Abstract
Background
Neuropeptide Y (NPY), a peptide neurotransmitter that regulates stress and anxiety, has been proposed to be a stress resilience factor in humans. Posttraumatic stress disorder (PTSD) is a stress-related anxiety disorder. We hypothesized that central nervous system NPY is dysregulated in PTSD and sought to redress the absence of central NPY data in the disorder.
Methods
We determined morning NPY concentrations in cerebrospinal fluid (CSF) from 10 male subjects with chronic combat-related PTSD and from 13 healthy men. Neuropeptide Y-like immunoreactivity was measured by enzyme immunoassay (EIA).
Results
As compared with the normal comparison subjects, PTSD patients had significantly lower concentrations of CSF neuropeptide Y (mean CSF NPY was 360.0 ± 17.7 pg/mL in control subjects but only 233.6 ± 28.7 pg/mL in PTSD patients [p = .0008]). Adjustments for age and body mass index (BMI) still revealed a highly significant reduction in CSF NPY in the PTSD group (p = .003).
Conclusions
Men with combat-related PTSD have low CSF concentrations of the putative resiliency hormone NPY, possibly related to the disorder or to extreme stress exposure per se.
Keywords: Cerebrospinal fluid (CSF), combat, neuropeptide Y (NPY), posttraumatic stress disorder (PTSD), resilience
The development of posttraumatic stress disorder (PTSD) has been associated with abnormalities in the major stress response systems of the body, the hypothalamic-pituitary-adrenal (HPA) axis, the central nervous system (CNS) noradrenergic system, and the sympathetic nervous system. Pathological, PTSD-related anxiety indicates an impairment in, or the inadequacy of, anxiety-regulating mechanisms in the CNS. A major neurotransmitter that is linked to the regulation of stress and anxiety is neuropeptide Y (NPY), which is increasingly regarded as a “stress resilience factor” (1).
Neuropeptide Y is a 36-residue peptide widely and abundantly distributed in the central and peripheral nervous systems (2,3). In the brain, NPY is expressed in forebrain and diencephalic limbic regions such as the hippocampus, amygdala, cortex, the bed nucleus of stria terminalis, and hypothalamus, as well as in the brain stem (2). These regions play an important role in stress response and regulation. Preclinical studies have shown stress resilience in transgenic rats overexpressing NPY (4), while an increased susceptibility to stress and anxiety is observed in NPY knockout mice (5).
In humans, a link between haplotype-driven NPY expression and individual differences in stress response and emotion has been observed (6). Additionally, data from American soldiers undergoing survival training demonstrated that acute uncontrollable stress significantly increases plasma NPY concentrations. Relatively higher NPY concentrations were associated with increased resilience and less psychological distress (7,8). Conversely, depleted plasma NPY levels correlated with poorer stress-handling ability and higher dissociation scores (7,8). With relevance to PTSD, baseline plasma NPY levels were found to be reduced (9) or unchanged (10) in PTSD patients as compared with healthy subjects. It has also been suggested that lower plasma NPY levels relate to trauma exposure rather than to PTSD per se in combat veterans (11).
Peripheral NPY concentrations may provide only limited information about central NPY. In this regard, plasma NPY levels in humans correlate poorly with those in the CNS (12); the origins and functions of the two NPY pools may be different. Currently, data on central NPY in PTSD are lacking. To address the information vacuum, we determined CSF concentrations of NPY in men with combat-related PTSD and in healthy comparison subjects.
Methods and Materials
This study was approved by the Institutional Review Board (IRB) of the University of Cincinnati and by the Research and Development Committee of the Cincinnati Veterans Affairs Medical Center (VAMC). Written, informed consent was obtained from participants before inclusion in the study. Subjects were enrolled in diverse continuous CSF sampling protocols of PTSD; those whose postlumbar puncture CSF (precatheter placement) samples were available were included in this study.
