Abstract
Cochlodinium polykrikoides is a cosmopolitan dinoflagellate that is notorious for causing fish-killing harmful algal blooms (HABs) across North America and Asia. While recent laboratory and ecosystem studies have definitively demonstrated that Cochlodinium forms resting cysts that may play a key role in the dynamics of its HABs, uncertainties regarding cyst morphology and detection have prohibited even a rudimentary understanding of the distribution of C. polykrikoides cysts in coastal ecosystems. Here, we report on the development of a fluorescence in situ hybridization (FISH) assay using oligonucleotide probes specific for the large subunit (LSU) ribosomal DNA (rDNA) of C. polykrikoides. The LSU rDNA-targeted FISH assay was used with epifluorescence microscopy and was iteratively refined to maximize the fluorescent reaction with C. polykrikoides and minimize cross-reactivity. The final LSU rDNA-targeted FISH assay was found to quantitatively recover cysts made by North American isolates of C. polykrikoides but not cysts formed by other common cyst-forming dinoflagellates. The method was then applied to identify and map C. polykrikoides cysts across bloom-prone estuaries. Annual cyst and vegetative cell surveys revealed that elevated densities of C. polykrikoides cysts (>100 cm−3) during the spring of a given year were spatially consistent with regions of dense blooms the prior summer. The identity of cysts in sediments was confirmed via independent amplification of C. polykrikoides rDNA. This study mapped C. polykrikoides cysts in a natural marine setting and indicates that the excystment of cysts formed by this harmful alga may play a key role in the development of HABs of this species.
INTRODUCTION
Resting cysts of dinoflagellates can be associated with genetic recombination, maintenance of blooms, termination of blooms, recurrence of annual blooms, resistance against unfavorable environmental conditions, protection from viruses, grazers, or parasite attacks, and geographical expansion of populations (1–10). Resting cysts, therefore, play an important role in the ecology of harmful algal blooms (HABs) caused by dinoflagellates (8, 11) and have been considered a fundamental attribute of dinoflagellate life cycles (12). About 200 marine and freshwater dinoflagellates are known to produce resting cysts, a small number relative to the ∼2,300 extant dinoflagellate species (5, 11, 13). More than 20 of these cyst-producing dinoflagellates are known to cause harmful algal blooms (HABs) (5), including Cochlodinium polykrikoides Margalef (10).
Cochlodinium polykrikoides Margalef is an unarmored dinoflagellate that has caused fish-killing HABs in locations across much of Asia and North America (14–17). The initiation and development of C. polykrikoides blooms have been shown to be related to multiple factors, including stimulation by nutrients such as nitrogen and vitamins (18, 19), mixotrophy (20), the production of extracellular toxins lethal to grazers (21–23), bacterial mutualism (24), and allelopathic effects on competing phytoplankton (25). Recently, definitive evidence of resting cyst production by North American clones of C. polykrikoides provided a mechanism to account for the recurrence of annual blooms in given locales as well as the global expansion of C. polykrikoides blooms during the past 2 decades (10).
The precise morphology and identity of cysts produced by C. polykrikoides have been a subject of some controversy. Some studies have reported on the existence of temporary cysts with hyaline membranes formed by modification of vegetative cells (26–28). Tang and Gobler (10) recently demonstrated the ability of North American strains of C. polykrikoides to produce resting cysts, via sexual fusion of vegetative cells, which can persist for several months and have a morphology that differs from that of hyaline cysts produced by the same cultures. Many prior studies have reported the identification of resting cysts of C. polykrikoides or Cochlodinium sp. from sediments (6, 29–34), but these studies have generally identified the cysts using previously published micrographs from Fukuyo (35) as Cochlodinium sp. 1, and from Matsuoka and Fukuyo (29), as Cochlodinium sp. 1 or C. cf. polykrikoides (a Cochlodinium sp. that looks like C. polykrikoides), which bear little resemblance to the cysts produced in cultures (10, 27, 28). Recently, Li et al. (36) reported on the recovery of a cyst from Korean coastal waters that germinated into a C. polykrikoides cell, confirmed via large subunit ribosomal DNA (LSU rDNA) sequencing, that exhibited a morphology distinct from that given in all prior reports, with a cyst body that was covered by reticulate ornaments and spines. While C. polykrikoides resting cysts have been observed in culture (10), thus far, identifying these cysts in the field using traditional microscopic methods or via PCR has proven difficult and has resulted in a series of putatively false-positive identifications (as reviewed in references 10 and 16).
Given the role of cyst beds as “seed banks” in the outbreak of toxic dinoflagellate blooms such as Alexandrium spp. (3, 37–39), the ability to establish the distribution of C. polykrikoides cysts in an ecosystem setting is highly desirable. The uncertainty of the morphology of C. polykrikoides cysts (as outlined above) and the likelihood of the alteration of morphology by exposure to bacteria (10), which are highly abundant in sediments, indicate that a method that does not rely exclusively on morphology for the identification of these cysts in an ecosystem setting is required. While molecular methods are a logical alternative to microscopic identification, simple amplification and sequencing of C. polykrikoides nucleic acids within sediments are likely to be of limited value. For example, Park and Park (40) detected C. polykrikoides via PCR in sediment samples, indicating the presence of C. polykrikoides, but because morphological information regarding cysts was not provided, the amplification of DNA from vegetative cells could not be excluded as a plausible explanation for those findings.
Here, we report on the development of a fluorescence in situ hybridization (FISH) assay using oligonucleotide probes that target the LSU rDNA gene of C. polykrikoides to quantify cysts from bloom-prone estuaries in New York, USA. The FISH assay was used in an epifluorescent setting and refined to maximize fluorescent reaction and minimize cross-reactivity with nontarget material. The final FISH assay was found to positively react with cysts made by North American isolates of C. polykrikoides added to a variety of natural sediments. The FISH assay was used in tandem with independent amplification of C. polykrikoides DNA to provide a robust confirmation of cyst presence. Finally, the method was used to create maps of C. polykrikoides cysts that provided insight regarding the temporal and spatial dynamics of C. polykrikoides blooms.
MATERIALS AND METHODS
Algal cultures and culturing conditions.
All Cochlodinium polykrikoides cultures (strains CP1, CPSB-1B, CPSB-1G, and CPSB-2A) used in this study were established from bloom water collected between 2006 and 2011 from the estuaries of Long Island, NY, USA. Several other dinoflagellate species were also used in this study, including Akashiwo sanguinea (clone ASNP), Prorocentrum minimum (clone CCMP696), Scrippsiella trochoidea(clone MS1), and two strains of Alexandrium fundyense (clones CCMP 2304 and NPB8), which were all isolated from Long Island, NY, USA, waters with the exception of CCMP 2304, which was isolated from the Bay of Fundy. Cultures were cultivated in either sterile GSe medium (41) or f/2 (-silicate, both Alexandrium strains (42) with a salinity of 32.5, made with autoclaved and 0.2-μm-filtered seawater, at 21°C in an incubator with a 12/12-h light-dark cycle, illuminated by a bank of fluorescent lights that provided ∼100 μmol quanta m−2 s−1 to cultures. An antibiotic solution (stock solution, Thermo Scientific HyClone penicillin [10,000 U ml−1] and streptomycin [10,000 μg ml−1] in 0.85% NaCl) was added into the medium immediately before inoculation, with a final concentration of 2% to discourage bacterial growth in cultures.
Cyst production in cultures.
Cochlodinium polykrikoides cysts were produced using strain CPSB-2A (nonclonal) (10). In six-well plates, ∼0.5 ml of dense stock culture was added to each well of the plate containing 8 ml GSe medium without nitrogen and phosphorus (GSe − N-P) and 3% antibiotics and incubated as above. Every week, the plates were observed for the presence of cysts under a Nikon Eclipse TS100, and antibiotics (3% final concentration) were added to each well to discourage microbial growth. The presence of resting cysts was determined based on the morphological description provided by Tang and Gobler (10): cysts were nonmotile, spherical with a cyst wall, 20 to 40 μm in diameter, and yellowish brown and contained a red accumulation body. When resting cysts were observed, they were isolated and preserved with 8% (wt/vol) of cold, freshly prepared paraformaldehyde (PFA) solution (in 1× phosphate-buffered saline solution [PBS]) in a 1:1 (vol/vol) ratio and processed as described below.
Development of Cochlodinium-specific oligonucleotide probes and FISH assays.
All Long Island Cochlodinium polykrikoides isolates used in this study were pelleted and extracted, and the LSU rDNA was sequenced as described by Gobler et al. (43). Sequences were aligned with other dinoflagellates covering different orders of Dinophyta using MEGA5.1, and oligonucleotide probes specific to the North American/Malaysian ribotype of C. polykrikoides were manually selected from the highly variable D2 domain of the LSU rDNA and then assessed for specificity of probe sequences using NCBI's BLASTn. All oligonucleotide probes were conjugated with Cy3 dye at the 5′ end and synthesized at Sigma-Aldrich.
