Abstract
Adaptation to stress by eukaryotic pathogens is often accompanied by a transition in cellular morphology. The human fungal pathogen Cryptococcus neoformans is known to switch between the yeast and the filamentous form in response to amoebic predation or during mating. As in the classic dimorphic fungal pathogens, the morphotype is associated with the ability of cryptococci to infect various hosts. Many cryptococcal factors and environmental stimuli, including pheromones (small peptides) and nutrient limitation, are known to induce the yeast-to-hypha transition. We recently discovered that secreted matricellular proteins could also act as intercellular signals to promote the yeast-to-hypha transition. Here we show that the secreted acyl coenzyme A (acyl-CoA)-binding protein Acb1 plays an important role in enhancing this morphotype transition. Acb1 does not possess a signal peptide. Its extracellular secretion and, consequently, its function in filamentation are dependent on an unconventional GRASP (Golgi reassembly stacking protein)-dependent secretion pathway. Surprisingly, intracellular recruitment of Acb1 to the secretory vesicles is independent of Grasp. In addition to Acb1, Grasp possibly controls the secretion of other cargos, because the graspΔ mutant, but not the acb1Δ mutant, is defective in capsule production and macrophage phagocytosis. Nonetheless, Acb1 is likely the major or the sole effector of Grasp in terms of filamentation. Furthermore, we found that the key residue of Acb1 for acyl binding, Y80, is critical for the proper subcellular localization and secretion of Acb1 and for cryptococcal morphogenesis.
INTRODUCTION
Adaptation to a changing environment by eukaryotic microbes is often accompanied by a transition in cellular morphology. The human fungal pathogen Cryptococcus neoformans causes devastating cryptococcal meningitis, which claims the lives of hundreds of thousands of people each year (1). Late diagnosis, limited options for antifungals, and the lack of vaccines to prevent cryptococcosis all contribute to the high mortality rate of this disease (2). C. neoformans typically grows as yeasts but can switch from yeasts to filaments (hyphae or pseudohyphae) in response to predation (e.g., by amoebae) or during sexual reproduction (3–7). As with many other fungal pathogens, the morphotype of C. neoformans shapes its interactions with various hosts (8). As we demonstrated recently, the hyphal form is associated with the attenuation of virulence in mouse models of cryptococcosis, because the hyphal morphotype elicits strong and protective host immune responses (9, 10). On the other hand, the hyphal morphotype assists the fungus in resisting predation from soil amoebae (8), increases its ability to explore the environment (11), and is linked to its unisexual and bisexual reproduction (3, 12–14). Thus, it is important to understand the factors that promote cryptococcal hyphal growth.
Many environmental stimuli and a few cryptococcal factors that promote hyphal growth in C. neoformans have been identified (12, 15–18). Pheromones are the most prominent cryptococcal molecules that stimulate mating and filamentation. We recently discovered that the matricellular and hypha-specific protein Cfl1, when released from the cell wall, can also act as an intercellular communication signal to stimulate the yeast-to-hypha transition (19, 20). Here we decided to investigate the potential role of the secreted protein Acb1 in filamentation and sexual reproduction in C. neoformans.
The acyl coenzyme A (acyl-CoA)-binding protein Acbp was first identified in mammals because its processed peptide inhibited the binding of diazepam to the gamma-aminobutyric acid (GABA) receptor, which gave rise to its name, diazepam binding inhibitor (DBI) (21). The homolog of DBI in Dictyostelium discoideum, called peptide signal spore differentiation factor 2 (SDF2), activates sporulation within the fruiting body (22). Similarly, SDF2 is processed from Dictyostelium AcbA (23, 24). In the absence of AcbA, D. discoideum fruiting bodies generate about 10% as many viable spores as the wild type (WT). Interestingly, coincubation of the acbAΔ mutant with wild-type cells restored the level of sporulation to that of the wild type (23). It was proposed that the AcbA secreted from the wild type was sufficient to complement the sporulation defect of the Dictyostelium acbAΔ mutant.
Acbps were later found to be widely distributed in the eukaryotic domain, and they play important roles in a wide range of biological processes (25–27). Among higher eukaryotes, there are multiple copies of Acbp-encoding genes in one genome, and these proteins differ in size and subcellular localization (28–34). Nonetheless, all Acbps are conserved in the acyl-CoA-binding domain (34). Not surprisingly, Acb1 in Saccharomyces cerevisiae helps transport newly synthesized acyl-CoA esters from the fatty acid synthase to acyl-CoA-consuming processes (35). Acb1 plays an important role in fatty acid elongation, membrane assembly, and protein trafficking in S. cerevisiae (36, 37).
Despite the predicted cytosolic localization of AcbA, due to the absence of a classical secretion signal or any transmembrane domain (38), it has been found to be located in puncta or vesicles in the cortical region in D. discoideum (39). The vast majority of AcbA proteins are intracellular; <5% are secreted extracellularly (39). However, extracellular secretion and postsecretion processing are critical for the signaling function of Dictyostelium AcbA in promoting spore generation (23, 39). The extracellular secretion of AcbA requires an unconventional pathway that is dependent on the Golgi apparatus-associated protein Grasp (Golgi reassembly stacking protein) in D. discoideum (38).
In the present study, we set out to investigate the role of secreted Acb1 in the yeast-to-hypha transition and sporulation in C. neoformans. We found that Acb1 contributes to the cryptococcal yeast-to-hypha transition. Interestingly, the secretion of Acb1 is dependent on its acyl-CoA-binding ability and on the Grasp protein in C. neoformans. Accordingly, mutation of the acyl-CoA-binding domain of Acb1 or deletion of the GRASP gene impairs cryptococcal hyphal growth.
MATERIALS AND METHODS
Media, strains, and in vitro phenotypic assay.
YPD medium (2% Bacto peptone, 1% yeast extract, and 2% glucose) was used for routine culture. For phenotypic assays, YNB medium (6.7 g/liter of yeast nitrogen base without amino acids or ammonium) without glucose was used as the base medium for testing the utilization of a specific nutrient source added to a final concentration of 2%, as indicated in the text and figures. For the phenotypic assays, wild-type and mutant cells were suspended at the same cell density. Cell suspensions with serial dilutions were spotted onto the relevant medium and were incubated for 2 to 3 days before photographs were taken. For the filamentation assay, we used V8 juice agar (50 ml V8 juice–0.5 g KH2PO4, in 1 liter [pH 5 or 7; adjusted with KOH]), YNB, or YPD medium. V8 juice medium is a commonly used mating medium in laboratory research (40). YNB is a minimal medium, and YPD is a nutrient-rich medium. All mutant strains were generated in the backgrounds of reference strains XL280 (serotype D) and H99 (serotype A). Both XL280 and H99 have publicly available genomes and congenic pairs (41, 42). The strains used in this study are listed in Table S1 in the supplemental material.
For bisexual mating, parental strains (mating types α and a) with equal number of cells were cocultured together on the YNB, V8 juice, or YPD medium in the dark at 22°C. Mating was examined microscopically for the formation of mating hyphae and spores. For unisexual mating (self-filamentation without a partner of an opposite mating type), individual isolates at the same cell density were dropped onto the YNB or V8 juice medium alone. Self-filamentation and sporulation were examined microscopically as described previously (14). XL280 is a hyperfilamentous strain, and it filaments robustly on V8 medium, which renders the reduction in the filamentation of the acb1Δ mutant less obvious. The reduction in the filamentation of the acb1Δ mutant is much more pronounced on the suboptimal YNB medium. In comparison, H99 strains filament poorly during bisexual mating on all these media, and the reduction in the filamentation of the acb1Δ mutant is evident irrespective of the medium used.
Confrontation assay.
The confrontation assay was used to test whether secreted products from the wild type could restore the bisexual mating defect of the acb1Δ mutant. The procedure was performed as described by us previously (19). Briefly, cocultured α and a cells of either the wild-type or the mutant strains at a 1:1 ratio were spotted onto the relevant medium (YNB or YPD medium) as donor strains. After the donor strains were incubated for 3 days, the recipient α-a cocultures (wild-type or mutant strains) were spotted onto the medium in close proximity to the donor cells (distance, <5 mm). After an additional 48 h of incubation, the cells were photographed in order to enable examination of colony morphology and the formation of hyphae in the recipients.
Gene deletion and complement.
The knockout and complementation constructs were generated as described previously (14, 43). To disrupt the ACB1 or GRASP gene, we amplified the 1-kb 5′ and 3′ flanking sequences of the coding region by using the genomic DNA isolated from strain XL280α or H99α as the template and the NEO or NAT dominant drug marker amplified from plasmid pAI1 or pJAF1, respectively. The knockout constructs with 5′ and 3′ flanking sequences bordering the selective marker gene were generated by overlap PCR as described by us previously (43). The knockout constructs were introduced into strains XL280α, XL280a, H99α, and KN99a by biolistic transformation as described previously (44). The resulting transformants were screened for gene replacement via homologous recombination events by PCR. The genetic linkage between the phenotype and the gene deletion was confirmed by analyzing the segregation pattern of the meiotic progeny generated from a bisexual cross between the mutant and a wild-type mating partner (43). For complementation, the wild-type genes with 1 to 1.5 kb upstream of their open reading frames (ORFs) were amplified by PCR, digested with proper restrictive digestion enzymes, and introduced into the pXL1-mCherry plasmid (9). The resulting plasmid, pXL1-ACB1-mCherry, was confirmed by enzyme digestion and gel electrophoresis. The plasmids were then linearized and were transformed into the relevant Cryptococcus strains through biolistic transformation or electroporation as described by us previously (43). The primers and plasmids used for this study are listed in Table S2 in the supplemental material.
