Abstract
Recreational and potable water supplies polluted with human wastewater can pose a direct health risk to humans. Therefore, sensitive detection of human fecal pollution in environmental waters is very important to water quality authorities around the globe. Microbial source tracking (MST) utilizes human fecal markers (HFMs) to detect human wastewater pollution in environmental waters. The concentrations of these markers in raw wastewater are considered important because it is likely that a marker whose concentration is high in wastewater will be more frequently detected in polluted waters. In this study, quantitative PCR (qPCR) assays were used to determine the concentrations of fecal indicator bacteria (FIB) Escherichia coli and Enterococcus spp., HFMs Bacteroides HF183, human adenoviruses (HAdVs), and polyomaviruses (HPyVs) in raw municipal wastewater influent from various climatic zones in Australia. E. coli mean concentrations in pooled human wastewater data sets (from various climatic zones) were the highest (3.2 × 106 gene copies per ml), followed by those of HF183 (8.0 × 105 gene copies per ml) and Enterococcus spp. (3.6 × 105 gene copies per ml). HAdV and HPyV concentrations were 2 to 3 orders of magnitude lower than those of FIB and HF183. Strong positive and negative correlations were observed between the FIB and HFM concentrations within and across wastewater treatment plants (WWTPs). To identify the most sensitive marker of human fecal pollution, environmental water samples were seeded with raw human wastewater. The results from the seeding experiments indicated that Bacteroides HF183 was more sensitive for detecting human fecal pollution than HAdVs and HPyVs. Since the HF183 marker can occasionally be present in nontarget animal fecal samples, it is recommended that HF183 along with a viral marker (HAdVs or HPyVs) be used for tracking human fecal pollution in Australian environmental waters.
INTRODUCTION
Direct monitoring of pathogenic microorganisms in water resources is likely to provide important information regarding public health risks. However, routine monitoring for a wide variety of pathogenic microorganisms can be expensive and challenging due to their uneven distribution among the host population and the affected waters. The microbiological quality of water is generally assessed by monitoring fecal indicator bacteria (FIB), such as Escherichia coli and Enterococcus spp., using culture-based methods (1, 2). These FIB are abundant in the feces of warm-blooded animals. The presence of elevated levels of FIB in environmental waters indicates not only the occurrence of fecal pollution but also the likely presence of pathogenic microorganisms that are capable of causing illnesses in exposed humans. For the remediation of polluted water bodies, it is vital for water utilities and regulators to identify the source(s) of the fecal pollution. However, monitoring FIB alone does not provide information regarding their origin due to their presence in all warm-blooded animals, including humans (3, 4). This major limitation can be resolved by application of microbial source tracking (MST) techniques, which can identify and quantify the source(s) of fecal pollution in environmental waters (5, 6).
Numerous MST techniques targeting bacteria (7–9), protozoa (10), and viruses (11, 12) have been reported in the literature. Among the bacterial targets, Bacteroides markers hold promise as alternative indicators of fecal pollution owing to a number of advantages, including short survival rates outside the hosts, exclusivity to the guts of warm-blooded animals, occurrence as a larger portion of fecal bacteria than to FIB, and inability to proliferate in the environment (7, 13, 14). A number of PCR- and quantitative PCR (qPCR)-based methods have been developed to detect and quantify human- and animal-associated Bacteroides markers in environmental waters (7, 15–18). Among the human-associated Bacteroides markers, HF183 has been studied extensively, and several PCR/qPCR assays have been developed to detect and quantify this marker in environmental waters (7, 19–21). Among the enteric viruses, human adenoviruses (HAdVs) and human polyomaviruses (HPyVs) have received significant attention as MST markers due to their high abundance in the feces and urine of hosts, high persistence in environmental waters, and strict host association (12, 22–24).
The successful field application of any MST marker depends on several performance characteristics, such as host specificity, host prevalence (also known as host sensitivity), evenness, and relevance to health risks (6, 25). Host specificity testing has been the focal point of many MST evaluation studies (26–29). The host specificity of the Bacteroides HF183 marker has been well studied around the globe (26-28, 30). However, host specificity is not an important issue for viral MST markers due to their strict host association (31).
Host prevalence is also considered an important performance characteristic because it is likely that a highly host-prevalent marker will be more frequently detected in polluted water samples. Many studies have reported the host prevalence values of the HF183, HAdV, and HPyV markers by analyzing individual fecal and wastewater samples using binary PCR (positive/negative) (8, 12, 26, 27, 32). The host prevalence value of a particular marker in the host population may vary geographically due to uneven distribution. It has been recommended that host prevalence be determined across a geographic region and verified in a new geographic region (6–9, 32, 33). In our previous studies, we determined the host prevalence (as a percentage) of the HF183, HAdV, and HPyV markers in subtropical Southeast Queensland, Australia, by testing raw wastewater samples using PCR (22, 34, 35). However, little is known regarding the concentrations of these markers in raw wastewater samples from different climatic zones in Australia. This information is important for identifying whether these makers can be reliably used for the detection of human fecal pollution in the surface waters in various climatic zones.
The aims of this study were (i) to determine the concentrations of FIB (E. coli and Enterococcus spp.) and human fecal markers (HFMs) Bacteroides HF183, HAdVs, and HPyVs in raw wastewater samples using qPCR assays, (ii) to examine the differences in the concentrations of FIB and HFMs in wastewater treatment plants (WWTPs) from three different climatic zones in Australia, and (iii) to determine any correlations that exist among FIB and HFMs in raw wastewater. Finally, the concentrations of HFMs in environmental water samples seeded with raw wastewater were used to support their usefulness for MST field studies across Australia.
MATERIALS AND METHODS
Human wastewater sampling.
WWTPs representing three different climatic zones, Brisbane, Perth, and Tasmania, were selected for this study (Table 1). WWTP A is located in Brisbane, Queensland, and treats human wastewater from approximately 250,000 people. The treatment process consists of a primary treatment, a secondary treatment (activated sludge), and disinfection with chlorine and UV prior to discharge of the wastewater into the Brisbane River. WWTP B is located in Perth, Western Australia, and treats human wastewater from approximately 600,000 people. The treatment process is similar to that in WWTP A. However, the wastewater is not subjected to UV disinfection as in WWTP A prior to being discharged into the Indian Ocean. WWTP C is located in Hobart, Tasmania, and treats human wastewater from 35,000 people. Prior to being discharged into the Coral River or Derwent River, chlorinated wastewater is passed through a 10-μm filter.
TABLE 1.