To confirm the specificity of oligonucleotide probes using multiple C. polykrikoides strains and cultures of cooccurring algae, four C. polykrikoides-specific LSU rRNA-targeted oligonucleotide probes were tested: P411, 5′-TTGCACTTTCAACGAAAGTG; P442, 5′-TCAATCGCCTTTCGCCTGAT; P505, 5′-ACCTTCAAAGGCATGGTAG; and P615, 5′-AGAACCAATCGGTTCTTGC. All probes were monolabeled with Cy3 at the 5′ end. Aliquots of multiple C. polykrikoides strains, CP1, CPSB-1B, CPSB-1G, and CPSB-2A, as well as multiple species of dinoflagellates that also form blooms in the New York estuaries that host C. polykrikoides blooms, including Akashiwo sanguinea (ASNP), Prorocentrum minimum (CCMP696), Scrippsiella trochoidea(MS1), and two strains of Alexandrium fundyense (CCMP 2304 and NPB8), were used to assess the specificity of these probes. A final concentration of 4% PFA in 1× PBS buffer was added to culture aliquots and incubated overnight (about 16 to 18 h) at 4°C. The samples were then centrifuged, and an appropriate volume of the mixture of 1:1 1× PBS–absolute ethanol was added. The samples with 1:1 PBS-ethanol were then centrifuged at 10,000 × g for 2 min, and supernatant was removed. One hundred microliters of a nonformamide hybridization buffer (0.9 M NaCl, 0.1% sodium dodecyl sulfate, 20 mM Tris-HCl, pH 7.2) with 5 μM probe was then added to the pellets. After being mixed, the samples were subjected to one-step, high-temperature FISH at 70°C for 60 min in a rotating incubator, which enhances the hybridization reaction (44). After FISH, the samples were centrifuged at 10,000 × g for 2 min, the pellets were resuspended in 500 μl of nonformamide hybridization buffer containing no probe, and the samples were then washed at 46°C for 30 min in a rotating incubator. After being centrifuged at 10,000 × g for 2 min, the pellets were resuspended in 100 μl of 1× PBS (pH 8.4) and put on ice until observation under a Nikon E800 epifluorescence microscope using a Cy3 filter set.
Experiments were performed to optimize hybridization time and temperature to maximize the signal intensity of the C. polykrikoides-specific probe. To optimize hybridization time, 100 μl of the nonformamide hybridization buffer (0.9 M NaCl, 0.1% sodium dodecyl sulfate, 20 mM Tris-HCl, pH 7.2) with 5 μM probe was added to vegetative C. polykrikoides cells preserved as above. After being mixed, the samples were subjected to one-step FISH at 46°C for 3 h (45), 6 h, 12 h, and 24 h in a rotating incubator. After FISH, the samples were processed as above using a centrifugation step of 3,300 × g for 2 min. After the optimum hybridization time was confirmed, the cells were hybridized with probes at temperatures of 35°C, 46°C, and 55°C for the same length of time in a rotating incubator. After FISH, the samples were processed as described above.
To optimize the hybridization protocol for use with Cochlodinium polykrikoides cysts from sediments, sediments from bloom-prone locations were collected using a Ponar grab. The top 3 cm of sediment was collected using a modified syringe, and a 5-ml sample was placed into a 50-ml centrifuge tube and refrigerated until further processing. In the laboratory, cold 0.2-μm-filtered seawater was added to the sediment core and well mixed, and an aliquot was sieved through a 100-μm mesh screen and then through a 20-μm mesh screen. The material caught on the 20-μm mesh was backwashed into a 15-ml tube and allowed to settle for 2 h in a refrigerator. Cysts from each sediment sample were extracted via the density gradient method (46) using sodium polytungstate (SPT) at a density of 1.3 g cm−3. The SPT method was initially utilized with two density gradients, 2.1 g cm−3 and 1.3 g cm−3. Consistent with the results of Bolch (46), the 1.3-g cm−3 gradient yielded far more cysts. While most cysts are known to secrete a mucilage that can ultimately aid in sinking via attaching to other sinking particles, we separated sediments through a series of sieves and while doing so generously washed each sieve with filtered seawater to dislodge any cysts that may have been attached to particles. We, however, cannot discount that some cysts may have been lost due to their attachment to sediments. The SPT-treated sediment samples (2 ml) were fixed with 8% (wt/vol) of cold, freshly prepared PFA solution (in 1× PBS) in a 1:1 (vol/vol) ratio, and the suspensions were mixed and incubated overnight (about 16 to 18 h) at 4°C. The samples were pelleted via centrifugation at 1,500 × g for 10 min (IEC CL31R multispeed centrifuge; Thermo) and washed with 1× PBS, and an appropriate volume of the mixture of 1:1 1× PBS–absolute ethanol was added. The samples were then transferred into 2-ml Eppendorf microcentrifuge tubes and stored at −20°C for several weeks until they were processed with the FISH method.
Samples stored in the microcentrifuge tubes with 1:1 PBS-ethanol were centrifuged at 10,000 × g for 4 min (microcentrifuge 5415D; Eppendorf). One hundred microliters of PBST solution (1× PBS with 0.5% [vol/vol] Triton X-100) was added to the pellets. After being mixed, the samples were incubated at 37°C for 10 min. The samples were then centrifuged at 10,000 × g for 4 min, and 100 μl of the proteinase K solution (1 μg ml−1 in 1× PBS) was added. After being mixed, the samples were incubated at room temperature for 1 h. After centrifugation at 10,000 × g for 4 min, the pellets were washed with 1× PBS, resuspended with a 4% PFA solution (4% [wt/vol] of PFA in 1× PBS), and fixed for 10 min on ice. After being centrifuged at 10,000 × g for 4 min, the pellets were washed with 1× PBS. One hundred microliters of the nonformamide hybridization buffer with 1 μM probe was then added to the pellets. After being mixed, the samples were subjected to a two-step FISH by being prewarmed at 90°C for 10 min in a water bath and then shifted to another water bath at 55°C for 16 to 18 h (44). After hybridization, the samples were centrifuged at 10,000 × g for 4 min, and the pellets were resuspended in 500 μl of nonformamide hybridization buffer and washed at 46°C for 30 min in a water bath. After being centrifuged at 10,000 × g for 4 min, the pellets were resuspended in 1,500 μl 1× PBS and put on ice until a 1-ml aliquot using a Sedgewick Rafter slide was observed by switching between epifluorescent and bright-field (to confirm positive fluorescent signals) settings on a Nikon E800.
The recovery of cysts after samples were brought through the FISH protocol and the percentage of cysts stained by the FISH protocol were quantified. Sediments were collected from a region (Northport Bay, NY, USA) (Fig. 1) not known to host C. polykrikoides blooms and were processed using the SPT protocol and preserved as described above. After preservation, these samples were spiked with culture-generated C. polykrikoides cysts preserved as described above. Prior to adding C. polykrikoides cysts to the environmental sample, 50 μl of C. polykrikoides cysts was placed in a Sedgewick-Rafter counting chamber, and the cysts were quantified via light microscopy in triplicate. Further, the specific aliquots of sediments tested were affirmed to contain no cysts. A known quantity of C. polykrikoides cysts (No) was added into the preserved Northport Bay sample and processed via the FISH protocol described above. After hybridization, 100 μl of sample was placed in a Sedgewick-Rafter counting chamber and observed via epifluorescence microscopy. All the cysts in bright-field mode (Nb) and those fluorescing under epifluorescence field mode (Nf) were counted, and the percentage of cysts stained and the original percent recovery were calculated as follows: percentage of cysts stained = Nf/Nb, where Nf is the fluorescing cysts counted in epifluorescence field mode and Nb is the cysts counted in bright-field mode; original percent recovery = Nf/No, where Nf is as defined above and No is the number of original cysts added into the sediments.
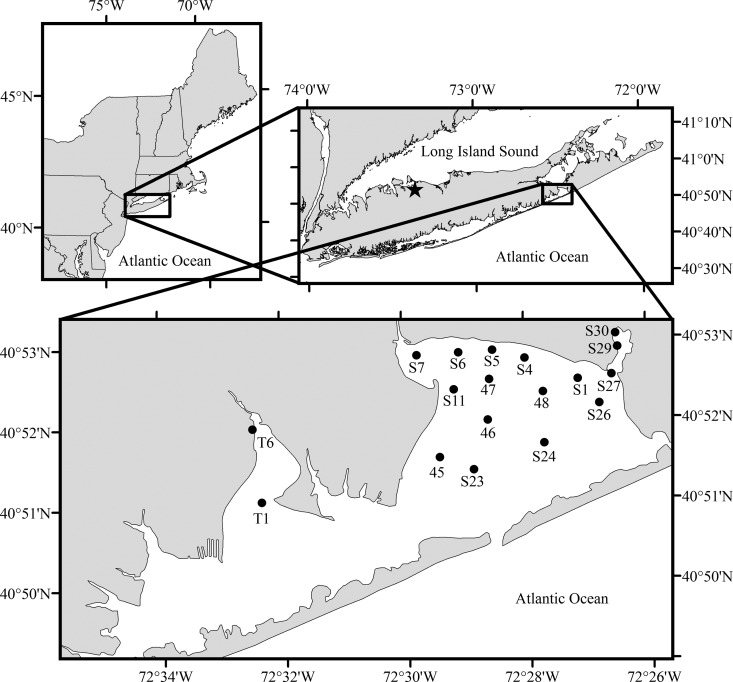
FIG 1.
Northeast United States and Long Island, NY, with Northport Bay indicated by a black star. The inset shows Shinnecock and Tiana Bay with black dots indicating cyst survey locations.