Target site-directed mutagenesis.
To mutate the acyl-CoA-binding site, the key residue of Acb1, Y80, was mutated to A (Y80A) by using a site-directed mutagenesis kit (QuikChange II; Agilent Technologies) according to the manufacturer's instructions. The fragment with the mutated allele of ACB1 and the 1-kb sequences upstream of the ACB1 ORF was ligated into plasmid PXL1-mCherry (9). The resulting plasmid, PXL1-Acb1(Y80A)-mCherry, was linearized and was transformed into XL280 or H99 as described above.
Microscopic examination.
To examine the subcellular localization of Acb1::mCherry or Acb1(Y80A)::mCherry, the relevant strains were cultured on YPD or YNB agar medium at 30°C for 24 h. Images were acquired and processed with a Zeiss M2 imaging system with the AxioCam MRm camera and Zen 11 software (Carl Zeiss Microscopy).
RNA extraction and qPCR.
RNA extraction and quantitative PCR (qPCR) were performed as described previously (9). Briefly, strains with opposite mating types were cocultured on YNB agar medium for the indicated durations (see Fig. 1C). Cells were harvested, washed with cold water, immediately frozen in liquid nitrogen, and then lyophilized. Cells were broken into a fine powder with glass beads, and total RNA was extracted with the PureLink RNA minikit (Life Technologies) according to the manufacturer's instructions. First-strand cDNA was synthesized with a SuperScript III cDNA synthesis kit (Invitrogen) according to the manufacturer's instructions. The housekeeping gene TEF1 was used as the endogenous control. The relative transcript levels were determined by using the comparative ΔΔCT method as described previously (9). Three biological replicates were performed for each sample, and their values were used to calculate the mean value and standard error.
FIG 1.
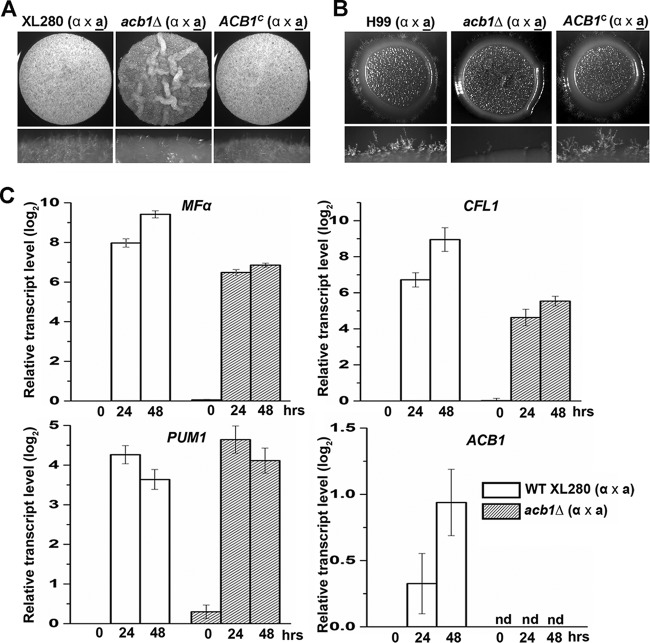
Deletion of ACB1 impairs filamentation during bisexual mating in Cryptococcus neoformans. (A) The a-α mating pairs of the WT XL280 strains, the acb1Δ mutants, and the ACB1-complemented strains were cultured on YNB medium for 48 h. (B) The a-α mating pairs of the WT H99 strains, the acb1Δ mutants, and the ACB1-complemented strains were cultured on YNB medium for 9 days. (C) Measurement of the transcript levels of MFα, CFL1, PUM1, and ACB1 by reverse transcription-quantitative PCR. The transcript level of each gene in the wild type at the 0-h time point was set at 1 (log2 value, 0) for comparison. RNA samples were extracted at 0 h, 24 h, and 48 h after the bisexual mating of WT XL280 and the acb1Δ mutants on YNB medium. nd, not detected.
Protein extraction and Western blotting.
Strains carrying Acb1-mCherry or Acb1(Y80A)-mCherry in the wild-type or graspΔ mutant background were cultured in YNB liquid medium with the proteinase inhibitors PMSF (phenylmethylsulfonyl fluoride) and protease inhibitor cocktail (Roche Inc.) for 48 h. The culture supernatant was separated from the cell pellet by centrifugation. The supernatant was concentrated with an Amicon Ultra-15 centrifugal filter (EMD Millipore) and was denatured with an SDS-containing loading buffer before electrophoresis in an SDS gel. The cell pellet was washed twice with cold phosphate-buffered saline (PBS) and was then lyophilized. The dried cells were disrupted by a cell disruptor (Next Advance) with glass beads. The total proteins were extracted with a lysis buffer (25 mM HEPES [pH 7.5], 300 mM NaCl, 2 mM EDTA, plus a proteinase inhibitor cocktail) and were then denatured with the SDS-containing loading buffer before electrophoresis in an SDS gel. Western blotting was carried out as described previously (19, 45). Briefly, the samples were separated on an SDS–12% PAGE gel and were transferred to a polyvinylidene difluoride (PVDF) membrane (Millipore) for 1 h at 30 V in a TE 70 ECL semidry transfer unit (GE Healthcare). The blots were incubated with an anti-mCherry primary antibody (dilution, 1/2,000), washed, and then incubated with a rabbit anti-mouse secondary antibody (dilution, 1/10,000) (Clontech Inc.). Signals were detected by using the enhanced chemiluminescence (ECL) system according to the instructions provided by the manufacturer (Pierce).
Phagocytosis assay.
The phagocytosis assay was performed as described by us previously (8). Briefly, the J774A.1 macrophage cell line (ATCC TIB-67) was cultured in Dulbecco's modified Eagle's medium (DMEM; catalog no. 30-2002) with 10% fetal bovine serum (FBS). Three hundred microliters of culture with 2.5 × 105 freshly grown J774A.1 cells was seeded into each well of a 24-well microtiter plate. The macrophages were cultured at 37°C under 5% CO2 overnight, and the medium was then replaced with fresh medium. Each well was inoculated with Cryptococcus cells to achieve a multiplicity of infection (MOI) of 3. After 30 s of mixing in a rocker, the cocultures were incubated at 37°C under 5% CO2 for an additional 3 h. The cocultures were then washed three times with warm PBS (500 μl/well) to remove the medium and nonadherent cells. Then PBS plus 0.1% Tween 20 was added to the culture, which was incubated for 10 min at 37°C to lyse the macrophages. The supernatant was then harvested, serially diluted, and spread onto YNB agar plates. The cryptococcal CFU was counted after 2 days of incubation at 30°C.
RESULTS
Deletion of the ACB1 gene reduced hyphal growth.
Based on our transcriptome-sequencing (RNA-seq) data, ACB1 is one of the abundantly expressed genes (46). Since secreted and processed Acb1 is involved in sexual reproduction as a signal molecule in other species (23, 39), we decided to test whether Acb1 is also important for Cryptococcus sexual reproduction. We first examined the transcript level of ACB1 during bisexual mating. We found that the expression of ACB1 was modestly increased during mating (Fig. 1C), suggesting a possible role for Acb1 in this biological process.
To examine the role of Acb1 in sexual reproduction and hypha formation in C. neoformans, the ACB1 gene (CNM01420) was deleted in the hyperfilamentous serotype D strain XL280α and its congenic strain XL280a (3, 47). No ACB1 transcript could be detected in the acb1Δ mutants (Fig. 1C), as expected. Under mating-inducing conditions on V8 juice agar medium, the a-α mating pair of the acb1Δ mutant showed dramatically reduced filamentation relative to that of the wild type, as reflected in the less white and fluffy mutant colony (Fig. 1A). Ectopic introduction of a wild-type allele of ACB1 into the acb1Δ mutant restored the defect (Fig. 1A), supporting the role of Acb1 in filamentation. Under mating-suppressing conditions on YPD agar medium, the colony derived from the wild-type a-α mating pair was wrinkled, with some degree of filamentation at the colony edge (see Fig. S1A in the supplemental material). In contrast, the colony derived from the acb1Δ a-α mating pair was smooth and almost barren at the edge (see Fig. S1A). During unisexual mating with only α cells, wild-type XL280α cells cultured on YNB medium produced a wrinkled colony with hyphae at the colony edge (see Fig. S1B). The acb1Δ mutant generated a smooth colony with very few hyphae at the colony edge (see Fig. S1B). Ectopic introduction of the wild-type ACB1 gene into the acb1Δ mutant restored self-filamentation to the wild-type level. These observations indicate that Acb1 also enhances hyphal growth during self-filamentation.