Description of the wastewater treatment plants that were selected for this study
| Wastewater treatment plant | Location | Climatic conditions | Treatment process | Vol of wastewater treated per day (Ml) |
|---|---|---|---|---|
| A | Brisbane | Subtropical (no dry season) | Activated sludge | 54 |
| B | Perth | Mediterranean (dry summer) | Activated sludge | 135 |
| C | Hobart | Temperate (mild summer) | Activated sludge | 6.6 |
Raw wastewater grab samples (approximately 100 ml each) were collected in sterile bottles from the influent of each WWTP. The samples were collected in triplicate using a telescopic bailer device from each of the WWTPs studied over a period of 11 weeks in early September to late November 2014. In total, 33 samples were collected from each WWTP. Samples were transported on ice to the laboratory and stored at 4°C.
DNA extraction.
DNA was extracted from an aliquot of 250 μl of raw wastewater sample using the MO Bio PowerSoil DNA isolation kit (Mo Bio Laboratories, Carlsbad, CA) with minor modifications (36). The extraction protocol was amended to allow the utilization of all supernatant at each step and, therefore, increased volumes of solutions C3 and C4 were added to compensate. Extracted DNA was eluted through the spin filter membrane by addition of 100 μl of solution C6, followed by storage at −80°C. Each DNA sample was quantified using a NanoDrop spectrophotometer (ND-1000; NanoDrop Technology, Wilmington, DE).
PCR inhibition testing.
To obtain information on the level of PCR inhibition, all wastewater DNA samples were spiked with 10 pg of Oncorhynchus keta DNA (Sigma Chemical Co., St. Louis, MO) and tested with the Sketa22 real-time PCR assay as described elsewhere (37). PCR inhibition was not detected in any of the DNA samples tested.
qPCR assays.
qPCR standards for E. coli 23S rRNA, Enterococcus 23S rRNA, and HAdVs were prepared from the genomic DNA of E. coli ATCC 35150, Enterococcus faecalis ATCC 19433, and HAdV strain 41 ATCC VR-930 as described elsewhere (38, 39). qPCR standards for HF183 and HPyVs were prepared from the plasmid DNA (26, 40). Next 10-fold dilutions ranging from 1 × 106 to 1 copy per μl of genomic and plasmid DNA standards were prepared and stored at −20°C. A 3-μl template from each dilution was used to prepare a standard curve for each qPCR assay. The primer sequences and amplification conditions for the qPCR assays used in this study are shown in Table S1 in the supplemental material. The E. coli, Enterococcus spp., HAdV, and HPyV qPCR assays were performed in 20-μl reaction mixtures using 10 μl of SsoAdvanced universal probe supermix (Bio-Rad Laboratories), 800 nM each primer and 80 nM probe (E. coli), 500 nM each primer and 400 nM probe (Enterococcus spp.), 250 nM each primer and 250 nM probe (HAdV) and 250 nM each primer and 200 nM probe (HPyV), and 3 μl of template DNA. The HF183 qPCR assays were performed in 20 μl of reaction mixture using 10 μl of iQ SYBR green supermix (Bio-Rad Laboratories), 300 nM each primer, and 3 μl of template DNA. To separate the specific product from nonspecific products, including primer dimers, melting curve analysis was performed for the HF183 qPCR. During melting curve analysis, the temperature was increased from 65 to 95°C at 0.5°C increments. All qPCRs were performed in triplicate. For each qPCR assay, a negative (sterile water) control was included.
qPCR performance characteristics.
The qPCR standards were analyzed in order to determine the amplification efficiencies (E) and the correlation coefficient (r2). The qPCR performance characteristics are shown in Table S2 in the supplemental material. The repeatability (intra-assay agreement) and reproducibility (interassay agreement) of each qPCR assay were assessed by determining the percent coefficient of variation (CV) (41). The CV values were calculated from the quantification cycle (Cq) values of each standard ranging from 3 × 106 to 3 gene copies. The intra-assay repeatability was calculated based on the Cq values by testing each dilution 10 times in the same qPCR run. The interassay reproducibility was calculated based on the Cq values by testing each standard on 5 different days. The mean intra-assay repeatability and interassay reproducibility CV for the qPCR assays are shown in Fig. S1 in the supplemental material. The qPCR lower limit of quantification (LLOQ) was also determined from the Cq values obtained for each standard. The smallest amount of diluted standard detected in 100% triplicate assays was considered the qPCR LLOQ. The LLOQ of the qPCR was determined to be 30 gene copies for all five assays.
Seeding experiment.
A 15-liter river water sample was collected from the Brisbane River at a site located in the lower portions of the river in a highly urbanized area. River water samples were stored at 4°C for no more than 1 h before processing. In addition, a 1-liter raw wastewater sample was collected from WWTP A. For the qPCR analysis of E. coli, Enterococcus spp., and HF183, 490-ml water samples (n = 3) were seeded with 10-ml raw wastewater samples. Water samples were serially diluted (10−1, 10−2, and 10−3) and filtered through 0.45-μm pore size (90-mm diameter) nitrocellulose membranes (Millipore, Tokyo, Japan). For the qPCR analysis of HAdVs and HPyVs, another batch of 490-ml water samples (n = 3) were also seeded with 10-ml raw wastewater samples and serially diluted (10−1, 10−2, and 10−3). HAdVs and HPyVs were concentrated using a previously published method (40). The method began with adjustment of each water sample pH to 3.5 using 2.0 N HCl. Water samples were then passed through 0.45-μm, 90-mm diameter negatively charged HA membranes (HAWP09000; Merck Millipore Ltd., Sydney, Australia) via a glass funnel and base (Merck Millipore Ltd.). All of the membranes were then placed in 50-ml PowerMax bead solution tubes. Nucleic acid was extracted directly from the membranes using a Mo Bio PowerMax soil DNA isolation kit. Extracted bacterial and viral nucleic acid was eluted through the spin filter membranes by addition of 2 ml of solution C6 and stored at −20°C until processed. The background concentrations of E. coli, Enterococcus spp., HF183, HAdVs, and HPyVs in river water samples were enumerated using qPCR assays as described above.
Statistical analysis.
The concentrations of FIB and HFMs in raw wastewater samples were not normally distributed (as determined by a Kolmogorov-Smirnov test). Therefore, the nonparametric Kruskal-Wallis one-way analysis of variance (ANOVA) with Dunn's posttest was performed to determine if there were any significant differences in FIB and HFM concentrations within and among WWTPs. The nonparametric Spearman rank correlation with a two-tailed P value was also used to establish the relationship between FIB and HFM concentrations in raw wastewater samples. In general, r = >0.7 was considered a strong positive correlation, r = >0.4 but <0.7 was moderate correlation, and r = >0.2 but <0.4 was weak correlation. GraphPad Prism 6 was used for statistical analysis (GraphPad Software, Inc.).