Patients and Healthy Volunteers
Ten male patients with chronic, combat-related PTSD were enrolled. Seven patients were Vietnam combat veterans (five Army, two Marines) and three fought in western Asia (all Army). All subjects were evaluated using the Structured Clinical Interview for the DSM-III-R in addition to exploratory clinical interviews. The posttraumatic syndrome was severe in all the patients. Forty percent of patients met diagnostic criteria for current major depressive disorder (60% lifetime) and 30% for panic disorder (30% lifetime). The day before CSF collection, the mean Clinician-Administered PTSD Scale (CAPS) (13) score was 78 ± 18 (mean ± SD) and the Hamilton Depression Scale (14) score averaged 16 ± 10. Thirteen healthy, nontraumatized comparison subjects scored zero on the CAPS and had a mean Hamilton Depression Scale score of 2 ± 2. Two healthy subjects were military veterans. The healthy subjects had no first-degree family members with an Axis I psychiatric diagnosis. Fifty percent of PTSD subjects smoked cigarettes; 14% of comparison subjects smoked cigarettes and another 21% were former smokers. Sixty percent of PTSD patients had histories of alcohol abuse or dependence but had all been abstinent for over 3 months before study (mean = 52.3 ± 37.8 months, range = 3–240 months). Fifty percent of patients had also abused marijuana or cocaine but had been abstinent for an average of 57.0 ± 45.7 months (range = 8–240 months).
All subjects were medication free for a minimum of more than five medication disappearance half-lives before undergoing CSF sampling. Forty percent of patients had never taken psychotropic medications.
Clinical Study Procedures
Subjects were admitted to the Clinical Psychobiology Unit of the Cincinnati VAMC the day before CSF collection. During the afternoon, standardized tests were administered. At 2000 hours, patients ate a standard 665-calorie mixed meal and fasted thereafter, with the exception of water ingestion until 2400 hours. At midnight, all subjects were confined to bed rest. In the case of the seven smoking participants, no further preprocedure smoking was permitted.
The next morning, using strict sterile technique and 1% intradermal and subcutaneous xylocaine anesthesia, CSF was obtained by lumbar puncture with subjects in the seated position. The first postlumbar puncture aliquot, 1 mL to 2 mL, was used for NPY assay.
CSF Neuropeptide Y Assay
Neuropeptide Y-like immunoreactivity was measured in duplicate CSF samples using a competitive enzyme immunoassay (EIA) (Peninsula Laboratories, Inc., Bachem, San Carlos, California) (for details, see Supplement 1).
Statistical Analysis
Mean CSF NPY concentrations at 1000 hours between groups were compared using the two-tailed Student t test. The p values < .05 were considered significant. To control for possible group differences, NPY concentrations were linearly modeled as a function of group, age, and body mass index (BMI). Group means of NPY concentration were then compared adjusting for age and BMI. These are known as least squares means and account for possible baseline difference in age and BMI. Neuropeptide Y data appear to follow a normal distribution (p value > .25 for the Shapiro-Wilk test).
Results
Age and Body Mass Index
Posttraumatic stress disorder patients were 46 ± 2.5 and the healthy subjects 36 ± 2.9 (mean ± SEM) years old (t = 2.4, df = 21, p = .03). The body mass indexes of the patients and healthy subjects averaged, respectively, 30.4 ± 1.1 and 26.5 ± 1.1; these were significantly different (t = 2.48, df = 21, p = .022). Cerebrospinal fluid NPY concentrations correlated with neither age (r2 = .024) nor BMI (r2 = .0027) (see Supplement 2 for comment).