To test whether the role of Acb1 in filamentation is conserved in other Cryptococcus subspecies, we deleted the ACB1 gene (CNAG_06140) in the serotype A reference strain H99α and the mating type a strain KN99a. As with XL280, the acb1Δ a-α mating pair in the H99 background showed a drastic reduction in filamentation when cultured on V8 juice agar medium, and robust filamentation could be restored by the introduction of a wild-type copy of ACB1 (Fig. 1B). Taken together, the results indicate that Acb1 has a conserved role in enhancing filamentation during bisexual mating in C. neoformans. Because sporulation is preceded by a yeast-to-hypha transition in C. neoformans (48), and because Acb1 in Dictyostelium discoideum is known to trigger sporulation within fruiting bodies (23), we decided to test if Acb1 is required for sporulation in C. neoformans. We examined spore production by a-α bisexual mating of the wild-type strain XL280 and the corresponding acb1Δ mutant on V8 juice medium. Although the level of filamentation was reduced in the acb1Δ mutant, both mating pairs produced 4 chains of basidiospores (Fig. 2A and B), and we found no apparent defect or drastic reduction in spore production.
FIG 2.
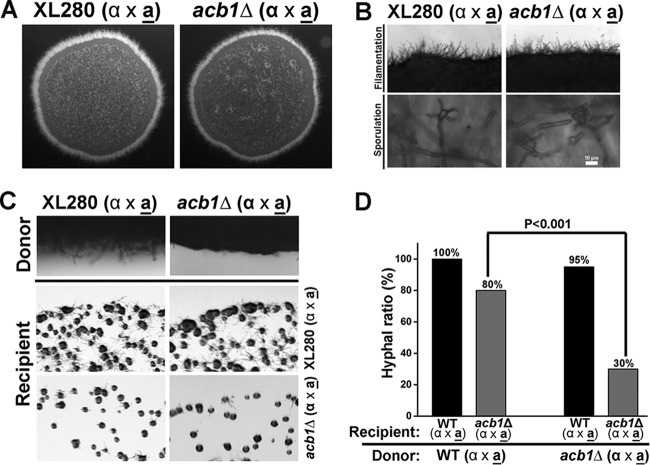
Secreted products from the wild type, but not from the acb1Δ mutant, enhanced filamentation in neighboring cells. (A) Images of colonies formed by a-α bisexual mating of WT XL280 and the corresponding acb1Δ mutant on V8 medium (pH 7). (B) Images of the colony edge (top) and basidiospores (bottom) generated by the bisexual mating of the WT strain or the acb1Δ mutant on V8 medium. (C) Confrontation assay using WT and mutant mating pairs as the donor or recipient. (D) Frequency of filament formation by recipient colonies at 48 h postinoculation. Eighty percent of the acb1Δ mutant recipient colonies formed hyphae when the donor was WT. In contrast, 30% of the acb1Δ mutant recipient colonies formed hyphae when confronted with an acb1Δ mutant donor.
Deletion of ACB1 reduced the transcript level of the pheromone gene MFα and the hypha-specific gene CFL1.
C. neoformans undergoes a yeast-to-hypha transition during both unisexual and bisexual mating. The pheromone signaling pathway initiates the process under mating-inducing conditions (14), and the activation of the filamentation pathway eventually leads to hyphal growth (9, 14). To understand how the loss of ACB1 affects filamentation, we decided to measure the impact of ACB1 deletion on the transcript levels of MFα, CFL1, and PUM1 during bisexual mating. The pheromone MFα is the initial signaling factor initiating mating (49). The secreted protein Cfl1 is a specific marker for filamentation (19). Pum1 is a genetic linker between filamentation and sporulation (48). The basal levels of all three transcripts at 0 h were similar for the wild type and the acb1Δ mutant (Fig. 1C). The transcript levels of both MFα and CFL1 were induced in the wild type as well as in the acb1Δ mutant during mating (Fig. 1C). However, the degrees of induction for both MFα and CFL1 were lower in the acb1Δ mutant than in the wild type (Fig. 1C). This is consistent with the reduced filamentation observed in the acb1Δ mutant. Interestingly, the transcript levels of PUM1, a gene that connects filamentation with sporulation in C. neoformans (48), were comparable for the wild type and the acb1Δ mutant at all three time points examined (Fig. 1C). Given that deletion of PUM1 causes the formation of barren basidial heads without spores (48), the finding that PUM1 expression is unaltered in the acb1Δ mutant is consistent with the observation that the acb1Δ mutant displays no specific defects in sporulation (Fig. 2B). Taken together, these observations indicate that secreted Acb1 contributes to the cryptococcal morphotype transition at least partly through its effects on the pheromone signaling and filamentation pathways.
Secreted products from the wild type, but not the acb1Δ mutant, could enhance hyphal formation in a nearby acb1Δ recipient strain.
In Dictyostelium discoideum, extracellular Acb1 secreted from wild-type cells acts as a signal and can compensate for the loss of ACB1 in nearby mutant cells in terms of sporulation (23). Since Acb1 is important for filamentation in C. neoformans and is highly expressed, we hypothesize that secreted Acb1 from the wild type may also act as a signal in promoting filamentation in the Cryptococcus acb1Δ mutant. To test this hypothesis, we performed confrontation assays, in which the donor and the recipient were placed in close proximity but not physically touching each other. The wild-type recipient filamented well regardless of whether the donor was the wild type or the acb1Δ mutant (Fig. 2C and D; see also Fig. S2 in the supplemental material). However, more acb1Δ recipient colonies formed filaments when the donor was the wild type rather than the acb1Δ mutant. Despite the increased frequency of hypha formation by acb1Δ recipient colonies with a wild-type donor, the hyphae formed by the mutant were rudimentary at the time point examined (see Fig. S2). Nonetheless, the evidence suggests that products secreted from the wild-type donor, but not from the acb1Δ mutant donor, enhanced the frequency of filamentation by the nearby acb1Δ recipient cells.
Acb1 promotes the utilization of alternative carbon sources.
As an acyl-CoA-binding protein, Acb1 regulates growth in different media and under different conditions, as demonstrated in S. cerevisiae (35–37, 50, 51). To our surprise, we found no apparent growth defect in the Cryptococcus acb1Δ mutants in either the rich YPD medium or the minimum YNB medium (Fig. 3; see also Fig. S3 and S4 in the supplemental material). The mutants also showed no difference from the corresponding wild-type strains in their tolerance of SDS and antifungal drugs such as caspofungin, polymyxin B, and fluconazole (not shown). In yeast and mammalian cells, the acb1Δ mutant showed a severe defect in long-chain fatty acid metabolism (35, 52). However, in C. neoformans, the acb1Δ mutant grew well on lipids as the sole carbon source, just like the wild type (see Fig. S5 in the supplemental material).
FIG 3.
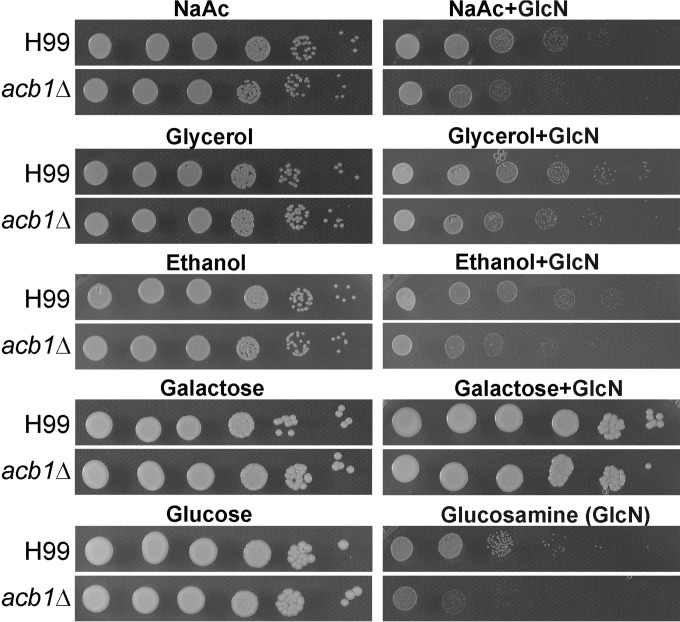
Growth of the acb1Δ mutant and the corresponding wild-type strain on different carbon sources in the absence and presence of glucosamine (GlcN).
More surprisingly, the acb1Δ mutant in either the XL280 or the H99 background grew equally well as the corresponding wild-type strains on media with different carbon sources (glucose, galactose, glycerol, sodium acetate [NaAc], or ethanol) or different nitrogen sources [(NH4)2SO4, NaNO3, glycine, aspartic acid, or thiamine] (Fig. 3; see also Fig. S3 and S4 in the supplemental material). This is, again, different from what was observed for the S. cerevisiae acb1Δ mutant, which showed growth defects with different carbon sources (35, 37, 50).
The presence of the preferred carbon source (usually glucose) represses the utilization of other carbon sources (53, 54). This is called catabolite repression. Catabolite repression can be observed with the addition of glucosamine, a glucose mimic (55, 56). The idea is that the presence of glucosamine (GlcN) suppresses the catabolism of other carbon sources and thus inhibits growth even when other carbon sources are available. Indeed, we found that the addition of glucosamine inhibited the utilization of NaAc, glycerol, and ethanol by the wild type (Fig. 3; see also Fig. S4 in the supplemental material). The acb1Δ mutant showed more-severe growth deficiency than the wild type in using NaAc or ethanol in the presence of glucosamine (Fig. 3; see also Fig. S4). This suggests that Acb1 in the wild type might be involved in relaxing catabolite repression, which could be useful for an organism found in soil and decaying vegetation, where complex carbon sources other than glucose are likely to be present.