Chemometric and statistical analyses were performed with SIMCA 14 (Umetrics AG, Umeå, Sweden). The cutoff level for significant features was kept at a false-discovery rate (FDR) (q value) of ≤0.1 and P values at ≤0.05. Unsupervised data were analyzed by principal-component analysis (PCA). Furthermore, in order to accommodate within-group analysis, partial least-squares discriminant analysis (PLS-DA) was employed.
RESULTS
Concentrations of FIB and HFMs in raw wastewater samples.
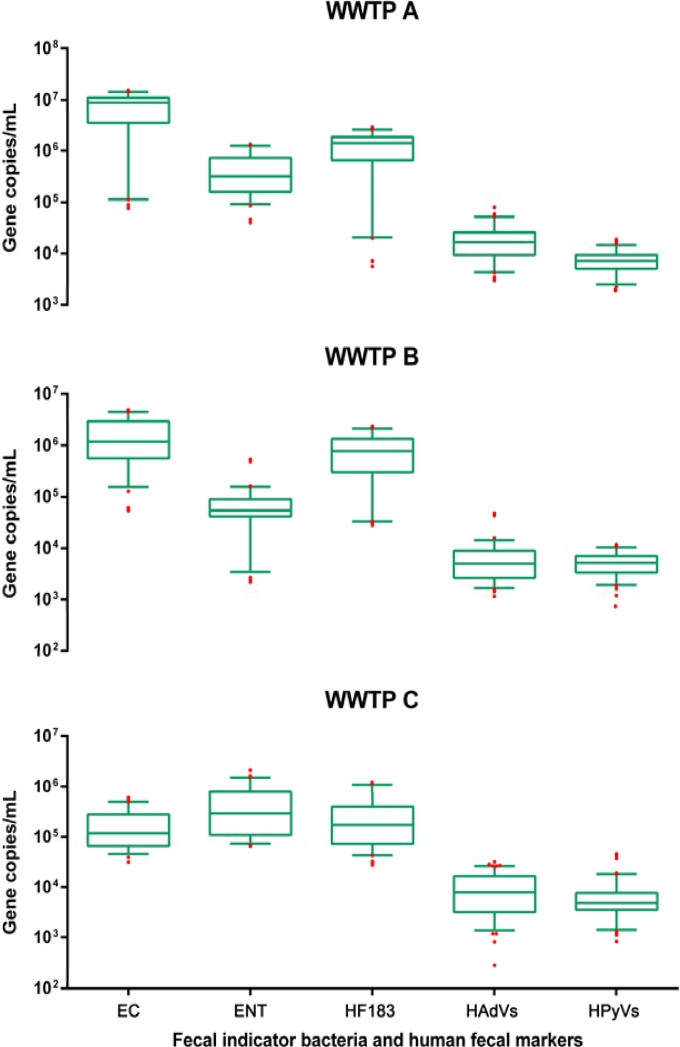
All wastewater DNA samples were determined to have concentrations of E. coli, Enterococcus spp., HF183, HAdVs, and HPyVs that were greater than the qPCR LLOQ. The concentrations of FIB and HFMs in raw wastewater samples from WWTP A, B, and C are shown in Fig. 1 in a box and whisker plot format. Among the five targets tested, the mean E. coli concentrations were the highest (7.6 × 106 gene copies per ml) in raw wastewater samples from WWTP A, followed by those of HF183 (1.3 × 106 gene copies per ml) and Enterococcus spp. (4.7 × 105 gene copies per ml). The mean concentrations of HAdVs (2.1 × 104 gene copies per ml) and HPyVs (7.7 × 103 gene copies per ml) were 2 to 3 orders of magnitude lower than those of FIB and HF183. Similar trends in FIB and HFM concentrations were also observed for WWTP B. However, the concentrations of FIB and HFMs in raw wastewater samples from WWTP C did not follow the same pattern. The mean concentration of Enterococcus spp. was the highest (5.2 × 105 gene copies per ml), followed by those of HF183 (2.9 × 105 gene copies per ml) and E. coli (1.8 × 105 per gene copies per ml). The mean concentrations of E. coli in pooled data sets (from three WWTPs) were the highest (3.2 × 106 gene copies per ml), followed by those of HF183 (8.0 × 105 gene copies per ml) and Enterococcus spp. (3.6 × 105 gene copies per ml). The HAdV and HPyV concentrations were 2 to 3 orders of magnitude lower than those of FIB and HF183.
FIG 1.
Box and whisker plots of the concentrations (gene copies per milliliter) of Escherichia coli (EC), Enterococcus spp. (ENT), sewage-associated Bacteroides (HF183), human adenoviruses (HAdVs), and human polyomaviruses (HPyVs) in raw wastewater samples collected from three wastewater treatment plants (WWTPs A, B, and C) in Australia. The upper and lower boxes denote the 75th and 25th percentiles, respectively. The upper and lower bars show the 95th and 5th percentiles, respectively, with outliers represented by red circles. Note the logarithmic vertical axis.
The Kruskal-Wallis one-way ANOVA was undertaken to determine if there are any significant differences in the FIB and HFM concentrations in each WWTP. Dunn's multiple comparisons posttest indicated that the concentrations of FIB and HFMs in raw wastewater samples collected from WWTP A were significantly different (P < 0.05) from each other except for the results for Enterococcus spp. versus HF183, which were not significant (P > 0.05) (see Table S3 in the supplemental material). For WWTP B, the concentrations of FIB and HFMs were significantly different (P < 0.05) for most of the comparisons except for E. coli versus Enterococcus spp. (P > 0.05) and E. coli versus HF183 (P > 0.05), which yielded nonsignificant results (P > 0.05). Similar results were also observed for WWTP C except for both FIB versus HF183 (P > 0.05) and HAdVs versus HPyVs (P > 0.05), which yielded nonsignificant results (P > 0.05). For the pooled data sets, the concentrations of FIB and HFMs were significantly different (P < 0.05) from each other except for Enterococcus spp. versus HF183, which yielded nonsignificant results (P > 0.05).
An ANOVA was also undertaken to determine if there are any significant differences in the FIB and HFM concentrations across the three WWTPs. The concentrations of E. coli, HF183, and HAdVs were significantly different across the WWTPs (P < 0.05). The concentrations of Enterococcus spp. were significantly different between WWTPs A and B (P < 0.05) and between WWTPs B and C (P < 0.05). No significant difference was observed between WWTPs A and C (P > 0.05) in terms of Enterococcus concentrations. The concentrations of HPyVs were significantly different between WWTPs A and B (P < 0.05) and between WWTPs A and C (P < 0.05). No significant difference was observed between WWTPs B and C in terms of HPyV concentrations (P > 0.05).