Ecosystem surveys of C. polykrikoides cells and cysts.
Cochlodinium polykrikoides is well known for displaying heterogeneous, patchy spatial distributions during bloom events with cell densities sometimes differing by 1 order of magnitude or more over small distances (∼100 m) (47, 48). However, georeferenced, continuous horizontal mapping of surface chlorophyll a fluorescence has proved to be a robust means for assessing the spatial distribution of blooms (47, 49). For this study, horizontal surveys were performed across Shinnecock Bay and its tributaries during hours of peak sun intensity on a weekly basis during August and September 2013 and 2014 to assess the spatial distribution of Cochlodinium blooms. During cruises, a continuous high-speed, flowthrough system was designed (modified from reference 50) to provide nonturbulent, underway, surface water monitoring of chlorophyll a fluorescence using a water intake and delivery system that transferred water from an ∼0.2-m depth across a YSI EXO2 multiparameter sensor array (∼0.5 liter s−1). All data were transmitted at 1 Hz and georeferenced using a TranSystems Inc. G-Log 760 commercial-grade global positioning system (GPS) receiver (RMC; NMEA). Discrete samples were randomly collected inline and preserved in Lugol's iodine and filtered onto a 0.2-μm polycarbonate filter. Filters were frozen, and chlorophyll a was later extracted and analyzed using standard fluorometric techniques (51). Discrete chlorophyll a values were georeferenced and used to normalize in situ fluorescence data (r2 = 0.94; P < 0.001). Samples were preserved in Lugol's iodine and quantified using a light microscope. During surveys, chlorophyll a fluorescence as measured via the EXO total algae sensor and Cochlodinium cell counts were highly correlated (r2 = 0.86; P < 0.001), affirming prior findings that chlorophyll a fluorescence is a good proxy for Cochlodinium cells during bloom events (47, 49). All sensor data collected during these bloom events were assimilated and used to generate maps of continuously measured levels of normalized chlorophyll a fluorescence by using ArcGIS 10.2 (ESRI, Redlands, CA).
In April 2014 and 2015, sediment surveys were conducted across Shinnecock Bay and its tributaries (Fig. 1, circles), during which no vegetative cells were present in the water column; Cochlodinium polykrikoides blooms in this region typically occur from August through October (43). Sediment samples collected during a previous sediment survey of Northport Bay, NY, USA (Fig. 1, star), were used as a negative control as this bay is not known to host C. polykrikoides blooms. In addition, following the termination of a C. polykrikoides bloom in Old Fort Pond, NY, sediment samples were collected weekly during October 2014 (Fig. 1; station S30). All sediment samples were collected and processed with the SPT and FISH assay protocols as described above and were counted using a Sedgewick-Rafter chamber under both bright-field and epifluorescent settings. Replicate samples were collected at site 29 (Table 1) to determine the reproducibility of the method (43 ± 9.8 cysts cm−3; relative standard deviation, 23%). The organic content of sediments was estimated via the mass loss following ignition for 5 h at 450°C in a furnace.
TABLE 1.
Quantification of Cochlodinium polykrikoides cysts from sediments collected in April 2014 from various New York water bodies, including Shinnecock Bay, Old Fort Pond, Tiana Bay, and Northport Baya
| Embayment and site ID | No. of Cochlodinium cysts cm−3 | PCR tested | % identity | BLASTn hit | GenBank accession no. |
|---|---|---|---|---|---|
| Shinnecock Bay | |||||
| S1 | 0 | ||||
| S4 | 24 | ||||
| S5 | 147 | ||||
| S6 | 101 | ||||
| S7 | 24 | ||||
| S11 | 9 | ||||
| S23 | 68 | ||||
| S24 | 7 | ||||
| S26 | 44 | ||||
| Old Fort Pond | |||||
| S27 | 26 | ||||
| S29.1 | 50 | ||||
| S29.2 | 36 | X | 97 | C. polykrikoides | AB295048 |
| S30 | 66 | X | 98 | C. polykrikoides | AB295048 |
| Tiana Bay | |||||
| T1 | 17 | ||||
| T6 | 95 | ||||
| Northport Bay | |||||
| N2 | 54 | ||||
| N18 | 78 | X | 99 | C. polykrikoides | AB295048 |
Select samples were confirmed via PCR, and positive PCR products were sequenced. Sequence similarity of positive products to existing sequences deposited in NCBI were determined using BLASTn.
PCR confirmation of C. polykrikoides cysts in sediments.
A subset of sediment samples with a range of cyst densities from the above surveys were used for independent PCR confirmation of the results obtained via FISH assays. Sediment samples were SPT treated (as described above), pelleted in 1.5-ml Eppendorf tubes, and frozen at −20°C until further processing. Following the method of Erdner et al. (52), 360 μl of ATL buffer (Qiagen) and 0.5-mm silica-zirconium beads were added to pellets and vortexed for 1 min. The resulting lysate was transferred to a clean tube, 40 μl of proteinase K solution (Qiagen) was added, and the samples were incubated at 56°C for 1 h. Thereafter, samples were processed according to the protocol provided in the Qiagen DNeasy blood and tissue kit, eluted twice with 50 μl of AE buffer (for a total of 100 μl), and stored at −20°C.
PCR amplification of LSU rDNA (28S) using primers specific to C. polykrikoides American/Malaysian ribotype was performed using the primers developed by Park et al. (53) (forward primer, AMCPSF, CTC AAT CGC CTT TCG CCT GAT, and reverse primer, AMCPSR, ACC GGA CAC CTC GGA TAT GAT), which were originally designed to positively identify New York clones of C. polykrikoides. PCR conditions were as described by Park et al. (53) with minor modifications, specifically using an initial denaturation at 95°C for 10 min, 60 cycles at 95°C for 20 s, 56°C for 20 s, and 72°C for 50 s, followed by a 5-min extension step at 72°C. Reaction mixtures (25 μl) were made with 12.5 μl of Go Taq Green (Promega), 10 μl of sample DNA (<20 ng), and 2.5 μl of forward and reverse primer mixture (4 μM). Alongside the sediment samples, both Alexandrium (NPB8) and Cochlodinium (CP1) genomic DNAs were amplified to serve as negative and positive controls, respectively. The presence of a PCR product was confirmed using a 2% agarose gel and visualized using UV light. Selected positive products were sequenced at Stony Brook University's DNA Sequencing Facility with an ABI 3730 genetic analyzer using the forward primer, AMCPSF. Sequences were trimmed in Geneious Pro 5.5.2 (A. J. Drummond, B. Ashton, S. Buxton, M. Cheung, A. Cooper, C. Duran, M. Field, J. Heled, M. Kearse, S. Markowitz, R. Moir, S. Stones-Havas, S. Sturrock, T. Thierer, A. Wilson, Geneious v5.5), and sequence similarities of all sediment samples to existing sequences deposited in NCBI were determined using BLASTn of NCBI.
RESULTS
Specificity of oligonucleotide probes.
Oligonucleotide probes P442 and P615 provided the brightest fluorescent labeling of C. polykrikoides strain CP1 of the four probes tested, and thus the other two were not evaluated further (Fig. 2). The hybridization of multiple strains of cooccurring dinoflagellates, including Akashiwo sanguinea (ASNP), Prorocentrum minimum (CCMP696), Scrippsiella trochoidea(MS1), and two strains of Alexandrium fundyense (CCMP 2304 and NPB8), with oligonucleotide probe P442 demonstrated that these probes were specific to C. polykrikoides, as no fluorescent signal was detected for any of the dinoflagellates examined (Fig. 3). Similarly, in a mixed sample of C. polykrikoides strain CPSB-1G, Akashiwo sanguinea, and Scrippsiella trochoidea, only C. polykrikoides reacted with probe P442 (Fig. 3, bottom panel). All strains of C. polykrikoides tested in this study (CP1, CPSB-1B, CPSB-1G, and CP2A) produced bright fluorescent signals with probe P442 (Fig. 4), with the relative intensity of the signal varying among the strains examined (CPSB-1B ≈ CPSB-1G > CPSB-2A ≈ CP1). Hence, only probe P442 was utilized for the remainder of this study.
FIG 2.
(A) Light microscopy image of vegetative cells of Cochlodinium polykrikoides strain CP1; (B) corresponding epifluorescent image using probe P442; (C) light microscope image of C. polykrikoides strain CP1; (D) corresponding epifluorescent image using probe P615.
Fig 3.