Cerebrospinal Fluid Neuropeptide Y Concentrations
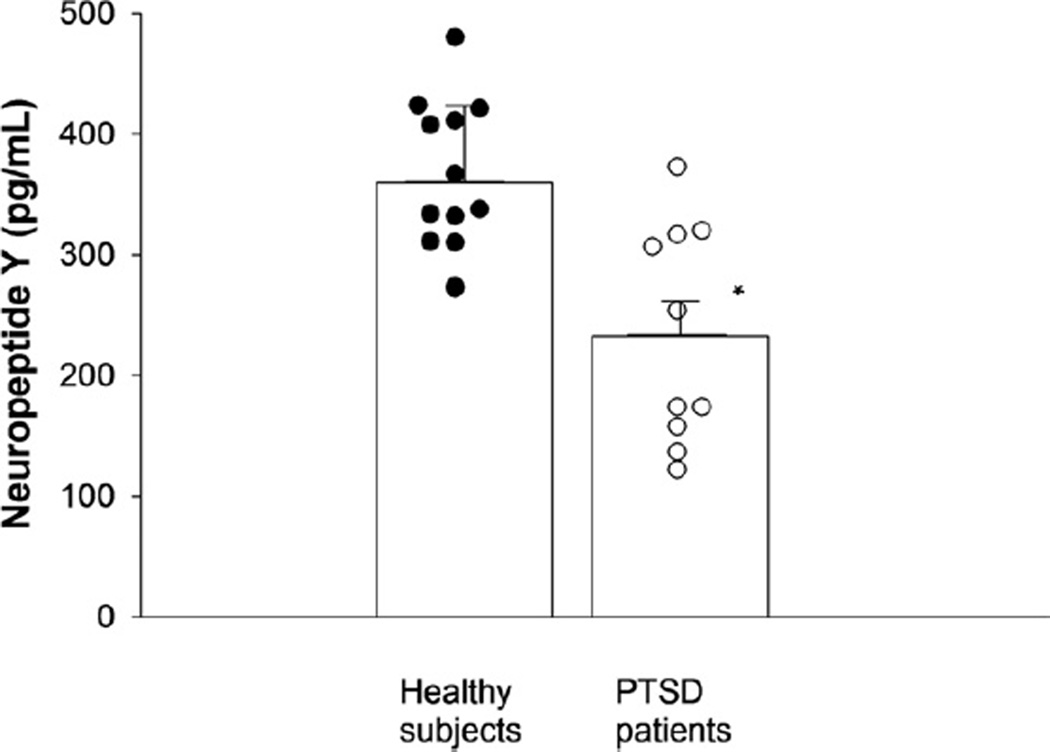
Cerebrospinal fluid NPY averaged 360.0 ± 17.7 pg/mL and 233.6 ± 28.7 pg/mL in the healthy control subjects and PTSD patients, respectively (Figure 1) (p = .0008, t = 3.924, df = 21). The overall linear model using CSF NPY as a function of group, age, and BMI was statistically significant (F value = 6.76, df = 3, 19, p value = .003); there was a lack of significance for age and BMI in the presence of the group variable (p values .592 and .121, respectively). Nevertheless, the group CSF NPY means were adjusted by age and BMI and these adjusted means, or least squares means, were statistically significant between the two groups (t value = 4.40, df = 19, p = .003).
Figure 1.
Cerebrospinal fluid concentrations of neuropeptide Y in posttraumatic stress disorder (PTSD) patients and healthy comparison subjects. *p < .0008 versus healthy subjects.
Discussion
These data reveal a striking diminution of NPY concentrations in the CSF of combat veterans with PTSD relative to healthy control subjects, raising the possibility that NPY, a putative resilience hormone, is a pathophysiological feature of the disorder. However, since the control subjects were not exposed to extreme trauma, the observed low CSF NPY levels could have been an outcome of exposure to extreme trauma per se (as previously reported for plasma NPY [11]).
Clinically, intrusive emotionality, anxiety, and sympathetic overdrive in PTSD patients may result from a dysregulation of the physiological balance between stress-regulatory transmitters in the CNS. In this regard, NPY has a reciprocal functional relationship with both norepinephrine (15) and corticotropin-releasing hormone (CRH) (16). Interestingly, our previous data showing elevated basal CRH and norepinephrine concentrations in the CSF of combat-related PTSD patients (17,18) are consistent with the current finding.
Of potential relevance to the food intake-modulating effects of NPY, our PTSD patients tended to have a higher mean BMI than the normal volunteers. However, it is unlikely that the higher mean body mass index of the PTSD subjects was responsible for their lower CSF NPY levels (see Supplement 2 for discussion).
One possible explanation for the low CSF NPY levels in the PTSD subjects is a blunted NPY response to the stress of the lumbar puncture procedure itself or to its anticipation. We have observed lumbar puncture-related effects for other neurochemicals (19). However, our recent observations using continuous CSF sampling reveal lower CSF NPY in PTSD patients, even following a several-hour period of relaxation during which time only a thin, flexible catheter remained (data collection in progress). This suggests that reduced CSF NPY concentrations in PTSD are not just the result of an impaired lumbar puncture-related NPY response.