Correlations between FIB and HFMs in raw wastewater samples.
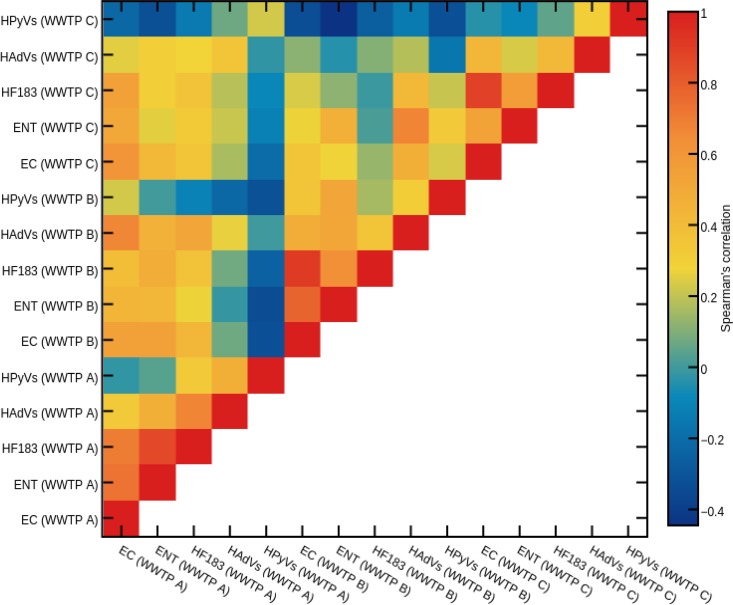
FIB (E. coli versus Enterococcus spp.) and HFMs (HF183 versus HAdVs versus HPyVs) and FIB versus HFMs showed significant cross-correlations with each other within and across WWTPs. A heat map of the Spearman rank order correlation matrix is shown in Fig. 2. Among the 105 bivariate comparisons, 11 (10.5%) showed strong positive correlations (r = 0.728 to 0.950; P < 0.0001) and 41 (39%) showed no or negative correlations (r = −0.023 to 0.171; P < 0.05) (see Table S4 in the supplemental material). Strong positive (r > 0.7) to weak (r > 0.2 but < 0.4) correlations were observed among FIB, HF183, and HAdVs in raw wastewater samples from WWTPs A and C. HPyVs did not correlate with FIB. Strong positive to weak correlations were also observed among FIB and HFMs in raw wastewater samples from WWTP B. For the pooled data sets, strong positive to weak correlations were observed among FIB, HF183, and HAdVs. However, HPyVs did not correlate with FIB (see Table S4).
FIG 2.
Spearman correlations among E. coli (EC), Enterococcus spp. (ENT), Bacteroides HF183, human adenoviruses (HAdVs), and human polyomaviruses (HPyVs) in raw wastewater samples collected from three wastewater treatment plants (WWTP A, B, and C).
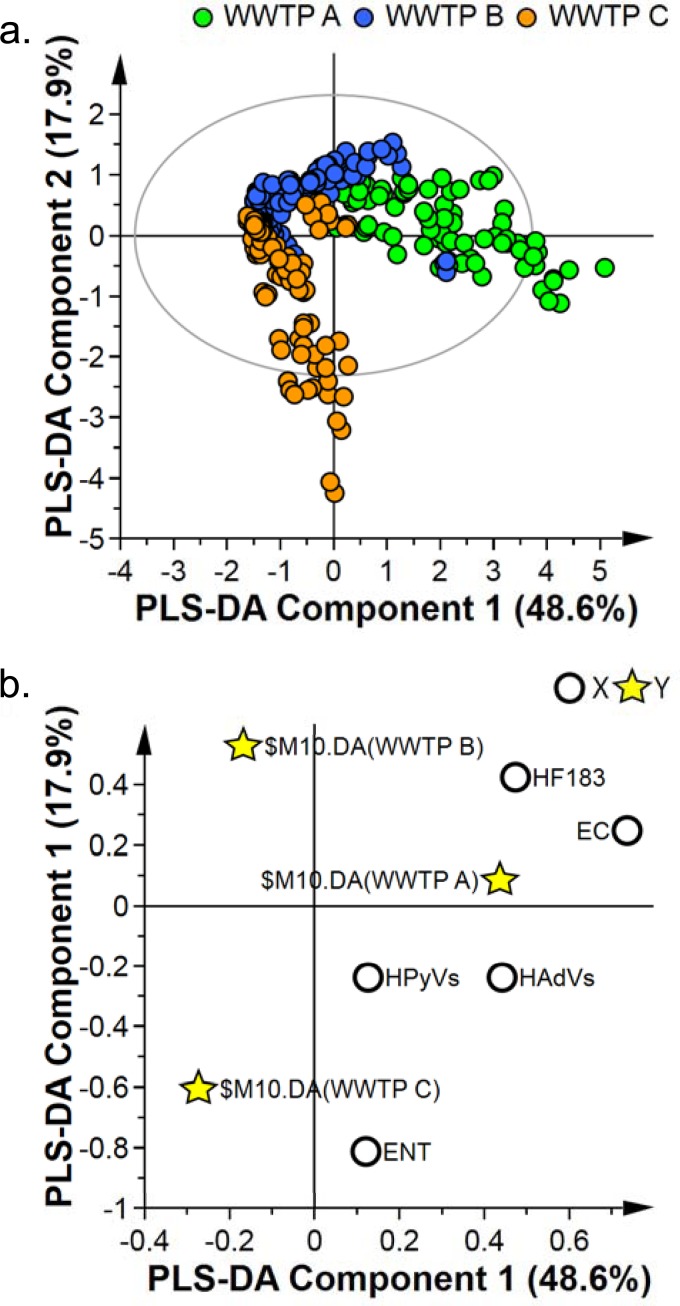
In order to explore the differences between the three WWTPs, a chemometric analysis of FIB and HFMs was undertaken. First, an unsupervised PCA plot was created and was observed to not discriminate samples. (No clusters or groups were observed, as evident in Fig. S2 in the supplemental material.) DCrit (critical value of DModX), derived from the F-distribution, calculates the size of the observational area under analysis. As illustrated in Fig. S3 in the supplemental material, the DModX plot of the PCA data indicates that there are no samples that exceed the threshold for rejecting a sample. The threshold for a moderate outlier is considered when the sample DModX value is twice the DCrit at 0.05, which in this instance was 3.425.