The Y80 residue in the acyl-CoA-binding domain is critical for the function and subcellular localization of Acb1.
The acyl-CoA-binding domain is highly conserved among Acb proteins (Fig. 4A), suggesting the importance of this domain to the function of Acb1. In this region, Y80 has been shown to be a conserved and important residue for binding acyl-CoA: a mutation of this residue can decrease a protein's acyl-binding ability 1,000-fold (28, 57–59).
FIG 4.
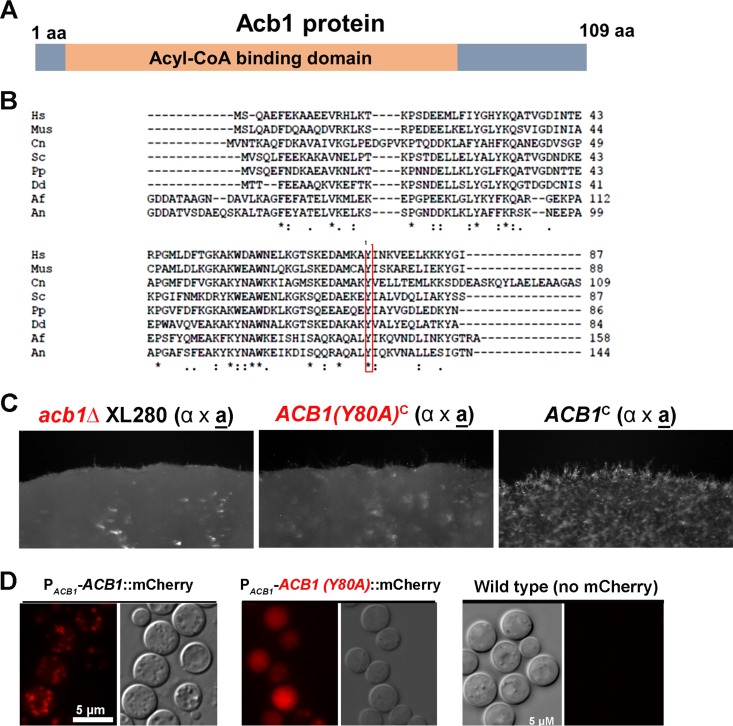
Mutation of the key residue Y80 affects the function and subcellular localization of Acb1. (A) Diagram of the Acb1 protein. (B) Acb1 is a highly conserved protein among different species. Shown is a multiple alignment of proteins from the following species: Hs, Homo sapiens; Mus, mouse species; Cn, Cryptococcus neoformans; Sc, Saccharomyces cerevisiae; Pp, Pichia pastoris; Dd, Dictyostelium discoideum; Af, Aspergillus fumigatus; An, Aspergillus nidulans. (C) The mutated Acb1(Y80A) strain could not restore the filamentation defect of the acb1Δ mutant. The acb1Δ mutant, the acb1Δ mutant transformed with the wild-type allele [ACB1c], and the acb1Δ mutant transformed with the Y80A allele [ACB1(Y80A)c] were cultured on YNB medium for 48 h. (D) Subcellular localization of Acb1-mCherry and Acb1(Y80A)-mCherry. The fluorescent image of nontransformed wild-type cells was used as the negative control.
To examine if the acyl-CoA-binding domain is critical for the function of Cryptococcus Acb1 in filamentation, we made a mutated (Y80A) allele of Acb1 through site-directed mutagenesis. The Acb1(Y80A) mutated allele, when introduced into the acb1Δ mutant, could not restore the mutant's filamentation defect, in contrast to the wild-type allele (Fig. 4B). This result suggests that acyl-CoA-binding ability is critical for the function of Acb1 in filamentation.
Acb1, despite its predicted cytosolic location, is known to be recruited to the secretory pathway in other organisms (28, 39). Under a wide-field epifluorescence microscope, the mCherry-labeled Acb1 in C. neoformans was located in intracellular puncta (Fig. 4C) that are consistent with secretory vesicles. In Dictyostelium discoideum, Acbp is also localized to intracellular puncta (39). Acb1(Y80A)-mCherry, however, showed a diffused cytoplasmic localization (Fig. 4C). The cytosolic localization of Acb1(Y80A) in C. neoformans is consistent with previous observations with S. cerevisiae and D. discoideum, where a decreased or abolished ability of Acbp to bind to acyl-CoA is associated with increased cytosolic localization (28, 39). Thus, previously published literature and our observation, taken together, indicate that the recruitment of Acb1 to the secretory pathway requires its acyl-CoA-binding capability. It is tempting to speculate that the binding partner of Acb1 might help bring this otherwise cytosolic protein to the secretory pathway.
In D. discoideum, a minor proportion of AcbA proteins was secreted extracellularly (39). This is also true in C. neoformans, where we found most of the Acb1 proteins in the total-cell lysate and some in the culture supernatant (Fig. 5B). Because Acb1(Y80A) is localized to the cytosol, we speculated that extracellular secretion of this mutated protein would be abolished. Indeed, we could not detect any Acb1(Y80A) in the culture supernatant, although we could easily detect the protein in the cell lysate. This suggests that the mutated Acb1(Y80A) protein was not released to the environment. Thus, the alteration of this key residue affects the function of Acb1 as well as its subcellular localization (Fig. 5B).
FIG 5.
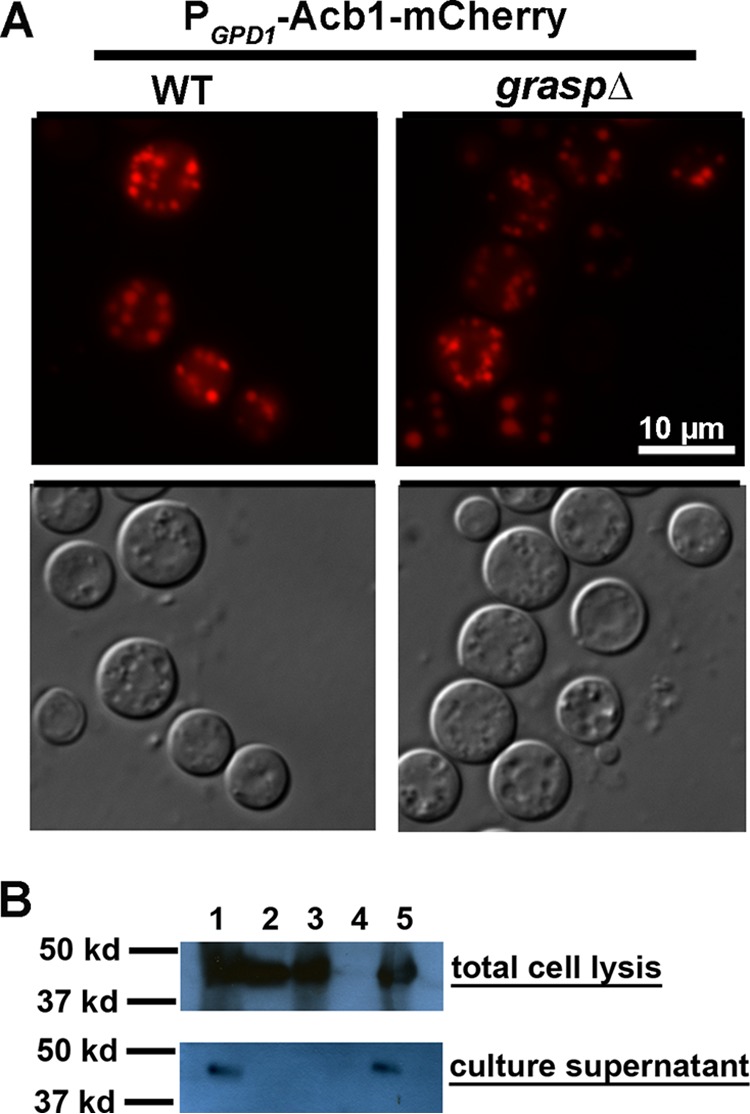
The subcellular localization of Acb1 is independent of Grasp, but the secretion of Acb1 requires Grasp. (A) Intracellular localization of Acb1-mCherry in the wild-type or graspΔ mutant background. (B) Western blots of supernatants from different strains. Lanes: 1, Acb1-mCherry in the acb1Δ mutant; 2, Acb1-mCherry in the graspΔ mutant; 3, Acb1(Y80A)-mCherry in the acb1Δ mutant; 4, XL280 without any mCherry, used as a negative control; 5, Acb1-mCherry in WT XL280.
The extracellular secretion of Acb1, but not its recruitment to the secretory pathway, is dependent on Grasp.