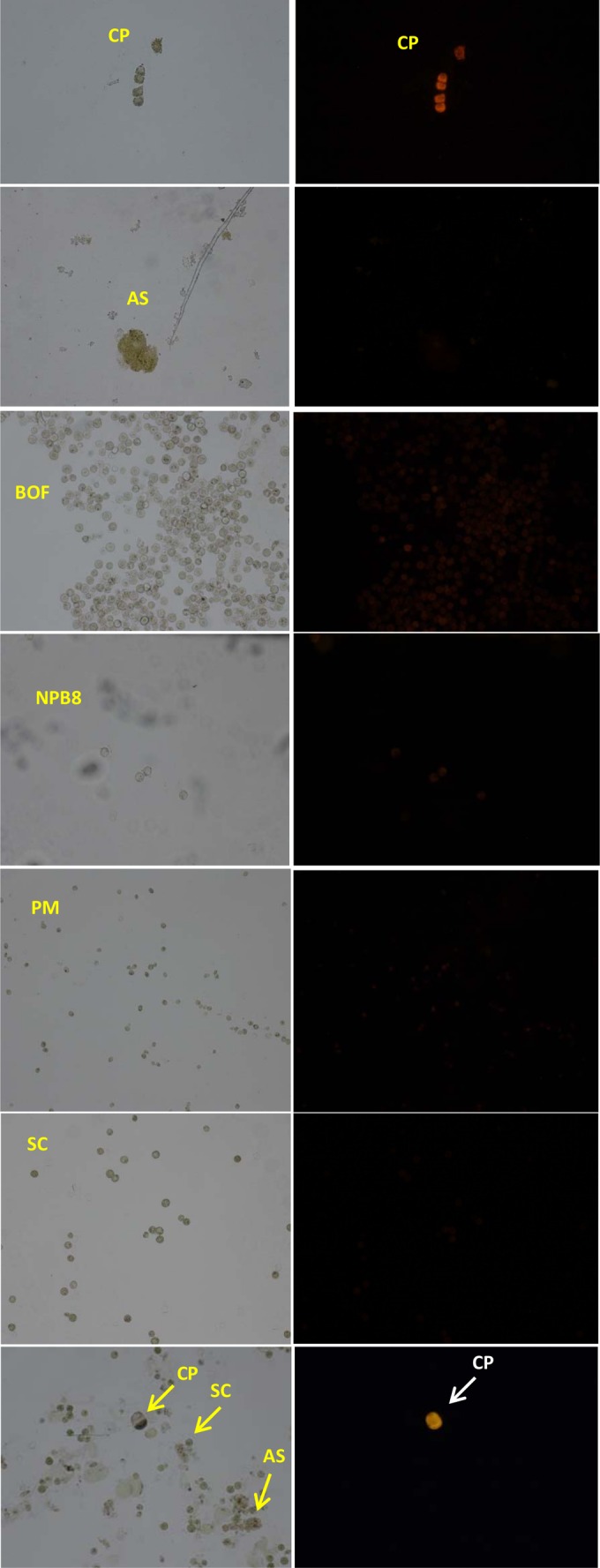
Light microscopy images (left) and corresponding epifluorescent images (right) of cultures Cochlodinium polykrikoides (CP), Akashiwo sanguinea (AS), Alexandrium fundyense (BOF), Alexandrium fundyense (NPB8), Prorocentrum minimum (PM), and Scrippsiella trochoidea (SC) hybridized with probe P442. All are vegetative cells.
FIG 4.

Light microscopy images (left) and corresponding epifluorescent images (right) of multiple strains (CPSB-1B, CPSB-1G, CP2A, and CP1) of Cochlodinium polykrikoides vegetative cells hybridized with probe P442.
Optimization of hybridization time and temperature.
The observed signal intensities generally increased with hybridization time (24 h ≈ 12 h > 6 h > 3 h) as well as temperature (55°C > 50°C > 46°C). Given the observed signal intensities, a hybridization temperature of 55°C and a hybridization time of 12 h were used for vegetative cells of C. polykrikoides. In addition, slight modifications of the methodology, including increasing the hybridization time to 16 to 18 h and prewarming samples at 90°C for 10 min prior to hybridization, were used to optimize the fluorescence of cysts.
Recovery of culture-generated C. polykrikoides cysts from sediments.
Stained C. polykrikoides cysts were easily distinguishable in sediment samples spiked with culture-generated cysts (Fig. 5A to D), whereas control sediments without cysts added revealed no cysts. From these samples spiked with culture-generated C. polykrikoides cysts, 95% ± 3% of cysts added and observed under bright-field microscopy were stained, and a lesser percentage of the cysts added (65% ± 1%) were recovered, indicating the loss of some cysts as they were brought through the entire FISH protocol. In sediment samples from various regions of Shinnecock Bay that were treated with FISH, C. polykrikoides cysts in addition to cysts of various dinoflagellates species, including Protoperidinium spp. (Fig. 5E and F) and Polykrikos spp. (Fig. 5I and J) were observed via bright-field microscopy, but only C. polykrikoides cysts fluoresced under epifluorescence microscopy. In rare cases, there was nonspecific binding of the oligonucleotide probe to pollen grains (Fig. 5G and H), while in other cases nonspecific binding to pollen grains was not observed (Fig. 5I and J). Hence, caution was still exercised when counting a fluorescing particle as a C. polykrikoides cyst, and only fluorescing particles resembling C. polykrikoides cysts in general morphology and size (as determined via bright-field microscopy) were quantified.
FIG 5.

Light microscopy images (left) and corresponding epifluorescent images (right). (A to D) Cochlodinium cyst observed in a Northport Bay sample spiked with culture-generated cysts. Magnification, ×100 (A and B) or ×400 (C and D). (E and F) C. polykrikoides (CP) and Protoperidinium spp. (Pr) cysts. (G and H) A pollen grain. (I and J) C. polykrikoides (CP) and Polykrikos spp. (Po) cysts and a pollen grain (Pg).
Shinnecock Bay vegetative cell and cyst surveys.
During the 2013 Cochlodinium survey, both low-density (<102 cells ml−1) and high-density (>104 cells ml−1) patches of vegetative cells were observed in the northeast (near S1) and northwest (near S6) regions and in the southwestern region (near S11 and 46), respectively, of Shinnecock Bay (Fig. 6A). This general spatial distribution persisted for the duration of the bloom, which was approximately 1 month. The sediment survey of Shinnecock Bay conducted in April 2014 revealed that C. polykrikoides cysts were present at concentrations of 7 to 147 cysts cm−3 of sediment (Fig. 6B; Table 1). The highest densities of C. polykrikoides cysts were found in the northern part of Shinnecock Bay (>100 cysts cm−3 of sediment), while moderate densities were observed in Old Fort Pond and the southwestern region of the survey (26 to 66 cysts cm−3 of sediment) (Fig. 6B; Table 1). During a 2014 vegetative cell survey, only moderate-density (103 cells ml−1) patches were observed in the northern region of Shinnecock Bay, while high densities (>3 × 104 cells ml−1) were observed in Old Fort Pond (Fig. 6C). The 2014 bloom was brief, ∼2 weeks, during which this spatial distribution of cells was generally maintained. Consistent with the September 2014 vegetative cell survey, the highest cyst densities found in the April 2015 sediment survey were in Old Fort Pond (>500 cysts cm−3 of sediment), while densities in Shinnecock Bay were substantially lower (<30 cysts cm−3 of sediment) (Fig. 6D; Table 2). There was no correlation between cyst densities in Shinnecock Bay and organic content of the sediment (r2 = 0.17; not significant [n.s.]); cysts were observed in various sediment types (clay, silt, sand) with a range of organic contents (1 to 14%). Sediment samples collected after the demise of the 2014 Cochlodinium bloom that occurred in Old Fort Pond demonstrated that cysts were present in the sediment soon after the end of the bloom (Table 3). Cyst densities ranged from 32 to 162 cysts cm−3 of sediment and varied over time (4 weeks), increasing in some cases while decreasing in others (Table 3).
FIG 6.
Cochlodinium vegetative cell and cyst surveys conducted in the Shinnecock Bay region. (A) 2013 vegetative cell survey (fluorescence-normalized chlorophyll a; μg liter−1); (B) April 2014 cyst survey (cysts cm−3 of sediment); (C) 2014 vegetative cell survey (fluorescence-normalized chlorophyll a; μg liter−1); (D) April 2015 cyst survey (cysts cm−3 of sediment).
TABLE 2.
Quantification of Cochlodinium polykrikoides cysts from sediments collected in April 2015 from Shinnecock Bay and Old Fort Pond, NY
| Embayment and site ID | No. of Cochlodinium cysts cm−3 |
|---|---|
| Shinnecock Bay | |
| S1 | 5 |
| S4 | 10 |
| S5 | 6 |
| S6 | 10 |
| S7 | 30 |
| S11 | 3 |
| S23 | 5 |
| S24 | 18 |
| S26 | 6 |
| 45 | 10 |
| 46 | 8 |
| 47 | 11 |
| 48 | 8 |
| Old Fort Pond | |
| S27 | 12 |
| S29 | 566a |
| S30 | 25 |
PCR confirmed; % identity, 99%; BLASTn hit, C. polykrikoides; GenBank accession no. AB295048.
TABLE 3.
Quantification of Cochlodinium cysts in Old Fort Pond, NY, sediments sampled toward the demise of the 2014 Cochlodinium blooma
| Site no. | No. of Cochlodinium cysts cm−3 of sediments sampled on: |
|||
|---|---|---|---|---|
| 1 Oct | 8 Oct | 15 Oct | 20 Oct | |
| 1 | 38 | 92 | 78 | 99 |
| 2 | 162b | 42 | 80 | 0 |
| 3 | 140 | 45 | 32 | 137 |
Sediments were sampled for a period of 4 weeks in October (Oct) 2015.
PCR confirmed; % identity, 95.3%; BLASTn hit, C. polykrikoides; GenBank accession no. AB295048.