In order to discriminate among the WWTPs further and investigate within-group variations, a PLS-DA model was applied to the same data set. The subsequent PLS-DA score scatter plot and PLS-DA loading scatter plot are presented in Fig. 3 and illustrate clear separation between the treatment plants, with R2X, R2Y, and Q2 values of 0.727, 0.514, and 0.503, respectively. This result is indicative of a model that moderately fits the data (R2X of >0.7) but has a poor predictive capability (Q2). As evident in the loading plot (Fig. 3b; see also Fig. S4 in the supplemental material) and subsequent one-way ANOVA (P < 0.05) with an applied Fisher least significant difference (LSD) post hoc analysis using a false-discovery rate (FDR) value (q value) of 0.1, it was observed that WWTP A > WWTP B > WWTP C in regard to E. coli concentrations (P = 1.283e−53 and FDR of 6.415e−53). Furthermore, WWTP C had elevated values of Enterococcus spp. (P = 3.5376e−17 and FDR of 4.4219e−17) and HAdVs (P = 2.0262e−17 and FDR of 3.77e−17) in comparison to those of WWTPs A and B. In addition, WWTPs A and B had elevated levels of HF183 compared to those of WWTP C (P = 7.5847e−23 and FDR of 1.8962e−22). Last, WWTP A was found to have slightly elevated levels of HPyVs in comparison to those of WWTP B, while WWTP C had greater HPyV concentrations than WWTP B (P = 0.04789 and FDR of 0.00479).
FIG 3.
Partial least-squares discriminant analysis (PLS-DA) of the concentrations (gene copies per milliliter) of Escherichia coli (EC), Enterococcus spp. (ENT), sewage-associated Bacteroides (HF183), human adenoviruses (HAdVs), and human polyomaviruses (HPyVs) in raw wastewater samples collected from three WWTPs (A, B, and C). (a) PLS-DA score scatter plot; (b) PLS-DA loading scatter plot. Each point on the scatter plot refers to a single sample, with R2X (cumulative) = 72.7%, R2Y (cumulative) = 51.4%, and Q2 (cumulative) = 50.3%. The ellipse represents the 95% confidence level.
To further investigate within-group comparisons, three additional within-group PLS-DA models were prepared to compare WWTP A to B (model 1), WWTP A to C (model 2), and WWTP B to C (model 3). In this analysis, it was observed that E. coli had fold changes of 4.4653 (P = 3.9544e−25), 42.752 (P = 1.77e-36), and 9.5742 (6.7289e−22) for model 1, model 2, and model 3, respectively. Enterococcus spp. had fold changes of 6.1651 (P = 2.6326e−20) and 0.14385 (4.7049e−15) for model 1 and model 3, respectively. HAdVs had fold changes of 3.0163 (P = 7.4878e−14) and 2.0539 (P = 4.3253e−9) for model 1 and model 2, respectively. Last, HF183 had fold changes of 4.2878 (P = 1.6386e−25) and 2.8671 (3.9245e−13) for model 2 and model 3, respectively.
Concentrations of FIB and HFMs in Brisbane River water samples seeded with raw wastewater.
HAdVs and HPyVs were not detected in ambient Brisbane River water samples. However, the samples were PCR positive for E. coli, Enterococcus spp., and HF183, but the results were not quantifiable (less than the LLOQ of 30 gene copies). The concentrations of FIB and HFMs in 3-μl river water (seeded with raw wastewater) DNA samples are shown in Table 2.
TABLE 2.
Concentrations of fecal indicator bacteria and human fecal markers in raw wastewater-seeded Brisbane River water DNA samples
| Amt of raw wastewater seeded | Concn (mean ± SD gene copies) of FIB and MST marker per 3-μl DNA samplea |
||||
|---|---|---|---|---|---|
| FIB |
HFMs |
||||
| E. coli | Enterococcus spp. | Bacteroides HF183 | HAdVs | HPyVs | |
| 10 ml | 9.4 × 104 ± 1.9 × 104 | 2.8 × 104 ± 2.8 × 103 | 3.0 × 104 ± 4.8 × 103 | 1.5 × 103 ± 1.5 × 103 | 9.6 × 102 ± 1.1 × 102 |
| 1 ml (10−1) | 7.8 × 103 ± 8.3 × 102 | 2.1 × 103 ± 5.7 × 102 | 2.2 × 103 ± 9.5 × 101 | 2.4 × 101 ± 1.6 × 101 | 8.1 × 101 ± 3.4 × 101 |
| 100 μl (10−2) | 1.0 × 103 ± 1.1 × 102 | 2.8 × 102 ± 5.6 × 101 | 4.5 × 102 ± 6.7 × 101 | NQ | NQ |
| 10 μl (10−3) | 1.1 × 102 ± 2.2 × 101 | ND | 1.8 × 102 ± 2.5 × 101 | ND | ND |
FIB, fecal indicator bacteria; HFM, human fecal markers; HAdVs, human adenoviruses; HPyVs, human polyomaviruses; ND, not detected; NQ, not quantifiable.
The mean concentration of E. coli was 1.1 × 102 gene copies per 3 μl of DNA at the dilution 10−3 (represents 10 μl of raw wastewater seeded into 490-ml water samples). However, at this dilution, Enterococcus spp. were not detected. Among the three HFMs, the mean concentration of HF183 was 1.8 × 102 gene copies per 3 μl of DNA at the dilution 10−3, whereas HAdV and HPyV concentrations were, respectively, 2.4 × 101 and 8.1 × 101 gene copies per 3 μl of DNA at the dilution 10−1 (represents 1 ml of sewage). HAdVs and HPyVs were detected at the dilution 10−2 (represents 100 μl of sewage) but the concentrations were not quantifiable.
DISCUSSION
Host prevalence is generally expressed as a percentage of samples from a given host that test PCR positive for a given marker (12, 27, 34, 42, 43). The closer the values are to 100%, the greater the prevalence and the better the performance of a marker. However, knowing the concentration of a marker in its host is important because it is likely that a marker whose concentration is high will be consistently and more easily detected in polluted water samples. In our previous studies, we have determined the host prevalences of the HF183, HAdV, and HPyV markers in a small number of individual fecal samples and in septic, raw, and treated wastewater samples collected from Southeast Queensland, Australia using binary PCR (22, 26, 35). In the current study, qPCR assays were used to determine the concentrations of HF183, HAdVs, and HPyVs along with two FIB in raw wastewater samples collected from three different climatic zones in Australia for 11 sampling events.