Acb1 lacks a signal peptide and is not a typical secretory protein that uses the conventional or the general secretion pathway. In S. cerevisiae and D. discoideum, the secretion of Acb1 was shown to be dependent on GRASPs (Golgi reassembly stacking proteins) (38, 60–62), which were originally identified as factors required for the stacking of Golgi cisternae and the tethering of vesicles destined to fuse with the Golgi apparatus (63, 64). In addition to Acb1, the secretion of other factors, such as integrin and CFTR (cystic fibrosis transmembrane conductance regulator), also depends on the unconventional Grasp-mediated pathway in other organisms (38, 65–68). Mammalian genomes encode two orthologs of GRASP genes (66), whereas only one GRASP gene has been found in C. neoformans and yeast previously (61, 69). It is noteworthy that in both fungal species, Grasp was shown not to be involved into Golgi stacking but to be important for molecular secretion (61, 69).
To test if Grasp in C. neoformans is involved in the recruitment of Acb1 to the secretory pathway and/or in its extracellular secretion, we deleted the GRASP gene. We then examined the localization and extracellular secretion of Acb1-mCherry in the graspΔ mutant background. Interestingly, we found that Acb1-mCherry was localized to vesicles in the graspΔ mutant, as observed in the wild type (Fig. 5A). This suggests that the recruitment of Acb1 to the secretory pathway is independent of Grasp.
Next, we tested whether the absence of Grasp affects the extracellular secretion of Acb1 in C. neoformans by using PACB1-ACB1-mCherry as the reporter. We detected a strong Acb1-mCherry signal from the cell lysate of the graspΔ mutant (Fig. 5B). This is consistent with our microscopic observation of Acb1-mCherry in intracellular vesicles in the wild type as well as in the graspΔ mutant. However, no Acb1-mCherry was detected in the supernatant derived from the graspΔ mutant (Fig. 5B), in contrast to its presence in the supernatant derived from the wild-type background. This suggests that extracellular release of Acb1-mCherry is abolished in the graspΔ mutant. Taking these findings together, we conclude that the recruitment of Acb1 to the secretory pathway is independent of Grasp but that its extracellular secretion requires Grasp.
The graspΔ mutant recapitulated the acb1Δ mutant phenotype in terms of filamentation.
The evidence presented above indicates that Acb1 proteins are predominantly localized intracellularly, with some being secreted extracellularly. Since Acb1 is important for filamentation, and since secreted products from the wild-type donor, but not the acb1Δ mutant donor, can enhance filamentation in nearby acb1Δ cells, we hypothesize that released extracellular Acb1 is important for filamentation. Since Grasp is required for the extracellular secretion of Acb1, but not for its production or intracellular localization, we decided to test our hypothesis using the graspΔ mutant. The graspΔ mutant in the H99 background showed reduced filamentation during bisexual mating on V8 medium, as observed for the acb1Δ mutant (Fig. 6A). Similarly, the graspΔ mutant in the XL280 background yielded a colony with smoother morphology and showed less-robust hyphal production than the wild type during bisexual mating on YNB medium, resembling the acb1Δ mutant (Fig. 6B). Consistently, the graspΔ a-α mixed culture gave rise to a smooth colony on YPD medium, in contrast to the wrinkled colony generated by the wild-type a-α mixed culture (Fig. 6C). Thus, the graspΔ mutant displayed the same colony morphology and filamentation phenotypes as the acb1Δ mutant. This suggests that Grasp regulates cryptococcal morphogenesis mainly through extracellularly secreted Acb1.
FIG 6.

The graspΔ mutant recapitulates the phenotype of the acb1Δ mutant in terms of colony morphology and filamentation. Shown are a-α cocultures of the wild type, the acb1Δ mutant, and the graspΔ mutant in the H99 background on V8 juice medium (pH 5) (A), in the XL280 background on YNB medium (B), and in the XL280 background on YPD medium (C). Panels A and B include images of the entire colonies and the colony edges. Panel C shows images of the entire colonies.
Deletion of GRASP, but not deletion of ACB1, affects capsule production and phagocytosis by macrophages.
We showed in the preceding section that the graspΔ mutant recapitulates the same colony morphology and filamentation phenotypes as the acb1Δ mutant. It was shown previously that the graspΔ mutant in C. neoformans is defective in capsule production and macrophage phagocytosis (69). To test if such defects in the graspΔ mutant are caused by its defect in the extracellular secretion of Acb1, we first examined the production of capsule and melanin in the acb1Δ mutant. To our surprise, deletion of ACB1 had no apparent impact on capsule production and melanization. In contrast, deletion of GRASP greatly reduced capsule size (Fig. 7A), a finding consistent with the previous report (69). There might be a slight reduction in the melanization of the graspΔ mutant (Fig. 7B). Next, we tested the phagocytosis of the acb1Δ mutant in both the XL280 and H99 backgrounds by J774A.1 murine macrophages. Although the levels of phagocytosis of the acb1Δ mutants might be slightly lower than those of the corresponding wild-type strains (Fig. 7C), the differences were not statistically significant. In contrast, the levels of phagocytosis of the graspΔ mutants were significantly lower than those of wild-type cells in both the XL280 and H99 backgrounds (Fig. 7C), as demonstrated previously for the graspΔ mutant in the H99 background (69). These results indicate that Acb1 is not the only effector of Grasp in C. neoformans but is likely the major, or the sole, effector of Grasp in terms of filamentation.
FIG 7.
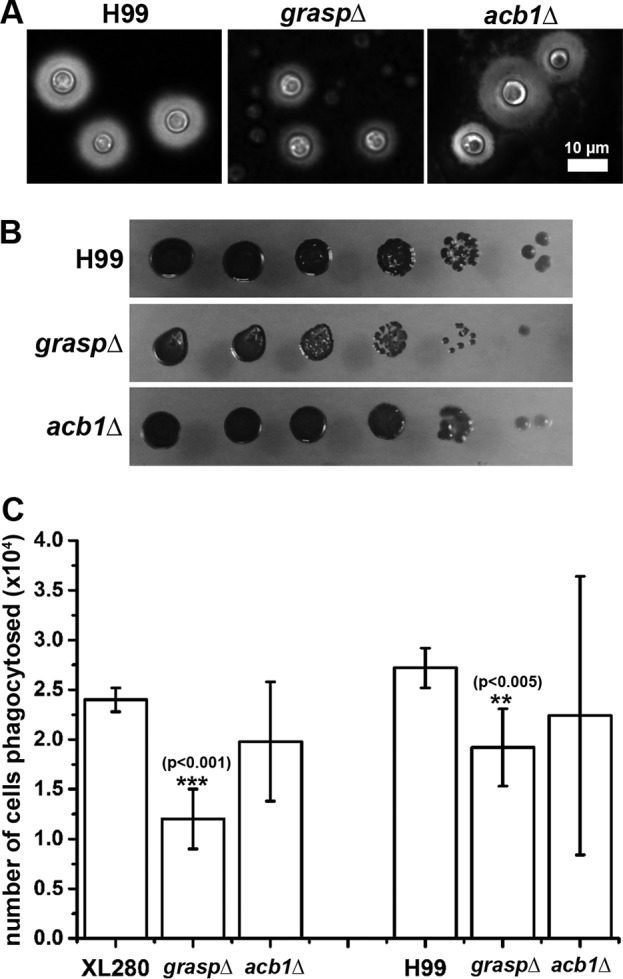
The graspΔ mutant showed slight deficiencies in multiple classic cryptococcal virulence traits. (A) The WT, the graspΔ mutant, and the acb1Δ mutant after culture on RPMI medium for 2 days. The capsule (halo surrounding the yeast cells) was visualized by negative staining with India ink. (B) The WT, the graspΔ mutant, and the acb1Δ mutant were cultured on l-3,4-dihydroxyphenylalanine medium for 3 days in order to test melanin formation. The dark pigment indicates melanin. (C) Macrophages were inoculated with the WT, the graspΔ mutant, and the acb1Δ mutant on RPMI medium and were cocultured for 3 h. The numbers of phagocytosed Cryptococcus cells were determined by CFU counting and were graphed.
DISCUSSION
We demonstrated previously that the secreted matricellular protein Cfl1, a downstream target of the global regulator Znf2 (9, 14), plays important roles in cellular and colony morphogenesis in the environmental fungal pathogen C. neoformans (19, 20). In this study, we investigated the role of the secretory protein Acb1 in the cryptococcal yeast-to-hypha morphological transition and in cryptococcal sporulation, given the importance of its ortholog in sporulation in Dictyostelium discoideum and Pichia pastoris (23, 62). In contrast to Acbp in D. discoideum (23), we found that Acb1 in Cryptococcus neoformans is not critical for sporulation per se but that secreted Acb1 is important for hyphal growth, which precedes sporulation during both unisexual and bisexual reproduction. The function of Acb1 in cellular and colony morphology is conserved in both serotype A and serotype D, two subspecies of the C. neoformans species complex (70). However, in contrast to CFL1, ACB1 is unlikely to be controlled by Znf2 at the transcript level, despite the modest increase in the number of ACB1 transcripts during bisexual mating. First, ACB1 is not among the genes in the ZNF2oe strain or the znf2Δ strain that are differentially expressed relative to the wild-type control (9, 14). Second, Znf2-controlled genes are typically expressed at low levels during yeast growth and are highly induced during filamentous growth (9, 19). This is not the case for ACB1. The transcript level of the ACB1 gene is high even during yeast growth in YPD medium. In fact, on the basis of our recent RNA-seq data analyses, ACB1 ranks in the top ∼5% among all cryptococcal genes expressed (46). However, whether Znf2 directly or indirectly affects the activity of Acb1 at other regulatory levels (e.g., translation, protein localization, secretion, or modification) has yet to be investigated.