Cochlodinium polykrikoides cysts were also observed in other regions of Long Island. Densities ranging from 17 to 95 cysts cm−3 of sediment were observed in Tiana Bay sediments (Western Shinnecock Bay) with the highest densities occurring in the northern part of Tiana Bay (Table 1). C. polykrikoides cyst densities of <78 cysts cm−3 of sediment were also found in Northport Bay sediments, an area not previously known to host blooms of C. polykrikoides (Table 1). Finally, five samples (2014 samples S29.2, S30, N18, and site 2 Oct 1 sample from the Old Fort Pond cyst deposition time series; 2015 sample S29) (Tables 1 to 3) that were positive for the presence of Cochlodinium polykrikoides cysts via the FISH assay were confirmed via PCR with Cochlodinium-specific primer sets and had BLASTn hits that were 95.3 to 99% identical to C. polykrikoides (GenBank accession number AB295048) (Tables 1 to 3).
DISCUSSION
While recent studies have affirmed the ability of Cochlodinium polykrikoides to form cysts in culture (10) and in the field (36), identifying these cysts in the field using traditional microscopic methods has proven difficult and has resulted in a series of potentially false-positive identifications (as reviewed in references 10 and 16). This study developed an assay to directly target C. polykrikoides cysts using fluorescent in situ hybridization (FISH) with oligonucleotide probes specific for the large subunit (LSU) rDNA of C. polykrikoides. The FISH assay positively identified culture-generated cysts made from North American C. polykrikoides isolates that were added to local sediments, and probes did not hybridize with any other local cyst-forming dinoflagellate. Further, using the FISH assay in combination with independent PCR confirmation, we conducted a C. polykrikoides cyst survey. Hence, this method provides the means to assess the potential vulnerability of coastal ecosystems to C. polykrikoides blooms.
Methodological advantages and limitations.
The reported morphologies for cysts of C. polykrikoides have been diverse, from spherical cysts with a cyst wall (10) to a cyst body that was covered by reticulate ornaments and spines (36). Such morphological differences may reflect ribotype-specific (36, 71, 72) or environmental differences among sites. Shin et al. (73) found that changes in pH can cause morphological differences in Scrippsiella trochoidea cysts with acidified environments, leading to the calcareous spines of S. trochoidea being lost (due to dissolution), resulting in a "naked type" cyst, a plausible outcome in the hypoxic sediments of our eutrophied New York estuaries (74). In this particular case, however, the former may be more likely, given that our C. polykrikoides cysts that were produced in culture and those found in New York sediments from regions where those cultures were originally isolated are highly similar in appearance (10; this study). Regardless, a molecular identification method that incorporates microscopy is highly advantageous and will greatly aid in the advancement of Cochlodinium research.
Given the vast morphological differences reported for Cochlodinium cysts in sediments (6, 29–34) and in culture (10, 27, 28) and the need to create maps of these cyst beds for fishery management (among other) purposes, the development of an accurate method for the detection of C. polykrikoides cysts is desirable. While Park and Park (40) detected C. polykrikoides in sediment samples using real-time PCR, the detection of vegetative cells or DNA residues of vegetative cells could not be excluded. The newly developed FISH assay reported here combines molecular and microscopic methods to ensure the accurate identification of C. polykrikoides resting cysts. Because all samples were evaluated under epifluorescent and bright-field microscopy, vegetative cells were easily distinguished from cysts. While both cell types react with fluorescent probes under epifluorescence, examination under bright-field microscopy allows users to clearly distinguish cell types via morphological differences. Additionally, sieving and rinsing sediment samples through a series of mesh sizes prior to our pretreatment of sediment samples with SPT to isolate cysts from the sediment reduces the possibility that cellular and DNA residues from sediments were amplified during PCR confirmation of samples. Further, we note that for the sediment samples that were collected in April, no vegetative cells were present and the bloom that “seeded” those cyst beds occurred 7 to 8 months prior to sediment collection; therefore, it is reasonable to assume that any residual DNA from that bloom had been degraded. It is notable that DNA from C. polykrikoides was amplified from sediment samples with FISH-identified cysts only after rigorous bead beating (data not shown), addition of proteinase K, and extraction with a Qiagen kit (52); thus, we conclude that the positive product was due to cysts rather than from vegetative cells as cultures used in initial direct PCR trials (data not shown) amplified without additional processing.
While there are many advantages to using this novel FISH assay for enumerating C. polykrikoides cysts from sediments, it also has limitations. While 95% ± 3% of C. polykrikoides cysts in sediment samples hybridized with the oligonucleotide probe, only 65% ± 1% of cysts were quantified when taken through the entire FISH methodology, demonstrating that there is some loss of cysts through the protocol. This is not surprising, given the fragile nature of C. polykrikoides cysts and their vulnerability to bacterial degradation (10). For example, only a fraction of laboratory-generated cysts survived to germinate (8 to 30%), with bacteria being responsible for degrading cysts that could not germinate (10). Because the state of bacterial degradation of cysts used in recovery experiments was unknown, it is likely that at least some of the cysts that were not quantitatively recovered through the FISH process were in a degraded state and also would not have germinated. Regardless, the less than 100% recovery of cysts via the FISH assay demonstrates that this method is semiquantitative.
While the FISH method was highly specific for C. polykrikoides and the probes never cross-reacted with any other dinoflagellate cell or cysts, we did observe on rare occasions that pollen grains fluoresced brightly, although in other cases they did not (Fig. 5). This is the advantage of using an approach combining a molecular probe and a microscopy setting whereby users can morphologically distinguish terrestrial particles from cysts. There was variation in the intensity of the fluorescent signal of the oligonucleotide probe P442 (Fig. 4) among different strains of vegetative cells of Cochlodinium that could be related to differences in the growth phase (i.e., lag, exponential, stationary) and associated copy numbers of rDNA and/or nutritional status that the cultures were experiencing at the time they were harvested for experimentation (54–56). Using flow cytometric analysis, Anderson et al. (54) found that the labeling intensity of nutrient-replete cultures of Alexandrium spp. was up to four times higher in exponential than in stationary phase and varied 3-fold depending on nutritional status. Further, Anderson et al. (55) state that life history phase (i.e., vegetative cells, temporary cysts, resting cysts) can contribute to variation in labeling intensity, as these phases may cause differences in overall cellular permeability. Given that cysts are known to have a cellular outer wall thicker than that of vegetative cells (6, 10) and therefore potentially limited permeability, we included a proteinase K step in our protocol to aid in increasing the permeability of the cysts and thus facilitating uptake of the oligonucleotide probe. Regardless, the ability to successfully identify C. polykrikoides cysts in sediments represents an important step to understanding the ecology of this bloom-forming, harmful dinoflagellate.
Cochlodinium cysts in natural sediments.
The novel FISH assay was successfully used to identify C. polykrikoides cyst bed locations and densities in bloom-prone regions such as Shinnecock Bay, Tiana Bay, and Old Fort Pond, NY, USA. During this study, cyst densities during spring of a given year were spatially consistent with regions of dense blooms the prior late summer and fall. For example, during 2013, the densest aggregations of vegetative Cochlodinium cells were found along the northern and western shores of Shinnecock Bay, and then in April 2014, the northwestern region hosted the greatest densities of Cochlodinium cysts. In 2014, Cochlodinium bloom patches were less dense within the greater Shinnecock Bay, but the most intense blooms observed during this study (3 × 104 cells ml−1) occurred within Old Fort Pond. The following spring, the greatest densities of Cochlodinium cysts were found in Old Fort Pond, whereas lower levels were present within Shinnecock Bay. These observed patterns suggest that cyst beds are representative of prior bloom events but are not necessarily predictive of future bloom intensity. This hypothesis is consistent with the relative densities of Cochlodinium cysts found in sediments that were low (tens to hundreds per cm3) relative to the densities of vegetative cells during blooms (thousands to tens of thousands per milliliter [18; this study]) that can persist for several weeks and can occupy the upper 0.5 m of the water column (48) and thus have the potential to yield elevated cyst densities. Low cyst densities quantified 6 months after high-density blooms may be indicative of a small portion of the vegetative population being involved in sexual reproduction (57), grazing of cysts in sediments (58, 59), bacterial degradation of cysts (10), and/or the movement of cysts/vegetative cells out of the bloom region via currents (60) and subsequent deposition of cysts elsewhere (61). Currently, there is only one other report of Cochlodinium cysts in sediments; however, abundances were reported in rDNA copies per cubic centimeter of wet sediment and thus not comparable (40). In regard to excystment, like Cochlodinium, other cyst-forming dinoflagellates such as Alexandrium can also produce high-density blooms (>106 cells liter−1) from low-density cyst beds (<50 cysts cm−3 [57, 62]). In these cases, the formation of dense vegetative blooms from modest cyst densities was shown to be caused by high rates of in situ, vegetative growth supported by high levels of anthropogenic nutrient loading and optimal temperatures. The highest cyst densities observed in Old Fort Pond (66 cysts cm−3) during 2014 would only yield a vegetative population of 440 cells liter−1 if all cysts emerged simultaneously into a 1.5-m water column; this is orders of magnitude lower than the densities in the 2014 bloom (3 × 104 cells ml−1). Gobler et al. (18) demonstrated that C. polykrikoides is nutritionally flexible and capable of using various nitrogenous compounds for growth, while Tang and Gobler (25) and Jiang et al. (21) have cited allelopathy and predator deterrence capabilities, respectively, as strategies that promote Cochlodinium blooms in these regions. The modest densities of cysts in Shinnecock Bay suggest that cyst beds are important as “seed banks” for initiating blooms and that the in situ growth of vegetative cells plays the dominant role in Cochlodinium bloom formation. More observations of Cochlodinium cyst distributions in other systems coupled with detailed hydrodynamic and growth rate studies will refine the ability to use cyst densities to predict future vegetative population densities and locations.