Notably, the concentrations of the E. coli 23S rRNA gene in WWTP C were 1 to 2 orders of magnitude lower than those in WWTPs A and B. This might be attributed to the fact that WWTP C treats a much lower volume of human wastewater from a smaller population than WWTPs A and B. It is also possible that WWTP C is located in a cool temperate region in Tasmania compared to WWTPs A and B, which are located in subtropical and Mediterranean-like climatic zones, respectively. E. coli cells are able to grow outside the intestine, especially in environmental waters in warmer tropical and subtropical climatic zones (44, 45). Given the potential for growth ability in tropical and subtropical climatic zones, E. coli concentrations can be artificially elevated above the expected level from fecal inputs alone. The concentrations of E. coli in WWTPs A and C were 1 to 1.5 orders of magnitude higher than those of Enterococcus spp. However, the E. coli concentrations in WWTP C were 0.5 order of magnitude lower than those of Enterococcus spp. The reason for such a discrepancy is not well understood; however, climatic variations may play a role.
Little is known regarding the effects of temporal and climatic variations on the concentrations of HFMs in raw wastewater samples in Australia. It has been reported that climatic variability may influence the prevalence and concentrations of Bacteroides markers (27, 46). If the concentration of a marker is highly variable in human wastewater, then it is likely that it may not be detected in environmental waters in the presence of human fecal pollution. The mean concentrations of the HF183 and HAdV markers across all three WWTPs were 8.0 × 105 and 1.3 × 104 gene copies per ml of raw wastewater, respectively. Similar levels of gene copies of these markers have been reported in raw wastewater samples from Spain, Japan, Italy, and the United States (26, 30, 47–50).
Little information on the concentrations of HPyVs in raw wastewater has been documented. McQuaig and colleagues (12) reported that the concentrations of HPyVs in raw wastewater might be as high as 4.7 × 104 gene copies per ml, which is comparable to the concentrations obtained in this study. The concentrations of all three HFMs in raw wastewater samples showed small or no temporal variations over the course of the study (see Table S5 in the supplemental material). Taken together, the high concentrations and small temporal variations of HFMs in raw wastewater samples from all three WWTPs across different climatic zones indicate that they might be useful for detecting human wastewater fecal pollution across Australia.
In this study, the correlations between FIB and HFMs in raw wastewater samples were determined. These are particularly important for establishing the fact that the concentrations of FIB can predict the concentrations of HFMs or vice versa. Strong positive correlations were observed between FIB and HF183 in raw wastewater samples from all three WWTPs. HPyVs showed no correlation or a negative correlation with E. coli or Enterococcus spp. McQuaig and colleagues (12) determined the correlations between FIB (fecal coliform bacteria, E. coli, and Enterococcus spp.) and HPyVs for human, disinfected, and septic wastewater samples. HPyVs were poorly or negatively correlated with all three FIB tested. Several factors such as the dilution effect, turbidity, differences in analytical methods, and decay may account for the lack of correlations observed. Poor correlations between FIB and viral markers may not necessarily hinder their application as MST tools if the objective of the study is to determine the sources of fecal pollution for the purpose of mitigation.
Sensitive detection of human fecal pollution in environmental waters is important for protecting public health risks because such pollution can impose a direct risk to humans. To identify the most sensitive marker of human fecal pollution, raw wastewater was seeded into river water samples and analyzed for FIB and HFMs. HF183 was quantifiable in the presence of 10 μl of sewage seeded into 500 ml of water. In contrast, HAdVs and HPyVs were quantifiable in the presence of 1 ml of sewage seeded into environmental waters. Both HAdVs and HPyVs were detected (but were not quantifiable) in the presence of 100 μl of sewage. The results of this study also indicate that the HF183 marker is the most sensitive marker compared to HAdVs and HPyVs. This was expected because the concentrations of HF183 in raw wastewater were 2 to 3 orders of magnitude higher than those of HAdVs and HPyVs. Although HF183 is more sensitive, HPyVs and HAdVs have the advantage of greater host specificity in Australia (22, 26, 35). Since the HF183 marker can occasionally be present in nontarget animal fecal samples (26), it is recommended that HF183 along with HAdVs or HPyVs should be used for human fecal pollution tracking in surface waters in Australia.
Supplementary Material
ACKNOWLEDGMENT
The information used in this paper comes from results of a research project funded by the Australian Centre of Excellence (NATVAL 2.2 subproject 3).
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.03765-15.
REFERENCES
- 1.US Environmental Protection Agency. 1978. Microbiological methods for monitoring the environment. EPA-600/8-78-017 US Environmental Protection Agency, Office of Research and Development, Washington, DC. [Google Scholar]
- 2.US Environmental Protection Agency. 2004. Water quality standards for coastal and great lakes recreational waters: final rule. Fed Regist 69:67218–67243. [Google Scholar]
- 3.Harwood VJ, Whitlock J, Withington V. 2000. Classification of antibiotic resistance patterns of indicator bacteria by discriminant analysis: use in predicting the source of fecal contamination in subtropical waters. Appl Environ Microbiol 66:3698–3704. doi: 10.1128/AEM.66.9.3698-3704.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.US Environmental Protection Agency. 2005. Microbial source tracking guide document. EPA/600-R-05-064 US Environmental Protection Agency, Cincinnati, OH. [Google Scholar]
- 5.Field KG, Samadpour M. 2007. Fecal source tracking, the indicator paradigm, and managing water quality. Water Res 41:3517–3538. doi: 10.1016/j.watres.2007.06.056. [DOI] [PubMed] [Google Scholar]