The cryptococcal genome carries two genes that encode proteins with an acyl-CoA-binding domain. One is CNAG_06140, which we named ACB1 in this study; it is predicted to encode a protein of a little over 100 amino acids (Fig. 4A). The other is CNAG_01191, which is predicted to encode a long-chain fatty acid transporter of 458 amino acids. Given that AcbP in D. discoideum and Acb1 in S. cerevisiae are composed of 84 and 87 amino acids, respectively (23, 26, 35, 71), we considered CNAG_06140/Acb1 in C. neoformans more likely to be an ortholog of Acb proteins. Furthermore, based on our RNA-seq data, the transcript level of ACB1 is about 10-fold higher than that of CNAG_01191 (46). Thus, we focused on ACB1 in this study. However, it is likely that the lack of any defect of the Cryptococcus acb1Δ mutant in utilizing various carbon sources could be due to functional redundancy of Acb1 and the protein encoded by CNAG_01191 in fatty acid metabolism.
The observation that secreted products from the wild type, but not the acb1Δ mutant, can partially restore the filamentation defect of a nearby acb1Δ mutant suggests that secreted Acb1 proteins can act intercellularly. Given that the filamentation of the acb1Δ mutant is not as robust as that of the wild type, even when it is confronted by the wild-type donor, Acb1 likely functions in a paracrine fashion. We hypothesize that it is the extracellular Acb1 proteins, and not the intracellular Acb1 proteins, that are critical for filamentation. This hypothesis is consistent with the predicted paracrine signaling function of Acb1 and is also corroborated by the finding that the graspΔ mutant, which is defective in secreting Acb1 to the environment but not in recruiting Acb1 to the secretory pathway, displays a drastic reduction in filamentation, similar to that of the acb1Δ mutant. The hypothesis is further supported by the observation that mislocalization of Acb1(Y80) to the cytosol and the consequent lack of protein secretion render the protein nonfunctional.
One interesting aspect of Acb1 is its unconventional recruitment to the secretory pathway. Although Grasp is critical for the extracellular secretion of Acb1, Grasp is not involved in recruiting Acb1 to the secretory vesicles intracellularly. Since the Y80 mutation is known to disrupt the acyl-binding property of Acb1 (58, 59, 72), and since Acb1(Y80A) showed diffused localization in the cytosol, it is tempting to speculate that the binding partner of Acb1, be it a lipid or a protein, might help recruit Acb1 to the vesicles through interaction with its acyl-binding domain. How Grasp recognizes Acb1 after Acb1 is recruited to secretory vesicles and how it assists in the extracellular secretion of Acb1 are unknown. It is clear, on the basis of this study and a previous study (69), that Grasp is involved in the secretion of Acb1 and additional factors in C. neoformans. In agreement with this idea, the secretion of integrin in Drosophila melanogaster and of CFTR in mammalian cells also depends on an unconventional Grasp-mediated mechanism (67, 68, 73). Given that many proteins that are found to be secreted extracellularly are atypical proteins that possess no signal peptide in fungi (74–78), it is important to continue the investigation into these atypical proteins and the corresponding unconventional secretory pathways.
In accord with the idea that Grasp is responsible for the secretion of factors in addition to Acb1, the graspΔ mutant showed drastically decreased capsule production (69; this study), while the acb1Δ mutant showed normal capsule production. Similarly, in contrast to the severe phagocytosis defect of the graspΔ mutant, the acb1Δ mutant behaves similarly to the wild type in the phagocytosis assay. Thus, Grasp likely affects the secretion of various molecules (e.g., capsule and possibly some cell wall proteins) that contribute to the phenotypic defects of the graspΔ mutant in various assays. Nonetheless, in terms of the effect of Grasp on hyphal growth, Acb1 appears to be the major factor, if not the sole factor.
Supplementary Material
ACKNOWLEDGMENTS
We thank Srijana Upadhyay for assistance with microscopic studies and members of the Lin lab for helpful suggestions.
Funding Statement
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.03691-15.
REFERENCES
- 1.Park BJ, Wannemuehler KA, Marston BJ, Govender N, Pappas PG, Chiller TM. 2009. Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. AIDS 23:525–530. doi: 10.1097/QAD.0b013e328322ffac. [DOI] [PubMed] [Google Scholar]
- 2.Idnurm A, Lin X. 2015. Rising to the challenge of multiple Cryptococcus species and the diseases they cause. Fungal Genet Biol 78:1–6. doi: 10.1016/j.fgb.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lin X, Hull CM, Heitman J. 2005. Sexual reproduction between partners of the same mating type in Cryptococcus neoformans. Nature 434:1017–1021. doi: 10.1038/nature03448. [DOI] [PubMed] [Google Scholar]
- 4.Lin X, Litvintseva A, Nielsen K, Patel S, Kapadia Z, Floyd A, Mitchell TG, Heitman J. 2007. αADα hybrids of Cryptococcus neoformans: evidence of same sex mating in nature and hybrid fitness. PLoS Genet 3:e186. doi: 10.1371/journal.pgen.0030186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin X, Patel S, Litvintseva AP, Floyd A, Mitchell TG, Heitman J. 2009. Diploids in the Cryptococcus neoformans serotype A population homozygous for the α mating type originate via unisexual mating. PLoS Pathog 5:e1000283. doi: 10.1371/journal.ppat.1000283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kwon-Chung KJ. 1975. A new genus, Filobasidiella, the perfect state of Cryptococcus neoformans. Mycologia 67:1197–1200. doi: 10.2307/3758842. [DOI] [PubMed] [Google Scholar]
- 7.Kwon-Chung KJ. 1976. Morphogenesis of Filobasidiella neoformans, the sexual state of Cryptococcus neoformans. Mycologia 68:821–833. doi: 10.2307/3758800. [DOI] [PubMed] [Google Scholar]
- 8.Lin J, Idnurm A, Lin X. 2015. Morphology and its underlying genetic regulation impact the interaction between Cryptococcus neoformans and its hosts. Med Mycol 53:493–504. doi: 10.1093/mmy/myv012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang L, Zhai B, Lin X. 2012. The link between morphotype transition and virulence in Cryptococcus neoformans. PLoS Pathog 8:e1002765. doi: 10.1371/journal.ppat.1002765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhai B, Wozniak KL, Masso-Silva J, Upadhyay S, Hole C, Rivera A, Wormley FL Jr, Lin X. 2015. Development of protective inflammation and cell-mediated immunity against Cryptococcus neoformans after exposure to hyphal mutants. mBio 6:e01433-15. doi: 10.1128/mBio.01433-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Phadke SS, Feretzaki M, Heitman J. 21 June 2013. Unisexual reproduction enhances fungal competitiveness by promoting habitat exploration via hyphal growth and sporulation. Eukaryot Cell doi: 10.1128/EC.00147-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alspaugh JA, Davidson RC, Heitman J. 2000. Morphogenesis of Cryptococcus neoformans. Contrib Microbiol 5:217–238. doi: 10.1159/000060352. [DOI] [PubMed] [Google Scholar]
- 13.Hull CM, Heitman J. 2002. Genetics of Cryptococcus neoformans. Annu Rev Genet 36:557–615. doi: 10.1146/annurev.genet.36.052402.152652. [DOI] [PubMed] [Google Scholar]
- 14.Lin X, Jackson JC, Feretzaki M, Xue C, Heitman J. 2010. Transcription factors Mat2 and Znf2 operate cellular circuits orchestrating opposite- and same-sex mating in Cryptococcus neoformans. PLoS Genet 6:e1000953. doi: 10.1371/journal.pgen.1000953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang L, Lin X. 2015. The morphotype heterogeneity in Cryptococcus neoformans. Curr Opin Microbiol 26:60–64. doi: 10.1016/j.mib.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 16.Fu C, Sun S, Billmyre RB, Roach KC, Heitman J. 2015. Unisexual versus bisexual mating in Cryptococcus neoformans: consequences and biological impacts. Fungal Genet Biol 78:65–75. doi: 10.1016/j.fgb.2014.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang P, Heitman J. 1999. Signal transduction cascades regulating mating, filamentation, and virulence in Cryptococcus neoformans. Curr Opin Microbiol 2:358–362. doi: 10.1016/S1369-5274(99)80063-0. [DOI] [PubMed] [Google Scholar]