While dense patches of vegetative cells were often found near regions that had higher (relative to the rest of the bay) cyst densities during the prior spring, there were also cases in which high-density patches were found in regions that had low Cochlodinium cyst densities the prior spring or vice versa. While in situ growth (18, 57, 62) could be responsible for such differences, physical attributes of the region are also likely to influence cyst distributions (61, 63). Hydrodynamics are key components in forecast models for HABs (63–65). Further, it has been suggested that cysts are more often found in silt and clay deposits (63, 66, 67). Given that there was no relationship between the number of cysts and sediment type or percent organic matter content of sediments during this study, other factors were more important for controlling cyst patterns (61). During our cyst deposition time series (in Old Fort Pond), we found that cyst densities varied within a short time frame (1 month) over ∼5-m sampling areas. Such variation likely reflects a combination of physical and biological factors, including the resuspension of cysts via wind and currents in this shallow system (61, 63), predation of cysts by benthic feeders (58, 59), and cyst degradation (10). Research utilizing sediment traps to assess Cochlodinium encystment rates and deposition as well as hydrodynamic and excystment rate studies are needed to gain further insight regarding the temporal and spatial dynamics of Cochlodinium cysts in bloom-prone regions.
While the novel FISH assay described here was useful for determining the abundance of cysts in bloom-prone regions, it was also useful for identifying C. polykrikoides cysts in regions with previously unreported blooms, specifically Northport Bay, NY. Northport Bay is hydrodynamically separated from regions known to host annual Cochlodinium blooms (Shinnecock Bay and Peconic Estuary) (18, 43) and whose only connection to these regions is through Long Island Sound, which exchanges with the Peconic Estuary but has had limited reports of Cochlodinium blooms (S. Lin, personal communication). While there has been robust monitoring of HABs in Northport Bay during April through July for nearly 1 decade (2007 to 2015) (62, 68), there has been only sporadic monitoring of this location during the months in which Cochlodinium forms HABs in the region (August through October). Hence, the identification of Cochlodinium cysts in Northport Bay indicates that this FISH method can be useful for the identification of regions that have hosted previously undocumented blooms or populations of C. polykrikoides as well as identifying the vulnerability of regions prone to future blooms.
Conclusions.
This novel FISH assay is an important advance in understanding the ecology of the globally distributed, ichthyotoxic dinoflagellate C. polykrikoides. This assay will allow the development of maps of cyst bed densities and distributions that, when coupled with vegetative cell distributions, should facilitate a greater understanding of Cochlodinium bloom evolution, expansion, and ecology. Mapping of Cochlodinium cysts may also have important managerial applications; for example, it may be useful for assessing the vulnerability of aquaculture sites to Cochlodinium blooms when data regarding vegetative cell abundances during months that blooms occur are not available. Finally, for decades, ballast water transport of dinoflagellate cysts by ocean-going vessels has been invoked as a mechanism facilitating the global spread of HABs (4, 69, 70), including those formed by Cochlodinium (10, 15). This novel FISH assay may also prove to be a useful monitoring tool for assessing the potential for ships' ballast water to transport C. polykrikoides.
ACKNOWLEDGMENTS
This work would not have been possible without the help of molecular assistance from Dianna L. Berry and field assistance from Yoonja Kang, Mark Lusty, and Brooke Morrell. We thank the Stony Brook Southampton Marine Science Center staff for logistical field assistance.
Funding Statement
This research was supported by New York Sea Grant Award R/CMB-40 to C.J.G. We also acknowledge the continued and generous support of the Laurie Landeau Foundation and Simons Foundation. The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
REFERENCES
- 1.Anderson DM, Morel FM. 1979. The seeding of two red tide blooms by the germination of benthic Gonyaulax tamarensis hypnocysts. Estuar Coast Mar Sci 8:279–293. doi: 10.1016/0302-3524(79)90098-7. [DOI] [Google Scholar]
- 2.Anderson DM. 1989. Cysts as factors in Pyrodinium bahamense ecology, p 81–88. In Hallegraeff GM, Maclean JL (ed), Biology, epidemiology and management of Pyrodinium red tides. ICLARM Conference Proceedings, vol. 21. Fisheries Department, Ministry of Development, Brunei Darussalam, and International Center for Living Aquatic Resources Management, Manila, Philippines. [Google Scholar]
- 3.Anderson DM, Wall D. 1978. Potential importance of benthic cysts of Gonyaulax tamarensis and G. excavata in initiating toxic dinoflagellate blooms. J Phycol 14:224–234. doi: 10.1111/j.1529-8817.1978.tb02452.x. [DOI] [Google Scholar]
- 4.Hallegraeff GM, Bolch CJ. 1991. Transport of toxic dinoflagellate cysts via ships' ballast water. Mar Pollut Bull 22:27–30. doi: 10.1016/0025-326X(91)90441-T. [DOI] [Google Scholar]
- 5.Nehring S. 1993. Mechanisms for recurrent nuisance algal blooms in coastal zones: resting cyst formation as life-strategy of dinoflagellates, p 454–467. In Sterr H, Hofstade J, Plag HP (ed), Interdisciplinary discussion of coastal research and coastal management issues and problems. Lang, Frankfurt, Germany. [Google Scholar]
- 6.Matsuoka K, Fukuyo Y. 2002. Technical guide for modern dinoflagellate cyst study. WESTPAC-HAB/WESTPAC/IOC Japan Society for the Promotion of Science, Tokyo, Japan. [Google Scholar]
- 7.Zingone A, Garces E, Wyatt T, Silvert B, Bolch CJ. 2002. The importance of life cycles in the ecology of harmful algal blooms, p 134–137. In Garces E, Zingone A, Montresor M, Reguera B, Dale B (ed), LIFEHAB: life histories of microalgal species causing harmful blooms. Office for the Official Publications of the European Community, Luxembourg, Luxembourg. [Google Scholar]
- 8.Figueroa RI, Rengefors K, Bravo I, Bensch S. 2010. From homothally to heterothally: mating preferences and genetic variation within clones of the dinoflagellate Gymnodinium catenatum. Deep Sea Res Part II Top Stud Oceanogr 57:190–198. doi: 10.1016/j.dsr2.2009.09.016. [DOI] [Google Scholar]
- 9.Anglès S, Garcés E, Reñé A, Sampedro N. 2012. Life-cycle alternations in Alexandrium minutum natural populations from the NW Mediterranean Sea. Harmful Algae 16:1–11. doi: 10.1016/j.hal.2011.12.006. [DOI] [Google Scholar]
- 10.Tang YZ, Gobler CJ. 2012. The toxic dinoflagellate Cochlodinium polykrikoides (Dinophyceae) produces resting cysts. Harmful Algae 20:71–80. doi: 10.1016/j.hal.2012.08.001. [DOI] [Google Scholar]
- 11.Matsuoka K, Fukuyo Y. 2003. Taxonomy of cysts, p 563–592. In Hallegraeff GM, Anderson DM, Cembella AD (ed), Manual on harmful marine microalgae manual and guides. International Oceanographic Commission of UNESCO, Paris, France. [Google Scholar]
- 12.Elbrăchter M. 2003. Dinophyte reproduction: progress and conflicts. J Phycol 39:629–632. doi: 10.1046/j.1529-8817.2003.39041.x. [DOI] [Google Scholar]
- 13.Gómez F. 2012. A checklist and classification of living dinoflagellates (Dinoflagellata, Alveolata). CICIMAR Oceánides 27:65–140. [Google Scholar]
- 14.Matsuoka K, Iwataki M, Kawami H. 2008. Morphology and taxonomy of chain-forming species of the genus Cochlodinium (Dinophyceae). Harmful Algae 7:261–270. doi: 10.1016/j.hal.2007.12.002. [DOI] [Google Scholar]