- 6.Stoeckel DM, Harwood VJ. 2007. Performance, design, and analysis in microbial source tracking studies. Appl Environ Microbiol 73:2405–2415. doi: 10.1128/AEM.02473-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bernhard AE, Field KG. 2000. Identification of nonpoint sources of fecal pollution in coastal waters by using host-specific 16S ribosomal DNA genetic markers from fecal anaerobes. Appl Environ Microbiol 66:1587–1594. doi: 10.1128/AEM.66.4.1587-1594.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scott TM, Jenkins TM, Lukasik J, Rose JB. 2005. Potential use of a host associated molecular marker in Enterococcus faecium as an index of human fecal pollution. Environ Sci Technol 39:283–287. doi: 10.1021/es035267n. [DOI] [PubMed] [Google Scholar]
- 9.Ufnar JA, Wang SY, Christiansen JM, Yampara-Iquise H, Carson CA, Ellender RD. 2006. Detection of the nifH gene of Methanobrevibacter smithii: a potential tool to identify sewage pollution in recreational waters. J Appl Microbiol 101:44–52. doi: 10.1111/j.1365-2672.2006.02989.x. [DOI] [PubMed] [Google Scholar]
- 10.Prystajecky N, Huck PM, Schreier H, Isaac-Renton JL. 2014. Assessment of Giardia and Cryptosporidium spp. as a microbial source tracking tool for surface water: application in a mixed-use watershed. Appl Environ Microbiol 80:2328–2336. doi: 10.1128/AEM.02037-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kirs M, Smith DC. 2007. Multiplex quantitative real-time reverse transcriptase PCR for F+-specific RNA coliphages: a method for use in microbial source tracking. Appl Environ Microbiol 73:808–814. doi: 10.1128/AEM.00399-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McQuaig SM, Scott TM, Lukasik JO, Paul JH, Harwood VJ. 2009. Quantification of human polyomaviruses JC virus and BK virus by TaqMan quantitative PCR and comparison to other water quality indicators in water and fecal samples. Appl Environ Microbiol 75:3379–3388. doi: 10.1128/AEM.02302-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kreader CA. 1995. Design and evaluation of Bacteroides DNA probes for the specific detection of human fecal pollution. Appl Environ Microbiol 61:1171–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sghir A, Gramet G, Suau A, Rochet V, Pochart P, Dore J. 2000. Quantification of bacterial groups within human faecal flora by oligonucleotide probe hybridization. Appl Environ Microbiol 66:2263–2266. doi: 10.1128/AEM.66.5.2263-2266.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kildare BJ, Leutenegger CM, McSwain BS, Bambic DG, Rajal VB, Wuertz S. 2007. 16S rRNA-based assays for quantitative detection of universal, human-, cow-, and dog-specific fecal Bacteroidales: a Bayesian approach. Water Res 41:3701–3715. doi: 10.1016/j.watres.2007.06.037. [DOI] [PubMed] [Google Scholar]
- 16.Layton A, McKay L, Williams D, Garrett V, Gentry R, Sayler G. 2006. Development of Bacteroides 16S rRNA gene TaqMan based real-time PCR assays for estimation of total, human, and bovine fecal pollution in water. Appl Environ Microbiol 72:4214–4224. doi: 10.1128/AEM.01036-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Okabe S, Okayama N, Savichtcheva O, Ito T. 2007. Identification and quantification of host-specific Bacteroides-Prevotella 16S rRNA genetic markers for assessment of fecal pollution in freshwaters. Appl Microbiol Biotechnol 74:890–901. doi: 10.1007/s00253-006-0714-x. [DOI] [PubMed] [Google Scholar]
- 18.Reischer GH, Kasper DC, Steinborn R, Farnleitner AH, Mach RL. 2007. A quantitative real-time PCR assay for the highly sensitive and specific detection of human faecal influence in spring water from a large alpine catchment area. Lett Appl Microbiol 44:351–356. doi: 10.1111/j.1472-765X.2006.02094.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Green HC, Haugland RA, Varma M, Millen HT, Borchardt MA, Field KG, Walters WA, Knight R, Kelty CA, Shanks OC. 2014. Improved HF183 quantitative real-time PCR assay for characterization of human fecal pollution in ambient surface waters samples. Appl Environ Microbiol 80:3086–3094. doi: 10.1128/AEM.04137-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haugland RA, Varma M, Kelty CA, Peed L, Sivaganesan M, Shanks OC. 2010. Evaluation of genetic markers from the 16S rRNA gene V2 region for use in quantitative detection of selected Bacteroidales species and human fecal waste by real-time PCR. Syst Appl Microbiol 33:348–357. doi: 10.1016/j.syapm.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 21.Seurinck S, Defoirdt T, Verstraete W, Siciliano SD. 2005. Detection and quantification of the human-specific HF183 Bacteroides 16S rRNA genetic markers with real-time PCR for assessment of human fecal pollution in freshwater. Environ Microbiol 7:249–259. doi: 10.1111/j.1462-2920.2004.00702.x. [DOI] [PubMed] [Google Scholar]
- 22.Ahmed W, Goonetilleke A, Gardner T. 2010. Human and bovine adenoviruses for the detection of source-specific fecal pollution in coastal waters in Australia. Water Res 44:4662–4673. doi: 10.1016/j.watres.2010.05.017. [DOI] [PubMed] [Google Scholar]
- 23.Fong TT, Griffin DW, Lipp EK. 2005. Molecular assays for targeting human and bovine enteric viruses in coastal waters and their application for library-independent source tracking. Appl Environ Microbiol 71:2070–2078. doi: 10.1128/AEM.71.4.2070-2078.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hundesa A, Maluquer de Motes C, Bofill-Mas S, Albinana-Gimenez N, Girones R. 2006. Identification of human and animal adenoviruses and polyomaviruses for determination of sources of fecal contamination in the environment. Appl Environ Microbiol 72:7886–7893. doi: 10.1128/AEM.01090-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harwood VJ, Staley C, Badgely BD, Borges K, Korajkic A. 2014. Microbial source tracking markers for detection of fecal contamination in environmental waters: relationships between pathogens and human health outcomes. FEMS Microbiol Rev 38:1–40. doi: 10.1111/1574-6976.12031. [DOI] [PubMed] [Google Scholar]
- 26.Ahmed W, Masters N, Toze S. 2012. Consistency in the host-specificity and host-sensitivity of the Bacteroides HF183 marker for sewage pollution tracking. Lett Appl Microbiol 55:283–289. doi: 10.1111/j.1472-765X.2012.03291.x. [DOI] [PubMed] [Google Scholar]
- 27.Gawler AH, Beecher JE, Brandão J, Carroll NM, Falcão L, Gourmelon M, Masterson B, Nunes B, Porter J, Rincė A, Rodrigues R, Thorp M, Walters JM, Meijer WG. 2007. Validation of host-specific Bacteriodales 16S rRNA genes as markers to determine the origin of faecal pollution in Atlantic Rim countries of the European Union. Water Res 41:3780–3784. doi: 10.1016/j.watres.2007.01.028. [DOI] [PubMed] [Google Scholar]
