450 superfamily. Appl Microbiol Biotechnol 64:671–674. doi: 10.1007/s00253-003-1529-7. [DOI] [PubMed] [Google Scholar]
- 27.Momoi K, Hofmann U, Schmid RD, Urlacher VB. 2006. Reconstitution of beta-carotene hydroxylase activity of thermostable CYP175A1 monooxygenase. Biochem Biophys Res Commun 339:331–336. doi: 10.1016/j.bbrc.2005.11.023. [DOI] [PubMed] [Google Scholar]
- 28.Brüggemann H, Chen C. 2006. Comparative genomics of Thermus thermophilus: plasticity of the megaplasmid and its contribution to a thermophilic lifestyle. J Biotechnol 124:654–661. doi: 10.1016/j.jbiotec.2006.03.043. [DOI] [PubMed] [Google Scholar]
- 29.Ramírez-Arcos S, Fernández-Herrero LA, Marín I, Berenguer J. 1998. Anaerobic growth, a property horizontally transferred by an Hfr-like mechanism among extreme thermophiles. J Bacteriol 180:3137–3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Degryse E, Glansdorff N, Piérard A. 1978. A comparative analysis of extreme thermophilic bacteria belonging to the genus Thermus. Arch Microbiol 117:189–196. doi: 10.1007/BF00402307. [DOI] [PubMed] [Google Scholar]
- 31.Sambrook J, Fritsch E, Maniatis T. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY. [Google Scholar]
- 32.Hanahan D. 1983. Studies on transformation of Escherichia coli with plasmids. J Mol Biol 166:557–580. doi: 10.1016/S0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- 33.Lasa I, de Grado M, de Pedro MA, Berenguer J. 1992. Development of Thermus-Escherichia shuttle vectors and their use for expression of the Clostridium thermocellum celA gene in Thermus thermophilus. J Bacteriol 174:6424–6431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de Grado M, Castán P, Berenguer J. 1999. A high-transformation-efficiency cloning vector for Thermus thermophilus. Plasmid 42:241–245. doi: 10.1006/plas.1999.1427. [DOI] [PubMed] [Google Scholar]
- 35.Yanisch-Perron C, Vieira J, Messing J. 1985. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene 33:103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- 36.de Grado M, Lasa I, Berenguer J. 1998. Characterization of a plasmid replicative origin from an extreme thermophile. FEMS Microbiol Lett 165:51–57. [DOI] [PubMed] [Google Scholar]
- 37.Hoffmann J, Bóna-Lovász J, Beuttler H, Altenbuchner J. 2012. In vivo and in vitro studies on the carotenoid cleavage oxygenases from Sphingopyxis alaskensis RB2256 and Plesiocystis pacifica SIR-1 revealed their substrate specificities and non-retinal-forming cleavage activities. FEBS J 279:3911–3924. doi: 10.1111/j.1742-4658.2012.08751.x. [DOI] [PubMed] [Google Scholar]
- 38.Liao H, McKenzie T, Hageman R. 1986. Isolation of a thermostable enzyme variant by cloning and selection in a thermophile. Proc Natl Acad Sci U S A 83:576–580. doi: 10.1073/pnas.83.3.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lasa I, Castón JR, Fernández-Herrero LA, de Pedro MA, Berenguer J. 1992. Insertional mutagenesis in the extreme thermophilic eubacteria Thermus thermophilus HB8. Mol Microbiol 6:1555–1564. doi: 10.1111/j.1365-2958.1992.tb00877.x. [DOI] [PubMed] [Google Scholar]
- 40.Dion M, Fourage L, Hallet JN, Colas B. 1999. Cloning and expression of a beta-glycosidase gene from Thermus thermophilus. Sequence and biochemical characterization of the encoded enzyme. Glycoconj J 16:27–37. [DOI] [PubMed] [Google Scholar]
- 41.Bradford MM. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 42.Park H-S, Kilbane JJ. 2004. Gene expression studies of Thermus thermophilus promoters PdnaK, Parg and Pscs-mdh. Lett Appl Microbiol 38:415–422. doi: 10.1111/j.1472-765X.2004.01512.x. [DOI] [PubMed] [Google Scholar]
- 43.Brock TD. 1984. Genus Thermus Brock and Freeze 1969, 295AL, p 333–337. In Krieg NR, Holt JG (ed), Bergey's manual of systematic bacteriology, vol 1 Williams & Wilkins, Baltimore, MD. [Google Scholar]
- 44.Yokoyama A, Shizuri Y, Hoshino T, Sandmann G. 1996. Thermocryptoxanthins: novel intermediates in the carotenoid biosynthetic pathway of Thermus thermophilus. Arch Microbiol 165:342–345. doi: 10.1007/s002030050336. [DOI] [PubMed] [Google Scholar]
- 45.Yokoyama A, Sandmann G, Hoshino T, Adachi K, Sakai M, Shizuri Y. 1995. Thermozeaxanthins, new carotenoid-glycoside-esters from thermophilic eubacterium Thermus thermophilus. Tetrahedron Lett 36:4901–4904. doi: 10.1016/00404-0399(50)0881C-. [DOI] [Google Scholar]
- 46.Koyama Y, Okamoto S, Furukawa K. 1990. Cloning of alpha- and beta-galactosidase genes from an extreme thermophile, Thermus strain T2, and their expression in Thermus thermophilus HB27. Appl Environ Microbiol 56:2251–2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moreno R, Zafra O, Cava F, Berenguer J. 2003. Development of a gene expression vector for Thermus thermophilus based on the promoter of the respiratory nitrate reductase. Plasmid 49:2–8. doi: 10.1016/S0147-619X(02)00146-4. [DOI] [PubMed] [Google Scholar]
- 48.Fujita A, Misumi Y, Honda S, Sato T, Koyama Y. 2013. Construction of new cloning vectors that employ the phytoene synthase encoding gene for color screening of cloned DNA inserts in Thermus thermophilus. Gene 527:655–662. doi: 10.1016/j.gene.2013.06.069. [DOI] [PubMed] [Google Scholar]
- 49.Fridjonsson O, Watzlawick H, Mattes R. 2000. The structure of the alpha-galactosidase gene loci in Thermus brockianus ITI360 and Thermus thermophilus TH125. Extremophiles 4:23–33. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.