- 18.Wang L, Lin X. 2011. Mechanisms of unisexual mating in Cryptococcus neoformans. Fungal Genet Biol 48:651–660. doi: 10.1016/j.fgb.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 19.Wang L, Tian X, Gyawali R, Lin X. 2013. Fungal adhesion protein guides community behaviors and autoinduction in a paracrine manner. Proc Natl Acad Sci U S A 110:11571–11576. doi: 10.1073/pnas.1308173110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tian X, Lin X. 2013. Matricellular protein Cfl1 regulates cell differentiation. Commun Integr Biol 6:e26444. doi: 10.4161/cib.26444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guidotti A, Forchetti CM, Corda MG, Konkel D, Bennett CD, Costa E. 1983. Isolation, characterization, and purification to homogeneity of an endogenous polypeptide with agonistic action on benzodiazepine receptors. Proc Natl Acad Sci U S A 80:3531–3535. doi: 10.1073/pnas.80.11.3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Richardson DL, Loomis WF, Kimmel AR. 1994. Progression of an inductive signal activates sporulation in Dictyostelium discoideum. Development 120:2891–2900. [DOI] [PubMed] [Google Scholar]
- 23.Anjard C, Loomis WF. 2005. Peptide signaling during terminal differentiation of Dictyostelium. Proc Natl Acad Sci U S A 102:7607–7611. doi: 10.1073/pnas.0501820102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cabral M, Anjard C, Loomis WF, Kuspa A. 2006. Genetic evidence that the acyl coenzyme A binding protein AcbA and the serine protease/ABC transporter TagA function together in Dictyostelium discoideum cell differentiation. Eukaryot Cell 5:2024–2032. doi: 10.1128/EC.00287-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Faergeman NJ, Wadum M, Feddersen S, Burton M, Kragelund BB, Knudsen J. 2007. Acyl-CoA binding proteins; structural and functional conservation over 2000 MYA. Mol Cell Biochem 299:55–65. doi: 10.1007/s11010-005-9040-3. [DOI] [PubMed] [Google Scholar]
- 26.Burton M, Rose TM, Faergeman NJ, Knudsen J. 2005. Evolution of the acyl-CoA binding protein (ACBP). Biochem J 392:299–307. doi: 10.1042/BJ20050664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Neess D, Bek S, Engelsby H, Gallego SF, Færgeman NJ. 2015. Long-chain acyl-CoA esters in metabolism and signaling: role of acyl-CoA binding proteins. Prog Lipid Res 59:1–25. doi: 10.1016/j.plipres.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 28.Hansen JS, Faergeman NJ, Kragelund BB, Knudsen J. 2008. Acyl-CoA-binding protein (ACBP) localizes to the endoplasmic reticulum and Golgi in a ligand-dependent manner in mammalian cells. Biochem J 410:463–472. doi: 10.1042/BJ20070559. [DOI] [PubMed] [Google Scholar]
- 29.Faergeman NJ, Knudsen J. 2002. Acyl-CoA binding protein is an essential protein in mammalian cell lines. Biochem J 368:679–682. doi: 10.1042/bj20021413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Futerman AH, Riezman H. 2005. The ins and outs of sphingolipid synthesis. Trends Cell Biol 15:312–318. doi: 10.1016/j.tcb.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 31.Lin MH, Khnykin D. 2014. Fatty acid transporters in skin development, function and disease. Biochim Biophys Acta 1841:362–368. doi: 10.1016/j.bbalip.2013.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Qian Z, Bilderback TR, Barmack NH. 2008. Acyl coenzyme A-binding protein (ACBP) is phosphorylated and secreted by retinal Muller astrocytes following protein kinase C activation. J Neurochem 105:1287–1299. doi: 10.1111/j.1471-4159.2008.05229.x. [DOI] [PubMed] [Google Scholar]
- 33.Xiao S, Chye ML. 2011. New roles for acyl-CoA-binding proteins (ACBPs) in plant development, stress responses and lipid metabolism. Prog Lipid Res 50:141–151. doi: 10.1016/j.plipres.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 34.Xiao S, Chye ML. 2009. An Arabidopsis family of six acyl-CoA-binding proteins has three cytosolic members. Plant Physiol Biochem 47:479–484. doi: 10.1016/j.plaphy.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 35.Schjerling CK, Hummel R, Hansen JK, Borsting C, Mikkelsen JM, Kristiansen K, Knudsen J. 1996. Disruption of the gene encoding the acyl-CoA-binding protein (ACB1) perturbs acyl-CoA metabolism in Saccharomyces cerevisiae. J Biol Chem 271:22514–22521. doi: 10.1074/jbc.271.37.22514. [DOI] [PubMed] [Google Scholar]
- 36.Gaigg B, Neergaard TB, Schneiter R, Hansen JK, Faergeman NJ, Jensen NA, Andersen JR, Friis J, Sandhoff R, Schroder HD, Knudsen J. 2001. Depletion of acyl-coenzyme A-binding protein affects sphingolipid synthesis and causes vesicle accumulation and membrane defects in Saccharomyces cerevisiae. Mol Biol Cell 12:1147–1160. doi: 10.1091/mbc.12.4.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Faergeman NJ, Feddersen S, Christiansen JK, Larsen MK, Schneiter R, Ungermann C, Mutenda K, Roepstorff P, Knudsen J. 2004. Acyl-CoA-binding protein, Acb1p, is required for normal vacuole function and ceramide synthesis in Saccharomyces cerevisiae. Biochem J 380:907–918. doi: 10.1042/bj20031949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kinseth MA, Anjard C, Fuller D, Guizzunti G, Loomis WF, Malhotra V. 2007. The Golgi-associated protein GRASP is required for unconventional protein secretion during development. Cell 130:524–534. doi: 10.1016/j.cell.2007.06.029. [DOI] [PubMed] [Google Scholar]
- 39.Cabral M, Anjard C, Malhotra V, Loomis WF, Kuspa A. 2010. Unconventional secretion of AcbA in Dictyostelium discoideum through a vesicular intermediate. Eukaryot Cell 9:1009–1017. doi: 10.1128/EC.00337-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kent CR, Ortiz-Bermudez P, Giles SS, Hull CM. 2008. Formulation of a defined V8 medium for induction of sexual development of Cryptococcus neoformans. Appl Environ Microbiol 74:6248–6253. doi: 10.1128/AEM.00970-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Janbon G, Ormerod KL, Paulet D, Byrnes EJ III, Yadav V, Chatterjee G, Mullapudi N, Hon CC, Billmyre RB, Brunel F, Bahn YS, Chen W, Chen Y, Chow EW, Coppee JY, Floyd-Averette A, Gaillardin C, Gerik KJ, Goldberg J, Gonzalez-Hilarion S, Gujja S, Hamlin JL, Hsueh YP, Ianiri G, Jones S, Kodira CD, Kozubowski L, Lam W, Marra M, Mesner LD, Mieczkowski PA, Moyrand F, Nielsen K, Proux C, Rossignol T, Schein JE, Sun S, Wollschlaeger C, Wood IA, Zeng Q, Neuveglise C, Newlon CS, Perfect JR, Lodge JK, Idnurm A, Stajich JE, Kronstad JW, Sanyal K, Heitman J, Fraser JA, Cuomo CA, Dietrich FS. 2014. Analysis of the genome and transcriptome of Cryptococcus neoformans var. grubii reveals complex RNA expression and microevolution leading to virulence attenuation. PLoS Genet 10:e1004261. doi: 10.1371/journal.pgen.1004261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Loftus BJ, Fung E, Roncaglia P, Rowley D, Amedeo P, Bruno D, Vamathevan J, Miranda M, Anderson IJ, Fraser JA, Allen JE, Bosdet IE, Brent MR, Chiu R, Doering TL, Donlin MJ, D'Souza CA, Fox DS, Grinberg V, Fu J, Fukushima M, Haas BJ, Huang JC, Janbon G, Jones SJ, Koo HL, Krzywinski MI, Kwon-Chung JK, Lengeler KB, Maiti R, Marra MA, Marra RE, Mathewson CA, Mitchell TG, Pertea M, Riggs FR, Salzberg SL, Schein JE, Shvartsbeyn A, Shin H, Shumway M, Specht CA, Suh BB, Tenney A, Utterback TR, Wickes BL, Wortman JR, Wye NH, Kronstad JW, Lodge JK, Heitman J, Davis RW, Fraser CM, Hyman RW. 2005. The genome of the basidiomycetous yeast and human pathogen Cryptococcus neoformans. Science 307:1321–1324. doi: 10.1126/science.1103773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lin X, Chacko N, Wang L, Pavuluri Y. 2015. Generation of stable mutants and targeted gene deletion strains in Cryptococcus neoformans through electroporation. Med Mycol 53:225–234. doi: 10.1093/mmy/myu083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Toffaletti DL, Rude TH, Johnston SA, Durack DT, Perfect JR. 1993. Gene transfer in Cryptococcus neoformans by use of biolistic delivery of DNA. J Bacteriol 175:1405–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Petrasovits LA. 2014. Protein blotting protocol for beginners. Methods Mol Biol 1099:189–199. doi: 10.1007/978-1-62703-715-0_16. [DOI] [PubMed] [Google Scholar]