- 15.Richlen ML, Morton SL, Jamali EA, Rajan A, Anderson DM. 2010. The catastrophic 2008-2009 red tide in the Arabian gulf region, with observations on the identification and phylogeny of the fish-killing dinoflagellate Cochlodinium polykrikoides. Harmful Algae 9:163–172. doi: 10.1016/j.hal.2009.08.013. [DOI] [Google Scholar]
- 16.Kudela RM, Gobler CJ. 2012. Harmful dinoflagellate blooms caused by Cochlodinium sp.: global expansion and ecological strategies facilitating bloom formation. Harmful Algae 14:71–86. doi: 10.1016/j.hal.2011.10.015. [DOI] [Google Scholar]
- 17.Margalef R. 1961. Hidrografia y fitoplancton de un area marina de la costa meridional de Puerto Rico. Invest Pesquera 18:76–78. [Google Scholar]
- 18.Gobler CJ, Burson A, Koch F, Tang YZ, Mulholland MR. 2012. The role of nitrogenous nutrients in the occurrence of harmful algal blooms caused by Cochlodinium polykrikoides in New York estuaries (USA). Harmful Algae 17:64–74. doi: 10.1016/j.hal.2012.03.001. [DOI] [Google Scholar]
- 19.Tang YZ, Koch F, Gobler CJ. 2010. Most harmful algal bloom species are vitamin B1 and B12 auxotrophs. Proc Natl Acad Sci U S A 107:20756–20761. doi: 10.1073/pnas.1009566107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jeong HJ, Yoo YD, Kim JS, Kim TH, Kim JH, Kang NS, Yih W. 2004. Mixotrophy in the phototrophic harmful alga Cochlodinium polykrikoides (Dinophycean): prey species, the effects of prey concentration, and grazing impact. J Eukaryot Microbiol 51:563–569. doi: 10.1111/j.1550-7408.2004.tb00292.x. [DOI] [PubMed] [Google Scholar]
- 21.Jiang XD, Tang YZ, Lonsdale DJ, Gobler CJ. 2009. Deleterious consequences of a red tide dinoflagellate Cochlodinium polykrikoides for the calanoid copepod Acartia tonsa. Mar Ecol Prog Ser 390:105–116. doi: 10.3354/meps08159. [DOI] [Google Scholar]
- 22.Tang YZ, Gobler CJ. 2009. Characterization of the toxicity of Cochlodinium polykrikoides isolates from Northeast US estuaries to finfish and shellfish. Harmful Algae 8:454–462. doi: 10.1016/j.hal.2008.10.001. [DOI] [Google Scholar]
- 23.Tang YZ, Gobler CJ. 2009. Cochlodinium polykrikoides blooms and clonal isolates from the northwest Atlantic coast cause rapid mortality in larvae of multiple bivalve species. Mar Biol 156:2601–2611. doi: 10.1007/s00227-009-1285-z. [DOI] [Google Scholar]
- 24.Park BS, Kim J-H, Kim JH, Gobler CJ, Baek SH, Han M-S. 2015. Dynamics of bacterial community structure during blooms of Cochlodinium polykrikoides (Gymnodiniales, Dinophyceae) in Korean coastal waters. Harmful Algae 48:44–54. [DOI] [PubMed] [Google Scholar]
- 25.Tang YZ, Gobler CJ. 2010. Allelopathic effects of Cochlodinium polykrikoides isolates and blooms from the estuaries of Long Island, New York, on co-occurring phytoplankton. Mar Ecol Prog Ser 406:19–31. doi: 10.3354/meps08537. [DOI] [Google Scholar]
- 26.Kim C-H, Cho H-J, Shin J-B, Moon C-H, Matsuoka K. 2002. Regeneration from hyaline cysts of Cochlodinium polykrikoides (Gymnodiniales, Dinophyceae), a red tide organism along the Korean coast. Phycologia 41:667–669. doi: 10.2216/i0031-8884-41-6-667.1. [DOI] [Google Scholar]
- 27.Kim C-J, Kim H-G, Kim C-H, Oh H-M. 2007. Life cycle of the ichthyotoxic dinoflagellate Cochlodinium polykrikoides in Korean coastal waters. Harmful Algae 6:104–111. doi: 10.1016/j.hal.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 28.Tomas CR, Smayda TJ. 2008. Red tide blooms of Cochlodinium polykrikoides in a coastal cove. Harmful Algae 7:308–317. doi: 10.1016/j.hal.2007.12.005. [DOI] [Google Scholar]
- 29.Matsuoka K, Fukuyo Y. 2000. Technical guide for modern dinoflagellate cyst study. WESTPAC-HAB/WESTPAC/IOC. IOC/WESTPAC-HAB. The University of Tokyo, Tokyo, Japan. [Google Scholar]
- 30.Rosales-Loessener F, Matsuoka K, Fukuyo Y, Sanchez EH. 1996. Cysts of harmful dinoflagellates found from Pacific coastal waters of Guatemala. In Yasumoto T, Oshima Y, Fukuyo Y (ed), Harmful and toxic algal blooms. International Oceanographic Commission of UNESCO, Paris, France. [Google Scholar]
- 31.Orlova TY, Morozova TV, Gribble KE, Kulis DM, Anderson DM. 2004. Dinoflagellate cysts in recent marine sediments from the east coast of Russia. Bot Mar 47:184–201. doi: 10.1515/BOT.2004.019. [DOI] [Google Scholar]
- 32.Seaborn DW, Marshall HG. 2008. Dinoflagellate cysts within sediment collections from the southern Chesapeake Bay and tidal regions of the James, York, and Rappahannock Rivers. Va J Sci 59:135–141. [Google Scholar]
- 33.Rubino F, Belmonte M, Caroppo C, Giacobbe M. 2010. Dinoflagellate cysts from surface sediments of Syracuse Bay (Western Ionian Sea, Mediterranean). Deep Sea Res Part II Top Stud Oceanogr 57:243–247. doi: 10.1016/j.dsr2.2009.09.011. [DOI] [Google Scholar]
- 34.Mohamed ZA, Al-Shehri AM. 2011. Occurrence and germination of dinoflagellate cysts in surface sediments from the Red Sea off the coasts of Saudi Arabia. Oceanologia 53:121–136. doi: 10.5697/oc.53-1.121. [DOI] [Google Scholar]
- 35.Fukuyo Y. 1982. Cysts of naked dinoflagellates, p 205–214. In Okaichi T. (ed), Fundamental studies of the effects of the marine environment on the outbreaks of red tides reports of environmental sciences, B 148-R14-8. Monbusho, Tokyo, Japan. [Google Scholar]
- 36.Li Z, Han M-S, Matsuoka K, Kim S-Y, Shin HH. 2015. Identification of the resting cyst of Cochlodinium polykrikoides Margalef (Dinophyceae, Gymnodiniales) in Korean coastal sediments. J Phycol 51:204–210. doi: 10.1111/jpy.12252. [DOI] [PubMed] [Google Scholar]
- 37.Anderson DM. 1998. Physiology and bloom dynamics of toxic Alexandrium species, with emphasis on life cycle transitions. Nato Asi Ser G Ecol Sci 41:29–48. [Google Scholar]
- 38.Anderson DM, Chisholm SW, Watras CJ. 1983. Importance of life cycle events in the population dynamics of Gonyaulax tamarensis. Mar Biol 76:179–189. doi: 10.1007/BF00392734. [DOI] [Google Scholar]
- 39.Dale B. 1977. Cysts of the toxic red-tide dinoflagellate Gonyaulax excavata (Braarud) Balech from Oslofjorden, Norway. Sarsia 63:29–34. [Google Scholar]
- 40.Park T-G, Park Y-T. 2010. Detection of Cochlodinium polykrikoides and Gymnodinium impudicum (Dinophyceae) in sediment samples from Korea using real-time PCR. Harmful Algae 9:59–65. doi: 10.1016/j.hal.2009.08.002. [DOI] [Google Scholar]
- 41.Doblin MA, Blackburn SI, Hallegraeff GM. 1999. Growth and biomass stimulation of the toxic dinoflagellate Gymnodinium catenatum (Graham) by dissolved organic substances. J Exp Mar Biol Ecol 236:33–47. doi: 10.1016/S0022-0981(98)00193-2. [DOI] [Google Scholar]
- 42.Guillard RR, Ryther JH. 1962. Studies of marine planktonic diatoms. I. Cyclotella nana Hustedt, and Detonula confervacea Cleve. Can J Microbiol 8:229–239. [DOI] [PubMed] [Google Scholar]
- 43.Gobler CJ, Berry DL, Anderson OR, Burson A, Koch F, Rodgers BS, Moore LK, Goleski JA, Allam B, Bowser P, Tang Y, Nuzzi R. 2008. Characterization, dynamics, and ecological impacts of harmful Cochlodinium polykrikoides blooms on eastern Long Island, NY, USA. Harmful Algae 7:293–307. doi: 10.1016/j.hal.2007.12.006. [DOI] [Google Scholar]
- 44.Tang YZ, Gin KYH, Lim TH. 2005. High-temperature fluorescent in situ hybridization for detecting Escherichia coli in seawater samples, using rRNA-targeted oligonucleotide probes and flow cytometry. Appl Environ Microbiol 71:8157–8164. doi: 10.1128/AEM.71.12.8157-8164.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fuchs BM, Wallner G, Beisker W, Schwippl I, Ludwig W, Amann R. 1998. Flow cytometric analysis of the in situ accessibility of Escherichia coli 16S rRNA for fluorescently labeled oligonucleotide probes. Appl Environ Microbiol 64:4973–4982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bolch CJ. 1997. The use of sodium polytungstate for the separation and concentration of living dinoflagellate cysts from marine sediments. Phycologia 36:472–478. doi: 10.2216/i0031-8884-36-6-472.1. [DOI] [Google Scholar]