Given the development of CNS-penetrating neuropeptide Y analogues potentially suitable for human use (20), the current data raise the possibility that the CNS NPY system could become a therapeutic target in PTSD. Our finding is potentially of high therapeutic relevance; therefore, it will be of clinical importance to replicate and extend our data using more definitive study designs. These would include larger subject numbers, new-onset PTSD patients, additional control groups (such as a traumatized but non-PTSD group), female subjects, control subjects for the stress of the lumbar puncture procedure, detailed temporal dynamics of CSF NPY, and the relationship between CSF and plasma NPY levels. The current results provide the rationale to proceed with future CNS studies of this stress-regulatory peptide in PTSD.
Supplementary Material
Acknowledgments
This work was supported by a Merit Review from the Department of Veterans Affairs (TDG).
We are indebted to the inpatient psychiatry nursing staff of the Cincinnati Veterans Affairs Medical Center (VAMC) for clinical assistance and support.
The authors disclose the following biomedical financial interests over the past 2 years: Dr. Sah has grant support from the National Institutes of Health and Department of Veterans Affairs. Dr. Geracioti receives grant support from the National Institutes of Health, the Department of Veterans Affairs, the Department of Defense, and, as a consultant, through the Research Council of the University of Cincinnati, and is the principle equity holder of RxDino, LLC, a pharmaceutical company that is developing dual corticosteroids for dermatological indications. Mr. Ekhator receives research support from the National Institutes of Health and the Department of Veterans Affairs and is a member/unit holder of RxDino, LLC. Dr. Horn receives grant/research support from the National Institutes of Health. Dr. Sallee receives grant support from the National Institutes of Health; he is a member of the board of directors and equity holder in Satiety Solutions, LLC, and P2Dinc; he also serves as a consultant to Impax Laboratories, Otsuka Pharmaceutical Development, and Shire Pharmaceutical. Dr. Baker acknowledges support from the Veterans Affairs (VA) Center of Excellence for Stress and Mental Health (CESAMH). In addition, she receives grant support from Veteran’s Affairs Health Services Research and Development Service (VA HSR&D), as well as support from the Department of Defense (Navy, Marine, Congressionally Directed Medical Research Programs (CDMRP).
Footnotes
Dr. Strawn reports no biomedical financial interests or potential conflicts of interest.
Supplementary material cited in this article is available online.
References
- 1.Yehuda R, Flory JD, Southwick SM, Charney D. Developing an agenda for translational studies of resilience and vulnerability following trauma exposure. Ann N Y Acad Sci. 2006;1071:379–396. doi: 10.1196/annals.1364.028. [DOI] [PubMed] [Google Scholar]
- 2.Hendry SHC. Organization of neuropeptide Y neurons in the mammalian central nervous system. In: Colmers WF, Wahlestedt C, editors. The Biology of Neuropeptide Y and Related Peptides. Totowa, NJ: Humana Press; 1993. pp. 65–135. [Google Scholar]
- 3.Sundler F, Bottcher G, Ekblad E, Hakanson R. PP, PYY and NPY: Occurrence and distribution in the periphery. In: Colmers WF, Wahlestedt C, editors. The Biology of Neuropeptide Y and Related Peptides. Totowa, NJ: Humana Press; 1993. pp. 170–183. [Google Scholar]
- 4.Thorsell A, Michalkiewicz M, Dumont Y, Quirion R, Caberlotto L, Rimondini R, et al. Behavioral insensitivity to restraint stress, absent fear suppression of behavior and impaired spatial learning in transgenic rats with hippocampal neuropeptide Y overexpression. Proc Natl Acad Sci U S A. 2000;97:12852–12857. doi: 10.1073/pnas.220232997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bannon AW, Seda J, Carmouche M, Francis JM, Norman MH, Karbon B, McCaleb ML. Behavioral characterization of neuropeptide Y knockout mice. Brain Res. 2000;868:79–87. doi: 10.1016/s0006-8993(00)02285-x. [DOI] [PubMed] [Google Scholar]