- 46.Chacko N, Zhao Y, Yang E, Wang L, Cai JJ, Lin X. 2015. The lncRNA RZE1 controls cryptococcal morphological transition. PLoS Genet 11:e1005692. doi: 10.1371/journal.pgen.1005692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhai B, Zhu P, Foyle D, Upadhyay S, Idnurm A, Lin X. 2013. Congenic strains of the filamentous form of Cryptococcus neoformans for studies of fungal morphogenesis and virulence. Infect Immun 81:2626–2637. doi: 10.1128/IAI.00259-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang L, Tian X, Gyawali R, Upadhyay S, Foyle D, Wang G, Cai JJ, Lin X. 2014. Morphotype transition and sexual reproduction are genetically associated in a ubiquitous environmental pathogen. PLoS Pathog 10:e1004185. doi: 10.1371/journal.ppat.1004185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Davidson RC, Moore TD, Odom AR, Heitman J. 2000. Characterization of the MFα pheromone of the human fungal pathogen Cryptococcus neoformans. Mol Microbiol 38:1017–1026. doi: 10.1046/j.1365-2958.2000.02213.x. [DOI] [PubMed] [Google Scholar]
- 50.Rijken PJ, Houtkooper RH, Akbari H, Brouwers JF, Koorengevel MC, de Kruijff B, Frentzen M, Vaz FM, de Kroon AI. 2009. Cardiolipin molecular species with shorter acyl chains accumulate in Saccharomyces cerevisiae mutants lacking the acyl coenzyme A-binding protein Acb1p: new insights into acyl chain remodeling of cardiolipin. J Biol Chem 284:27609–27619. doi: 10.1074/jbc.M109.016311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Feddersen S, Neergaard TB, Knudsen J, Faergeman NJ. 2007. Transcriptional regulation of phospholipid biosynthesis is linked to fatty acid metabolism by an acyl-CoA-binding-protein-dependent mechanism in Saccharomyces cerevisiae. Biochem J 407:219–230. doi: 10.1042/BJ20070315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee L, DeBono CA, Campagna DR, Young DC, Moody DB, Fleming MD. 2007. Loss of the acyl-CoA binding protein (Acbp) results in fatty acid metabolism abnormalities in mouse hair and skin. J Invest Dermatol 127:16–23. doi: 10.1038/sj.jid.5700511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McGoldrick EM, Wheals AE. 1989. Controlling the growth rate of Saccharomyces cerevisiae cells using the glucose analogue d-glucosamine. J Gen Microbiol 135:2407–2411. [DOI] [PubMed] [Google Scholar]
- 54.Nevado J, Heredia CF. 1996. Galactose induces in Saccharomyces cerevisiae sensitivity of the utilization of hexoses to inhibition by d-glucosamine. Can J Microbiol 42:6–11. doi: 10.1139/m96-002. [DOI] [PubMed] [Google Scholar]
- 55.Mountain HA, Sudbery PE. 1990. The relationship of growth rate and catabolite repression with WHI2 expression and cell size in Saccharomyces cerevisiae. J Gen Microbiol 136:733–737. doi: 10.1099/00221287-136-4-733. [DOI] [PubMed] [Google Scholar]
- 56.Furst A, Michels CA. 1977. An evaluation of d-glucosamine as a gratuitous catabolite repressor of Saccharomyces carlsbergensis. Mol Gen Genet 155:309–314. doi: 10.1007/BF00272810. [DOI] [PubMed] [Google Scholar]
- 57.Zhang YM, Wu B, Zheng J, Rock CO. 2003. Key residues responsible for acyl carrier protein and beta-ketoacyl-acyl carrier protein reductase (FabG) interaction. J Biol Chem 278:52935–52943. doi: 10.1074/jbc.M309874200. [DOI] [PubMed] [Google Scholar]
- 58.Kragelund BB, Osmark P, Neergaard TB, Schiødt J, Kristiansen K, Knudsen J, Poulsen FM. 1999. The formation of a native-like structure containing eight conserved hydrophobic residues is rate limiting in two-state protein folding of ACBP. Nat Struct Biol 6:594–601. doi: 10.1038/9384. [DOI] [PubMed] [Google Scholar]
- 59.Kragelund BB, Poulsen K, Andersen KV, Baldursson T, Kroll JB, Neergard TB, Jepsen J, Roepstorff P, Kristiansen K, Poulsen FM, Knudsen J. 1999. Conserved residues and their role in the structure, function, and stability of acyl-coenzyme A binding protein. Biochemistry 38:2386–2394. doi: 10.1021/bi982427c. [DOI] [PubMed] [Google Scholar]
- 60.Duran JM, Anjard C, Stefan C, Loomis WF, Malhotra V. 2010. Unconventional secretion of Acb1 is mediated by autophagosomes. J Cell Biol 188:527–536. doi: 10.1083/jcb.200911154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Levi SK, Bhattacharyya D, Strack RL, Austin JR II, Glick BS. 2010. The yeast GRASP Grh1 colocalizes with COPII and is dispensable for organizing the secretory pathway. Traffic 11:1168–1179. doi: 10.1111/j.1600-0854.2010.01089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Manjithaya R, Anjard C, Loomis WF, Subramani S. 2010. Unconventional secretion of Pichia pastoris Acb1 is dependent on GRASP protein, peroxisomal functions, and autophagosome formation. J Cell Biol 188:537–546. doi: 10.1083/jcb.200911149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Barr FA, Puype M, Vandekerckhove J, Warren G. 1997. GRASP65, a protein involved in the stacking of Golgi cisternae. Cell 91:253–262. doi: 10.1016/S0092-8674(00)80407-9. [DOI] [PubMed] [Google Scholar]
- 64.Short B, Preisinger C, Korner R, Kopajtich R, Byron O, Barr FA. 2001. A GRASP55-rab2 effector complex linking Golgi structure to membrane traffic. J Cell Biol 155:877–883. doi: 10.1083/jcb.200108079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Malhotra V. 2013. Unconventional protein secretion: an evolving mechanism. EMBO J 32:1660–1664. doi: 10.1038/emboj.2013.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vinke FP, Grieve AG, Rabouille C. 2011. The multiple facets of the Golgi reassembly stacking proteins. Biochem J 433:423–433. doi: 10.1042/BJ20101540. [DOI] [PubMed] [Google Scholar]
- 67.Schotman H, Karhinen L, Rabouille C. 2009. Integrins mediate their unconventional, mechanical-stress-induced secretion via RhoA and PINCH in Drosophila. J Cell Sci 122:2662–2672. doi: 10.1242/jcs.039347. [DOI] [PubMed] [Google Scholar]
- 68.Gee HY, Noh SH, Tang BL, Kim KH, Lee MG. 2011. Rescue of ΔF508-CFTR trafficking via a GRASP-dependent unconventional secretion pathway. Cell 146:746–760. doi: 10.1016/j.cell.2011.07.021. [DOI] [PubMed] [Google Scholar]
- 69.Kmetzsch L, Joffe LS, Staats CC, de Oliveira DL, Fonseca FL, Cordero RJ, Casadevall A, Nimrichter L, Schrank A, Vainstein MH, Rodrigues ML. 2011. Role for Golgi reassembly and stacking protein (GRASP) in polysaccharide secretion and fungal virulence. Mol Microbiol 81:206–218. doi: 10.1111/j.1365-2958.2011.07686.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lin X, Heitman J. 2006. The biology of the Cryptococcus neoformans species complex. Annu Rev Microbiol 60:69–105. doi: 10.1146/annurev.micro.60.080805.142102. [DOI] [PubMed] [Google Scholar]
- 71.Knudsen J, Faergeman NJ, Skott H, Hummel R, Borsting C, Rose TM, Andersen JS, Hojrup P, Roepstorff P, Kristiansen K. 1994. Yeast acyl-CoA-binding protein: acyl-CoA-binding affinity and effect on intracellular acyl-CoA pool size. Biochem J 302(Part 2):479–485. doi: 10.1042/bj3020479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Teilum K, Olsen JG, Kragelund BB. 2011. Protein stability, flexibility and function. Biochim Biophys Acta 1814:969–976. doi: 10.1016/j.bbapap.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 73.Schotman H, Karhinen L, Rabouille C. 2008. dGRASP-mediated noncanonical integrin secretion is required for Drosophila epithelial remodeling. Dev Cell 14:171–182. doi: 10.1016/j.devcel.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 74.Karkowska-Kuleta J, Kozik A. 21 July 2015. Cell wall proteome of pathogenic fungi. Acta Biochim Pol doi: 10.18388/abp.2015_1032. [DOI] [PubMed] [Google Scholar]
- 75.de Groot PW, de Boer AD, Cunningham J, Dekker HL, de Jong L, Hellingwerf KJ, de Koster C, Klis FM. 2004. Proteomic analysis of Candida albicans cell walls reveals covalently bound carbohydrate-active enzymes and adhesins. Eukaryot Cell 3:955–965. doi: 10.1128/EC.3.4.955-965.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Castillo L, Calvo E, Martinez AI, Ruiz-Herrera J, Valentin E, Lopez JA, Sentandreu R. 2008. A study of the Candida albicans cell wall proteome. Proteomics 8:3871–3881. doi: 10.1002/pmic.200800110. [DOI] [PubMed] [Google Scholar]
- 77.Asif AR, Oellerich M, Amstrong VW, Riemenschneider B, Monod M, Reichard U. 2006. Proteome of conidial surface associated proteins of Aspergillus fumigatus reflecting potential vaccine candidates and allergens. J Proteome Res 5:954–962. doi: 10.1021/pr0504586. [DOI] [PubMed] [Google Scholar]
- 78.Vallejo MC, Nakayasu ES, Matsuo AL, Sobreira TJ, Longo LV, Ganiko L, Almeida IC, Puccia R. 2012. Vesicle and vesicle-free extracellular proteome of Paracoccidioides brasiliensis: comparative analysis with other pathogenic fungi. J Proteome Res 11:1676–1685. doi: 10.1021/pr200872s. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.