- 47.Morse RE, Mulholland MR, Hunley WS, Fentress S, Wiggins M, Blanco-Garcia JL. 2013. Controls on the initiation and development of blooms of the dinoflagellate Cochlodinium polykrikoides Margalef in lower Chesapeake Bay and its tributaries. Harmful Algae 28:71–82. doi: 10.1016/j.hal.2013.05.013. [DOI] [Google Scholar]
- 48.Koch F, Burson A, Tang YZ, Collier JL, Fisher NS, Sañudo-Wilhelmy S, Gobler CJ. 2014. Alteration of plankton communities and biogeochemical cycles by harmful Cochlodinium polykrikoides (Dinophyceae) blooms. Harmful Algae 33:41–54. doi: 10.1016/j.hal.2014.01.003. [DOI] [Google Scholar]
- 49.Mulholland MR, Morse RE, Boneillo GE, Bernhardt PW, Filippino KC, Procise LA, Blanco-Garcia JL, Marshall HG, Egerton TA, Hunley WS, Moore KA, Berry DL, Gobler CJ. 2009. Understanding causes and impacts of the dinoflagellate, Cochlodinium polykrikoides, blooms in the Chesapeake Bay. Estuaries Coasts 32:734–747. doi: 10.1007/s12237-009-9169-5. [DOI] [Google Scholar]
- 50.Madden CJ, Day JW Jr. 1992. An instrument system for high-speed mapping of chlorophyll a and physico-chemical variables in surface waters. Estuaries 15:421–427. doi: 10.2307/1352789. [DOI] [Google Scholar]
- 51.Parsons TR, Maita Y, Lalli CM. 1984. A manual of chemical and biological methods for seawater analysis. Pergamon Press, Elmsford, NY. [Google Scholar]
- 52.Erdner DL, Percy L, Keafer B, Lewis J, Anderson DM. 2010. A quantitative real-time PCR assay for the identification and enumeration of Alexandrium cysts in marine sediments. Deep Sea Res Part II Top Stud Oceanogr 57:279–287. doi: 10.1016/j.dsr2.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Park BS, Wang P, Kim JH, Kim J-H, Gobler CJ, Han M-S. 2014. Resolving the intra-specific succession within Cochlodinium polykrikoides populations in southern Korean coastal waters via use of quantitative PCR assays. Harmful Algae 37:133–141. doi: 10.1016/j.hal.2014.04.019. [DOI] [Google Scholar]
- 54.Anderson DM, Kulis DM, Keafer BA, Berdalet E. 1999. Detection of the toxic dinoflagellate Alexandrium fundyense (Dinophyceae) with oligonucleotide and antibody probes: variability in labeling intensity with physiological condition. J Phycol 35:870–883. doi: 10.1046/j.1529-8817.1999.3540870.x. [DOI] [Google Scholar]
- 55.Anderson DM, Kulis DM, Keafer BA, Gribble KE, Marin R, Scholin CA. 2005. Identification and enumeration of Alexandrium spp. from the Gulf of Maine using molecular probes. Deep Sea Res I 52:2467–2490. [Google Scholar]
- 56.Mikulski C, Park Y, Jones K, Lee C, Lim W, Lee Y, Scholin C, Doucette G. 2008. Development and field application of rRNA-targeted probes for the detection of Cochlodinium polykrikoides Margalef in Korean coastal waters using whole cell and sandwich hybridization formats. Harmful Algae 7:347–359. doi: 10.1016/j.hal.2007.12.015. [DOI] [Google Scholar]
- 57.Anglès S, Garcés E, Hattenrath-Lehmann TK, Gobler CJ. 2012. In situ life-cycle stages of Alexandrium fundyense during bloom development in Northport Harbor (New York, USA). Harmful Algae 16:20–26. doi: 10.1016/j.hal.2011.12.008. [DOI] [Google Scholar]
- 58.Persson A, Rosenberg R. 2003. Impact of grazing and bioturbation of marine benthic deposit feeders on dinoflagellate cysts. Harmful Algae 2:43–50. doi: 10.1016/S1568-9883(03)00003-9. [DOI] [Google Scholar]
- 59.Persson A, Smith BC, Wikfors GH, Quilliam M. 2006. Grazing on toxic Alexandrium fundyense resting cysts and vegetative cells by the eastern oyster (Crassostrea virginica). Harmful Algae 5:678–684. doi: 10.1016/j.hal.2006.02.004. [DOI] [Google Scholar]
- 60.Matsuoka K, Mizuno A, Iwataki M, Takano Y, Yamatogi T, Yoon YH, Lee J-B. 2010. Seed populations of a harmful unarmored dinoflagellate Cochlodinium polykrikoides Margalef in the East China Sea. Harmful Algae 9:548–556. doi: 10.1016/j.hal.2010.04.003. [DOI] [Google Scholar]
- 61.Genovesi B, Mouillot D, Laugier T, Fiandrino A, Laabir M, Vaquer A, Grzebyk D. 2013. Influences of sedimentation and hydrodynamics on the spatial distribution of Alexandrium catenella/tamarense resting cysts in a shellfish farming lagoon impacted by toxic blooms. Harmful Algae 25:15–25. doi: 10.1016/j.hal.2013.02.002. [DOI] [Google Scholar]
- 62.Hattenrath TK, Anderson DM, Gobler CJ. 2010. The influence of anthropogenic nitrogen loading and meteorological conditions on the dynamics and toxicity of Alexandrium fundyense blooms in a New York (USA) estuary. Harmful Algae 9:402–412. doi: 10.1016/j.hal.2010.02.003. [DOI] [Google Scholar]
- 63.Anderson DM, Stock CA, Keafer BA, Bronzino Nelson A, Thompson B, McGillicuddy DJ, Keller M, Matrai PA, Martin J. 2005. Alexandrium fundyense cyst dynamics in the Gulf of Maine. Deep Sea Res Part II Top Stud Oceanogr 52:2522–2542. doi: 10.1016/j.dsr2.2005.06.014. [DOI] [Google Scholar]
- 64.McGillicuddy DJ, Anderson DM, Lynch DR, Townsend DW. 2005. Mechanisms regulating large-scale seasonal fluctuations in Alexandrium fundyense populations in the Gulf of Maine: results from a physical–biological model. Deep Sea Res Part II Top Stud Oceanogr 52:2698–2714. doi: 10.1016/j.dsr2.2005.06.021. [DOI] [Google Scholar]
- 65.Stock CA, McGillicuddy DJ, Solow AR, Anderson DM. 2005. Evaluating hypotheses for the initiation and development of Alexandrium fundyense blooms in the western Gulf of Maine using a coupled physical–biological model. Deep Sea Res Part II Top Stud Oceanogr 52:2715–2744. doi: 10.1016/j.dsr2.2005.06.022. [DOI] [Google Scholar]
- 66.Anglès S, Jordi A, Garcés E, Basterretxea G, Palanques A. 2010. Alexandrium minutum resting cyst distribution dynamics in a confined site. Deep Sea Res Part II Top Stud Oceanogr 57:210–221. doi: 10.1016/j.dsr2.2009.09.002. [DOI] [Google Scholar]
- 67.Dale B. 1983. Dinoflagellate resting cysts: benthic plankton, p 69–136. In Fryxell GA. (ed), Survival strategies of the algae. Cambridge University Press, Cambridge, England. [Google Scholar]
- 68.Hattenrath-Lehmann TK, Marcoval MA, Berry DL, Fire S, Wang Z, Morton SL, Gobler CJ. 2013. The emergence of Dinophysis acuminata blooms and DSP toxins in shellfish in New York waters. Harmful Algae 26:33–44. doi: 10.1016/j.hal.2013.03.005. [DOI] [Google Scholar]
- 69.Hallegraeff GM. 1993. A review of harmful algal blooms and their apparent global increase. Phycologia 32:79–99. doi: 10.2216/i0031-8884-32-2-79.1. [DOI] [Google Scholar]
- 70.Smayda TJ. 1997. Harmful algal blooms: their ecophysiology and general relevance to phytoplankton blooms in the sea. Limnol Oceanogr 42:1137–1153. doi: 10.4319/lo.1997.42.5_part_2.1137. [DOI] [Google Scholar]
- 71.Iwataki M, Kawami H, Mizushima K, Mikulski CM, Doucette GJ, Relox JR Jr, Anton A, Fukuyo Y, Matsuoka K. 2008. Phylogenetic relationships in the harmful dinoflagellate Cochlodinium polykrikoides (Gymnodiniales, Dinophyceae) inferred from LSU rDNA sequences. Harmful Algae 7:271–277. doi: 10.1016/j.hal.2007.12.003. [DOI] [Google Scholar]
- 72.Reñé A, Garcés E, Camp J. 2013. Phylogenetic relationships of Cochlodinium polykrikoides Margalef (Gymnodiniales, Dinophyceae) from the Mediterranean Sea and the implications of its global biogeography. Harmful Algae 25:39–46. doi: 10.1016/j.hal.2013.02.004. [DOI] [Google Scholar]
- 73.Shin HH, Jung SW, Jang MC, Kim YO. 2013. Effect of pH on the morphology and viability of Scrippsiella trochoidea cysts in the hypoxic zone of a eutrophied area. Harmful Algae 28:37–45. doi: 10.1016/j.hal.2013.05.011. [DOI] [Google Scholar]
- 74.Wallace RB, Baumann H, Grear JS, Aller RC, Gobler CJ. 2014. Coastal ocean acidification: the other eutrophication problem. Estuar Coast Shelf Sci 148:1–13. doi: 10.1016/j.ecss.2014.05.027. [DOI] [Google Scholar]