- 6.Zhou Z, Zhu G, Hariri AR, Enoch MA, Scott D, Sinha R, et al. Genetic variation in human NPY expression affects stress response and emotion. Nature. 2008;452:997–1001. doi: 10.1038/nature06858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morgan CA, Rasmusson A, Wang S, Hoyt G, Hauger RL, Hazlett G. Neuropeptide Y, cortisol, and subjective distress in humans exposed to acute stress: Replication and extension of previous report. Biol Psychiatry. 2002;52:136–142. doi: 10.1016/s0006-3223(02)01319-7. [DOI] [PubMed] [Google Scholar]
- 8.Morgan CA, Wang S, Southwick SM, Rasmusson A, Hazlett G, Hauger RL, Charney DS. Plasma neuropeptide Y concentrations in humans exposed to military survival training. Biol Psychiatry. 2000;47:902–909. doi: 10.1016/s0006-3223(99)00239-5. [DOI] [PubMed] [Google Scholar]
- 9.Rasmusson AM, Hauger RL, Morgan CA, Bremner JD, Charney D, Southwick SM. Low baseline and yohimbine-stimulated plasma neuropeptide Y (NPY) levels in combat-related PTSD. Biol Psychiatry. 2000;47:526–539. doi: 10.1016/s0006-3223(99)00185-7. [DOI] [PubMed] [Google Scholar]
- 10.Yehuda R, Brand S, Yang R-K. Plasma neuropeptide Y concentrations in combat exposed veterans: Relationship to trauma exposure, recovery from PTSD, and coping. Biol Psychiatry. 2006;59:660–663. doi: 10.1016/j.biopsych.2005.08.027. [DOI] [PubMed] [Google Scholar]
- 11.Morgan CA, 3rd, Rasmusson AM, Winters B, Hauger RL, Morgan J, Hazlett G, Southwick SM. Trauma exposure rather than posttraumatic stress disorder is associated with reduced baseline plasma neuropeptide-Y levels. Biol Psychiatry. 2003;54:1087–1091. doi: 10.1016/s0006-3223(03)00433-5. [DOI] [PubMed] [Google Scholar]
- 12.Dotsch J, Adelmann M, Englaro P, Dotsch A, Hanze J, Blum WF, et al. Relation of leptin and neuropeptide Y in human blood and cerebrospinal fluid. J Neurol Sci. 1997;151:185–188. doi: 10.1016/s0022-510x(97)00116-0. [DOI] [PubMed] [Google Scholar]
- 13.Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, et al. The development of a Clinician-Administered PTSD scale. J Trauma Stress. 1995;8:75–90. doi: 10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- 14.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1962;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morris MJ, Hastings JA, Pavia JM. Central interactions between noradrenaline and neuropeptide Y in the rat: Implications for blood pressure control. Clin Exp Hypertens. 1997;19:619–630. doi: 10.3109/10641969709083174. [DOI] [PubMed] [Google Scholar]
- 16.Sajdyk TJ, Shekhar A, Gehlert DR. Interactions between NPY and CRF in the amygdala to regulate emotionality. Neuropeptides. 2004;38:225–234. doi: 10.1016/j.npep.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 17.Baker DG, West SA, Nicholson WE, Ekhator NN, Kasckow JW, Hill KK, et al. Serial CSF corticotropin-releasing hormone levels and adrenocortical activity in combat veterans with posttraumatic stress disorder. Am J Psychiatry. 1999;156:585–588. doi: 10.1176/ajp.156.4.585. [DOI] [PubMed] [Google Scholar]
- 18.Geracioti TD, Jr, Baker DG, Ekhator NN, West SA, Hill KK, Bruce AB, et al. CSF norepinephrine concentrations in posttraumatic stress disorder. Am J Psychiatry. 2001;158:1227–1230. doi: 10.1176/appi.ajp.158.8.1227. [DOI] [PubMed] [Google Scholar]
- 19.Hill KK, West SA, Ekhator NN, Bruce AB, Wortman MD, Baker DG, Geracioti TD., Jr The effect of lumbar puncture stress on dopamine and serotonin metabolites in human cerebrospinal fluid. Neurosci Lett. 1999;276:25–28. doi: 10.1016/s0304-3940(99)00778-8. [DOI] [PubMed] [Google Scholar]
- 20.Hallschmid M, Benedict C, Born J, Fehm HL, Kern W. Manipulating central nervous mechanisms of food intake and body weight regulation by intranasal administration of neuropeptides in man. Physiol Behav. 2004;83:55–64. doi: 10.1016/j.physbeh.2004.07.023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.