- 28.Gourmelon M, Caparis MP, Segura R, Le Mennec C, Lozach S, Piriou JY, Rince A. 2007. Evaluation of two-library independent microbial source tracking methods to identify sources of fecal contamination in French Estuaries. Appl Environ Microbiol 73:4857–4866. doi: 10.1128/AEM.03003-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Griffith JF, Weisberg SB, McGee CD. 2003. Evaluation of microbial source tracking methods using mixed fecal sources in aqueous test samples. J Water Health 1:141–151. [PubMed] [Google Scholar]
- 30.Van De Werfhorst LC, Sercu B, Holden PA. 2011. Comparison of the host specificities of two Bacteroidales quantitative PCR assays used for tracking human fecal contamination. Appl Environ Microbiol 77:6258–6260. doi: 10.1128/AEM.00239-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wong K, Fong T-T, Bibby K, Molina M. 2012. Application of enteric viruses for fecal pollution source tracking in environmental waters. Environ Int 45:151–164. doi: 10.1016/j.envint.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 32.Harwood VJ, Brownell M, Wang S, Lepo J, Ellender RD, Ajidahun A, Hellein KN, Kennedy E, Ye X, Flood C. 2009. Validation and field testing of library-independent microbial source tracking methods in the Gulf of Mexico. Water Res 43:4812–4819. doi: 10.1016/j.watres.2009.06.029. [DOI] [PubMed] [Google Scholar]
- 33.McQuaig SM, Scott TM, Harwood VJ, Farrah SR, Lukasik JO. 2006. Detection of human-derived fecal pollution in environmental waters by use of a PCR-based human polyomavirus assay. Appl Environ Microbiol 72:7567–7574. doi: 10.1128/AEM.01317-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ahmed W, Stewart J, Powell D, Gardner T. 2008. Evaluation of Bacteroides markers for the detection of human faecal pollution. Lett Appl Microbiol 46:237–242. [DOI] [PubMed] [Google Scholar]
- 35.Ahmed W, Wan C, Goonetilleke A, Gardner T. 2010. Evaluating sewage-associated JCV and BKV polyomaviruses for sourcing human fecal pollution in a coastal river in southeast Queensland, Australia. J Environ Qual 39:1743–1750. doi: 10.2134/jeq2010.0062. [DOI] [PubMed] [Google Scholar]
- 36.Gyawali P, Sidhu JPS, Ahmed W, Jagals P, Toze S. 2015. Rapid concentration and sensitive detection of hookworm ova in wastewater matrices using a real-time PCR method. Exp Parasitol 159:5–12. doi: 10.1016/j.exppara.2015.08.009. [DOI] [PubMed] [Google Scholar]
- 37.Ahmed W, Gyawali P, Toze S. 2015. Quantitative PCR measurements of Escherichia coli including Shiga toxin producing E. coli (STEC) in animal feces and environmental waters. Environ Sci Technol 49:3084–3090. doi: 10.1021/es505477n. [DOI] [PubMed] [Google Scholar]
- 38.Ahmed W, Richardson K, Sidhu JPS, Toze S. 2012. Escherichia coli and Enterococcus spp. in rainwater tank samples: comparison of culture-based methods and 23S rRNA gene quantitative PCR assays. Environ Sci Technol 46:11370–11376. doi: 10.1021/es302222b. [DOI] [PubMed] [Google Scholar]
- 39.Sidhu JPS, Ahmed W, Toze S. 2013. Sensitive detection of human adenoviruses from small volume of primary wastewater samples by quantitative PCR. J Virol Methods 187:395–400. doi: 10.1016/j.jviromet.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 40.Ahmed W, Harwood VJ, Gyawali P, Sidhu JPS, Toze S. 2015. Comparison of concentration methods for quantitative detection of sewage-associated viral markers in environmental waters. Appl Environ Microbiol 81:2042–2049. doi: 10.1128/AEM.03851-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, Kubista M, Mueller R, Nolan T, Pfaffl MW, Shipley GL, Vandesompele J, Wittwer CT. 2009. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem 55:611–622. doi: 10.1373/clinchem.2008.112797. [DOI] [PubMed] [Google Scholar]
- 42.Hundesa A, Bofill-Mas S, Maluquer de Motes C, Rodriguez-Manzano J, Bach A, Casas M, Girones R. 2010. Development of a quantitative PCR assay for the quantification of bovine polyomavirus as a microbial source-tracking tool. J Virol Methods 163:385–389. doi: 10.1016/j.jviromet.2009.10.029. [DOI] [PubMed] [Google Scholar]
- 43.Odagiri M, Schriewer A, Hanley K, Wuertz S, Misra PR, Panigrahi P, Jenkins MW. 2015. Validation of Bacteroidales quantitative PCR assays targeting human and animal fecal contamination in the public and domestic domains in India. Sci Total Environ 502:462–470. doi: 10.1016/j.scitotenv.2014.09.040. [DOI] [PubMed] [Google Scholar]
- 44.Byappanahalli MN, Fujioka RS. 1998. Evidence that tropical soil environment can support the growth of Escherichia coli. Water Sci Technol 38:171–174. doi: 10.1016/S0273-1223(98)00820-8. [DOI] [Google Scholar]
- 45.Solo-Gabriele HM, Wolfert MA, Desmarais TR, Palmer CJ. 2000. Sources of Escherichia coli in a coastal subtropical environment. Appl Environ Microbiol 66:230–237. doi: 10.1128/AEM.66.1.230-237.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shanks OC, Kelty CA, Sivaganesan M, Varma M, Haugland RA. 2009. Quantitative PCR for genetic markers of human fecal pollution. Appl Environ Microbiol 75:5507–5513. doi: 10.1128/AEM.00305-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Albinana-Gimenez N, Clemente-Casares P, Bofill-Mas S, Hundesa A, Ribas F, Girones R. 2006. Distribution of human polyomaviruses, adenoviruses, and hepatitis E viruses in the environment and in a drinking water treatment plant. Environ Sci Technol 40:7416–7422. doi: 10.1021/es060343i. [DOI] [PubMed] [Google Scholar]
- 48.Haramoto E, Katayama H, Oguma K, Ohgaki S. 2007. Quantitative analysis of human enteric adenoviruses in aquatic environments. J Appl Microbiol 103:2153–2159. doi: 10.1111/j.1365-2672.2007.03453.x. [DOI] [PubMed] [Google Scholar]
- 49.Kuo DH-W, Simmons FJ, Blair C, Hart E, Rose JB, Xagoraraki I. 2010. Assessment of human adenovirus removal in a full-scale membrane bioreactor treating municipal wastewater. Water Res 44:1520–1530. doi: 10.1016/j.watres.2009.10.039. [DOI] [PubMed] [Google Scholar]
- 50.Muscillo M, Pourshaban M, Laconelli M, Fontana S, Grazia AD, Manzara S, Fadda S, Santangelo R, Rosa GL. 2008. Detection and quantification of human adenoviruses in surface waters by nested PCR, TaqMan real-time PCR and cell culture assays. Water Air Soil Pollut 191:83–93. doi: 10.1007/s11270-007-9608-5. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.